Abstract
Approximately 400 liver transplants are performed in Canada every year and close to 6000 per year in the United States. Forty per cent to 45% of all liver transplants are performed for patients with underlying hepatitis C virus (HCV)-related liver disease. These patients have a different natural history, new complication risks and different treatment efficacy than nontransplant HCV patients. Every effort must be made to identify those patients at highest risk for progressive liver disease post-transplant. Recurrent HCV is an Achilles’ heel to transplant hepatology. The true natural history of this disease is only starting to unravel and many questions remain unanswered on the optimal management of these patients after liver transplantation. The present report summarizes the literature and ongoing research needs that are specific to HCV-related liver transplantation.
Keywords: Management, Recurrent hepatitis C, Research, Risk factors, Transplantation
Abstract
Environ 400 transplantations du foie sont pratiquées, chaque année, au Canada, et près de 6000 le sont annuellement aux États-Unis. Les maladies du foie sous-jacentes, liées au virus de l’hépatite C (VHC) justifient de 40 à 45 % des transplantations. Les patients touchés se distinguent de ceux qui sont atteints du VHC mais qui n’ont pas subi de greffe du foie par l’évolution naturelle de la maladie, les risques de complications et l’efficacité des traitements. Il faut donc tout mettre en œuvre pour repérer les patients qui connaissent les risques les plus élevés d’hépatopathie évolutive après la transplantation. La réapparition de l’infection au VHC est un point faible du traitement en hépatologie de la transplantation. On commence à peine à comprendre la véritable évolution naturelle de la maladie, et de nombreuses questions restent encore sans réponse sur le traitement optimal des patients après une transplantation du foie. Le présent rapport fait une synthèse de la documentation sur les transplantations du foie liées au VHC et expose brièvement la recherche future dans le domaine.
Canadian transplant hepatologists representing each of the major centres in Canada gathered in Halifax, Nova Scotia, to attend a workshop to discuss hepatitis C virus (HCV) post-transplantation and develop research goals for the collaborative group. A review of the current literature and a summary of these proceedings is provided.
BACKGROUND
HCV-related cirrhosis is the leading indication for liver transplantation (LT). Approximately 400 liver transplants are performed in Canada every year and close to 6000 per year in the United States. Forty per cent to 45% of all LTs are performed for patients with underlying HCV-related liver disease (1,2). That translates into 150 Canadian and 2500 American HCV patients transplanted each year. These patients represent a new cohort of HCV patients with a new or different natural history, new complication risks, different treatment efficacy and, potentially, a life sentence in certain circumstances.
Recurrence of HCV infection in the transplanted organ is universal and the consequences are still being unravelled (2–6). The natural history of recurrent HCV is quite variable and ranges from rapidly progressive liver failure within months of transplantation to a more benign hepatitis, which can slowly progress over years (4). Evidence supports an overall increased rate of liver fibrosis, with approximately 20% of HCV-related transplants developing allograft cirrhosis within five years (3,6–10). These patients subsequently progress to end-stage liver disease (ESLD) at a more rapid rate than patients with HCV before transplantation (5,7,9).
Retransplantation is an option for patients with ESLD of their transplanted liver. However, survival after retransplantation is significantly lower than after a primary transplant and is particularly poor for HCV patients (1). Many centres are reluctant to offer retransplantation to patients with recurrent HCV underlying their allograft failure, due to the poor overall survival rate in that cohort of patients. Thus, every effort must be made to identify those patients at the highest risk for progressive liver disease post-transplant.
Patient survival after primary transplant for HCV throughout the 1990s was similar to most other indications for transplantation (10–12). However, more recent studies showed an increased rate of death and allograft failure in HCV-positive recipients compared with HCV-negative recipients (13,14). Concern has also been raised about the increasing rate of progressive fibrosis in more recent time frames compared with the early and mid-1990s (15).
Discussion
A clear definition of recurrent HCV is required to interpret current and future studies. The natural history of recurrent disease requires further study and can be better clarified by long-term prospective studies using protocol biopsies.
Recommendations
A clear definition of recurrent hepatitis needs to be established by literature review and pathology consensus.
Several centres have already instituted protocol biopsies at varying time intervals. Protocol biopsies have not been mandated but each centre is strongly encouraged to use protocol biopsies (uniform minimum biopsies at six and 12 months, and annually thereafter). The use of protocol biopsies and the development of a nationwide database are necessary to better understand the natural history of the disease and allow for larger patient enrollment in future studies.
The data for worse outcomes overall, over the more recent years, has not been reproduced in Canada. A study to determine mortality rates over the years in Canadian patients should be looked into.
RISK FACTORS
Risk factors listed below have been associated with poor outcome post-transplantation for HCV-related cirrhosis and attempts have been made to create a model for risk assessment (16–20). These models are impractical and, thus far, we have not been able to easily identify a cohort of HCV patients at highest risk for poor outcomes (severe recurrent disease, progression to cirrhosis/ESLD and mortality). Many of these risk factors for poor outcomes in recurrent HCV are similarly risk factors for poor outcomes in non-HCV-related liver disease post-transplantation. Factors such as race, high viral load pretransplant, high viral load post-transplant, early recurrence within months of transplant and year of transplantation have been reasonably well established to be associated with more severe HCV recurrence after liver transplant (2,3,7,10,12,14,15,18).
Some risk factors thought to have an impact on recurrent HCV severity are not as well established and these will be addressed as separate topics in the present paper (living donor [LD] liver transplant), immunosuppression and cytomegalovirus [CMV] infection).
Two of the risk factors that seem to recur throughout the literature are donor age and ischemic time. Donor age cutoffs as low as 40 years have been shown to have higher mortality in HCV patients (21). The use of older donors (older than 55 years of age) has increased over the years (22), without definitive consequence in the non-HCV patient population. Unfortunately, because donor age does not appear to be the only factor relating to poor outcomes, recommendations on donor age restrictions have not been established.
Cold ischemia and, more importantly, warm ischemia time during implantation are thought to be risk factors for a more aggressive course of recurrent HCV post-transplantation (10,18,23). In fact, early post-transplant biopsy findings of preservation injury may identify HCV patients at significant risks for poor outcomes (24). However, prospective validation of this finding is needed.
Discussion
Who is at the highest risk of progressive fibrosis and most in need of treatment of HCV is one of the most important questions in this area of transplant medicine. Not all patients with HCV post-transplant have an aggressive course, and treatment of the infection may be worse than the disease itself in some cases. Determining which patients are at highest risk of aggressive disease has been elusive.
Recommendations
There is great need for a nationwide prospective database for liver transplant patients in Canada. Future studies will depend on this.
LD LIVER TRANSPLANTS IN HCV PATIENTS
Concerns had initially been raised that patients with HCV had poorer outcomes after a LD liver transplant than after deceased donor (DD) liver transplants. It was speculated that the process of regeneration might result in a worse reinfection of the graft, which ultimately might lead to a poor outcome. One retrospective study (25) and several abstracts appeared in transplantation literature in 2001 to 2003, showing disease recurrence was more severe and outcomes more inferior in patients who received LD liver transplants.
