Abstract
Mannose-binding lectin (MBL), a pattern recognition innate immune molecule, selectively binds distinct chemical patterns, including carbohydrates expressed on Group B streptococcus (GBS). MBL interacts with IgM, resulting in the activation of MBL-associated serine proteases (MASPs), thus is initiating a lectin complement pathway. Complement proteins and IgM modulate production of antigen specific antibody. In this study, we investigated the relative effect of MBL in antibody response against tetanus toxoid-conjugated GBS polysaccharide vaccines (GBS PS-TT) by comparing wild type and null mice for MBL, complement 3 (C3), IgM, MBL/C3, and MBL/IgM. We found that GBS PS specific IgG response was upregulated in MBL deficient mice following immunization with GBS PS-TT but not GBS PS. B1 cells were expanded in peritonium but not in spleen of MBL null mice. The mechanisms of heightened IgG response in MBL null mice were related to C3, and share the same pathway with IgM.
Keywords: Mannose-binding lectin, complement, IgM, vaccine, antibody response, Goup B streptococcus, polysaccharide
Introduction
Group B Steptococcus (GBS) can cause serious infection in newborns and infants. Serotype III GBS, among 9 serotypes distinguished by their capsular polysaccharides (PS), is the most prevalent. At present, the most widely utilized protective measure is vaccination of the mother during pregnancy, however, a wide range of efficacy [1; 2] leads to additional challenges for clinical therapy. Even after enhancement of the vaccine by conjugation of GBS capsular PS with tetanus toxid (TT), there are still individuals with poor immunological response as assessed by specific antibody production [1; 2]. This finding suggests that the immune response and antibody production may be influenced by host factors involving genetic and immunologic factors. In this regard, it has been found that mice lacking complement 3 (C3) are impaired in IgG responses to GBS PS, which is characterized as T cell independent type 2 antigen (TI2 antigen) while TT-conjugated GBS PS is a T cell dependent antigen (TD antigen) [3].
Mannose-binding lectin (MBL), a pattern recognition molecule of the innate immune system, selectively binds a wide rang of chemical motifs, including carbohydrates expressed on a variety of human microbial pathogens. The human MBL (hMBL) gene has polymorphisms in the coding region, promoter and untranslated 3′ region, combinations of which produce aberrant protein and/or reduce blood concentration. Low MBL serum levels and low MBL secretory haplotypes have been associated with increased susceptibility to infections in many clinical cohorts [4] [5; 6]. Some of these clinical observations have been confirmed by murine infection studies using MBL deficient mice that we have generated [4]. Recent studies have demonstrated that MBL cooperates with other molecules of the innate immune system, suggesting a broader role for MBL in immunity and inflammation [7]. MBL interacts with IgM and the complement system to induce tissue damage, and through activation of MBL-associated serine proteases (MASPs) the lectin complement pathway, distinct from the classical or alternative complement pathways, is initiated. Soluble innate immune molecules, including several complement proteins and IgM, have been shown to influence antibody productions [8]. A salient question that then arises is whether MBL can affect the immune response in terms of antibody production, given that MBL 1) Activates the complement cascade; 2) Selectively recognizes carbohydrates; and 3) Binds to IgM [7; 9; 10; 11].
Of note, our previous research has demonstrated that the B1b cell population among peritoneal cells is expanded in naïve MBL null mice [12]. Additionally, it has been found that mice lacking a soluble form of IgM (sIgM) also had expanded peritoneal B1b cells, and the IgG response to TD antigen was reportedly impaired in these mice [8]. These observations led us to investigate the role of MBL in antibody production in response to GBS PS vaccines and to explore the possible involvement of other molecules of the innate immune system, including C3 and sIgM. In order to obtain direct evidence, we compared antibody responses to GBS vaccines in mice that genetically lack MBL, C3, sIgM, MBL and C3, and MBL and sIgM.
Materials and methods
Mice
Mice lacking MBL (MBL null) and both MBL and C3 (MBL/C3 null) were generated as described previously [13; 14]. Mice lacking MBL and a soluble form of IgM (sIgM) were generated by crossing MBL null and sIgM null mice. C3 null and sIgM null mice were kindly provided by M. C. Carroll at the Center for Blood Research, Harvard Medical School and J. Chen at the Center for Cancer Research and the Department of Biology, Massachusetts Institute of Technology. All mice were maintained on a mixed background of 129 × C57Bl/6J. Only female mice (6-8 weeks of age) were used in each experiment. All animal experiments were performed under a protocol approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital.
