Abstract
AIM:
To determine the test characteristics and the optimal cut-off point for the 13C urea breath test (13C UBT) in a Canadian community laboratory setting.
METHODS:
Of 2232 patients (mean age ± SD: 51±21 years, 56% female) who completed a 13C UBT, 1209 were tested to evaluate the primary diagnosis of Helicobacter pylori infection and 1023 were tested for confirmation of eradication following treatment. Cluster analysis was performed on the 13C UBT data to determine the optimal cut-off point and the risk of false-positive and false-negative results. Additionally, 176 patients underwent endoscopic biopsy to allow validation of the sensitivity and specificity of the 13C UBT against histology and microbiology using the calculated cut-off point.
RESULTS:
The calculated cut-off points were 3.09 δ‰ for the whole study population (n=2232), 3.09 δ‰ for the diagnosis group (n=1209) and 2.88 δ‰ for the post-treatment group (n=1023). When replacing the calculated cut-off points by a practical cut-off point of 3.0 δ‰, the risk of false-positive and false-negative results was lower than 2.3%. The 13C UBT showed 100% sensitivity and 98.5% specificity compared with histology and microbiology (n=176) for the diagnosis of active H pylori infection.
CONCLUSIONS:
The 13C UBT is an accurate, noninvasive test for the diagnosis of H pylori infection and for confirmation of cure after eradication therapy. The present study confirms the validity of a cutoff point of 3.0 δ‰ for the 13C UBT when used in a large Canadian community population according to a standard protocol.
Keywords: 13C urea breath test, Cut-off point, Helicobacter pylori
Abstract
OBJECTIF :
Déterminer les caractéristiques et les limites d’inclusion optimales du test respiratoire à l’urée marquée au 13C (TRUM 13C) dans un laboratoire communautaire canadien.
MÉTHODOLOGIE :
Des 2 232 patients (âge moyen de 51±21 ans, 56 % de sexe féminin) qui ont effectué un TRUM 13C, 1 209 avaient subi le test pour évaluer un diagnostic primaire d’infection par Helicobacter pylori et 1 023 pour en confirmer l’éradication après traitement. On a exécuté une analyse typologique des données du TRUM 13C pour déterminer la limite d’inclusion optimale et le risque de résultats faux positifs et faux négatifs. De plus, 176 patients ont subi une biopsie endoscopique afin de valider la sensibilité et la spécificité du TRUM 13C par rapport à l’histologie et à la microbiologie, au moyen de la limite d’inclusion calculée.
RÉSULTATS :
Les limites d’inclusion calculées étaient de 3,09 δ‰ pour toute la population à l’étude (n=2 232), de 3,09 δ‰ pour le groupe diagnostiqué (n= 1 209) et de 2,88 δ‰ pour le groupe déjà traité (n= 1023). Lorsqu’on remplaçait les limites d’inclusion calculées par une limite d’inclusion pratique de 3,0 δ‰, le risque de résultats faux positifs et faux négatifs était inférieur à 2,3 %. Le TRUM 13C s’associe à une sensibilité de 100 % et à une spécificité de 98,5 % par rapport à l’histologie et à la microbiologie (n=176) pour le diagnostic d’infection par H pylori active.
CONCLUSIONS :
Le TRUM 13C est un test précis et non effractif pour diagnostiquer l’infection par H pylori et en confirmer la guérison après une thérapie d’éradication. La présente étude confirme la validité d’utiliser une limite d’inclusion de 3,0 δ‰ pour le TRUM 13C au sein d’une vaste population du Canada, selon un protocole standard.
Helicobacter pylori infection is one of the most common human infections worldwide (1). This organism has been shown to infect over 50% of the world’s population, with a prevalence of 20% to 40% in the Canadian population and up to 80% in developing countries (2–5).
