Abstract
BACKGROUND:
Physician nonadherence to colorectal cancer (CRC) screening recommendations contributes to underuse of screening.
OBJECTIVE:
To assess physicians’ knowledge of CRC screening guidelines for average-risk individuals, perceived barriers to screening and practice behaviours.
METHODS:
Between October 2004 and March 2005, staff physicians working in three university-affiliated hospitals in Montreal, Quebec, were surveyed. Self-administered questionnaires assessed knowledge of risk classification and current guidelines for average-risk individuals, as well as perceptions of barriers to screening and practice behaviours.
RESULTS:
All 65 invited physicians participated in the survey, including 46 (70.8%) family medicine physicians and 19 (29.2%) general internists. Most physicians knew that screening should begin at 50 years of age, all knew to screen men and women and 92% said they screened average-risk patients. Fifty-seven (87.7%) physicians correctly identified three common characteristics associated with high risk for developing CRC. Physicians who screened average-risk patients preferred fecal occult blood testing (88.3%) and colonoscopy (88.3%) to flexible sigmoidoscopy (10.0%) and double-contrast barium enema (30.0%). Most physicians knew the correct screening periodicity for fecal occult blood testing (87.6%), but only 40% or fewer could identify correct screening periodicities for the other modalities. Barriers and facilitators focused on health care delivery system improvements, better evidence on which to base recommendations and development of practical screening modalities.
CONCLUSIONS:
Physicians lacked knowledge of the recommended screening modalities and periodicities to appropriately screen average-risk individuals. Because CRC screening can reduce mortality, efforts to improve physician delivery should focus on physician knowledge and changes to the health care delivery system.
Keywords: Colorectal cancer, Guidelines, Primary care, Screening
Abstract
HISTORIQUE :
La non-adhésion des médecins aux recommandations de dépistage du cancer colorectal (CCR) contribue à une sous-utilisation du dépistage.
OBJECTIF :
Évaluer les connaissances des lignes directrices du dépistage du CCR par le médecin pour les personnes à risque moyen, les obstacles perçus au dépistage et les comportements de pratique.
MÉTHODOLOGIE :
Entre octobre 2004 et mars 2005, une enquête a été menée auprès des médecins de trois hôpitaux universitaires de Montréal, au Québec. Des questionnaires auto-administrés ont permis d’évaluer les connaissances de la classification des risques et les lignes directrices à jour pour les personnes à risque moyen, ainsi que leurs perceptions des obstacles au dépistage et leurs comportements de pratique.
RÉSULTATS :
Les 65 médecins invités ont participé à l’enquête, soit 46 (70,8 %) médecins de famille et 19 (29,2 %) internistes. La plupart des médecins savaient que le dépistage devrait commencer à 50 ans, tous savaient qu’il fallait procéder au dépistage à la fois chez les hommes et chez les femmes, et 92 % ont déclaré dépister les patients à risque moyen. Cinquante-sept (87,7 %) médecins ont pu nommer trois caractéristiques courantes associées à un risque élevé de CCR. Les médecins qui procédaient au dépistage des patients à risque moyen préféraient la recherche de sang occulte dans les selles (88,3 %) et la coloscopie (88, 3 %) à la sigmoïdoscopie flexible (10,0 %) et au lavement baryté à double contraste (30,0 %). La plupart des médecins connaissaient la périodicité du dépistage par repérage de sang occulte dans les selles (87,6 %), mais seulement 40 % ou moins pouvaient indiquer la périodicité du dépistage par les autres tests. Les obstacles et les catalyseurs étaient axés sur les améliorations aux systèmes de prestation des soins, de meilleurs éléments probants sur lesquels fonder les recommandations et l’élaboration de modalités de dépistage pratiques.
CONCLUSIONS :
Les médecins n’étaient pas assez au courant des modalités de dépistage recommandées et de la périodicité convenable du dépistage des personnes à risque moyen. Puisque le dépistage du CCR peut réduire le taux de mortalité, les efforts en vue d’améliorer leur utilisation par le médecin devraient être orientés sur les connaissances des médecins et les modifications au système de prestation des soins.