Immunization protocol
Type III and V GBS PS (GBS III PS and GBS V PS, respectively) without and with TT-conjugates (GBS III PS, GBS III PS-TT, GBS V PS and GBS V PS-TT, respectively) were prepared as described previously [15; 16]. The polysaccharide structure of GBS III and V are shown in Figure 1 [15; 16]. Mice were intraperitoneally injected with 0.8 or 8 μg of GBS III PS-TT, 8 μg of GBS III PS, 0.8 or 8 μg GBS V PS-TT, 8 μg of GBS V PS, 0.08 or 0.8 μg of TT, or saline in 0.2 ml on days 0 and 21 and also day 41 in some experiments as we will indicate in the figure legends. No adjuvant was used at any immunization in this study. Serum was collected from tail bleed on days −5, 5, 11 and thereafter every 10 days till day 31 or day 61 after immunization till day 21 or 41, respectively. Studies to examine the effects of sIgM and C3 in addition to MBL on IgG responses and IgG isotypes, were performed using samples on day 31 after two immunizations.
Figure 1.
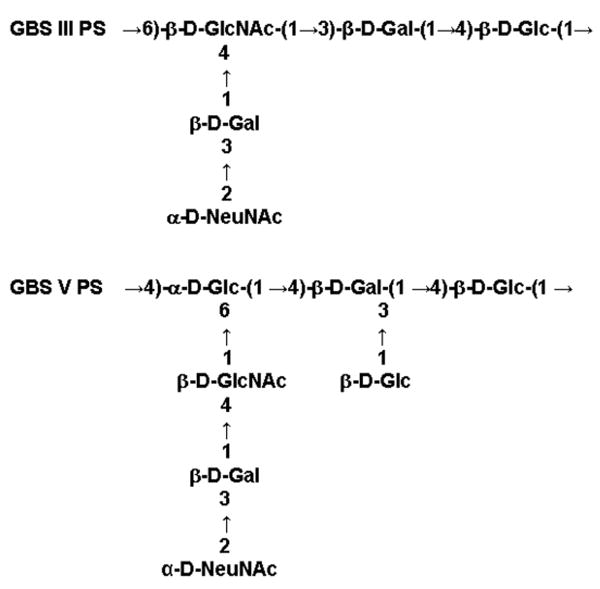
Structures of antigenic carbohydrates on GBS III-PS and GBS V-PS.
Titration and isotype determination of antibodies
Concentrations of immunoglobulin specific for GBS PS or TT were determined by ELISA as described previously [17]. Total Igs and Ig isotypes were assayed using a commercially available ELISA kit (Bethyl Labs, Texas, US). All titers were expressed as mean ± standard error. IgG isotypes were determined using sera from day 31 after two immunizations and from day 60 after three immunizations, as described in the immunization protocol.
Phenotyping of peritoneal cells and splenocytes
Peritoneal cells and splenocytes were harvested on day 14 after one immunization with GBS III PS-TT (8 μg/mouse, i.p.). Four wild type and four MBL null mice were used. For FACS analysis, all antibodies used were described previously [12] and were from BD Pharmingen except F4/80-allophycocyanin (Caltag Laboratories).
Statistical analysis
Data for antibody titers was analyzed by ANOVA or Wilcoxon/Kruskal-Wallis using Statview or JMP5 software (SAS Institute).
Results
Lack of MBL increases GBS III-PS specific IgG response
In order to assess the effect of MBL on the immune response to vaccination, WT and MBL null mice were intraperitoneally immunized three times with 0.8 or 8 μg of GBS III-PS-TT or saline. Subsequently, titers of IgG and IgM antibody specific for GBS III-PS were determined on various days. GBS III-PS specific IgM levels were increased to similar levels following immunization with 8 μg of GBS III PS-TT in both WT and MBL null mice, while there was no response to saline control (Figure 2). In contrast, GBS III-PS specific IgG was increased almost 2 orders of magnitude in MBL null mice compared with WT mice after as little as a single immunization (Figure 2). The IgG increase continued in MBL null mice following multiple immunizations, while it reached a plateau and even slightly decreased in WT mice after the third immunization on day 41 (Figure 2). Immunization with low dose (0.8 μg) of GBS III-PS-TT still increased GBS III-PS specific IgG response in MBL null mice although there was no statistical significance (data not shown).