H pylori is the primary cause of gastritis and peptic ulcer disease, and has been associated with gastric lymphoma and adenocarcinoma (6,7). Since the discovery of its pivotal role in many human gastroduodenal pathologies, several diagnostic tests, both invasive and noninvasive, have been developed. Recently, it has been recommended that subjects younger than 45 years of age with abdominal discomfort should undergo a noninvasive and rapid diagnosis of H pylori infection, followed by pharmacological treatment if the test is positive (8). The 13C urea breath test (13C UBT), with a specificity of 98% and a sensitivity of 97%, allows the diagnosis of an active H pylori infection without the need of costly and invasive endoscopic testing (9). Thus, the 13C UBT has become the noninvasive test of choice in many jurisdictions for diagnosis and confirmation of eradication following treatment, as recommended by a number of clinical guidelines (8,10,11). The 13C UBT also has the advantage of assessing the global presence of H pylori throughout the stomach, whereas endoscopy-based tests are limited to focal assessments (at the site of biopsy) with the consequent risk of false-negative tests due to sampling errors (12).
The 13C UBT detects the presence of gastric H pylori urease, which hydrolyzes orally administered 13C-labelled urea (a stable isotope) and produces ammonia and 13C-labelled carbon dioxide (13CO2). The 13CO2 diffuses into the blood and is excreted by the lungs; therefore it can be detected in the breath using various methods. To distinguish between positive and negative results, a diagnostic cut-off value is defined at a specified time point after ingestion of a substrate. This cut-off point is generally determined by comparison with the ‘gold standard’ diagnostic technique (usually, histology and culture) in the affected population (13). However, there is still controversy about the value of the best cut-off point. The 13C UBT value may, for example, be affected by sociodemographic factors, concomitant medication and bacterial and host factors (14–18), leading to the possibility that the optimal cut-off point could be quite variable. Thus, before a test such as this is widely adopted, a comprehensive reassessment of the cut-off point is needed in the appropriate populations. Despite the need to validate the clinical performance of the 13C UBT in a Canadian population, until now, there have been no large-scale data available for this purpose. Validation of the 13C UBT’s performance in a community setting is essential before it is adopted as part of a primary care-based ‘test and treat’ strategy for dyspepsia management (19).
The objective of the present study was to determine the optimal cut-off value for the 13C UBT by cluster analysis in a large dataset from Canadian patients who had undergone testing in community laboratories, and to support it by independent validation against histology and culture in a Canadian setting using the same study protocol.
PATIENTS AND METHODS
The protocol of the present study was approved by the McMaster University Research Ethics Board. Results of 13C UBT performed on 2232 patients (mean age ± SD: 51±21 years, 56% female) were analyzed. Among them, 1209 patients (mean age: 49±17 years, 54% female) were tested to evaluate the primary diagnosis of H pylori infection (diagnosis group), and 1023 patients (mean age: 53±25 years, 57% female) were tested for confirmation of eradication following treatment (post-treatment group). Samples were collected through community laboratories (MDS Diagnostic Services, Toronto, Ontario) and analyzed at the McMaster University Medical Centre, Hamilton, Ontario. An additional 176 patients from the McMaster University Medical Centre had both 13C UBT and endoscopic biopsies to determine their H pylori status.
Using a standardized questionnaire, patients were asked about their use of antibiotics and acid-suppressive treatment during the four weeks before testing. Exclusion criteria included the use of proton pump inhibitors, bismuth compounds or antibiotics within 14 days before the 13C UBT. The 13C UBT was performed after an overnight fast by obtaining two breath samples, one before (T0) and the second 30 min after (T30) oral administration of 75 mg 13C-labelled urea (Helikit, Isotechnika Diagnostics, Canada) in 100 mL of citric acid solution. The samples were obtained by having patients gently exhale through a plastic straw, with its distal tip placed at the bottom of a 10 mL glass tube. The tube was sealed with a stopper immediately after patient exhalation. All samples were analyzed by a gas isotope ratio mass spectrometer (BreathMAT, Finnigan MAT GmbH, Germany). The difference between values at 30 min and at baseline (T30–T0) is expressed as delta (δ) over baseline (DOB, δ‰).