In 2004, many studies (most in abstract form) showed that there was no difference in patient outcomes (disease recurrence or survival) between these patient populations (26,27). During this time, there was also a shift to studies using protocol biopsies to determine the actual frequency and outcomes of disease recurrence. Recently, a larger database review showed no difference in graft or patient survival for LD liver transplants in the HCV population (28).
A recent study in Toronto, Ontario, of 154 patients with HCV (29 LD liver transplant recipients and 125 DD liver transplant recipients) with protocol biopsies at three, six and 12 months, and yearly thereafter were followed. There was a trend for better survival in LD liver transplant patients beyond the first year and a much higher proportion of DD liver transplant recipients actually had stage 3 to 4 fibrosis within two years than the LD liver transplant recipients (29). Thus, data within the last few years have shifted to support the use of LD liver transplant in the HCV cohort of transplant patients. As experience grows in this aspect of transplantation, larger prospective protocol biopsy-driven studies will be required.
Discussion
LD liver transplant patients are generally healthier with lower Model End-stage Liver Disease (MELD) scores, have younger donors on average and the shortest ischemic time. Thus, we have corrected as much as we can to improve outcomes in recurrent HCV. The fact that they have, at best, equivalent outcomes to cadaveric donors in the more recent studies, may be interpreted as a negative effect. Certainly, larger multicentred studies using protocol biopsies are needed.
Recommendations
A study to determine the frequency of significant fibrosis, that would have otherwise been missed if protocol biopsies were not performed, would be useful to perform.
A study to determine if the chosen immunosuppression regimen affects outcome would also be of great value.
A study looking into the feasibility of preoperative treatment of HCV before LD liver transplant may be a future endeavor.
EFFECTS OF STEROID USE ON POST-TRANSPLANT HCV RECURRENCE
HCV viremia after transplant initially increases rapidly to approximately 10 to 100 times the pretransplant level (30,31). The magnitude of elevation of HCV levels appears to be associated with the dose and duration of steroid exposure (32,33). High virus levels before transplant have been shown to be a predictor of severe post-transplant HCV recurrence (32,34). The concept that bolus high-dose steroids appear detrimental to the outcome of these patients is supported by several studies (15,21).
The evidence for an impact of low-dose, long-term steroids on HCV recurrence and graft, and patient survival is variable. Several studies suggest worse outcomes and others suggest no effect or even better outcomes. Attempts at steroid-free regimens are now being reported (35–37). No definitive answers can be ascertained yet due to small sample sizes and/or limited duration of follow-up. Careful assessments of these studies are required to ensure these patients are truly steroid-free without any intraoperative induction steroids or rejection treatments. Although the recent studies are suggestive of better outcome, no definitive answers can be ascertained with these small studies.
Discussion
Many studies claim to be using a steroid-free protocol but are actually not, and these study patients do receive steroids. Careful review of the data is required in this field. Certainly, the evidence is convincing that bolus steroids are detrimental to these patients. More studies are underway to determine if there is an advantage to steroid-free regimens.
Recommendations
A study to determine the viral kinetics in the steroid-free patient compared with the nonsteroid-free patient should be performed.
Large multicentre studies using a truly steroid-free regimen are needed.
TACROLIMUS VERSUS CYCLOSPORINE IN THE MANAGEMENT OF HCV PATIENTS POST-TRANSPLANT
Several studies reported over the past five years have suggested worse outcomes for patients transplanted for HCV infection compared with 10 to 15 years ago. This suggestion first originated from Berenguer et al (15), who reported that HCV patients transplanted at their respective centres had lower survival rates in the more recent years. Many factors, including the use of marginal donors, donor age and severity of liver disease before transplantation were suggested as potential causes of the so called ‘Berenguer effect’, and the choice of calcineurin inhibitor was also a potential candidate because cyclosporine and tacrolimus were clearly associated with the two different eras. Tacrolimus has emerged as the more commonly used immunosuppressant in recent years but it appears that cyclosporine may have antiviral effects.
Independent from the effects of calcineurin inhibition, cyclosporine appears to have antiviral effects against HIV, herpes simplex virus and many other viral agents in vitro. For the immunosuppression activity, tacrolimus binds to peptidyl-prolyl isomerases known as FK-binding protein and cyclosporine binds cyclophilins; both these complexes affect downstream calcineurin inhibition. However, cyclophilins have been shown to enable HIV particle assembly and, more recently, cyclophilin B has been implicated as an essential factor in mediating HCV replication by activating NS5B (38). Accordingly, cyclosporine has been shown to inhibit HCV replication in vitro, whereas tacrolimus has no such effect (38). The antiviral effects of cyclosporine in vivo have been more difficult to demonstrate. For example, a study of viral kinetics with cyclosporine treatment showed that patients treated for 24 weeks with cyclosporine had diminished alanine aminotransferase levels but the treatment had little effect on HCV RNA levels (39). However, it was interesting to note that HCV RNA levels did not rise with immunosuppression. In contrast, combination treatment with interferon-alpha (IFN-α) and cyclosporine showed sustained virological response (SVR) rates to IFN of 32% and to IFN plus cyclosporine of 55% (40). Not only was this finding statistically significant but the SVR responders were predominantly those with high HCV RNA and genotype 1, the most difficult patients to treat (40). This group is now doing a multicentre study to verify these findings.
The results of several recent studies comparing the effects of cyclosporine versus tacrolimus in transplant patients with HCV infection have not provided any meaningful data. This is likely due to the short duration of follow-up because graft and patient survival rates for the HCV cohort tend to fall off after three to five years and these study end points were before three years. A small randomized controlled trial from the University of California at Los Angeles (USA) showed no difference in HCV outcomes (recurrence or survival) with cyclosporine and tacrolimus; however, patients with cyclosporine therapy had a higher serum HCV RNA ratio following transplant as compared with baseline (41). In contrast, the Liver International Study of 2 h Neoral versus Tacrolimus Pre-dose (LIS2T) study (42) showed better outcomes for HCV patients with cyclosporine than those on tacrolimus with regard to graft loss and death, 6% versus 15%, respectively. Clearly more studies are required to delineate advantages to immunosuppressive regimens.
Discussion
The issue of tacrolimus versus cyclosporine in HCV transplant patients has yet to be resolved. Now that we are entering a period where tailored immunosuppression directed toward specific disorders is more commonplace, a fresh look at whether the antiviral effect of cyclosporine, so elegantly illustrated in vitro, translates into better outcomes for transplant recipients with HCV infection in vivo remains to be resolved.
Recommendations
A prospective natural history study comparing cyclosporine and tacrolimus in HCV patients is needed in the transplant community.
A study of the viral kinetics in tacrolimus- versus cyclosporine-treated patients would also be of significant value.
CMV AND HCV RECURRENCE POST-LT: A SYNERGISTIC INTERACTION?