Figure 2.
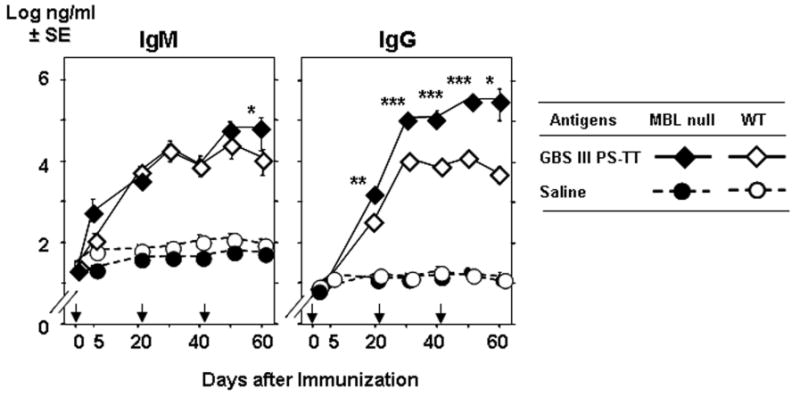
Increased GBS III-PS specific IgG response in MBL null mice. Mice were intraperitoneally immunized on days 0, 21 and 41 (as indicated by arrows). IgG titers in serum were determined as described in the Materials and Methods. Seven mice were used in each group. *, p <0.01; **, p <0.005; ***, p <0.0001.
We next examined whether the increase in GBS-III PS specific IgG response was a result of an increase in total IgG in naïve MBL null mice. Total IgG in naïve WT and MBL null mice was 1.39 ± 0.28 mg/ml (n = 14) and 1.47 ± 0.13 mg/ml (n = 8), respectively. Thus, total IgG concentration was not affected by lack of MBL. We also examined the concentration of IgM in native mice because IgM is known to regulate IgG, although a high concentration of IgM generally inhibits IgG, unlike our case in which there was an increase in IgG titer. The concentration of total IgM in WT and MBL null mice was 0.23 ± 0.04 mg/ml (n = 14) and 0.27 ± 0.06 mg/ml (n = 7), respectively. These data demonstrate that lack of MBL did not affect production of IgM.
Taken together, these results suggest that 1) MBL did not have an effect on total immunoglobulin levels in naïve mice; and that 2) Lack of MBL resulted in an increased GBS III-PS specific IgG secondary response following three immunizations with GBS III PS-TT vaccine.
Increased peritoneal B1 cells in MBL null mice
We hypothesized that the increase in IgG response may be due to an expanded B cell population and/or activation of germinal center B cells in the peritoneum or spleen. Germinal center B cells are thought to be required to produce antigen specific IgG. Another source of IgG is peritoneal B cells, in particular B1 cells, which are able to produce IgG upon maturation and stimulation. As vaccination in this study was performed intraperitoneally, B1 cells were suspected to play a significant role. In order to test these hypotheses we immunized WT and MBL null mice with 8 μg of GBS III-PS-TT on day 0 and harvested splenocytes and peritoneal cells on day 14 to perform FACS analysis. In spleen, B1 cells that are characterized by B220highCD23neg were not expanded in MBL null mice compared with WT mice as mean% (± SD) of these cells were 27. 2% (± 4.0) and 21.8% (± 2.4) respectively. Total B cells, B220high were also unchanged between the two groups, 53.4 ± 2.9% in WT vs. 60.3 ± 7.6% in MBL null mice.
In contrast, total B220high cells in peritoneal lavage were expanded 1.6 fold in MBL null mice compared with WT mice (Figure 3a). Further analysis of this B cell population, B1 cells that are characterized by B220highIgMhighIgDlow [18; 19] were average 3-fold increase in MBL null mice (58.1 ± 11.5) compared with WT mice (16.9 ± 5.0) (Figure 3b). This is two-fold increase from our previous finding in naïve WT and MBL null mice that peritoneal B1 cells were expanded by 1.5-fold in MBL null mice compared to WT mice [12].
Figure 3.
