Abstract
INTRODUCTION:
Data on small bowel abnormalities in patients with portal hypertension (PHT) are limited. Bleeding from the gastrointestinal tract and anemia are common complications in these patients. Capsule endoscopy (CE) was used to evaluate small bowel (SB) pathology in patients with PHT and anemia, and possible associations with various parameters were examined.
METHODS:
Thirty-five patients with PHT referred for CE investigation of the SB for anemia were prospectively enrolled in the study, as well as 70 age- and sex-matched control patients with anemia, normal liver function and no evidence of PHT who underwent CE.
RESULTS:
Findings compatible with portal hypertensive enteropathy (PHE) were detected in 65.7% of the patients and in 15.7% of the controls (χ2=26.641, P=0.000). Abnormalities in PHT patients included varices in 25.7%, diffuse changes of mucosa with inflammatory-like appearance in 42.9%, and angiodysplasias and/or spider angiomas in 22.9% of cases. The presence of PHE was significantly associated only with the presence of severe portal hypertensive gastropathy, while the presence of SB varices alone was significantly associated with the presence of severe portal hypertensive gastropathy, larger esophageal varices and the presence of colonic varices.
CONCLUSIONS:
Varices, diffuse changes of mucosa with inflammatory-like appearance, and angiodysplasias and/or spider angiomas are detected more often in patients with PHT than in controls, and probably constitute the endoscopic characteristics of PHE. CE of the SB added a significant number of likely important findings to those detected by conventional endoscopic techniques for the clinical management of patients with PHT and anemia.
Keywords: Capsule endoscopy, Portal hypertension, Small bowel
Abstract
INTRODUCTION :
Les données sur les anomalies de l’intestin grêle chez les patients souffrant d’hypertension portale (HTP) sont limitées. Les saignements du tractus digestif et l’anémie sont des complications fréquentes chez ces patients. L’endoscopie par capsule (EC) a permis d’é-valuer la pathologie du grêle chez des patients atteints d’HTP et les liens possibles avec divers paramètres ont été analysés.
MÉTHODE :
Trente-cinq patients atteints d’HTP adressés pour EC du grêle en raison d’anémie ont été inscrits de manière prospective à l’étude, de même que 70 patients témoins, assortis selon l’âge et le sexe, atteints d’anémie, mais ne présentant aucune dysfonction hépatique ni signe d’HTP et ayant subi une EC.
RÉSULTATS :
On a noté des signes d’entéropathie hypertensive portale (EHP) chez 65,7 % des patients et chez 15,7 % des témoins (χ2 = 26,641, p = 0,000). Les anomalies observées chez les patients atteints d’HTP incluaient : varices chez 25,7 %, anomalies diffuses de la muqueuse avec signes pseudo-inflammatoires chez 42,9 % et angiodysplasies et/ou angiomes stellaires chez 22,9 %. La présence d’EHP a été significativement associée uniquement à la présence de gastropathie hypertensive portale sévère, tandis que la présence de varices au niveau du grêle seulement a été significativement associée à la gastropathie hypertensive portale sévère, à la présence de varices œsophagiennes plus volumineuses et à la présence de varices au niveau du côlon.
CONCLUSION :
Les varices, les anomalies diffuses de la muqueuse d’aspect pseudo-inflammatoire et les angiodysplasies et/ou angiomes stellaires s’observent plus souvent chez les patients atteints d’HTP que chez les témoins et constituent probablement les caractéristiques endoscopiques de l’EHP. L’EC du grêle a révélé un nombre significatif de caractéristiques probablement importantes en plus des signes mis au jour par les techniques endoscopiques habituelles pour la prise en charge clinique des patients qui souffrent d’HTP et d’anémie.
The term portal hypertension (PHT) was first introduced by Gilbert and Carnot in 1902 for patients with ascites, splenomegaly and esophageal hemorrhage. Bleeding from the gastrointestinal tract is a common complication of PHT, and acute or chronic blood loss may contribute to the appearance of anemia, a common hematological complication in these patients. Gastroesophageal varices can be found in up to 70% of patients with cirrhosis and 30% of these varices will bleed within two years of diagnosis (1). Portal hypertensive gastropathy (PHG), first described in 1985 (2), accounts for 10% to 20% of acute bleeding, but it has been mainly identified as a cause of chronic blood loss. Nonvariceal bleeding from peptic ulcers, gastric erosions and the Mallory-Weiss syndrome is not uncommon. Less common and less significant causes of blood loss are hemorrhoids and portal hypertensive colopathy (PHC), a term comprising anorectal varices, spider angiomas and inflammatory changes with or without spontaneous mucosal bleeding (3–7).