Since 1997, there have been several studies (43–48) reporting both direct and indirect evidence of a deleterious post-transplant interaction between CMV and HCV. These studies generally report an increased risk of HCV-associated graft loss and worse histological progression of disease compared with CMV-negative HCV recipients. Problems with the reported studies include small patient numbers (43,44) and, in one large database review (45), only indirect evidence with the finding of pretransplant CMV serology as a significant variable in a multivariate analysis. Stronger evidence has been reported in two Mayo clinic studies (46,47) and a Canadian study (48). The two Mayo clinic studies reported a high risk of graft failure in those who had CMV infection (both CMV disease and subclinical infection), approaching 50% compared with less than 20% in those who were CMV-negative (46,47). Likewise, a higher proportion of CMV-infected recipients progressed to fibrosis stage 2 of 4, at four months post-transplant compared with CMV-negative recipients (45% versus 16.4%, P=0.01), although there was no histological difference at one year post-transplant (46). The only Canadian study to date (48) similarly reported an increased fibrosis score in CMV-infected patients compared with CMV-negative patients (P=0.016) and a trend toward more severe fibrosis (at least fibrosis stage 2 of 4), although there was no difference in the proportion who developed histological HCV recurrence between groups.
Not all studies have reported an interaction between CMV and HCV. A large database review (49) that investigated factors associated with the progression of recurrent HCV specifically included CMV infection among multiple variables and did not find CMV infection to be a significant determinant. Two smaller studies (50,51) also failed to find an association. Although the lack of an association in the latter two studies may have been a type II error, an aggressive ganciclovir treatment protocol may have ameliorated the effect of CMV on HCV recurrence.
Discussion
The body of evidence does suggest that CMV infection may be associated with worse post-transplant HCV outcomes. Given the known deleterious effects of CMV infection post-transplant in general, it behooves the liver transplant community to monitor and treat CMV aggressively in this recipient group.
Recommendations
Previous multicentred studies performed in Canada have CMV data available for analysis. These data should be analyzed.
PEGYLATED IFN AND RIBAVIRIN FOR RECURRENT HCV AFTER LT
HCV is difficult to treat in the transplant population because of immunosuppression, pre-existing cytopenias and renal dysfunction from the calcineurin inhibitors. There are several strategies to deal with the problem of recurrent HCV. Treatment of HCV can be attempted in cirrhotic patients before LT as preemptive therapy in the early post-transplant period or once the recurrent disease has been established.
There are no published studies on the use of pegylated (PEG) IFN with or without ribavirin (RBV) for pretransplant prophylaxis. Everson (52) has reported on the use of standard IFN and RBV in a low accelerated dose regimen in well-compensated cirrhotic patients (mean Child-Turcotte-Pugh [CTP] score was approximately seven) awaiting LT. Reasonable SVR rates were reported and all eight patients who had an SVR before their LT remained HCV RNA-negative after transplant (53). Unfortunately, more recent data suggest that some people who go into LT HCV RNA-negative will relapse after transplantation (53). A multicentre study in the United States also has treated patients (mean CTP scores of approximately 12) on the LT waiting list with IFN and RBV (54). None of these patients became HCV RNA-negative before transplantation and, after two deaths from sepsis, the study was terminated early. The International Liver Transplant Society (ILTS) consensus panel concluded that treatment can be considered for cirrhotic patients with a CTP score of seven or less or a MELD score less than 18, and is contraindicated when the CTP score is greater than 11 or the MELD score is greater than 25 (55).
Preemptive strategies attempt to prevent HCV infection in the allograft. There is only one study examining PEG IFN in this setting. This multicentre, randomized control trial used PEG IFN-α2a monotherapy treatment within three weeks of transplantation and had an SVR of only 8% (56). However, there were lower hepatic activity index scores at the end of treatment and at week 72 in the PEG IFN-treated patients compared with untreated controls, but it did not reach significance. In this study, acute cellular rejection (ACR) was seen in 21% of untreated controls and only 12% of the PEG IFN group (56).
Focusing on treatment of established disease, this same paper examined PEG IFN-α2a monotherapy started between six and 60 months after LT for histologically proven recurrence (56). Only 68% of patients completed therapy and the end of treatment response was 45%, with an SVR of only 12%. ACR occurred in 12% of PEG IFN subjects versus none in the untreated group. After 48 weeks, there was improvement in hepatic activity index scores in PEG IFN subjects compared with controls but these differences were less apparent at week 72 (56).
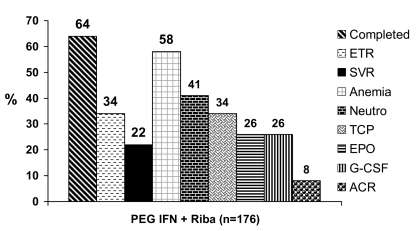
The published literature (excluding abstracts) on combination therapy for recurrent HCV consists of seven single-centre, small case series using PEG IFN-α2b in combination with RBV (57–63). Immunosuppression, the histological stage of subjects and the use of growth factor (GF) support was also highly variable. Table 1 summarizes the seven published series for recurrent HCV. The majority of the patients are genotype 1 (range 63% to 100%). Ross et al (60) switched standard IFN and RBV nonresponders to PEG IFN and RBV (accounting for their low response rates). Figure 1 illustrates a pooled analysis of this published experience. On average, 64% of subjects completed therapy, end of treatment response was seen in 34% and SVR occurred in 22% of the 176 treated subjects. Anemia occurs in approximately 60% of patients, neutropenia in approximately 40%, thrombocytopenia in approximately one-third and, overall, approximately one-quarter of this population has received GFs. The average rate of rejection (ACR) on PEG IFN therapy was 8%.
TABLE 1.
Summary of published studies using pegylated interferon (PEG IFN) and ribavirin for treatment of established recurrent hepatitis C virus after liver transplant
| References | Number of patients | PEG IFN dose (μg/kg) | Ribavirin dose (mg) | Duration (weeks) | CT (%) | ACR (%) | ETR (%) | SVR (%) | EPO (%) | G-CSF (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mukherjee et al (57) | 39 | Goal 1.5 | Goal 800 | 24/48 | 46 | 0 | 38 | 31 | 0 | 0 |
| Dumortier et al (58) | 20 | 0.5→1.0 | 400→1200 | 48 | 80 | 25 | 55 | 45 | 0 | 0 |
| Rodriguez-Luna et al (59) | 19 | 0.5→1.5 | 400→1000 | 48 | 51 | 5 | 37 | 26 | 74 | 47 |
| Ross et al (60) | 16 | 1.5 | 1200 | 48+ | 100 | 0 | 38 | 0 | 39 | 88 |
| Neff et al (61) | 57 | 1.5 | 400→600 | 48 | 68 | – | 25 | 14 | 44 | 39 |
| Toniutto et al (62) | 12 | 0.5 | 600→800 | 48 | 58 | 0 | 17 | 8 | 0 | 0 |
| Babatin et al (63) | 13 | 1.0→1.5 | ~600→ | 48+ | 46 | 23 | 39 | 31 | 8 | 0 |
ACR Acute cellular rejection; CT Completion of treatment; EPO Erythropoietin; ETR End of treatment response; G-CSF Granulocyte colony-stimulating factor; SVR Sustained virological response
Figure 1).