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in men and women and a leading cause of cancer deaths in industrialized countries (1–5). Screening for CRC reduces its incidence through removal of premalignant polyps, and CRC morbidity and mortality through early detection and treatment (6–9). Current Canadian guidelines recommend fecal occult blood testing (FOBT) every other year as a primary screening for CRC in average-risk individuals (50 years of age and older who are without risk factors for the development of CRC and do not have any personal or family history of colon polyps or CRC) (10–12). American guidelines make different recommendations for the same group of patients. They suggest annual FOBT, flexible sigmoidoscopy (FS) every five years, double-contrast barium enema (DCBE) every five years or colonoscopy every 10 years (13–15). The Quebec Association of Gastroenterology recommends DCBE every five to 10 years or colonoscopy every 10 years, further confusing the issue. Despite the proliferation of CRC screening guidelines or perhaps because of the lack of consensus, only an estimated 10% to 50% of the eligible population have been screened (16–20). Given that primary care practices are major sites for providing health promotion and screening services, some of the underutilization may be attributable to physician nonadherence to CRC screening guidelines.
In the absence of a mass screening program, individuals rely on their physicians to provide CRC screening. Studies consistently find associations between increased CRC screening and receipt of a physician’s recommendation to patients for CRC screening, as well as having health care coverage or a regular physician (21–29). Yet, although physicians’ self-report to offering CRC screening to 36% to 90% of their eligible patients (4,30,31) when patient medical chart data are examined, the proportion of screen-eligible patients who actually received documented screening is overestimated (4,30). For example, 74% primary care physicians in Alberta recommended that asymptomatic patients undergo screening but only 36% actually offered screening to at least 75% of their average-risk patients (30). Physician underuse of CRC screening may be a result of the perception that the evidence to support screening is inconclusive (32), lack of familiarity with CRC screening guidelines (4,31,33,34), failure to routinely assess CRC risk (35), inappropriate use of CRC screening (33) or of procedures such as digital rectal examinations that are not recommended (36), or absence of a screening policy (32,37). Moreover, the physician’s decision to screen, as well as choice of screening modality is influenced by perceptions of barriers (31,38) such as scheduling difficulties for large bowel procedures (30,36), lack of consultation time (30,36), uncertainty about which modality to offer (30) and patient comorbidity (36,39).
Because CRC is one of the few cancers that can be cured when detected early (40), increased physician delivery of screening would lead to reductions in CRC incidence, morbidity and mortality. Therefore, the aim of the present study was to identify physicians’ knowledge of CRC risk classification and screening guidelines, their self-reported screening practice and the barriers and facilitators to implementing screening. The first step toward improving delivery of CRC screening is to document physicians’ knowledge of screening guidelines, practice behaviours, and perceived barriers and potential facilitators to screening.
METHODS
Recruitment
A purposeful sample of physicians working in three primary care outpatient clinics affiliated with McGill University (Montreal, Quebec) were approached to participate in the survey. Data collection occurred between October 2004 and March 2005 using a self-administered questionnaire. Most physicians completed the survey during staff meetings before an information session on CRC screening. Physicians not in attendance were approached by the study coordinator at a later date. Staff physicians affiliated with each study site were eligible for inclusion. Residents and physician assistants were excluded because they often see patients in consultation with staff physicians.
Survey instrument
The physician questionnaire was designed to provide information on knowledge of current CRC screening guidelines, perceived barriers to CRC screening and practice regarding CRC screening. The survey was pilot-tested on five medical residents and pretested on five physicians.
Risk stratification:
To successfully implement screening guidelines in average-risk patients, physicians needed to understand risk classification to discern average- from high risk. One question on the questionnaire asked physicians to select from a list of characteristics that would put individuals at high risk for CRC. Response choices included inflammatory bowel disease, irritable bowel syndrome, personal family history of CRC and familial polyposis.
Knowledge of guidelines:
Seven questions assessed clinicians’ knowledge of guidelines for average-risk individuals. These included, age when screening should begin (30, 35, 40, 45, 50, 55 or 60 years), who should be screened (men, women) and frequency of screening for digital rectal examination, FOBT, FS, DCBE and colonoscopy (every one, two, three, five or 10 years, or not appropriate).
Usual practice:
Physicians were asked whether they would recommend that average-risk patients undergo screening for CRC, and to indicate the screening modalities usually recommended (digital rectal examination, FOBT, FS, DCBE, colonoscopy or preference depending on patient status).
Barriers to screening:
For each of the four screening modalities currently recommended, physicians were asked to indicate the barriers that would prevent them from recommending the procedure. Potential barriers included uncertainty about the efficacy of screening, lack of training or experience, poor patient compliance, unavailability of equipment, scheduling difficulty, lack of consultation time, absence of practice nurses and patients with comorbid conditions.
