Abstract
Objectives
To study the association between preeclampsia and cancer incidence.
Study Design
The Jerusalem Perinatal Study is a population-based cohort of all births to 41,206 residents of Western Jerusalem in 1964-76. Cancer incidence to 2004 was assessed by linkage of the cohort with the Israel Cancer Registry. Cox’s proportional hazards models were constructed to estimate the hazard ratio (HR) for cancer among women who had had preeclampsia.
Results
Preeclampsia was associated with a 1.23-fold increased risk of cancer at all sites, a 37% increased risk of breast cancer, and more than a doubling of ovarian cancer risk. Analysis by morphology yielded significantly increased risks for malignancies classed as cystic mucinous and serous (RR:1.96, 95% Confidence interval:1.00-3.83), and for ductal, lobular and medullary carcinomas (1.40, 1.07-1.83). No differential association was observed by sex of offspring.
Conclusions
Our study suggests that the previously-described protective effect of preeclampsia on cancer is not universal.
Keywords: preeclampsia, cancer, risk, cohort, gender, morphology
Introduction
A growing body of evidence suggests an association between obstetric events and long-term morbidity and mortality of mothers. Several case-control studies from the US 1–4 and a cohort study from Norway 5 suggested a decrease in breast cancer risk for women with a history of preeclampsia. Recently, two reports suggested that this protective effect was evident only among preeclamptic women who gave birth to male offspring 6, 7. Another report from Norway suggested a decreased risk of all-cancer mortality for women who were diagnosed with preeclampsia and had a preterm delivery 8. Similarly, in a recently published retrospective cohort study from Utah9, preeclampsia was associated with an 8% decrease in risk of cancer at all sites, and a statistically decreased risk of papillary and squamous cell carcinomas (Hazard Ratio (HR): 0.74, 95% Confidence Interval (CI): 0.58-0.94), irrespective of site.
In contrast to these reports, our group previously published findings from the Jerusalem Perinatal Study 10, suggesting that women who experienced preeclampsia were at increased risk of cancer at all sites (HR: 1.27, 95% CI: 1.03-1.57), as well as cancers of the breast (1.38, 1.00-1.89), ovary, (2.32, 1.01-5.34), stomach (3.10, 1.23-7.84), and lung or larynx (2.81, 1.12-7.05).
We recently updated our cancer incidence data up to December 31 2004, adding 175,625 person-years of follow up. In view of the recent studies 6, 7, 9, we aimed to study the association between preeclampsia and cancer incidence and to further our analysis by morphologic tumor type and gender of offspring.
Materials and Methods
The Jerusalem Perinatal Study is a population-based research cohort of all births to residents of Western Jerusalem and its surroundings between 1964 and 1976. The database includes demographic, obstetric and neonatal information on 92,408 births and 41,206 mothers. Detailed information on data collection has been previously described 11. Briefly, information on all births was copied from birth notifications, and for 92% of participants data were also abstracted from maternity ward logbooks. Thus, information on pregnancy complications was complete for 37,927 women. During the cohort inception preeclampsia was defined as hypertension (systolic blood pressure of >140mmHg and/or diastolic blood pressure of >90mmHg), proteinuria, and edema. Information was recorded per birth with mother’s and offspring’s identity numbers (IDs). These IDs enabled data linkage with the Israel Population Registry to ascertain vital status and to validate demographic characteristics of mothers and offspring. These IDs also enabled linkage with the Israel Cancer Registry to ascertain cancer diagnosis as of December 31, 2004. The Israel Cancer Registry receives notification on all malignancies (except for non-melanoma skin cancer) throughout the country. Since 1981, reporting of cases has been mandatory by law, but reporting was considered relatively complete even before this. Cancer diagnoses were coded according to the International Classification of Diseases—Oncology (ICDO-3).
Statistical Analysis
To compare the risk of cancer between women with and without a history of preeclampsia, we constructed Cox proportional hazards models. Time of follow up was counted from first birth in the cohort until diagnosis of cancer, death, or December 31, 2004. We analyzed the incidence of all-cancers as well as site-specific cancer, where the number of events exceeded 30. These included malignancies of the stomach (ICDO codes 16.0-16.9), colon and rectum (18.0-20.9), pancreas (25.0-25.9), lung or larynx (32.0-34.9), malignant melanoma (morphologic codes 87202-87743), breast (ICDO codes 50.0-50.9), uterus (54.0-55.9), uterine cervix (53.0-53.9), ovary (56.0-56.9), bladder (67.0-67.9), kidney (64.0-64.9), brain (70.0, 71.0-72.9, 75.1, 75.2, ICDO code 30.0 with morphologic code 95223, and ICDO code 75.3 with morphologic code 93611), thyroid (73.0-73.9), Hodgkin’s lymphoma (morphologic codes 96503-96673), non-Hodgkin lymphoma (morphologic codes 95903-96502, 96674-97143, 97273), and leukemia (morphologic codes 98003-99403).