Data were examined visually by plotting the logarithmic transformation of measurements taken from 13C UBT. The distribution of the natural logarithms (logn) of the DOB values for each breath sample test interval identified two normal subpopulations that were considered to represent H pylori-positive and H pylori-negative patients. The normal distributions of the positive and negative populations allowed cluster analysis to be performed on these data to determine the minimal interclass variance, and thus, the logn (T30–T0) value, which best separated the presumed H pylori-negative and H pylori-positive populations. Thereafter, the parameters (mean DOB [δ‰], and SD) for the H pylori-negative and H pylori-positive populations were established. The cut-off value was calculated as the point equidistant between the mean DOB values of the H pylori-negative and H pylori-positive populations. This was the basis for determining the probability of an H pylori-negative patient producing a 13C UBT result above the cut-off point (ie, a false-positive result), and the probability of an H pylori-positive patient producing a 13C UBT result below the cut-off point (ie, a false-negative result). This analysis was performed on the whole patient group and on the subgroups (primary diagnosis and confirmation of cure after eradication therapy).
To compare the 13C UBT with histology and microbiology, upper gastrointestinal endoscopy was performed on 176 patients before the 13C UBT was administered. During an examination, three biopsy specimens each from the gastric antrum and corpus were obtained for histology and bacterial culture. Silver staining (Warthin-Starry) was used to identify H pylori in the specimens. Culture samples were inoculated in Brucella blood agar, Mueller-Hinton sheep blood agar and egg yolk emulsion; the plates were incubated at 35°C for four days. H pylori growth was confirmed by Gram-staining, a rapid urea test, an oxidase test and a wet preparation for motility. Negative cultures were reincubated and examined seven to 10 days later. H pylori status was determined when both methods (histology and culture) produced concordant results. Sensitivity, specificity and positive and negative predictive values for the 13C UBT were calculated using the optimal cut-off point.
RESULTS
Cut-off point determined by cluster analysis
Data were examined visually by plotting the logarithmic transformation of the DOB values obtained from 2232 13C UBT results. It was evident that the normal distributions of the DOB values could describe two distinct classes of results: H pylori-negative and H pylori-positive populations (Figure 1). From this, the means and SDs of the logn (T30–T0) for the presumed subpopulations were calculated. The point equidistant between the mean values of the subpopulations was determined to be an appropriate cut-off point. The calculated cutoff point for the whole study population was 3.09 δ‰. The distances (normal deviate) between the cut-off point and the means of the negative and positive H pylori distributions were calculated. The normal deviates were found to be greater than 2 SD (normal deviate = 2.28 SD), indicating that by comparison with the table of proportions of the normal curve, the proportions of H pylori-negative and H pylori-positive populations producing a 13C UBT result greater or smaller than the cut-off point were always lower than 2.3%. Thus, the risk of a false-positive or a false-negative result from the 13C UBT for a diagnosis of H pylori infection was lower than 2.3% when using the cut-off point 3.09 δ‰. Using a practical cut-off point of 3.0 δ‰, the corresponding normal deviates for the H pylori-negative and H pylori-positive distributions were still greater than 2 SD; therefore, according to the table of the proportions of the normal curve, the risks of error were still lower than 2.3%. Using a cut-off point of 3.0 δ‰, 73.3% of the population studied was negative and 26.7% was positive for H pylori infection.
Figure 1).