Facilitators to screening:
One open-ended question asked physicians for three things that would make it easier for them to provide CRC screening to their patients.
RESULTS
Description of physicians
All 65 primary care physicians who were approached completed the survey. The sample included 46 (70.8%) family medicine (FM) physicians and 19 (29.2%) general internists (GIs) (Table 1). Thirty-seven (56.9%) were men and 28 (43.1%) were women; there was no difference in sex by specialty (P=0.2249). The mean number of years since graduation from medical school was 21.5 (SD=8.6) and ranged from three to 35 years.
TABLE 1.
Physician characteristics (n=65)
| Characteristic | Mean or frequency | SD or % |
|---|---|---|
| Sex | ||
| Female | 28 | 43.1 |
| Male | 37 | 56.9 |
| Discipline | ||
| Family medicine | 46 | 70.8 |
| Internal medicine | 19 | 29.2 |
| Years since graduation from medical school | 21.5 | 8.6 |
Risk status
Of the 65 physicians, most indicated that inflammatory bowel disease (92.3%), family history (96.9%) and familial polyposis (98.5%) influenced CRC risk classification. No one selected irritable bowel syndrome. Fifty-seven (87.7%) physicians correctly identified the three common risk factors for developing CRC, inflammatory bowel disease, family history and familial polyposis. Knowledge of risk factors did not differ by physician specialty (all P>0.2621).
Guidelines
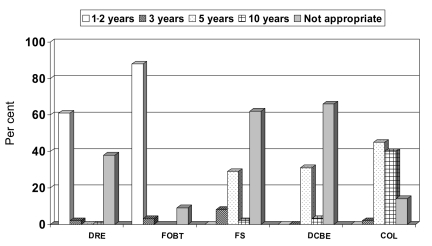
Most (90.6%) physicians correctly selected 50 years as the age at which to begin screening for CRC in average-risk individuals. All (100%) physicians were aware that both men and women should be screened. As seen in Figure 1, the majority (87.6%) of physicians correctly chose performance of FOBT every one or two years, two (3.1%) said it should be every three years and six (9.2%) indicated that it was not an appropriate screening method. For FS, 36.9% of physicians correctly indicated the periodicity was three or five years, one (1.5%) said 10 years and 40 (61.5%) physicians said it was not an appropriate screening method. Twenty-two (33.8%) physicians correctly chose every five or 10 years for performance of DCBE and 43 (66.2%) indicated that it was not appropriate. For colonoscopy, 26 (40%) physicians correctly reported an interval of 10 years, 30 (46.2%) said it should be performed more frequently and nine (13.8%) said it was not an appropriate screening modality for average-risk patients.
Figure 1).
Knowledge of recommended screening periodicities according to modality (n=65). COL Colonoscopy; DCBE Double-contrast barium enema; DRE Digital rectal examination (not recommended); FOBT Fecal occult blood test; FS Flexible sigmoidoscopy
Practice
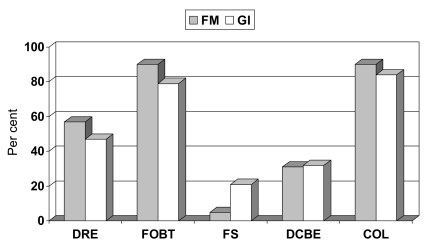
A total of 61 (93.8%) physicians reported screening average-risk patients. As seen in Figure 2, FOBT (88.3%) and colonoscopy (88.3%) were preferred to FS (10.0%) and DCBE (30.0%). Digital rectal examination was used by 32 (52.5%) physicians. Of the 61 physicians who screened average-risk patients, proportionally more GIs compared with FM physicians used FS (21.1% versus 4.8%, respectively, P=0.0694, Fisher’s exact test); although this difference did not reach statistical significance.
Figure 2).