Pooled analysis of seven published studies using pegylated interferon (PEG IFN) and ribavirin (Riba) for treatment of established recurrent hepatitis C virus after liver transplant. ACR Acute cellular rejection; EPO Erythropoietin; ETR End of treatment response; G-CSF Granulocyte colony-stimulating factor; Neutro Neutropenia; SVR Sustained virological response; TCP Thrombocytopenia
In summary, minimizing immunosuppression before starting therapy is important while being mindful that rejection can occur. Treatment can be undertaken before transplant in carefully selected patients. Preempted therapy in the early post-transplant period requires further study. Most centres will wait for established disease before introducing therapy and although response rates are lower in the post-transplant setting, the combination of PEG IFN and RBV is the most effective therapy for patients with recurrent HCV. Adjusting the RBV dose according to renal function is necessary (starting with lower doses and using dose acceleration when possible). The use of GFs to support the hemoglobin and neutrophil count may improve compliance and success of antiviral therapy after transplant.
Discussion
The transplant community struggles with many research questions in this area. What is the ultimate duration of therapy? Is it a set duration such as 48 weeks or do we treat patients until polymerase chain reaction (PCR) becomes negative? How long do we continue to treat patients after PCR becomes negative, 24 weeks, 48 weeks? Is 24 weeks of treatment in genotypes 2 and 3 post-transplant adequate? What is the value of an early viral response at 12 weeks, particularly when stepwise treatment is the current approach? When do you stop therapy due to nonresponse, because some data suggest delayed responses occur? What is the frequency of delayed response? Is IFN less effective in immunosuppressed patients or are they just not getting enough drugs, or is it both? Has previous exposure to treatment created resistance to treatment or worsened responses?
Studies are ongoing throughout the transplant community to answer these questions. Most studies are small and include patients with cirrhosis and fibrosing cholestatic disease (FCH). These patients undoubtedly have a different natural history than patients with less severe disease and should be distinguished in analyses of these studies.
Recommendations
In addition to all of the questions listed above, the transplant community needs to look into several other important aspects.
A study to determine the benefit of low-dose combination treatment with PEG IFN/RBV long-term in nonresponders treated post-transplantation, to determine effects on disease progression.
A study of RBV level monitoring during treatment to address toxicity in the postorthotopic LT setting.
Patients with FCH have been noted to have rapid recurrence with discontinuation of treatment, thus a long-term study looking at continuous treatment is needed.
Development of a FCH registry would allow for larger cohort data and will address significant deficits in the literature in this patient population. All trials incorporating treatment of recurrent HCV should distinguish FCH and severe cirrhotic patients from the less severe cases.
THE USE OF ERYTHROPOIETIN AND GRANULOCYTE COLONY-STIMULATING FACTOR IN THE TREATMENT OF HCV RECURRENCE AFTER LT
The use of GFs in conjunction with combination therapy with PEG IFN/RBV for HCV recurrence after LT is addressed in only four publications, each with small sample sizes (61,64–66). In fact, in three of the publications there is only a brief citation that the GFs erythropoietin (EPO) and granulocyte colony-stimulating factor (G-CSF) were used as adjuncts (61,65,66). Only in the paper by Shergill et al (64) was there an attempt to draw some conclusions in using GFs. Here, the authors state that the use of G-CSF corrected low neutrophil counts and allowed for the use of doses of PEG IFN that were originally aimed for. On the other hand, the use of EPO allowed the continuation of RBV without dose reduction but did not allow for much dose increase. They observed no difference in SVR rates between the two groups of patients, but the patient numbers were small.
Therefore, if we are going to draw any conclusions as to the use of these factors in the treatment of HCV, we have to turn toward the nontransplantation literature. In general, the conventional management of cytopenias during therapy has been dose reduction, and these reductions occur in approximately 20% of treatments. However, it has been shown that patients who take PEG IFN/RBV as close as possible to the target dose have greater SVR rates than those who do not (67). It seems to be very important to maximize the dose, particularly in the first 12 weeks of therapy.
Two main factors used to stimulate EPO, epoetin-alpha and darbepoetin-alpha, are synthetic glycoproteins that act like endogenous EPO. In HCV treatment, there has been no case of pure red cell aplasia with the use of darbepoetin but there is one recent report with epoetin-alpha (68). It is generally thought to be safe to use these GFs in treating patients with HCV. In a randomized trial with patients being treated for HCV who had a reduction in hemoglobin to 120 mg/L or less, patients were randomly assigned to receive 40,000 U epoetin-alpha subcutaneously once a week versus placebo. Patients receiving epoetin-alpha were found to be more capable of maintaining their dose of RBV (69). An improvement in the quality of life of patients was also noted (70). Similar findings were also found with the use of darbepoetin in a small dose study (71).
Data for using G-CSF are even less plentiful. Two forms of G-CSF exist, namely filgrastim (Neupogen, Amgen, USA) and PEG filgrastim, which has a longer half-life. In the only publication thus far, 15 patients were treated with Neupogen with an increase in peak of white blood cell count but no change in nadir counts. However, there was no difference in SVR noted (72).
In summary, there is little literature in the liver transplant setting for the use of these GFs. Our approach has mostly been borrowed from the nonliver transplant literature where there is limited medical literature. In the liver transplant setting, the majority of patients are unable to tolerate maximal doses of medications and cannot adhere to the ‘80/80/80 rule’ of therapy, and this would be an opportunity to observe if maximizing the dose early on in therapy could improve SVR and quality of life for these patients.
Discussion
Viral loads are much higher post-transplant than they are pretransplant and evidence supports this. In general it is thought that previous treatment with PEG IFN/RBV may have induced resistance, and the escalating risk of the dose regimen that many are forced to do, due to intolerance to the medication without GFs, may increase emerging resistance. Thus, the group feels that using epoetin-alpha to increase the tolerable dose of RBV would be an advantage. RBV levels would be required because these patients often have coexistent renal dysfunction. Studies looking at the ability to maximize the dose of medication received and comparing the SVR are needed desperately by the transplant community. Nationwide access to epoetin-alpha for these patients would be required to perform these studies to avoid single-centre study biases and accrue the patient numbers needed to perform these studies effectively.
Recommendations
Studies are needed to determine the RBV levels of patients postliver transplant on combination therapy.
More robust data is needed with respect to the use of GFs in these patients. Studies looking into the effectiveness of EPO in optimizing RBV dosing and potential effect on SVR are needed.
RENAL TRANSPLANTATION IN PATIENTS WITH UNDERLYING HCV
HCV is present in 22% to 60% of hemodialysis patients (73). Dialysis patients with well-compensated cirrhosis are generally not offered renal transplant due to the risk of progressive liver disease. However, patients with decompensated liver disease are able to have combined LT and kidney transplantation. However, more often, these HCV dialysis patients do not have cirrhosis or clinical evidence of advanced liver disease (74).
Approximately 1000 renal transplants occur in Canada per year. After renal transplant, there is a greater incidence of alanine aminotransferase elevations, increased viral replication and progressive liver disease (75). There have been case reports of fibrosing cholestatic hepatitis and rapidly progressive liver failure after renal transplant in HCV patients (76). Although initial studies suggested no worse short-term graft or patient survival in HCV-positive patients, more recent studies have shown a significant difference in graft and patient survival at 10 years in HCV-positive patients compared with HCV-negative patients (77,78). These studies suggest that we treat these patients before they get transplanted. Other studies suggest approximately only one-half of these HCV patients develop significant disease, making it difficult to establish which patients are at highest risk of disease progression (75).