Data on small bowel (SB) abnormalities in patients with portal hypertensive enteropathy (PHE) are limited, due to the difficulty of exploring the whole length of the SB (2,8–12). In view of the scarcity of information, we conducted the present study and used capsule endoscopy (CE) to evaluate SB pathology in patients with PHT. This novel method is well tolerated and allows complete visual investigation of the SB (13,14). Moreover, the diagnostic yield of CE in anemia is well documented and significantly higher than that of any other method, including push enteroscopy, small bowel follow-through, computed tomography, angiography, colonoscopy and gastroscopy (15). To our knowledge, there is only one study published in this field (16).
PATIENTS AND METHODS
The present prospective study was conducted from April 2004 until December 2007, and included consecutive patients with PHT and iron deficiency anemia referred to the General Hospital of Athens ‘Helena Venizelou’ (Athens, Greece) for SB investigation with CE. An open-access system was followed (ie, any physician who wished to refer a patient to the hospital could do so. All patients had been recently investigated with esophagogastroduodenoscopy (EGD) and colonoscopy. Exclusion criteria were congestive heart disease, liver transplantation, hepatocellular carcinoma, history of abdominal surgery and the use of acetylsalicylic acid or nonsteroidal anti-inflammatory drugs.
Patients’ clinical characteristics, including sex, age, etiology of PHT, Child-Pugh score, prior history of upper gastrointestinal and variceal bleeding, and endoscopic intervention (sclerotherapy or ligation), were recorded. Biochemical test results (ferrum, ferritin, liver function), prothrombin time and hemoglobin levels were obtained. In EGD, esophageal varices were graded according to the system proposed by the North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices (17), and congestive gastropathy was classified according to McCormack et al (2).
Age- and sex-matched patients with anemia, negative EGD and colonoscopy, normal liver function and no evidence of PHT who were investigated with CE during the same period, were used as controls for the interpretation of findings.
The M2A capsule (Given Imaging, Israel) was used in the present study. Contraindications for the CE procedure were the generally accepted contraindications described previously (18). Written informed consent was obtained in all cases. Patients’ preparation and CE procedure followed the generally recommended guidelines (19). All patients were advised to abstain from solid food on the day before the procedure and to ingest a 1 L solution of polyethylene glycol. Prokinetic medications were not administered. Nine hours after ingestion, the sensory array and recording device were removed.
CE videos were studied and all SB abnormal findings were recorded. A careful search was performed for signs of PHE, which, according to Misra et al (12), include diffuse hyperemia and edema, spider angiomata, patchy hyperemia and severe acute enteritis with spontaneous bleeding from the mucosa. SB varices were also considered as part of the spectrum of PHE.
For the interpretation of CE results, a single gastroenterologist initially screened all videos and selected images of potential abnormalities. Then, two gastroenterologists experienced in interpreting CE independently reviewed the selected images. All videos were extensively discussed; findings identified by both reviewers were considered as definitive and were included in the report. The procedure was defined as complete or incomplete depending on the passage of the capsule into the cecum throughout the duration of the examination.
Then, a search was conducted for any association between the presence of PHE and the following parameters: sex, age, etiology of cirrhosis, Child-Pugh class, size of esophageal varices, presence and severity of PHG, history of upper gastrointestinal bleeding, history of endoscopic intervention on esophageal varices and presence of colonic varices or PHC.
Statistical analysis
The SPSS program, version 13.0 (SPSS Inc, USA) was used for statistical analysis. Continuous data with normal distributions are presented as mean ± SEM. Differences between groups were evaluated by the χ2 test or Fisher’s exact test for qualitative variables. Student’s t test was used to compare quantitive variables. P<0.05 was considered to be statistically significant.