Choice of colorectal cancer screening modalities for average-risk patients according to physician specialty (n=61). COL Colonoscopy; DCBE Double-contrast barium enema; DRE Digital rectal examination (not recommended); FM Family medicine physicians; FOBT Fecal occult blood test; FS Flexible sigmoidoscopy; GI General internists
Barriers
Physicians reported a mean of 5.9 (SD=3.7) barriers to providing or recommending the four currently accepted screening modalities. Table 2 shows the physicians’ responses to barriers to screening according to modality. Uncertainty about the efficacy of the modality was salient for FOBT, FS and DCBE. Lacking experience was relevant mainly for FS. Scheduling difficulties and comorbidity were major concerns for colonoscopy. Having sufficient consultation time was most important for colonoscopy. Concern about patient compliance was of similar importance for all modalities. Barriers were similar across physician specialty. Lack of equipment was of most concern for FS and colonoscopy; lack of practice nurse was of minimal importance for all modalites.
TABLE 2.
Perceived barriers to implementing screening recommendations (n=65)
| Barrier | FOBT n (%) | FS n (%) | DCBE n (%) | COL n (%) |
|---|---|---|---|---|
| Uncertainty about efficacy | 26 (40.0) | 28 (43.1) | 23 (35.4) | 6 (9.2) |
| Lack of experience | 1 (1.5) | 23 (35.4) | 7 (10.8) | 6 (9.2) |
| Patient compliance | 14 (21.5) | 12 (18.5) | 15 (23.1) | 17 (26.2) |
| Lack of equipment | 0 (0.00) | 12 (18.5) | 8 (12.3) | 12 (18.5) |
| Scheduling difficulties | 0 (0.00) | 15 (23.1) | 13 (20.0) | 32 (49.2) |
| Insufficient consultation time | 2 (3.1) | 9 (13.9) | 6 (9.2) | 17 (26.2) |
| Lack of practice nurse | 2 (3.1) | 3 (4.6) | 0 (0.00) | 1 (1.5) |
| Concern about comorbidity | 5 (7.7) | 14 (21.5) | 19 (29.2) | 37 (56.9) |
COL Colonoscopy; DCBE Double-contrast barium enema; FOBT Fecal occult blood test; FS Flexible sigmoidoscopy
Facilitators
When asked what would facilitate screening, physicians said they would like easier access to gastroenterologists (61.5%), having educational information to give to patients (23.1%), simpler screening modalities (21.5%), clearer guidelines (13.9%), more time to counsel patients about the various modalities (13.9%), reminder systems (10.8%), specialized screening clinics (10.8%) and better scientific evidence to support screening recommendations (7.7%). A larger proportion of FM physicians wanted clearer guidelines (19.6% versus 0%, P=0.0491) compared with GIs.
DISCUSSION
The present study of academic FM physicians and GIs assessed knowledge of risk classification and screening recommendations for individuals at average risk for developing CRC, screening practice, and perceived barriers and facilitators to screening. Nearly all of the physicians were familiar with the common risk factors for developing CRC, suggesting that recommended screening could be offered to the majority of average-risk patients seeking health care. Physicians were knowledgeable about who should receive CRC screening and at what age to begin; most said they screened average-risk patients. Digital rectal examination was commonly used to screen for CRC despite the lack of endorsement as a screening strategy, an approach also used in other studies (31,41). Of the recommended strategies, FOBT and colonoscopy were preferred to FS and DCBE. Most physicians knew the correct screening periodicity for FOBT. However, 9% indicated that it was not an appropriate screening method despite the evidence to support its efficacy at reducing mortality from CRC (7,8). Correct periodicities for FS and DCBE were reported by fewer than 40% of physicians, with the majority indicating these procedures were not appropriate screening methods. Of note, only 40% of physicians correctly indicated the appropriate periodicity for colonoscopy; 46.2% said it should be performed more frequently and nine (13.8%) said it was not an appropriate screening modality for average-risk individuals. Given that many physicians endorsed more frequent colonoscopic screening than is currently recommended, if translated into practice, this could unnecessarily expose average-risk patients to increased risk for severe consequences from colonoscopy (eg, bowel perforation, hemorrhage and death) and could also increase health care costs.