All hazard ratios (HR, equivalent to relative risk) were adjusted for age at first birth in the cohort as a continuous variable, except for breast cancer where there was an additional adjustment for parity (categorized into 1, 2-3, and >4 children). We further conducted an analysis of all cancer by morphology (irrespective of cancer site), as described by Aagaard-Tillery et al. 9.
Restricting our study population to primiparous women, we repeated our models for the association between preeclampsia in the first pregnancy and the later development of cancer, stratified by gender of the first offspring. We also constructed unstratified models including gender of offspring and an interaction term between sex of offspring and preeclampsia in determining the HR of cancer.
The study was approved by the Institutional Review Boards in Hadassah-Hebrew University and Columbia University.
Results
During 1,271,313 person-years of follow up (mean: 33.52, SD: 6.03 years), 3,914 mothers were diagnosed with cancer. Compared with mothers who had not developed cancer, mothers who were diagnosed with cancer were older at first birth (28.1+5.9 vs. 26.0+5.6 years) and had a higher parity by the end of cohort assembly (37.5% vs. 32.4% had > 4 children).
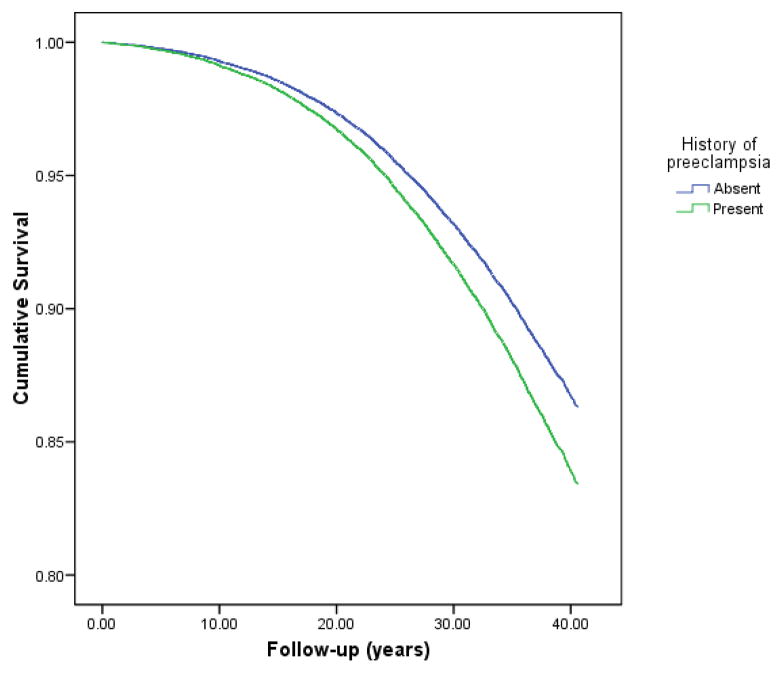
Survival curves by time from first birth and by history of preeclampsia are presented in Figure. Mothers who had preeclampsia (n=1,107) were at increased risk of cancer at any site (HR: 1.23, 95% CI: 1.05-1.45). Preeclampsia was associated with a 37% increase in risk of breast cancer (1.37, 1.06-1.78), and more than a doubled risk for ovarian cancer (2.59, 1.35-4.94). Our previous findings of increased risks for cancer of the stomach, lung and bronchus were attenuated [Table].
Figure.
Survival curves for women followed from birth, by history of preeclampsia, according to the Cox Proportional Hazards model, controlling for age at first birth
Table.