RESULTS
A total of 35 patients fulfilled the inclusion criteria during the study period. There were 33 cirrhotic patients and two patients with portal vein thrombosis. The control group consisted of 70 patients. Demographic and clinical characteristics of patients, as well as findings of upper and lower gastrointestinal tract endoscopies are listed in Table 1.
TABLE 1.
Demographic, clinical and endoscopic characteristics of patients and controls
| Characteristic | Patients with PHT | Controls |
|---|---|---|
| Patients, n | 35 | 70 |
| Male:female | 28:7 | 56:14 |
| Age, years, mean ± SEM | 53.0±1.7 | 52.9±1.1 |
| Hemoglobin, g/L, mean ± SEM | 81.0±0.2 | 84.0±0.1 |
| Cirrhosis/portal vein thrombosis, n | 33/2 | 0 |
| Etiology of cirrhosis, n (%) | NA | |
| Alcohol | 15 (45.4) | |
| Hepatitis C | 7 (21.2) | |
| Hepatitis B | 6 (18.2) | |
| Cryptogenic | 3 (9.1) | |
| Primary biliary cirrhosis | 2 (6.1) | |
| Child-Pugh class, n (%) | NA | |
| A | 8 (24.2) | |
| B | 17 (51.6) | |
| C | 8 (24.2) | |
| History of upper GI bleeding, n (%) | 19 (54.3) | 10 (14.3) |
| Variceal bleeding | 13 (37.1) | 0 |
| Nonvariceal bleeding | 6 (17.1) | 10 (14.3) |
| History of endoscopic intervention, n (%) | 14 (40.0) | NA |
| Endoscopic findings of upper and lower GI tract related to PHT | NA | |
| Esophageal varices, n (%) | 30 (85.7) | |
| Small | 10 | |
| Medium | 18 | |
| Large | 2 | |
| Gastric varices, n (%) | 5 (14.3) | |
| Duodenal varices, n (%) | 1 (2.9) | |
| Portal gastropathy, n (%) | 28 (80.0) | |
| Mild | 16 | |
| Severe | 12 | |
| Colonic varices, n (%) | 9 (25.7) | |
| Portal colopathy, n (%) | 16 (45.7) | |
Some patients showed more than one finding. GI Gastrointestinal; NA Not applicable; PHT Portal hypertension
All patients completed the procedure uneventfully. No cases of capsule retention were observed and the exit of the capsule was confirmed in all cases. Complete visualization of the SB was achieved in 29 of 35 patients with PHT and in 55 of 70 controls (χ2=0.268, degrees of freedom =1, P=0.605). Causes of failure of the capsule to reach the colon within the recording time among patients with PHT were slow gastric passage (n=1), presence of food that impaired capsule progression (n=1) and no clear reason (n=4). Gastric emptying time ranged from 4 min to 198 min (median 13 min) and SB transit time ranged from 139 min to 438 min (median 278 min).