Perceived barriers that prevented physicians from providing or recommending CRC screening varied by modality. Some barriers suggested gaps in physician knowledge. For example, uncertainty about efficacy was important for FOBT and FS but not for colonoscopy. These findings are inconsistent with the scientific evidence in support of screening FOBT (7,8) and FS (42,43) in reducing mortality from CRC, and the lack of evidence for colonoscopy. Nonetheless, use of FS has declined while colonoscopy use has increased (44), owing to recent studies (45,46) and media coverage (47) documenting the advantages of colonoscopy, as well as the backing of organizations that develop the guidelines (48). Other barriers either were not or should not have been associated with reported practice behaviours. Physicians reported more barriers to colonoscopy than to other screening modalities even though colonoscopy was one of the two most commonly recommended screening modalities. In addition, lack of experience was an important barrier to providing FS but should not have prevented physicians from recommending it as a screening strategy because this examination is widely performed by gastroenterologists. Finally, approximately one-quarter of physicians reported that concern about patient compliance to all screening modalities prevented them from offering CRC screening, despite the inconsistent evidence that patient compliance is a barrier to CRC screening (8,45,49–51). These findings point to a need to educate physicians of the benefits of appropriate screening, that CRC is one of the few cancers that is detectable in the precancerous state and that early detection and treatment can reduce the incidence of morbidity and mortality from CRC.
Reported facilitators to screening provide insight to potential interventions that may increase physician adherence to screening recommendations. The main area targeted for improvement was the health care delivery system, with many physicians indicating that having better access to or availability of specialists, more consultation time to explain CRC screening, educational material for patients, reminder systems and specialized screening clinics would improve their ability to offer CRC screening. Some of these improvements would require additional resources to create new innovations (eg, reminder systems and screening clinics), while others would benefit from increased efficiency (eg, having nonmedical personnel to explain screening and available educational material). The second and third targets for improvement were the screening guidelines and modalities themselves, which need to be easier to follow for both physicians and their patients. Physicians expressed concern that guidelines need to be clear and consistent across organizations. In addition, they confirmed what is already known about screening in general – that the success of delivering screening rests on the characteristics of the examination, such as its availability, ease of administration and accurate test results (52). Given that none of the current screening modalities meets these criteria, the search for an optimal screening strategy is ongoing (53–55). In the meantime, changes targeted at office systems have shown to be effective at increasing rates of CRC screening (56).
The increasing awareness of the importance of CRC screening and the movement to establish mass screening programs may help to explain why greater proportions of physicians in the present study said they screened average-risk patients, knew the recommended age at which to begin screening, and knew the recommended periodicities for colonoscopy and DCBE compared with results from the previous surveys (30,37,41,57). Yet, CRC screening guidelines are only one of countless guidelines that primary care physicians need to know about and implement with their patients. For example, the National Guideline Clearinghouse web site (58) currently contains 1718 individual summaries. Primary care physicians may indeed feel overwhelmed by the amount of literature they need to integrate into practice. Clear and consistent CRC screening guidelines across organizations would instill greater confidence in the recommendations, as well as reduce the amount of information that physicians need to manage.
The present survey was part of a larger research project aimed at evaluating the determinants of CRC screening in primary care. One study strength is the 100% response rate that could be attributed in part to the fact that at least one influential physician at each study site was a coinvestigator on the project. These physicians not only contributed to the conceptualization and design of the study but also generated enthusiasm for participation among staff physicians. Study limitations worth mentioning were that all data were obtained by self-report and no attempts were made to compare physician responses with actual practice. In addition, generalizability of our findings may be limited because all participants were affiliated with one large, metropolitan university and may have been more knowledgeable of current CRC screening guidelines compared with physicians in rural and nonteaching hospitals.
CONCLUSION
Academic FM physicians and GIs demonstrated awareness of CRC guidelines but lacked specific knowledge about the recommended modalities and periodicities to correctly implement screening guidelines in average-risk patients. Of particular concern were physicians’ perceptions that FOBT and FS were not effective screening methods and the shorter-than-recommended periodicity for colonoscopy. Barriers and facilitators to providing CRC screening focused on health care delivery system improvements, better evidence upon which to base recommendations and development of practical screening modalities. Having guidelines that are consistent across organizations may promote physician uptake. In as much as CRC screening can reduce mortality, efforts to increase physician offers of screening should be aimed at physician knowledge and changes to the health care delivery system.
Acknowledgments
The present research was supported by a grant from the Fonds de la Recherche en Santé du Québec (FRSQ). Maida J Sewitch is supported as a Research Scientist of the Canadian Cancer Society through an award from the National Cancer Institute of Canada. A particular tribute is paid to the study coordinator, Ms Caroline Fournier, who recruited the physicians for the study.