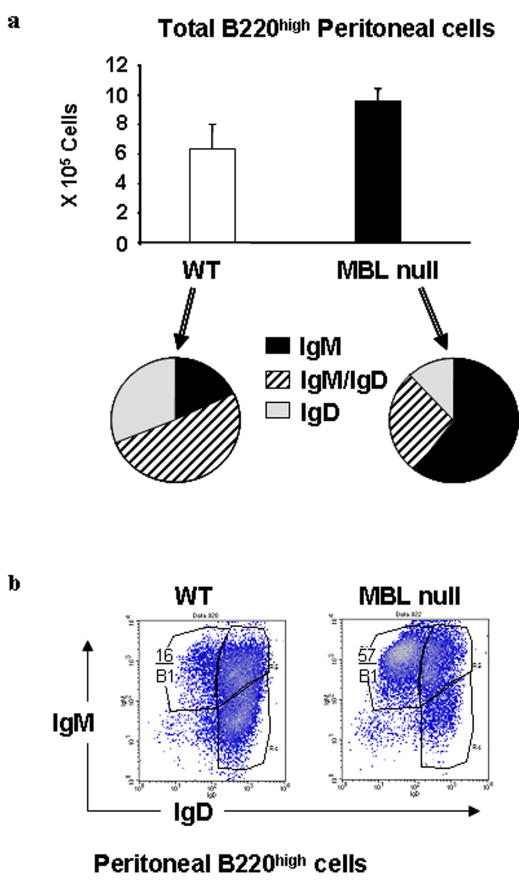
B1 cell expansion in MBL null mice. Peritoneal cells were phenotyped by FACS analysis. 3a. Summary of FACS phenotyping of peritoneal B cells that are characterized by B220high is shown. Bars indicate mean ± SD. 3b. Representative FACS density plots of peritoneal B cells is shown. Percentages of B1 cells, which are characterized as IgMhighIgDlow are shown. Four WT and three MBL null mice were used.
MBL and IgM share the same pathway in IgG response
The increased peritoneal B1 cells phenotype was also observed in mice lacking soluble IgM (sIgM), and in these mice IgG response to TD antigen, NP-KLH, was impaired [8] relative to MBL null mice. This observation suggested that IgM might have some role in IgG response to TD antigen. Therefore, we designed experiments to determine whether IgM is required for the increased IgG response in MBL null mice. We compared GBS III-PS specific IgG titers in mice lacking MBL, mice lacking sIgM, and mice lacking both MBL and sIgM (MBL/sIgM). On day 31, following immunization with 8 μg of GBS III-PS-TT on days 0 and 21, GBS III PS specific IgG titers were comparable to each other among MBL null (22.5 ± 8.2 μg/ml), sIgM null (23.5 ± 6.9 μg/ml) and MBL/sIgM null (16.0 ± 4.4 μg/ml) mice. However, these titers were significantly increased compared with WT mice (4.1 ± 3.3 μg/ml, p<0.005) (Figure 4). These data suggest that lack of sIgM, MBL, or both had similar effects on increased production of antigen specific IgG. Since the effect of eliminating both IgM and MBL together is equivalent to the effect of eliminating either IgM or MBL alone, these data further suggest that both MBL and sIgM share the same pathway in inhibiting IgG response to GBS III PS-TT.
Figure 4.
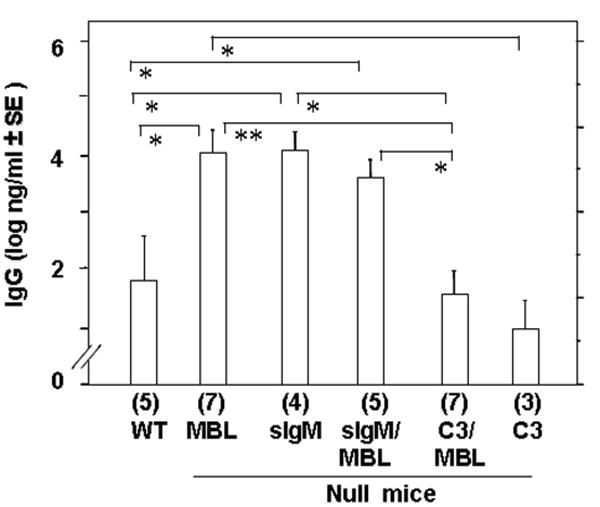
Effect of sIgM and C3 in combination with MBL in GBS III-PS specific antibody response. Bars indicate GBS III-PS specific IgG and IgM titers in the top panel and the lower panel, respectively. The antibody titer was determined on day 31 after two immunizations, on days 0 and 21. The titer was determined as described in the Materials and Methods. Numbers in parentheses under each bar indicate the number of mice in each group. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.005.