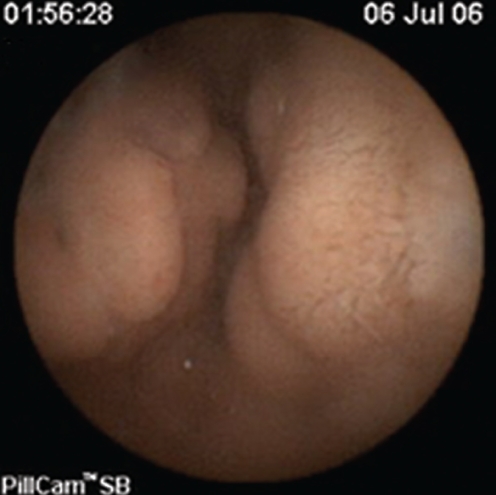
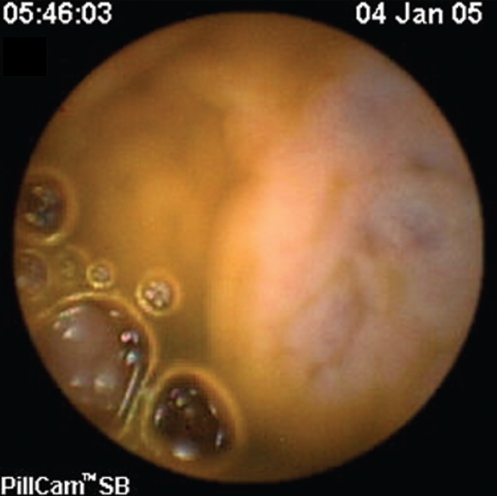
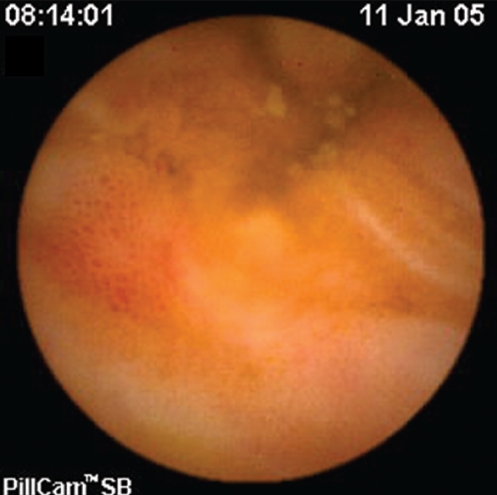
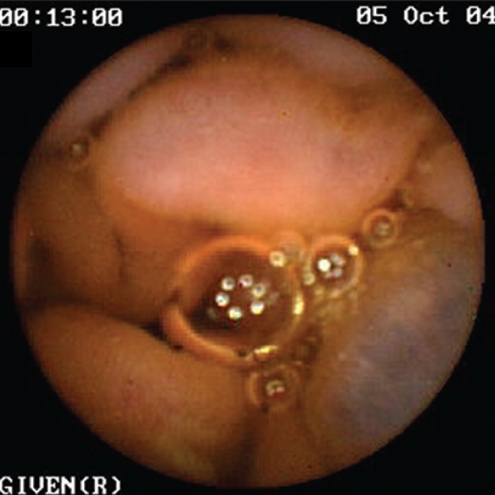
SB findings detected by CE in both patients and controls are listed in Table 2. Twenty-three of the 35 patients with PHT (65.7%) were found to have signs of PHE. SB varices (Figures 1 and 2) were evident in nine of 35 patients (25.7%), diffuse changes of mucosa with inflammatory-like appearance in 15 of 35 (42.9%) (Figures 3 and 4), and angiodysplasias and/or spider angiomas in eight of 35 patients (22.9%). In eight patients with PHT, CE revealed more than one abnormality. The jejunum and ileum were equally involved. In the control group, no patient had evidence of SB varices, while diffuse changes of the mucosa with inflammatory-like appearance were revealed in three patients (4.3%) and angiodysplastic lesions were revealed in eight (11.4%). Findings compatible with PHE were detected in 65.7% of the patients and in 15.7% of the controls (χ2=26.641, P=0.000).
TABLE 2.
Small bowel findings detected by capsule endoscopy
| Small bowel findings compatible with portal hypertension | Patients with portal hypertension (n=35) | Controls (n=70) | Statistics |
|---|---|---|---|
| Varices, n (%) | 9 (25.7) | 0 (0) | χ2=19.688,
df=1, P=0.000 |
| Duodenum | 1 | ||
| Jejunum | 3 | ||
| Ileum | 4 | ||
| Jejunum and ileum | 1 | ||
| Diffuse changes of mucosa with inflammatory-like appearance, n (%) | 15 (42.9) | 3 (4.3) | χ2=24.440,
df=1, P=0.000 |
| Duodenum | 1 | 0 | |
| Jejunum | 5 | 1 | |
| Ileum | 3 | 2 | |
| Jejunum and ileum | 6 | 0 | |
| Spider angiomas and/or angiodysplasias, n (%) | 8 (22.9) | 8 (11.4) | χ2=2.360,
df=1, P=0.125 |
| Jejunum | 3 | 4 | |
| Ileum | 2 | 2 | |
| Jejunum and ileum | 3 | 2 | |
| Total, n (%) | 23 (65.7) | 11 (15.7) | χ2=26.641,
df=1, P=0.000 |
| Other small bowel findings, n (%) | NA | ||
| Scalloping, fissures | 0 (0) | 1 (1.4) | |
| Single ulcer | 0 (0) | 2 (2.9) | |
| Ulceration – cobblestoning 0 (0) | 1 (1.4) | ||
| Multiple diverticulae | 0 (0) | 1 (1.4) | |
| Submucosal mass | 0 (0) | 2 (2.9) | |
| Polyp/tumour | 0 (0) | 2 (2.9) |
Eight patients with portal hypertension showed more than one finding. df Degrees of freedom; NA Not applicable
Figure 1).