REFERENCES
- 1.Faivre J, Bouvier AM, Bonithon-Kopp C. Epidemiology and screening of colorectal cancer. Best Pract Res Clin Gastroenterol. 2002;16:187–99. doi: 10.1053/bega.2001.0280. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute of Canada . Percentage distribution of estimated new cases and deaths for selected cancer sites, males and females. Toronto: National Cancer Institute of Canada; 2001. [Google Scholar]
- 3.National Cancer Institute of Canada. Canadian Cancer Statistics . Toronto: National Cancer Institute of Canada; 2004. 2004. [Google Scholar]
- 4.Schattner A, Gilad A. Primary care physicians’ awareness and implementation of screening guidelines for colorectal cancer. Prev Med. 2002;35:447–52. doi: 10.1006/pmed.2002.1101. [DOI] [PubMed] [Google Scholar]
- 5.Scholefield JH. ABC of colorectal cancer: Screening. BMJ. 2000;321:1004–6. doi: 10.1136/bmj.321.7267.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ransohoff DF. Colon cancer screening in 2005: Status and challenges. Gastroenterology. 2005;128:1685–95. doi: 10.1053/j.gastro.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: Effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91:434–7. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 8.Hardcastle JD, Chamberlain JO, Robinson MHE, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 9.Doria-Rose VP, Newcomb PA, Levin TR. Incomplete screening flexible sigmoidoscopy associated with female sex, age, and increased risk of colorectal cancer. Gut. 2005;549:1273–8. doi: 10.1136/gut.2005.064030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canadian Task Force on Preventive Health Care Colorectal cancer screening. Recommendation statement from the Canadian Task Force on Preventive Health Care. CMAJ. 2001;165:206–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health Agency of Canada: Reducing Canadian colorectal cancer mortality through screening<http://www.phac-aspc.gc.ca/publicat/ncccs-cndcc/ccsrec_e.html> (Version current at July 24, 2006).
- 12.Canadian Cancer Society: Screening for colorectal cancer<http://www.cancer.ca/ccs/internet/standard/0,3182,3649_10175_74549480_langId-en,00.html> (Version current at July 24, 2006).
- 13.US Preventive Services Task Force Screening for colorectal cancer: Recommendations and rationale. Ann Intern Med. 2002;137:129–31. doi: 10.7326/0003-4819-137-2-200207160-00003. [DOI] [PubMed] [Google Scholar]
- 14.American Cancer Society Colon and rectum cancer guidelines.<http://www.cancer.org/docroot/cri/content/cri_2_4_3x_can_colon_and_rectum_cancer_be_found_early.asp> (Version current at July 24, 2006).
- 15.Winawer S, Fletcher R, Rex D, et al. Gastrointestinal Consortium Panel Colorectal cancer screening and surveillance: Clinical guidelines and rationale – Update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 16.Levenson D. CDC says colorectal cancer screening rates remain low. Rep Med Guidel Outcomes Res. 2003;14:10, 12. [PubMed] [Google Scholar]
- 17.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 18.Ioannou GN, Chapko MK, Dominitz JA. Predictors of colorectal cancer screening participation in the United States. Am J Gastroenterol. 2003;98:2082–91. doi: 10.1111/j.1572-0241.2003.07574.x. [DOI] [PubMed] [Google Scholar]
- 19.Nadel MR, Blackman DK, Shapiro JA, Seeff LC. Are people being screened for colorectal cancer as recommended? Results from the National Health Interview Survey. Prev Med. 2002;35:199–206. doi: 10.1006/pmed.2002.1070. [DOI] [PubMed] [Google Scholar]
- 20.Chao A, Connell CJ, Cokkinides VE, Jacobs EJ, Calle E, Thun MJ. Underuse of screening sigmoidoscopy and colonoscopy in a large cohort of US adults. Am J Public Health. 2004;94:1775–81. doi: 10.2105/ajph.94.10.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross CP, Mead LA, Ford DE, Klag MJ. Physician, heal thyself? Regular source of care and use of preventive health services among physicians. Arch Intern Med. 2000;160:3209–14. doi: 10.1001/archinte.160.21.3209. [DOI] [PubMed] [Google Scholar]