A meta-analysis of 14 small clinical control trials using monotherapy IFN in hemodialysis patients showed an SVR range of 20% to 68%, but the average SVR was 37% (79). PEG IFN has limited data but dose reduction is recommended (79,80). Treatment of HCV after renal transplant has largely been denied due to concerns regarding IFN induction of rejection. Large studies are needed to determine if this is warranted.
Discussion
The literature in inconclusive in regard to treating postrenal transplant patients without the risk of allograft rejection.
Recommendation
A registry for nonliver transplant patients undergoing HCV treatment would be of value. At this point in time, a randomized study may not be ethical with the published data available.
HEPATOCELLULAR CARCINOMA AS A COMPLICATION IN HCV PATIENTS
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide. In the United States, there has been a 70% increase in HCC over a 20-year period thought to be secondary to the HCV epidemic (81). In Canada, it is likely very similar. The development of cirrhosis in these HCV patients will peak in the coming years. In those with cirrhosis there is a transition to HCC of 1.5% to 4% per year (82). Currently, more than 50% of HCC patients have underlying HCV. As projected in a recent Canadian study, the burden of disease will increase dramatically in the coming years (Figure 2) (83).
Figure 2).
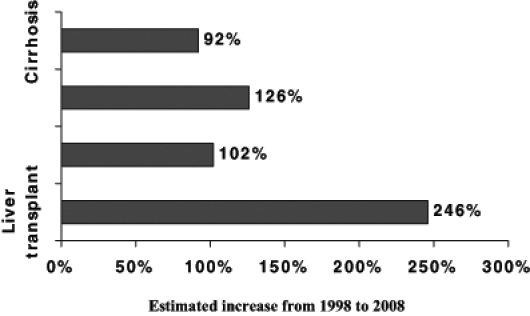
Future disease burden in Canada. Adapted from reference 83
As tumours increase in size and number, recurrence-free survival decreases. The main reason for that is that as size increases, so does the likelihood of vascular invasion. Vascular invasion is the most important prognostic factor for HCC and is present either macroscopically or microscopically in up to 50% of tumours greater than 5 cm (84). The Milan criteria (85), looking at early-stage tumours defined by a single lesion less than or equal to 5 cm or three lesions all less than 3 cm with no evidence of extrahepatic disease, vascular invasion or lymphadenopathy is what has been accepted as criteria for LT. Patient survival is 75% at four years with a recurrence rate of less than 10% (85). Yao et al (86) have shown in a retrospective cohort (based on explant findings) that a single lesion up to 6.5 cm or three lesions not greater than 4.5 cm or a total tumour diameter of no greater than 8 cm, also has a 72% five-year survival, which is quite acceptable. This has led other investigators to cautiously extend criteria for accepting HCC as an indication for LT. This continues to be investigated and, at present, Milan criteria are used in most centres, outside of studies.
Immunosuppression may also impact the recurrence of HCC. Data supporting this concept are minimal. Cyclosporine has been shown, in vitro, to promote cancer by the promotion of angiogenesis and by increasing the vascular invasion of tumour cells (87), whereas sirolimus may inhibit some cancers by inhibition of angiogenesis (88). In a study by Kneteman et al (89), patients transplanted for HCC were tapered off steroids and calcineurin inhibitors and maintained on sirolimus monotherapy for three to six months. They used extended criteria of single tumours 7.5 cm or smaller or multiple tumours each 5 cm or smaller (with no absolute limit on tumour number) with no extrahepatic malignancy, lymph node metastases or vascular invasion. The extended criteria patients enjoyed a 78% disease-free survival at four years; there was a 16% recurrence rate.
Clearly there remains potential for progression of tumours while patients are awaiting LT. The wait list dropout rate due to progression of HCC beyond acceptable criteria is between 5% and 20%. Is there a role for neoadjuvant therapy? Transarterial chemoembolization and radio frequency ablation have been used in this population. Evidence for the benefit of neoadjuvant therapy is variable with many studies supporting its use and many refuting its benefit. It is not within the scope of the present paper to review these data. Currently, the use of neoadjuvant therapy is centre-dependent.
Discussion
Criteria for transplantation for HCC has been largely based on the Milan criteria but extended criteria are currently being heavily investigated. With the expected rise in HCV/HCC prevalence, this can easily overwhelm the transplant community. Careful assessment of survival outcomes is mandatory for optimal use of scarce donor organs.
Recommendation
A multicentre study to determine the outcomes of Canadian patients transplanted for HCC within the Milan criteria compared with those transplanted outside the criteria is needed.
Acknowledgments
This endeavour was a physician/investigator-initiated activity. The authors thank the Institute of Infection and Immunity from the Canadian Institutes of Health Research, the Canadian Liver Foundation, the Capital District Health Authority and Atlantic Hepatology Services Inc for their support and encouragement of this activity. Special thanks also goes to Roche Canada and its four divisions that provided an unrestricted educational grant.
Footnotes
DISCLOSURES: Dr Andrew Mason currently has a financial grant from Novartis Pharmaceuticals. No other contributors to this meeting have disclosures.