Jejunal varix in a patient with portal hypertension
Figure 2).
Ileal varix in a patient with portal hypertension
Figure 3).
Portal hypertensive enteropathy in the ileum of a patient with portal hypertension
Figure 4).
Portal hypertensive enteropathy and a varix in the jejunum of a patient with portal hypertension
The presence of PHE was significantly associated only with the presence of severe PHG. No association was found with sex, age, etiology of PHT, Child-Pugh score, history of upper gastrointestinal bleeding, size of esophageal varices, history of endoscopic intervention, PHC and colonic varices (Table 3). Considering SB varices separately, their presence was significantly associated with the presence of severe PHG, larger esophageal varices and colonic varices (Table 4).
TABLE 3.
Factors associated with portal hypertensive enteropathy
| Factor | χ2 | df | P |
|---|---|---|---|
| Sex | 0.127 | 1 | 1.000 |
| Age | t=0.334 | 33 | 0.740 |
| Etiology of cirrhosis | 5.507 | 4 | 0.239 |
| Child-Pugh class | 0.017 | 2 | 0.991 |
| Size of esophageal varices | 5.213 | 3 | 0.157 |
| Grade of PHG | 7.947 | 2 | 0.019 |
| History of upper GI bleeding | 0.135 | 1 | 0.713 |
| History of endoscopic intervention | 0.338 | 1 | 0.721 |
| Colonic varices | 0.783 | 1 | 0.450 |
| PHC | 1.128 | 1 | 0.288 |
df Degrees of freedom; GI Gastrointestinal; PHC Portal hypertensive colopathy; PHG Portal hypertensive gastropathy
TABLE 4.
Factors associated with small bowel varices
| Factor | χ2 | df | P |
|---|---|---|---|
| Sex | 0.598 | 1 | 0.648 |
| Age | t=1.541 | 33 | 0.153 |
| Etiology of cirrhosis | 1.082 | 4 | 0.897 |
| Child-Pugh class | 0.850 | 2 | 0.654 |
| Size of esophageal varices | 9.348 | 3 | 0.025 |
| Grade of PHG | 6.534 | 2 | 0.038 |
| History of upper GI bleeding | 0.008 | 1 | 1.000 |
| History of endoscopic intervention | 3.590 | 1 | 0.112 |
| Colonic varices | 5.648 | 1 | 0.030 |
| PHC | 2.143 | 1 | 0.245 |
df Degrees of freedom; GI Gastrointestinal; PHC Portal hypertensive colopathy; PHG Portal hypertensive gastropathy
DISCUSSION
Increased resistance to portal blood flow due to liver cirrhosis or thrombosis of the portal vein leads to PHT. PHT results in the development of gastroesophageal and/or ectopic (colonic, enteric) varices, as well as other mucosal lesions in the stomach, small intestine and colon. They are referred to under the term ‘portal hypertensive intestinal vasculopathy’ (including PHG, PHC and PHE) (10,20). While PHG and PHC are the commonly recognized components of the spectrum, data on PHE are limited. There are only a few reports on PHE concerning the duodenum, the upper jejunum, and the terminal ileum (9–12,21). This lack of information reflects the inability to examine the SB in the era before CE introduction in clinical practice. The above-mentioned studies were based on partial endoscopic examination with push enteroscopy and ileocolonoscopy and, in some cases, on histology.