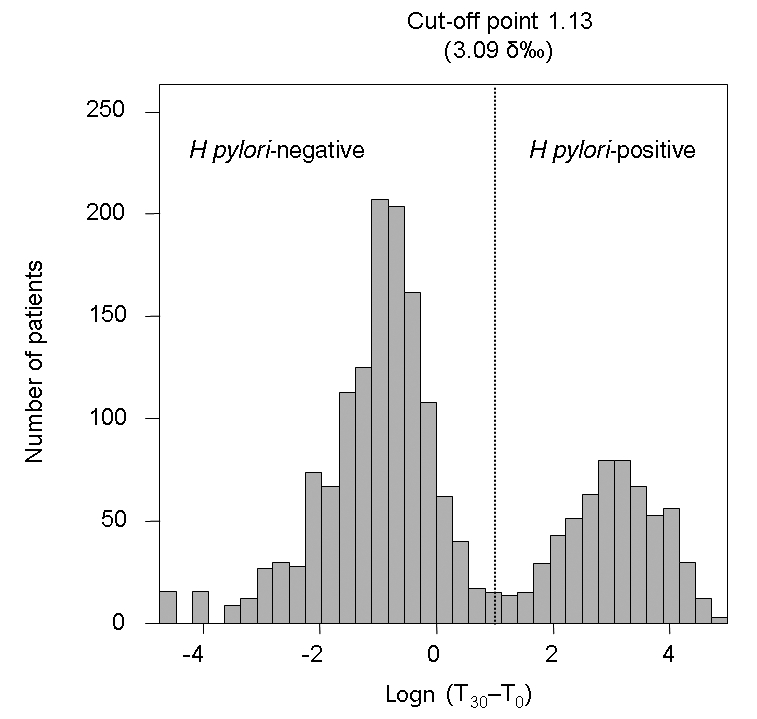
Cluster analysis in the whole study population. The histogram of the logarithmically transformed 13C urea breath test values shows two distinct populations: Helicobacter pylori-negative and H pylori-positive. The cut-off value was calculated as the point equidistant from the means of the H pylori-negative and H pylori-positive populations. Logn Natural logarithm; T0 Time (0 min) before oral administration; T30 Time (30 min) after oral administration
These calculations were also performed for the diagnosis and post-treatment groups. The calculated cut-off point for the 13C UBT was 3.09 δ‰ for the diagnosis group and 2.89 δ‰ for the post-treatment group (Figure 2). Replacing the cut-off points obtained in the two groups with 3.0 δ‰ (as determined for the whole population), the normal deviates for the H pylori-negative and H pylori-positive distributions in both groups were greater than 2 SD in all cases, which is again indicative of a risk lower than 2.3% for false-positive or false-negative results. Overall, 29.4% of patients were positive for H pylori infection in the diagnosis group and 22.4% were positive in the post-treatment group. There was no difference between women and men when a cut-off point of 3.0 δ‰ was used; the risk of error was lower than 2.3% in both groups.
Figure 2).
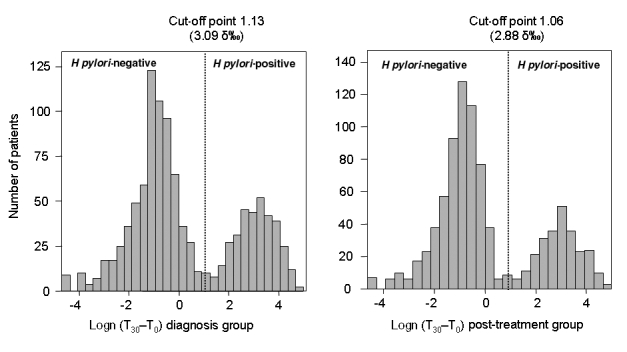
Cluster analysis in the diagnosis (left) and post-treatment (right) groups. The histograms of the logarithmically transformed 13C urea breath test values show two distinct populations: Helicobacter pylori-negative and H pylori-positive. The cut-off value was calculated as the point equidistant from the means of the H pylori-negative and H pylori-positive populations. Logn Natural logarithm; T0 Time (0 min) before oral administration; T30 Time (30 min) after oral administration
A cut-off point was also determined for different age groups. Although DOB values increased with age (Figure 3), using a cut-off point of 3.0 δ‰ showed a risk of error lower than 2.3% in the population older than 16 years of age. The cut-off point was not determined in patients younger than 16 years of age because of insufficient data.