- 22.Carlos RC, Fendrick AM, Patterson SK, Bernstein SJ. Associations in breast and colon cancer screening behavior in women. Acad Radiol. 2005;12:451–8. doi: 10.1016/j.acra.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian S, Amonkar MM, Hunt TL. Use of colonoscopy for colorectal cancer screening: Evidence from the 2000 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2005;14:409–16. doi: 10.1158/1055-9965.EPI-03-0493. [DOI] [PubMed] [Google Scholar]
- 24.Friedman LC, Webb JA, Richards S, Plon SE. Psychological and behavioral factors associated with colorectal cancer screening among Ashkenazim. Prev Med. 1999;29:119–25. doi: 10.1006/pmed.1999.0508. [DOI] [PubMed] [Google Scholar]
- 25.Brenes GA, Paskett ED. Predictors of stage of adoption for colorectal cancer screening. Prev Med. 2000;31:410–6. doi: 10.1006/pmed.2000.0729. [DOI] [PubMed] [Google Scholar]
- 26.Michielutte R, Dignan MB, Smith BL. Psychosocial factors associated with the use of breast cancer screening by women age 60 years or over. Health Educ Behav. 1999;26:625–47. doi: 10.1177/109019819902600505. [DOI] [PubMed] [Google Scholar]
- 27.Manne S, Markowitz A, Winawer S, et al. Understanding intention to undergo colonoscopy among intermediate-risk siblings of colorectal cancer patients: A test of a mediational model. Prev Med. 2003;36:71–84. doi: 10.1006/pmed.2002.1122. [DOI] [PubMed] [Google Scholar]
- 28.Zapka JG, Puleo E, Vickers-Lahti M, Luckmann R. Healthcare system factors and colorectal cancer screening. Am J Prev Med. 2002;23:28–35. doi: 10.1016/s0749-3797(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 29.Madlensky L, Esplen MJ, Gallinger S, McLaughlin JR, Goel V. Relatives of colorectal cancer patients: Factors associated with screening behavior. Am J Prev Med. 2003;25:187–94. doi: 10.1016/s0749-3797(03)00202-2. [DOI] [PubMed] [Google Scholar]
- 30.McGregor SE, Hilsden RJ, Murray A, Bryant HE. Colorectal cancer screening: Practices and opinions of primary care physicians. Prev Med. 2004;39:279–85. doi: 10.1016/j.ypmed.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 31.Schroy PC, III, Geller AC, Crosier Wood M, et al. Utilization of colorectal cancer screening tests: A 1997 survey of Massachusetts internists. Prev Med. 2001;33:381–91. doi: 10.1006/pmed.2001.0903. [DOI] [PubMed] [Google Scholar]
- 32.Asano TK, Toma D, Stern HS, McLeod RS. Current awareness in Canada of clinical practice guidelines for colorectal cancer screening. Can J Surg. 2004;47:104–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma VK, Vasudeva R, Howden CW. Colorectal cancer screening and surveillance practices by primary care physicians: Results of a national survey. Am J Gastroenterol. 2000;95:1551–6. doi: 10.1111/j.1572-0241.2000.02093.x. [DOI] [PubMed] [Google Scholar]
- 34.Zack DL, DiBaise JK, Quigley EM, Roy HK. Colorectal cancer screening compliance by medicine residents: Perceived and actual. Am J Gastroenterol. 2001;96:3004–8. doi: 10.1111/j.1572-0241.2001.04678.x. [DOI] [PubMed] [Google Scholar]
- 35.Barrison AF, Smith C, Oveido J, Heeren T, Schroy PC., III Colorectal cancer screening and familial risk: A survey of internal medicine residents’ knowledge and practice patterns. Am J Gastroenterol. 2003;98:1410–6. doi: 10.1111/j.1572-0241.2003.07481.x. [DOI] [PubMed] [Google Scholar]
- 36.Lemon SC, Zapka JG, Estabrook B, Erban S, Luckmann R. Screening for colorectal cancer on the front line. Am J Gastroenterol. 2003;98:915–23. doi: 10.1111/j.1572-0241.2003.07360.x. [DOI] [PubMed] [Google Scholar]
- 37.Mack LA, Stuart H, Temple WJ. Survey of colorectal cancer screening practices in a large Canadian urban centre. Can J Surg. 2004;47:189–94. [PMC free article] [PubMed] [Google Scholar]
- 38.Feldman GE, McCord CW, Bassett MT, Frieden TR. Screening for colorectal cancer. JAMA. 2003;290:191. doi: 10.1001/jama.290.2.191-a. [DOI] [PubMed] [Google Scholar]
- 39.Fontana SA, Baumann LC, Helberg C, Love RR. The delivery of preventive services in primary care practices according to chronic disease status. Am J Pub Health. 1997;87:1190–6. doi: 10.2105/ajph.87.7.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bresalier RS, Kim YS. Malignant neoplasms of the large intestine In: Feldman M, Sleisenger MH, eds Gastrointestinal and Liver Disease: Pathophysiology/Diagnosis/Management. Philadelphia: WB Saunders Company; 1998. pp. 1906–42. [Google Scholar]