Although there are occasional reports on SB lesions in patients with PHT, their true prevalence, as well as their implication in overt and occult bleeding are unknown (9–12,22–27). In the present study, we used CE to investigate the whole length of the SB in patients with PHT and anemia. Our main objective was to search for SB findings that could be of potential clinical significance among patients with PHT. Signs of PHE – varices, diffuse changes of mucosa with inflammatory-like appearance, and angiodysplasias and/or spider angiomas – were found in65.7% of our patients. This figure is in accordance with a study reported by De Palma et al in 2005 (16). These endoscopic findings were more common among patients with PHT than among controls. The increased prevalence of SB varices in our study, compared with the study of De Palma et al (25.7% versus 8.1%), could be possibly explained by the presence of esophageal varices in the vast majority of our patients (85.7% versus 32.4%).
Other studies confirm the presence of PHE, but the reported incidence rates are conflicting. Pennazio et al (28), in a study published recently in abstract form, reported a 10% incidence of SB varices (also detected with CE) in a small number of cirrhotic patients. On the other hand, Misra et al (12) reported a high incidence (18%) of ileal varices in patients with PHT using ileocolonoscopy, and considered that this is a probable underestimation, because they examined only a short segment of the ileum (12).
Diffuse changes of SB mucosa with inflammatory-like appearance (erythema, edema, granularity and/or friability) as part of the spectrum of PHE, were predominant among our patients (42.9%), followed by SB varices (25.7%) and spider angiomas or angiodysplasias (22.9%). These diffuse lesions probably reflect mucosal alterations similar to that of the gastric mucosa in patients with PHG (21).
Based on the findings of this and the previously mentioned studies, the endoscopic characteristics of PHE are well documented. Nevertheless, the question of whether they can be the cause of overt or occult bleeding in patients with PHT remains unanswered. Our study included patients with anemia only and, consequently, failed to identify actively bleeding lesions. The assumption that PHE lesions are implicated in gastrointestinal bleeding is based on reports of bleeding SB varices and other PHE lesions (11,22–27,29,30), as well as on the knowledge that pathogenetically similar lesions, like esophagogastric or colonic varices, PHG and PHC, are well-established causes of bleeding.
PHE affected both the jejunum and the ileum equally, and a significant number of findings were beyond the reach of conventional endoscopic techniques, including push enteroscopy. Although, theoretically, these lesions could be diagnosed by means of double-balloon enteroscopy, this novel method is not yet widely available and is clearly more invasive. The safe and well-tolerated CE technique is currently the preferable method to image areas of the gut such as the distal jejunum and the proximal ileum.
We examined possible associations between PHE and various parameters, and found that the presence of PHE was significantly associated only with the severity of PHG. No association was found with sex, age, etiology of PHT, Child-Pugh score, history of upper gastrointestinal bleeding, size of esophageal varices, history of endoscopic intervention, PHC and colonic varices. The absence of any association between PHE and Child-Pugh class was a surprise to us, although both Misra et al (12) and Pennazio et al (18) found the same. We have also been surprised by the lack of association with history of endoscopic intervention of esophageal varices, because it is well known that variceal ligation and sclerotherapy are predisposing factors for PHG and ectopic varices formation (31,32). SB varices were more common in patients with severe PHG. They were also significantly associated with the presence of larger esophageal varices and colonic varices. Although the pathophysiological basis for, and the clinical importance of these findings needs to be further explored, we can conclude that PHE represents the enteric manifestations of PHT, and that it shares a common pathogenesis with PHG and esophageal varices.
CONCLUSIONS
The present study reinforces the views that the SB in patients with PHT manifests macroscopically similar changes as the rest of the gut and that these changes (varices and mucosal alterations) most probably share, as a common pathophysiological mechanism, the impaired enteric venous drainage through the portal system. CE of the SB proved to be a useful tool in the investigation of patients with PHT and anemia, and added a significant number of likely important findings to those detected by conventional endoscopic techniques. Our findings did not alter the ongoing management of the patients (ie, treatment with beta-blockers and conventional endoscopic follow-up) because we did not identify patients with active bleeding. However, in case of such a finding, the next step would be to perform push enteroscopy or double-balloon enteroscopy and provide endoscopic treatment for the bleeding lesion. Additionally, patients with already identified PHE lesions could be treated accordingly in the case of a future episode of obscure gastrointestinal bleeding (negative upper and lower endoscopy).