Number of cases, Hazard-Ratios of cancer (overall, by site, and by morphology) by history of preeclampsia among women who gave birth in West Jerusalem in 1964–76 and were followed until 2004.
| History of Preeclampsia | Age-adjusted HR | 95% CI | P | ||
|---|---|---|---|---|---|
| Absent | Present | ||||
| Person years | 1234847 | 36552 | |||
| Cases (n) | Cases (n) | ||||
| All malignant neoplasms | 3767 | 147 | 1.23 | 1.05–1.45 | 0.013 |
| By site | |||||
| Breast* | 1561 | 63 | 1.37 | 1.06–1.78 | 0.016 |
| Colon/Rectum | 354 | 11 | 0.94 | 0.52–1.71 | 0.84 |
| Malignant Melanoma | 212 | 5 | 0.77 | 0.32–1.89 | 0.56 |
| Uterus | 178 | 5 | 0.90 | 0.37–2.19 | 0.82 |
| Brain | 169 | 4 | 0.78 | 0.29–2.10 | 0.61 |
| Thyroid | 168 | 5 | 1.01 | 0.41–2.45 | 0.99 |
| Non-Hodgkin lymphoma | 146 | 6 | 1.34 | 0.59–3.04 | 0.48 |
| Ovary | 120 | 10 | 2.59 | 1.35–4.94 | 0.004 |
| Lung or larynx | 112 | 7 | 1.95 | 0.91–4.19 | 0.09 |
| Cervix | 86 | 0 | - | - | - |
| Stomach | 72 | 5 | 2.11 | 0.85–5.23 | 0.11 |
| Leukemia | 58 | 0 | - | - | - |
| Pancreas | 51 | 3 | 1.62 | 0.50–5.20 | 0.42 |
| Bladder | 48 | 3 | 1.91 | 0.61–6.15 | 0.28 |
| Kidney | 46 | 2 | 1.25 | 0.30–5.15 | 0.76 |
| Hodgkin’s lymphoma | 28 | 2 | 2.49 | 0.59–10.5 | 0.21 |
| By Morphology | |||||
| Neoplasia NOS | 140 | 4 | 0.97 | 0.36–2.61 | 0.95 |
| Epithelial NOS | 196 | 11 | 1.90 | 1.04–3.49 | 0.04 |
| Papillary and Squamous | 233 | 7 | 1.02 | 0.48–2.16 | 0.96 |
| Transitional | 12 | 0 | - | - | - |
| Adenomas and adenocarcinomas | 738 | 28 | 1.28 | 0.88–1.87 | 0.20 |
| Cystic mucinous and serous neoplasms | 156 | 9 | 1.96 | 1.00–3.83 | 0.05 |
| Ductal, lobular and medullary | 1338 | 55 | 1.40 | 1.07–1.83 | 0.02 |
| Complex epithelial neoplasms | 5 | 0 | - | - | - |
| Malignant melanoma | 213 | 5 | 0.79 | 0.33–1.93 | 0.61 |
| Fibromatous neoplasms | 13 | 0 | - | - | - |
| Myomatous neoplasms | 20 | 0 | - | - | - |
| Complex mixed and stromal neoplasms | 18 | 2 | 3.79 | 0.88–16.3 | 0.08 |
| Glioma | 39 | 3 | 2.62 | 0.81–8.47 | 0.11 |
| Lymphoma, Hodgkin, nodular or follicular | 120 | 7 | 1.97 | 0.92–4.22 | 0.08 |
| Plasma cell | 2 | 0 | - | - | - |
| Leukemias | 52 | 0 | - | - | - |
HR – Hazard Ratio
CI – Confidence Interval
Stratifying our analysis by ethnic origin, according to birthplace of women’s father, preeclampsia was associated with an increased risk of cancer especially among Jewish women of Western-Asian origin (HR: 1.37, 95% CI: 1.02-1.83) and Israeli origin (1.53, 1.03-2.29). Jewish women of North-African origin as well as Non-Jewish women who had preeclampsia had non-significantly increased risks for cancer [HRs of 1.21, 0.82-1.81, and 1.60, 0.38-6.80, respectively]. No association between preeclampsia and risk of cancer was found among women of European origin (1.04, 0.78-1.39) (p for heterogeneity across origin groups=0.565).
Analysis of all cancer by morphology code, yielded significantly increased risks for malignancies classed as epithelial NOS (HR:1.90, 95% CI: 1.04-3.49), cystic mucinous and serous (1.96, 1.00-3.83), as well as for ductal, lobular and medullary carcinomas (1.40, 1.07-1.83). No association was found between preeclampsia and the incidence of papillary and squamous cell carcinomas (1.02, 0.48-2.16) [Table].
Restricting our analysis to primiparous women (n=23,204), women who had had preeclampsia in their first pregnancy (n=394) had an increased risk of cancer at any site (1.53, 1.16-2.00) as well as of cancer of the breast (1.38, 0.91-2.11), and ovary (3.93, 1.58-9.79). Women who experienced preeclampsia in their first pregnancy and gave birth to a male offspring had a 45% increased risk of cancer at any site (1.45, 0.99-2.11), while those who had a female offspring had a 63% increased risk (1.63, 1.10-2.40; p for interaction=0.639). Similarly, no differential association was found between preeclampsia and risk of breast cancer by gender of the first offspring (male offspring:1.20, 0.66-2.19; female offspring: 1.62, 0.89-2.95; p for interaction=0.529).