REFERENCES
- 1.Watt KD, Lyden ER, McCashland TM. Poor survival after liver retransplantation: Is hepatitis C to blame? Liver Transpl. 2003;9:1019–24. doi: 10.1053/jlts.2003.50206. [DOI] [PubMed] [Google Scholar]
- 2.Rosen H, Gretch DR, Oehlke M, et al. Timing and severity of initial hepatitis C recurrence as predictors of long-term liver allograft injury. Transplantation. 1998;65:1178–82. doi: 10.1097/00007890-199805150-00006. [DOI] [PubMed] [Google Scholar]
- 3.Gane EJ, Portmann BC, Naoumov NV, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815–20. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein JS, Poterucha JJ, Zein N, Wiesner RH, Persing DH, Rakela J. Epidemiology and natural history of hepatitis C infections in liver transplant recipients. J Hepatol. 1995;22(Suppl 1):154–9. [PubMed] [Google Scholar]
- 5.Testa G, Crippin JS, Netto GJ, et al. Liver transplantation for hepatitis C: Recurrence and disease progression in 300 patients. Liver Transpl. 2000;6:553–61. doi: 10.1053/jlts.2000.9741. [DOI] [PubMed] [Google Scholar]
- 6.Sheiner P. Hepatitis C after liver transplantation. Semin Liver Dis. 2000;20:201–9. doi: 10.1055/s-2000-9942. [DOI] [PubMed] [Google Scholar]
- 7.Berenguer M, Lopez-Labrador FX, Wright TL. Hepatitis C and liver transplantation. J Hepatol. 2001;35:666–78. doi: 10.1016/s0168-8278(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Fueyos A, Restrepo JC, Quinto L, et al. Impact of the recurrence of hepatitis C infection after liver transplantation on the long term viability of the graft. Transplantation. 2002;73:56–63. doi: 10.1097/00007890-200201150-00010. [DOI] [PubMed] [Google Scholar]
- 9.Berenguer M. Natural history of recurrent hepatitis C. Liver Transpl. 2002;8(Suppl 1):S14–S8. doi: 10.1053/jlts.2002.35781. [DOI] [PubMed] [Google Scholar]
- 10.Charlton M, Seaberg E, Wiesner R, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology. 1998;28:823–30. doi: 10.1002/hep.510280333. [DOI] [PubMed] [Google Scholar]
- 11.Ghobrial RM, Farmer DG, Baquerizo A, et al. Orthotopic liver transplantation for hepatitis C: Outcome, effect of immunosuppression and causes of retransplantation during an eight year single center experience. Ann Surg. 1999;6:824–33. doi: 10.1097/00000658-199906000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casavilla FA, Rakela J, Kapur S, et al. Clinical outcome of patients infected with hepatitis C virus infection on survival after primary liver transplantation under tacrolimus. Liver Transpl Surg. 1998;4:448–54. doi: 10.1002/lt.500040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–96. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 14.Velidedeoglu E, Mange KC, Frank A, et al. Factors differentially correlated with the outcome of liver transplantation in HCV+ and HCV– recipients. Transplantation. 2004;77:1834–42. doi: 10.1097/01.tp.0000130468.36131.0d. [DOI] [PubMed] [Google Scholar]
- 15.Berenguer M, Ferrell L, Watson J, et al. HCV-related fibrosis progression following liver transplantation: Increase in recent years. J Hepatol. 2000;32:673–84. doi: 10.1016/s0168-8278(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 16.Prieto M, Berenguer M, Rayon JM, et al. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: Relationship with rejection episodes. Hepatology. 1999;29:250–6. doi: 10.1002/hep.510290122. [DOI] [PubMed] [Google Scholar]
- 17.Rosen H, Martin P. Hepatitis C infection in patients undergoing liver retransplantation. Transplantation. 1998;66:1612–6. doi: 10.1097/00007890-199812270-00007. [DOI] [PubMed] [Google Scholar]
- 18.Ghobrial RM, Steadman R, Gornbein J, et al. A ten year experience of liver transplantation for hepatitis C: Analysis of factors determining outcome in over 500 patients. Ann Surg. 2001;234:384–94. doi: 10.1097/00000658-200109000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghobrial RM, Gornbein J, Steadman R, et al. Pretransplant model to predict posttransplant survival in liver transplant patients. Ann Surg. 2002;236:315–23. doi: 10.1097/00000658-200209000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen HR, Madden JP, Martin P. A model to predict survival following liver retransplantation. Hepatology. 1999;29:365–70. doi: 10.1002/hep.510290221. [DOI] [PubMed] [Google Scholar]
- 21.Neumann UP, Berg T, Bahra M, et al. Long-term outcome of liver transplants for chronic hepatitis C: A 10-year follow-up. Transplantation. 2004;77:226–31. doi: 10.1097/01.TP.0000101738.27552.9D. [DOI] [PubMed] [Google Scholar]
- 22.Canadian Organ Replacement Register/Canadian Institute for Health Information. 2002.
- 23.Baron PW, Sindram D, Higdon D, et al. Prolonged rewarming time during allograft implantation predisposes to recurrent hepatitis C infection after liver transplantation. Liver Transpl. 2000;6:407–12. doi: 10.1053/jlts.2000.7581. [DOI] [PubMed] [Google Scholar]
- 24.Watt KD, Lyden ER, Gulizia JM, McCashland TM. Recurrent hepatitis C post transplant: Early preservation injury may predict poor outcome. Liver Transpl. 2006;12:134–9. doi: 10.1002/lt.20583. [DOI] [PubMed] [Google Scholar]
- 25.Gaglio PJ, Malireddy S, Levitt BS, et al. Increased risk of cholestatic hepatitis C in recipients of grafts from living versus cadaveric liver donors. Liver Transpl. 2003;9:1028–35. doi: 10.1053/jlts.2003.50211. [DOI] [PubMed] [Google Scholar]
- 26.Bozorgzadeh A, Jain A, Ryan C, et al. Impact of hepatitis C viral infection in primary cadaveric liver allograft versus primary living-donor allograft in 100 consecutive liver transplant recipients receiving tacrolimus. Transplantation. 2004;77:1066–70. doi: 10.1097/01.tp.0000122142.00818.9e. [DOI] [PubMed] [Google Scholar]
- 27.Shiffman ML, Stravitz RT, Contos MJ, et al. Histologic recurrence of chronic hepatitis C virus in patients after living donor and deceased donor liver transplantation. Liver Transpl. 2004;10:1248–55. doi: 10.1002/lt.20232. [DOI] [PubMed] [Google Scholar]
- 28.Russo MW, Galanko J, Beavers K, Fried MW, Shrestha R. Patient and graft survival in hepatitis C recipients after adult living donor liver transplantation in the United States. Liver Transpl. 2004;10:340–6. doi: 10.1002/lt.20090. [DOI] [PubMed] [Google Scholar]
- 29.Girgrah N, Lilly L, Chen D, et al. Recurrence of hepatitis C following living donor vs deceased donor liver transplantation. Am J Transplant. 2005;5:1450A. (Abst) [Google Scholar]
- 30.Gane EJ, Naoumov NV, Qian KP, et al. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology. 1996;110:167–77. doi: 10.1053/gast.1996.v110.pm8536853. [DOI] [PubMed] [Google Scholar]
- 31.Magy N, Cribier B, Schmitt C, et al. Effects of corticosteroids on HCV infection. Int J Immunopharmacol. 1999;21:253–61. doi: 10.1016/s0192-0561(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 32.Papatheodoridis GV, Barton SG, Andrew D, et al. Longitudinal variation in hepatitis C virus (HCV) viraemia and early course of HCV infection after liver transplantation for HCV cirrhosis: The role of different immunosuppressive regimens. Gut. 1999;45:427–34. doi: 10.1136/gut.45.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Retortillo M, Forns X, Feliu A, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680–7. doi: 10.1053/jhep.2002.31773. [DOI] [PubMed] [Google Scholar]