REFERENCES
- 1.Bornman PC, Krige JE, Terblanche J. Management of oesophageal varices. Lancet. 1994;343:1079–84. doi: 10.1016/s0140-6736(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 2.McCormack TT, Sims J, Eyre-Brook I, et al. Gastric lesions in portal hypertension: Inflammatory gastritis or congestive gastropathy? Gut. 1985;26:1226–32. doi: 10.1136/gut.26.11.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozarek RA, Botoman VA, Bredfeldt JE, Roach JM, Patterson DJ, Ball TJ. Portal colopathy: Prospective study of colonoscopy in patients with portal hypertension. Gastroenterology. 1991;101:1192–7. doi: 10.1016/0016-5085(91)90067-u. [DOI] [PubMed] [Google Scholar]
- 4.Chen LS, Lin HC, Lee FY, Hou MC, Lee SD. Portal hypertensive colopathy in patients with cirrhosis. Scand J Gastroenterol. 1996;31:490–4. doi: 10.3109/00365529609006770. [DOI] [PubMed] [Google Scholar]
- 5.Hosking SW, Smart HL, Johnson AG, Triger DR. Anorectal varices, haemorrhoids and portal hypertension. Lancet. 1989;1:349–52. doi: 10.1016/s0140-6736(89)91724-8. [DOI] [PubMed] [Google Scholar]
- 6.Ganguly S, Sarin SK, Bhatia V, Lahoti D. The prevalence and spectrum of colonic lesions in patients with cirrhotic and noncirrhotic portal hypertension. Hepatology. 1995;21:1226–31. [PubMed] [Google Scholar]
- 7.Misra SP, Dwivedi M, Misra V. Prevalence and factors influencing hemorrhoids, anorectal varices and colopathy in patients with portal hypertension. Endoscopy. 1996;28:340–5. doi: 10.1055/s-2007-1005477. [DOI] [PubMed] [Google Scholar]
- 8.Tarnawski A, Sarfeh IJ, Stachura J, et al. Microvascular abnormalities of the portal hypertensive gastric mucosa. Hepatology. 1988;8:1488–94. doi: 10.1002/hep.1840080604. [DOI] [PubMed] [Google Scholar]
- 9.Nagral AS, Joshi AS, Bhatia SJ, Abraham P, Mistry FP, Vora IM. Congestive jejunopathy in portal hypertension. Gut. 1993;34:694–7. doi: 10.1136/gut.34.5.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viggiano TR, Gostout CJ. Portal hypertensive intestinal vasculopathy: A review of the clinical, endoscopic, and histopathologic features. Am J Gastroenterol. 1992;87:944–54. [PubMed] [Google Scholar]
- 11.Ohtani T, Kajiwara E, Suzuki N, et al. Ileal varices associated with recurrent bleeding in a patient with liver cirrhosis. J Gastroenterol. 1999;34:264–8. doi: 10.1007/s005350050255. [DOI] [PubMed] [Google Scholar]
- 12.Misra SP, Dwivedi M, Misra V, Gupta M. Ileal varices and portal hypertensive ileopathy in patients with cirrhosis and portal hypertension. Gastrointest Endosc. 2004;60:778–83. doi: 10.1016/s0016-5107(04)02049-8. [DOI] [PubMed] [Google Scholar]
- 13.Meron GD. The development of the swallowable video capsule (M2A) Gastrointest Endosc. 2000;52:817–9. doi: 10.1067/mge.2000.110204. [DOI] [PubMed] [Google Scholar]
- 14.Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 15.Friedman S. Comparison of capsule endoscopy to other modalities in small bowel. Gastrointest Endosc Clin N Am. 2004;14:51–60. doi: 10.1016/j.giec.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 16.De Palma G, Rega M, Masone S, et al. Mucosal abnormalities in patients with cirrhosis and portal hypertension: A capsule endoscopy study. Gastrointest Endosc. 2005;62:529–34. doi: 10.1016/s0016-5107(05)01588-9. [DOI] [PubMed] [Google Scholar]
- 17.Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices A prospective multicenter study. The North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. N Engl J Med. 1988;319:983–9. doi: 10.1056/NEJM198810133191505. [DOI] [PubMed] [Google Scholar]