- 41.Federici A, Rossi PG, Bartolozzi F, Farchi S, Borgia P, Guastcchi G. Survey on colorectal cancer screening knowledge, attitudes, and practices of general practice physicians in Lazio, Italy. Prev Med. 2005;41:30–5. doi: 10.1016/j.ypmed.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Atkin WS, Edwards R, Wardle J, et al. Design of a multicentre randomised trial to evaluate flexible sigmoidoscopy in colorectal cancer screening. J Med Screen. 2001;8:137–44. doi: 10.1136/jms.8.3.137. [DOI] [PubMed] [Google Scholar]
- 43.Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–7. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 44.Hilsden RJ. Patterns of use of flexible sigmoidoscopy, colonoscopy and gastroscopy: A population-based study in a Canadian province. Can J Gastroenterol. 2004;18:213–9. doi: 10.1155/2004/276149. [DOI] [PubMed] [Google Scholar]
- 45.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 46.Schoenfeld P, Cash B, Flood A, et al. for the CONCeRN Study Investigators Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352:2061–8. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 47.Cram P, Fendrick M, Inadomi J, Cowen ME, Carpenter D, Vijan S. The impact of a celebrity promotional campaign on the use of colon cancer screening: The Katie Couric effect. Arch Intern Med. 2003;163:1601–5. doi: 10.1001/archinte.163.13.1601. [DOI] [PubMed] [Google Scholar]
- 48.Barkun AN, Jobin G, Cousineau G, et al. The Quebec Association of Gastroenterology position paper on colorectal cancer screening – 2003. Can J Gastroenterol. 2004;18:509–19. doi: 10.1155/2004/327858. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg D, Schiff GD, McNutt R, Furumoto-Dawson A, Hammerman M, Hoffman A. Mailings timed to patients’ appointments: A controlled trial of fecal occult blood test cards. Am J Prev Med. 2004;26:431–5. doi: 10.1016/j.amepre.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Scott RG, Edwards JT, Fritschi L, Foster NM, Mendelson RM, Forbes GM. Community-based screening by colonoscopy or computed tomographic colography in asymptomatic average-risk subjects. Am J Gastroenterol. 2004;99:1145–51. doi: 10.1111/j.1572-0241.2004.30253.x. [DOI] [PubMed] [Google Scholar]
- 51.Denberg TD, Melhado TV, Coombes JM, et al. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med. 2005;20:989–95. doi: 10.1111/j.1525-1497.2005.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hennekens CH, Buring JE. Screening. In: Mayrent SL, editor. Epidemiology in Medicine. Boston: Little, Brown and Co; 1987. pp. 327–47. [Google Scholar]
- 53.Imperiale TF. Can computed tomographic colonography become a “good” screening test? Ann Intern Med. 2005;142:669–70. doi: 10.7326/0003-4819-142-8-200504190-00017. [DOI] [PubMed] [Google Scholar]
- 54.Woolf SH. A smarter strategy? Reflections on fecal DNA screening for colorectal cancer. N Engl J Med. 2004;351:2755–8. doi: 10.1056/NEJMe048259. [DOI] [PubMed] [Google Scholar]
- 55.Imperiale TF, Ransohoff DF, Itkowitz SH, Turnbull BA, Ross ME, Colorectal Cancer Study Group Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;351:2704–14. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 56.Wei EK, Ryan CT, Dietrich AJ, Colditz GA. Improving colorectal cancer screening by targeting office systems in primary care practices: Disseminating research results into clinical practice. Arch Intern Med. 2005;165:661–6. doi: 10.1001/archinte.165.6.661. [DOI] [PubMed] [Google Scholar]
- 57.Klabunde CN, Frame PS, Meadow A, Jones E, Nadel M, Vernon SW. A national survey of primary care physicians’ colorectal cancer screening recommendations and practices. Prev Med. 2003;36:352–62. doi: 10.1016/s0091-7435(02)00066-x. [DOI] [PubMed] [Google Scholar]
- 58.National Guideline Clearinghouse<http://www.guideline.gov/browse/guideline_index.aspx> (Version current at July 24, 2006)