Figure 3).
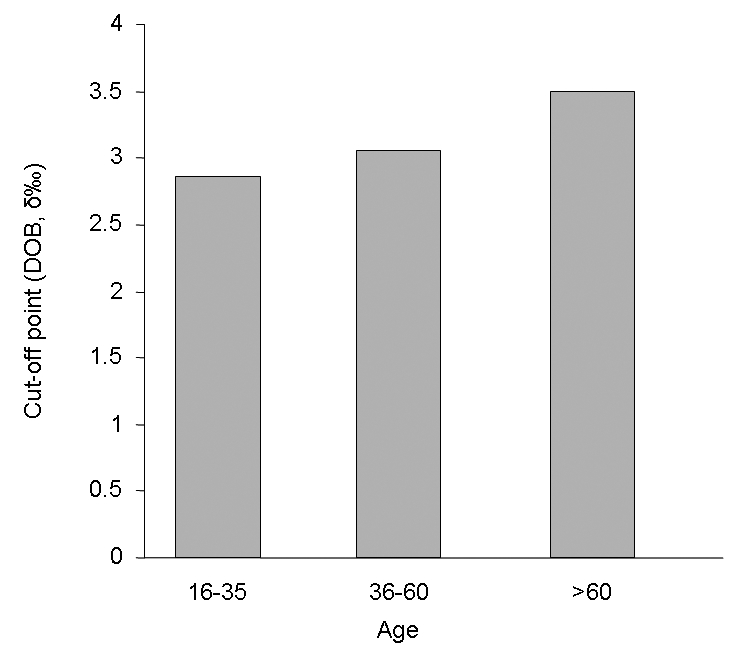
Cut-off points in different age (years) groups. The delta over baseline (DOB) value increased with age. When a cut-off point of 3.0 δ‰ in all age groups was used, the risk of error was lower than 2.3%. The cut-off point was not determined for patients younger than 16 years of age due to insufficient data
Validation of 13C UBT by histology and microbiology
In 176 patients, the 13C UBT results at a cut-off point of 3.0 δ‰ were compared with histology and culture (considered to be the gold standard). In this analysis, the 13C UBT showed a sensitivity of 100% and a specificity of 98.5% for the diagnosis of H pylori. Based on an H pylori incidence of 20% to 40% in the Canadian population (16), the positive predictive value of the 13C UBT would be 94.5% to 97.9% and the negative predictive value would be 100% (Figure 4).
Figure 4).
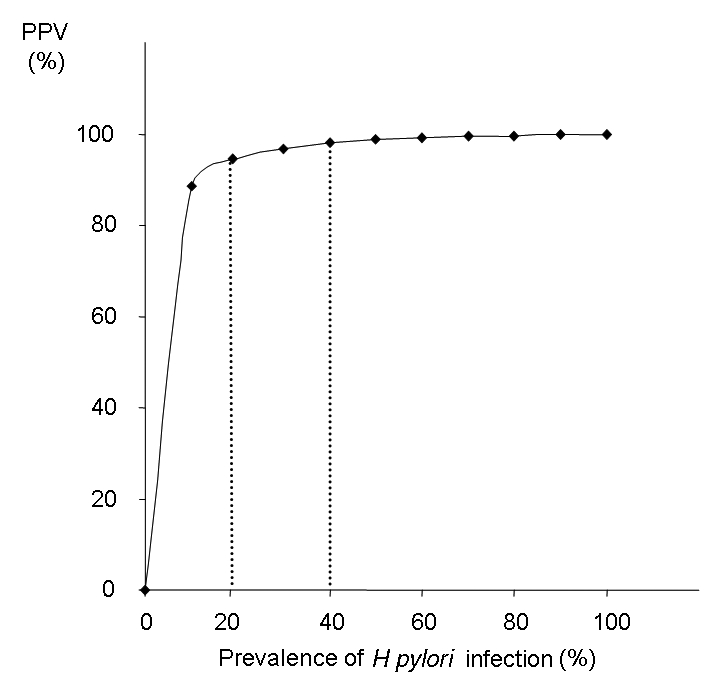
Positive predictive value (PPV) and negative predictive value for 13C urea breath test. Based on a prevalence between 20% to 40% of Helicobacter pylori infection in the Canadian population, the PPV would be between 94.5% and 97.9%, and the negative predictive value would be 100%
DISCUSSION
The 13C UBT is now widely used to document H pylori infection (20). The test has been recommended as the preferred method for epidemiological studies and for screening patients with dyspeptic symptoms (19). Because it is a test for active infection, it is also recognized as the best noninvasive test to assess the efficacy of anti-H pylori treatments (21,22).