- 18.Mishkin DS, Chuttani R, Croffie J, et al. Technology Assessment Committee American Society for Gastrointestinal Endoscopy ASGE: Technology Status Evaluation Report: Wireless capsule endoscopy. Gastrointest Endosc. 2006;63:539–45. doi: 10.1016/j.gie.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Gay G, Delvaux M, Rey JF. The role of video capsule endoscopy in the diagnosis of digestive diseases: A review of current possibilities. Endoscopy. 2004;36:913–20. doi: 10.1055/s-2004-825868. [DOI] [PubMed] [Google Scholar]
- 20.Marrero R, Barkin JS. Wireless capsule endoscopy and portal hypertensive intestinal vasculopathy. Gastrointest Endosc. 2005;62:535–7. doi: 10.1016/j.gie.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 21.Misra V, Misra SP, Dwivedi M, Gupta SC. Histomorphometric study of portal hypertensive enteropathy. Am J Clin Pathol. 1997;108:652–7. doi: 10.1093/ajcp/108.6.652. [DOI] [PubMed] [Google Scholar]
- 22.Lewis P, Warren BF, Bartolo DC. Massive gastrointestinal haemorrhage due to ileal varices. Br J Surg. 1990;77:1277–8. doi: 10.1002/bjs.1800771126. [DOI] [PubMed] [Google Scholar]
- 23.Arst HF, Reynolds JD. Acute ileal variceal hemorrhage secondary to esophageal sclerotherapy. J Clin Gastroenterol. 1986;8:603–4. doi: 10.1097/00004836-198610000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Hojhus JH, Pedersen SA. Cirrhosis and bleeding ileal varices without previous intraabdominal surgery. A case report. Acta Chir Scand. 1986;152:479–80. [PubMed] [Google Scholar]
- 25.Kobayashi K, Yamaguchi J, Mizoe A, et al. Successful treatment of bleeding due to ileal varices in a patient with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2001;13:63–6. doi: 10.1097/00042737-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Varanasi RV, Fleisher AS, Darwin PE, King CE, Haluszka O. Colonoscopic sclerotherapy of ileal varices. Gastrointest Endosc. 2000;52:109–11. doi: 10.1067/mge.2000.106538. [DOI] [PubMed] [Google Scholar]
- 27.Guth E, Katz MD, Hanks SE, Teitelbaum GP, Ralls P, Korula J. Recurrent bleeding from ileal varices treated by transjugular intrahepatic shunt: Value of Doppler ultrasonography in diagnosis and follow-up. J Ultrasound Med. 1996;15:67–9. [PubMed] [Google Scholar]
- 28.Pennazio M, Repici A, Ottobrelli A, Barbon V, De Lio A, Rizzetto M. Capsule endoscopy in cirrhotic patients: Prevalence and spectrum of small bowel lesions. Proceedings of the 4th International Conference on Capsule Endoscopy; Miami. 2005. March 7 to 8, 2005. (Abst) [Google Scholar]
- 29.Tang SJ, Zanati S, Dubcenco E, et al. Diagnosis of small-bowel varices by capsule endoscopy. Gastrointest Endosc. 2004;60:129–35. doi: 10.1016/s0016-5107(04)01458-0. [DOI] [PubMed] [Google Scholar]
- 30.Thiruvengadam R, Gostout CJ. Congestive gastroenteropathy – an extension of nonvariceal upper gastrointestinal bleeding in portal hypertension. Gastrointest Endosc. 1989;35:504–7. doi: 10.1016/s0016-5107(89)72898-4. [DOI] [PubMed] [Google Scholar]
- 31.Sarin SK, Govil A, Jain AK, et al. Prospective randomized trial of endoscopic sclerotherapy versus variceal band ligation for esophageal varices: Influence on gastropathy, gastric varices and variceal recurrence. J Hepatol. 1997;26:826–32. doi: 10.1016/s0168-8278(97)80248-6. [DOI] [PubMed] [Google Scholar]
- 32.Primignani M, Carpinelli L, Preatoni P, et al. Natural history of portal hypertensive gastropathy in patients with liver cirrhosis. The New Italian Endoscopic Club for the study and treatment of esophageal varices (NIEC) Gastroenterology. 2000;119:181–7. doi: 10.1053/gast.2000.8555. [DOI] [PubMed] [Google Scholar]