- 34.Sreekumar R, Gonzalez-Koch A, Maor-Kendler Y, et al. Early identification of recipients with progressive histologic recurrence of hepatitis C after liver transplantation. Hepatology. 2000;32:1125–30. doi: 10.1053/jhep.2000.19340. [DOI] [PubMed] [Google Scholar]
- 35.Filipponi F, Callea F, Salizzoni M, et al. Double-blind comparison of hepatitis C histological recurrence Rate in HCV+ Liver transplant recipients given basiliximab + steroids or basiliximab + placebo, in addition to cyclosporine and azathioprine. Transplantation. 2004;78:1488–95. doi: 10.1097/01.tp.0000140881.07208.4e. [DOI] [PubMed] [Google Scholar]
- 36.Langrehr JM, Neumann UP, Lang M, et al. First results from a prospective randomized trial comparing steroid-free induction therapy with tacrolimus and MMF versus tacrolimus and steroids in patients after liver transplantation for HCV. Transplant Proc. 2002;34:1565–6. doi: 10.1016/s0041-1345(02)03024-5. [DOI] [PubMed] [Google Scholar]
- 37.Kato T, Yoshida H, Sadfar K, et al. Steroid-free induction and preemptive antiviral therapy for liver transplant recipients with hepatitis C: A preliminary report from a prospective randomized study. Transplant Proc. 2005;37:1217–9. doi: 10.1016/j.transproceed.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa M, Sakamoto N, Tanabe Y, et al. Suppression of hepatitis C virus replication by cyclosporine a is mediated by blockade of cyclophilins. Gastroenterology. 2005;129:1031–41. doi: 10.1053/j.gastro.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 39.Kakumu S, Takayanagi M, Iwata K, et al. Cyclosporine therapy affects aminotransferase activity but not hepatitis C virus RNA levels in chronic hepatitis C. J Gastroenterol Hepatol. 1997;12:62–6. doi: 10.1111/j.1440-1746.1997.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 40.Inoue K, Sekiyama K, Yamada M, Watanabe T, Yasuda H, Yoshiba M. Combined interferon alpha2b and cyclosporine A in the treatment of chronic hepatitis C: Controlled trial. J Gastroenterol. 2003;38:567–72. doi: 10.1007/s00535-002-1104-5. [DOI] [PubMed] [Google Scholar]
- 41.Martin P, Busuttil RW, Goldstein RM, et al. Impact of tacrolimus versus cyclosporine in hepatitis C virus-infected liver transplant recipients on recurrent hepatitis: A prospective, randomized trial. Liver Transpl. 2004;10:1258–62. doi: 10.1002/lt.20222. [DOI] [PubMed] [Google Scholar]
- 42.Levy G, Villamil F, Samuel D, et al. LIS2T Study Group Results of LIS2T, a multicenter, randomized study comparing cyclosporine microemulsion with C2 monitoring and tacrolimus with C0 monitoring in de novo liver transplantation. Transplantation. 2004;77:1632–8. doi: 10.1097/01.tp.0000129095.51031.42. [DOI] [PubMed] [Google Scholar]
- 43.Rosen HR, Chou S, Corless CL, et al. Cytomegalovirus viremia: risk factor for allograft cirrhosis after liver transplantation of hepatitis C. Transplantation. 1997;64:721–26. doi: 10.1097/00007890-199709150-00010. [DOI] [PubMed] [Google Scholar]
- 44.Chopra K, Demetris AJ, Blakolmer K, et al. Progression of liver fibrosis in patients with chronic hepatitis C after orthotopic liver transplantation. Transplantation. 2003;76:1487–91. doi: 10.1097/01.TP.0000088668.28950.7C. [DOI] [PubMed] [Google Scholar]
- 45.Charlton M, Ruppert K, Belle SH, et al. Long-term results and modeling to predict outcomes in recipients with HCV infection: Results of the NIDDK liver transplantation database. Liver Transpl. 2004;10:1120–30. doi: 10.1002/lt.20211. [DOI] [PubMed] [Google Scholar]
- 46.Burak KW, Kremers WK, Batts KP, et al. Impact of cytomegalovirus infection, year of transplantation, and donor age on outcomes after liver transplantation for hepatitis C. Liver Transpl. 2002;8:362–9. doi: 10.1053/jlts.2002.32282. [DOI] [PubMed] [Google Scholar]
- 47.Razonable RR, Burak KW, van Cruijsen H, et al. The pathogenesis of hepatitis C virus is influenced by cytomegalovirus. Clin Inf Dis. 2002;35:974–81. doi: 10.1086/342911. [DOI] [PubMed] [Google Scholar]
- 48.Humar A, Kumar D, Raboud J, et al. Interactions between cytomegalovirus, human herpesvirus-6 and the recurrence of hepatitis C after liver transplantation. Am J Transpl. 2002;2:461–6. doi: 10.1034/j.1600-6143.2002.20511.x. [DOI] [PubMed] [Google Scholar]
- 49.Firpi RJ, Abdelmalek MF, Soldevila-Pico C, et al. One-year protocol liver biopsy can stratify fibrosis progression in liver transplant recipients with recurrent hepatitis C infection. Liver Transpl. 2004;10:1240–7. doi: 10.1002/lt.20238. [DOI] [PubMed] [Google Scholar]
- 50.Teixeira R, Pastacladi S, Davies S, et al. The influence of cytomegalovirus viraemia on the outcome of recurrent hepatitis C after liver transplantation. Transplantation. 2000;70:1454–8. doi: 10.1097/00007890-200011270-00010. [DOI] [PubMed] [Google Scholar]
- 51.Singh N, Husain S, Carrigan DR, et al. Impact of human herpesvirus-6 on the frequency and severity of recurrent hepatitis C virus hepatitis in liver transplant recipients. Clin Transpl. 2002;16:92–6. doi: 10.1034/j.1399-0012.2002.1o096.x. [DOI] [PubMed] [Google Scholar]
- 52.Everson GT. Treatment of patients with hepatitis C virus on the waiting list. Liver Transpl. 2003;9:S90–4. doi: 10.1053/jlts.2003.50247. [DOI] [PubMed] [Google Scholar]
- 53.Everson GT, Trotter J, Forman L, et al. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255–62. doi: 10.1002/hep.20793. [DOI] [PubMed] [Google Scholar]
- 54.Crippin JS, McCashland T, Terrault N, Sheiner P, Charlton MR. A pilot study of the tolerability and efficacy of antiviral therapy in hepatitis C virus-infected patients awaiting liver transplantation. Liver Transpl. 2002;8:350–5. doi: 10.1053/jlts.2002.31748. [DOI] [PubMed] [Google Scholar]
- 55.Wiesner RH, Sorrell M, Villamil F, International Liver Transplantation Society Expert Panel Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9:S1–S9. doi: 10.1053/jlts.2003.50268. [DOI] [PubMed] [Google Scholar]
- 56.Chalasani N, Manzarbeitia C, Ferenci P, et al. Pegasys Transplant Study Group Peginterferon alfa-2a for hepatitis C after liver transplantation: Two randomized, controlled trials. Hepatology. 2005;41:289–98. doi: 10.1002/hep.20560. [DOI] [PubMed] [Google Scholar]
- 57.Mukherjee S, Rogge J, Weaver L, Schafer D. Pilot study of pegylated interferon alfa-2b and ribavirin for recurrent hepatitis C after liver transplantation. Transpl Proc. 2003;35:3042–4. doi: 10.1016/j.transproceed.2003.10.083. [DOI] [PubMed] [Google Scholar]
- 58.Dumortier J, Scoazec JY, Chevallier P, Boillot O. Treatment of recurrent hepatitis C after liver transplantation: A pilot study of peginterferon alfa-2b and ribavirin combination. J Hepatol. 2004;40:669–74. doi: 10.1016/j.jhep.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez-Luna H, Khatib A, Sharma P, et al. Treatment of recurrent hepatitis C infection after liver transplantation with combination of pegylated interferon alfa 2b and ribavirin: An open-label series. Transplantation. 2004;77:190–4. doi: 10.1097/01.TP.0000100481.14514.BB. [DOI] [PubMed] [Google Scholar]