Considering the increasing application of the 13C UBT and the fact that different test meals, fasting states, nationalities, bacterial and host factors, and concomitant medication use may affect this test, we assessed a large set of Canadian patients with dyspeptic symptoms to determine the cut-off point for the 13C UBT in community practice (14–18,23–25).
Using cluster analysis, we found that the optimal cut-off point for the 13C UBT for our population was 3.09 δ‰, which showed 100% sensitivity and 98.5% specificity compared with histology and culture. This is a lower cut-off point than that reported in earlier studies (T30–T0=5.0 δ‰) (26,27), but it has been confirmed by others in comparison with histology (28) and by cluster analysis in a large set of data (29). The risk of false-negative or false-positive results was less than 2.3% when a practical cut-off point of 3.0 δ‰ was used, a more than adequate efficacy for a noninvasive test. The risk of error was less than 2.3% when the same cut-off point was used in the groups that came for diagnosis and for confirmation of cure; thus, depending on the clinical situation (ie, before or after treatment), it appears unnecessary to use different cut-off points. Like others (29), we recommend an indeterminate zone (2.5 δ‰ to 3.5 δ‰) for the 13C UBT, in which a second test would be recommended to assess a patient’s H pylori status more accurately rather than adhering to a very strict cut-off point. This intermediate zone would take into account spontaneous variations of respiratory 13CO2 related to fasting, a patient’s metabolism and the limits of analytical precision of 13CO2 measurements. Only 0.62% (14/2232) of patient results fell in this indeterminate zone (Table 1).
TABLE 1.
Calculated cut-off point and risk of error in the whole study population and in the diagnosis and post-treatment groups
| Patients, n | Optimal cut-off point, δ‰ | Risk of error with optimal cut-off point, % | Risk of error with cut-off point at 3.0 δ‰, % | Number of results in zone 2.5–3.5 δ‰, n (%) | |
|---|---|---|---|---|---|
| All patients | 2232 | 3.09 | <2.3 | <2.3 | 14 (0.62) |
| Diagnosis group | 1209 | 3.09 | <2.3 | <2.3 | 10 (0.82) |
| Post-treatment group | 1023 | 2.88 | <2.3 | <2.3 | 4 (0.39) |
CONCLUSION
The 13C UBT is an accurate, noninvasive test for the diagnosis of H pylori infection and for the confirmation of cure after eradication therapy. The present study confirms the validity of a cut-off point of 3.0 δ‰ for a Canadian community population using a standard protocol. Thus, the 13C UBT is a practical and accurate test on which to base a ‘test and treat’ strategy for the management of dyspepsia in community practice in Canada. These data provide further support for making the 13C UBT more widely available in community practice because it is convenient, accurate and likely more cost-effective than endoscopy or H pylori serology (30).
Acknowledgments
The authors thank Dr Fiona Smaill and Pamela Lyn for their invaluable assistance. The work of author Markad Kamath was supported by the DeGroote Foundation and the Natural Sciences and Engineering Research Council of Canada (NSERC).