Comment
As opposed to the protective effect seen in Norway and the United States, our repeated analysis suggested an increased risk of cancer among Israeli women who experienced preeclampsia. This increased risk was found to be considerably high for ovarian cancer.
We did not replicate the results of Aagaard-Tillery et al. 9 regarding papillary or squamous carcinoma, and in fact found an overall increased risk of ductal, lobular and medullary carcinomas that was similar in magnitude and attributed mainly to the increased risk of breast cancer shown in the analysis by cancer site. Similarly, the increased risk of cystic mucinous and serous neoplasms mostly represents the increased risk of ovarian cancer.
In this study, a differential association was not found between preeclampsia and risk of breast cancer by gender of offspring. Thus, the results of Troisi et al. 6 and Vatten et al.7 were not confirmed. However, Troisi et al 6 found a differential association by gender only among women who had their first birth after the age of 30 years, whereas for the whole population there was no differential association. In our study, only six percent of primiparous women gave birth after the age of 30 and there was no differential association in this age group [not shown].
Some of the differences between this and other studies might be attributed to differences in classifications of exposure (or definitions of preeclampsia). In this study, women with preeclampsia were compared to women without preeclampsia, and other hypertensive disorders were not considered as exposures, unlike the cohort study from Norway 5. The association between hypertensive disorders of pregnancy and risk of subsequent cancer might be different for the various diseases, as been suggested by a case-control study of breast cancer and hypertensive diseases of pregnancy 12, where preeclamptic women had a non-significant OR of 1.31, whereas hypertension of pregnancy alone suggested a decreased risk (OR: 0.79, 95% CI 0.40–1.57).
Alternatively, these discrepancies between studies might stem from differences in study populations and differential risks among populations. Previously-studied populations were predominantly of European origin; ours, while also Caucasian, includes somewhat distinct ethnic groups, consisting predominantly of Jews who arrived to Israel from communities in various countries – mainly from West-Asia, North-Africa, and Europe - where they had been genetically isolated for hundreds of years. These ethnic groups have different incidence rates of breast and ovarian cancers 13, 14. The genetic susceptibility for breast and ovarian cancer among Ashkenazi Jews has been well documented, especially regarding mutations in the BRCA1/2 genes15, 16, however, we have recently reported an increased risk of breast cancer among young women of West-Asian origin which was about twice the risk of women of European-American origin 17. Interestingly, the effect of preeclampsia on cancer risk in Jewish women of European origin was null in our study, whereas it seemed to be the strongest among women of West-Asian origin. It is therefore possible that there are genetic differences and founder effects in the different cohorts which may be related on one hand to preeclampsia and on the other hand to cancer incidence.
Data on smoking and obesity were available for less than half of the women, precluding adjustment by these variables. However, for a subcohort (n=15036 mothers) for which data on smoking existed, inclusion of smoking in the models did not affect the results (not shown). Lack of data on smoking and obesity is also a limitation of the other cohort studies 5, 8, 9; therefore, residual confounding as a source for the discrepancies between studies cannot be excluded.
Limitations of this study include a possible misclassification of exposure, as the criteria used to diagnose preeclampsia included edema; therefore, it is possible that women who experienced preeclampsia according to the current definition were included in the control, unexposed, group in our analysis. These women were probably included in a group labeled as ‘other maternal conditions’, together with women who had had other diseases and other hypertensive disorders during pregnancy. To try and assess the effect of such misclassification, we conducted an analysis excluding women with’other maternal conditions’, and the results were not materially altered [not shown]. We also lacked data on gestational age for the majority of study population, precluding analysis by term/preterm preeclampsia. Using birthweight as a proxy for preterm deliveries, the exclusion of births with low-birthweight (<2500gr) offspring did not affect the results [not shown].
Our study’s main strength is in the population-based nature of data, the active surveillance for preeclampsia during cohort assembly, and the longitudinal design.
In summary, the protective effect of preeclampsia on cancer incidence is not universal. Whether genetic factors play a role in the association of preeclampsia and cancer is yet to be determined. Future studies from Middle-Eastern and non-European countries are warranted to clarify this issue.
Acknowledgments
This study was funded by the National Institute of Health (RO1 CA80197).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.