- 60.Ross AS, Bhan AK, Pascual M, Thiim M, Benedict Cosimi A, Chung RT. Pegylated interferon alpha-2b plus ribavirin in the treatment of post-liver transplant recurrent hepatitis C. Clin Transplant. 2004;18:166–73. doi: 10.1046/j.1399-0012.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 61.Neff GW, Montalbano M, O’Brien CB, et al. Treatment of established recurrent hepatitis C in liver-transplant recipients with pegylated interferon-alfa-2b and ribavirin therapy. Transplantation. 2004;78:1303–7. doi: 10.1097/01.tp.0000129811.93072.1c. [DOI] [PubMed] [Google Scholar]
- 62.Toniutto P, Fabris C, Fumo E, et al. Pegylated versus standard interferon-alpha in antiviral regimens for post-transplant recurrent hepatitis C: Comparison of tolerability and efficacy. J Gastroenterol Hepatol. 2005;20:577–82. doi: 10.1111/j.1440-1746.2005.03795.x. [DOI] [PubMed] [Google Scholar]
- 63.Babatin M, Schindel L, Burak KW. Pegylated-interferon alpha 2b and ribavirin for recurrent hepatitis C after liver transplantation: From a Canadian experience to recommendations for therapy. Can J Gastroenterol. 2005;19:359–65. doi: 10.1155/2005/745197. [DOI] [PubMed] [Google Scholar]
- 64.Shergill AK, Khalili M, Straley S, et al. Applicability, tolerability and efficacy of preemptive antiviral therapy in hepatitis C-infected patients undergoing liver transplantation. Am J Transplant. 2005;5:118–24. doi: 10.1111/j.1600-6143.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 65.Bizollon T, Adham M, Pradat P, et al. Triple antiviral therapy with amantadine for IFN-ribavirin nonresponders with recurrent post transplantation hepatitis C. Transplantation. 2005;79:325–9. doi: 10.1097/01.tp.0000149499.78996.b3. [DOI] [PubMed] [Google Scholar]
- 66.Beckebaum S, Cicinnati VR, Zhang X, et al. Combination therapy with peginterferon alpha-2B and ribavirin in liver transplant recipients with recurrent HCV infection: Preliminary results of an open prospective study. Transplant Proc. 2004;36:1489–91. doi: 10.1016/j.transproceed.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 67.McHutchison JG, Manns M, Patel K, et al. International Hepatitis Interventional Therapy Group Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–9. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 68.Stravitz RT, Chung H, Sterling RK, et al. Antibody-mediated pure red cell aplasia due to epoetin alfa during antiviral therapy of chronic hepatitis C. Am J Gastroenterol. 2005;100:1415–9. doi: 10.1111/j.1572-0241.2005.41910.x. [DOI] [PubMed] [Google Scholar]
- 69.Afdhal NH, Dieterich DT, Pockros PJ, et al. Proactive Study Group Epoetin alfa maintains ribavirin dose in HCV-infected patients: A prospective, double-blind, randomized controlled study. Gastroenterology. 2004;126:1302–11. doi: 10.1053/j.gastro.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 70.Dieterich DT, Wasserman R, Brau N, et al. Once-weekly epoetin alfa improves anemia and facilitates maintenance of ribavirin dosing in hepatitis C virus-infected patients receiving ribavirin plus interferon alfa. Am J Gastroenterol. 2003;98:2491–9. doi: 10.1111/j.1572-0241.2003.08700.x. [DOI] [PubMed] [Google Scholar]
- 71.Younossi ZM, Ong JP, Collantes R, et al. Darbepoetin-alfa for ribavirin induced anemia in patients with chronic hepatitis C treated with pegylated interferon and ribavirin: A preliminary analysis. Gastroenterology. 2004;126:A666. (Abst) [Google Scholar]
- 72.Van Thiel DH, Faruki H, Friedlander L, et al. Combination treatment of advanced HCV associated liver disease with interferon and G-CSF. Hepatogastroenterology. 1995;42:907–12. [PubMed] [Google Scholar]
- 73.Fabrizi F, Poordad FF, Martin P. Hepatitis C infection and the patient with end-stage renal disease. Hepatology. 2002;36:3–10. doi: 10.1053/jhep.2002.34613. [DOI] [PubMed] [Google Scholar]
- 74.Meyers CM, Seeff LB, Stehman-Breen CO, Hoofnagle JH. Hepatitis C and renal disease: An update. Am J Kidney Dis. 2003;42:631–57. doi: 10.1016/s0272-6386(03)00828-x. [DOI] [PubMed] [Google Scholar]
- 75.Kamar N, Rostaing L, Selves J, et al. Natural history of hepatitis C virus-related liver fibrosis after renal transplantation. Am J Transplant. 2005;5:1704–12. doi: 10.1111/j.1600-6143.2005.00918.x. [DOI] [PubMed] [Google Scholar]
- 76.Morales JM. Hepatitis C virus infection and renal disease after renal transplantation. Transplant Proc. 2004;36:760–2. doi: 10.1016/j.transproceed.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 77.Mathurin P, Mouquet C, Poynard T, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29:257–63. doi: 10.1002/hep.510290123. [DOI] [PubMed] [Google Scholar]
- 78.Bruchfeld A, Wilczek H, Elinder CG. Hepatitis C infection, time in renal-replacement therapy, and outcome after kidney transplantation. Transplantation. 2004;78:745–50. doi: 10.1097/01.tp.0000131948.29742.24. [DOI] [PubMed] [Google Scholar]
- 79.Fabrizi F, Dulai G, Dixit V, Bunnapradist S, Martin P. Meta-analysis: Interferon for the treatment of chronic hepatitis C in dialysis patients. Aliment Pharmacol Ther. 2003;18:1071–81. doi: 10.1046/j.1365-2036.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- 80.Teta D, Luscher BL, Gonvers JJ, Francioli P, Phan O, Burnier M. Pegylated interferon for the treatment of hepatitis C virus in haemodialysis patients. Nephrol Dial Transplant. 2005;20:991–3. doi: 10.1093/ndt/gfh747. [DOI] [PubMed] [Google Scholar]
- 81.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase of hepatocellular carcinoma in the United Status: An update. Ann Intern Med. 2003;139:817. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 82.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: A retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 83.Zou S, Tepper M, El Saadany S. Prediction of hepatitis C burden in Canada. Can J Gastroenterol. 2000;14:575–80. doi: 10.1155/2000/642707. [DOI] [PubMed] [Google Scholar]
- 84.Figueras J, Ibanez L, Ramos E, et al. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: Results of a multicenter study. Liver Transpl. 2001;7:877–83. doi: 10.1053/jlts.2001.27856. [DOI] [PubMed] [Google Scholar]
- 85.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 86.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 87.Vivarelli M, Cucchetti A, Piscaglia F, et al. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: Key role of immunosuppression. Liver Transpl. 2005;11:497–503. doi: 10.1002/lt.20391. [DOI] [PubMed] [Google Scholar]
- 88.Law BK. Rapamycin: An anti-cancer immunosuppressant? Crit Rev Oncol Hematol. 2005;56:47–60. doi: 10.1016/j.critrevonc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 89.Kneteman NM, Oberholzer J, Al Saghier M, et al. Sirolimus-based immunosuppression for liver transplantation in the presence of extended criteria for hepatocellular carcinoma. Liver Transpl. 2004;10:1301–11. doi: 10.1002/lt.20237. [DOI] [PubMed] [Google Scholar]