REFERENCES
- 1.The EUROGAST Study Group Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. Gut. 1993;34:1672–6. doi: 10.1136/gut.34.12.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 3.Rothenbacher D, Brenner H. Burden of Helicobacter pylori and H. pylori-related diseases in developed countries: Recent developments and future implications. Microbes Infect. 2003;5:693–703. doi: 10.1016/s1286-4579(03)00111-4. [DOI] [PubMed] [Google Scholar]
- 4.Frenck RW, Jr, Clemens J. Helicobacter in the developing world. Microbes Infect. 2003;5:705–13. doi: 10.1016/s1286-4579(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 5.Hunt R, Fallone C, Veldhuyzen van Zanten S, et al. CHSG 2004 participants. Canadian Helicobacter Study Group Consensus Conference: Update on the management of Helicobacter pylori – an evidence-based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infection. Can J Gastroenterol. 2004;18:547–54. doi: 10.1155/2004/326767. [DOI] [PubMed] [Google Scholar]
- 6.McColl KE, el-Omar E. Helicobacter pylori and disturbance of gastric function associated with duodenal ulcer disease and gastric cancer. Scand J Gastroenterol. 1996;215(Suppl 31):32–7. doi: 10.3109/00365529609094531. [DOI] [PubMed] [Google Scholar]
- 7.Imrie C, Rowland M, Bourke B, Drumm B. Is Helicobacter pylori infection in childhood a risk factor for gastric cancer? Pediatrics. 2001;107:373–80. doi: 10.1542/peds.107.2.373. [DOI] [PubMed] [Google Scholar]
- 8.Malfertheiner P, Megraud F, O’Morain C, et al. European Helicobacter Pylori Study Group Current concepts in the management of Helicobacter pylori infection – The Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–80. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 9.Logan RP. Breath tests used to detect Helicobacter pylori. In: Goodwin CS, Worsley BW, editors. Helicobacter pylori: Biology and Clinical Practice. Boca Raton: CRC Press; 1993. pp. 307–27. [Google Scholar]
- 10.Hunt RH, Fallone CA, Thomson AB, Canadian Helicobacter Study Group Canadian Helicobacter pylori Consensus Conference update: Infections in adults. Can J Gastroenterol. 1999;13:213–7. doi: 10.1155/1999/180751. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Masaoka T, Nomura S, et al. Current consensus on the diagnosis and treatment of H pylori-associated gastroduodenal disease. Keio J Med. 2003;52:163–73. doi: 10.2302/kjm.52.163. [DOI] [PubMed] [Google Scholar]
- 12.Koletzko S, Haisch M, Seeboth I, et al. Isotope selective non-dispersive infrared spectrometry for detection of Helicobacter pylori infection with 13C-urea breath test. Lancet. 1995;345:961–2. doi: 10.1016/s0140-6736(95)90704-1. [DOI] [PubMed] [Google Scholar]
- 13.Graham DY, Klein PD. Accurate diagnosis of Helicobacter pylori: 13C-urea breath test. Gastroenterol Clin North Am. 2000;29:885–93. doi: 10.1016/s0889-8553(05)70156-4. [DOI] [PubMed] [Google Scholar]
- 14.Perri F, Ricciardi R, Merla A, et al. Appropriateness of urea breath test: A prospective observational study based on Maastricht 2000 guidelines. Aliment Pharmacol Ther. 2002;16:1443–7. doi: 10.1046/j.1365-2036.2002.01315.x. [DOI] [PubMed] [Google Scholar]
- 15.Bode G, Rothenbacher D, Brenner H, Adler G. Variation in the 13C-urea breath test value by nationality in Helicobacter pylori-infected children. Scand J Gastroenterol. 1998;33:468–72. doi: 10.1080/00365529850172016. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Haruma K, Kamada T, et al. Factors that affect results of the 13C urea breath test in Japanese patients. Helicobacter. 2000;5:98–103. doi: 10.1046/j.1523-5378.2000.00015.x. [DOI] [PubMed] [Google Scholar]
- 17.Adachi K, Fujishiro H, Mihara T, Komazawa Y, Kinoshita Y. Influence of lansoprazole, famotidine, roxatidine and rebamipide administration on the urea breath test for the diagnosis of Helicobacter pylori infection. J Gastroenterol Hepatol. 2003;18:168–71. doi: 10.1046/j.1440-1746.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- 18.Lai YC, Wang TH, Huang SH, et al. Density of Helicobacter pylori may affect the efficacy of eradication therapy and ulcer healing in patients with active duodenal ulcers. World J Gastroenterol. 2003;9:1537–40. doi: 10.3748/wjg.v9.i7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veldhuyzen van Zanten SJ, Bradette M, Chiba N, et al. Canadian Dyspepsia Working Group Evidence-based recommendations for short- and long-term management of uninvestigated dyspepsia in primary care: An update of the Canadian Dyspepsia Working Group (CanDys) clinical management tool. Can J Gastroenterol. 2005;19:285–303. doi: 10.1155/2005/674607. [DOI] [PubMed] [Google Scholar]
- 20.Atherton JC, Spiller RC. The urea breath test for Helicobacter pylori. Gut. 1994;35:723–5. doi: 10.1136/gut.35.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slomianski A, Schubert T, Cutler AF. [13C]-urea breath test to confirm eradication of Helicobacter pylori. Am J Gastroenterol. 1995;90:224–6. [PubMed] [Google Scholar]
- 22.Mion F, Rousseau M. Diagnostic test to document Helicobacter pylori eradication. Gastroenterology. 1996;110:324–5. doi: 10.1053/gast.1996.v110.agast960324b. [DOI] [PubMed] [Google Scholar]
- 23.Kato C, Sugiyama T, Sato K, et al. Appropriate cut-off value of 13C-urea breath test after eradication of Helicobacter pylori infection in Japan. J Gastroenterol Hepatol. 2003;18:1379–83. doi: 10.1046/j.1440-1746.2003.03193.x. [DOI] [PubMed] [Google Scholar]
- 24.Logan RP, Polson RJ, Misiewicz JJ, et al. Simplified single-sample 13Carbon urea breath test for Helicobacter pylori: Comparison with histology, culture, and ELISA serology. Gut. 1991;32:1461–4. doi: 10.1136/gut.32.12.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gatta L, Vakil N, Ricci C, et al. A rapid, low-dose, 13C urea tablet for the detection of Helicobacter pylori infection before and after treatment. Aliment Pharmacol Ther. 2003;17:793–8. doi: 10.1046/j.1365-2036.2003.01490.x. [DOI] [PubMed] [Google Scholar]
- 26.Eggers RH, Kulp A, Tegeler R, et al. A methodological analysis of the 13C-urea breath test for the detection of Helicobacter pylori infections: High sensitivity and specificity with 30 min using 75 mg of 13C-urea. Eur J Gastroenterol Hepatol. 1990;2:437–44. [Google Scholar]
- 27.Logan R, Dill S, Bauer F, et al. The European 13C-urea breath test for the detection of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1991;3:915–21. [Google Scholar]
- 28.Mion F, Delecluse HJ, Rosseau M, Berger F, Brazier JL, Minaire Y. [13C-urea breath test for the diagnosis of Helicobacter pylori infection. Comparison with histology] Gastroenterol Clin Biol. 1994;18:1106–11. [PubMed] [Google Scholar]
- 29.Mion F, Rosner G, Rousseau M, Minaire Y. 13C-urea breath test for Helicobacter pylori: Cut-off point determination by cluster analysis. Clin Sci (Lond) 1997;93:3–6. doi: 10.1042/cs0930003. [DOI] [PubMed] [Google Scholar]
- 30.Marshall JK, Armstrong D, O’Brien BJ. Test and treat strategies for Helicobacter pylori in uninvestigated dyspepsia: A Canadian economic analysis. Can J Gastroenterol. 2000;14:379–88. doi: 10.1155/2000/978035. [DOI] [PubMed] [Google Scholar]