C3 is required for enhanced IgG response in hosts lacking MBL
Next, we wished to examine the involvement of C3 in IgG response in MBL deficient mice for three reasons: (1) C3 has been implicated in B cell receptor signaling [20]; (2) MBL activates the lectin complement pathway [7]; and (3) IgG response to GBS III PS was impaired in C3 null mice [17]. We compared GBS III PS specific IgG response in mice lacking MBL, C3, both MBL and C3 (MBL/C3), and WT mice. Interestingly, the IgG titers in C3 null mice (2.1 ± 1.0 μg/ml) were comparable to that of WT mice suggesting that there was no effect of C3 on IgG response against GBS III PS-TT. In contrast, IgG titers in MBL/C3 null mice (3.4 ± 1.3 μg/ml) were significantly (p<0.05) lower than that of MBL null mice, similar to that of C3 null mice (Figure 4). These results suggest that C3 is required for the elevated IgG response to GBS III PS-TT in MBL deficient hosts.
Increased GBS III PS specific IgG response is due to increase in GBS III PS specific IgG
We next identified IgG isotypes in order to determine if there was a class of IgG that was preferentially increased in MBL null mice and whether IgM and C3 have any role in possible IgG class switching. Isotypes were analyzed using day 31 serum after two immunizations with GBS III PS-TT. The isotypes in MBL null mice were revealed to be predominantly IgG1 as shown in the Table 1. The IgG1 isotype was also predominant in immunized sIgM null and MBL/sIgM null mice (Table 1). These data suggest that lack of IgM did not have an effect on isotypes in MBL null mice and vise versa. Unexpectedly, IgG3 was significantly increased in sIgM null mice compared with other strains of mice (p < 0.01, Table 1), suggesting that sIgM is inhibitory in IgG3 production. Moreover, the IgG3 response was significantly reduced in MBL/sIgM null mice (p < 0.005, Table 1) compared with sIgM null mice, suggesting that MBL is required for elevated IgG3 production in hosts lacking sIgM. Taken together, these data suggest that MBL and IgM: (1) Share a common pathway in IgG1 production; but (2) Use different pathways or have different influence in the production of IgG3. Additionally these data suggest that MBL is required to produce IgG3 in sIgM deficient hosts.
Table 1.
IgG isotypes in mice immunized with GBS III-PS-TT (8 μg/mouse).
| Immunoglobulin isotypes (± SE μg/ml) | ||||
|---|---|---|---|---|
| Genotype | IgG1 | IgG2a | IgG2b | IgG3 |
| WT | 1.03 ± 1.00 (5) | 0.75 ± 0.58 (5) | 0.53 (2) | 0.12 (2) |
| MBL null | 10.19 ± 2.35 (7) | 0.87 ± 0.27 (7) | 0.95 ± 0.27 (7) | 0.01 ± 0.05 (7) |
| MBL/sIgM null | 7.93 ± 4.39 (5) | 1.34 ± 0.73 (5) | 1.08 ± 0.28 (5) | 0.06 ± 0.02 (5) |
| sIgM null | 14.30 ± 4.13 (4) | 2.31 ± 1.00 (4) | 1.08 ± 0.33 (4) | 0.52 ± 0.24 (4) |
| MBL/C3 null | 1.23 ± 0.51 (7) | 0.76 ± 0.85 (7) | 0.56 ± 0.20 (7) | 0.17 ± 0.05 (7) |
| C3 null | 1.01 ± 0.17 (3) | 0.64 ± 0.46 (3) | 0.11 ± 0.07 (3) | 0.09 ± 0.06 (3) |
Note, bold faced values are significantly different (p <0.05) compared with WT mice. Numbers in parentheses indicate the numbers of mice used for each group.
Increase in IgM and IgG response against GBS V PS-TT in MBL null mice
We also examined IgG response to GBS V PS-TT to test if the increased antibody response could be achieved using other serotype of GBS. The chief differences in the carbohydrate structure is that GBS V PS has β–D-GlcNAc in its side chain, unlike that in the glucan chain of GBS III PS, and GBS V PS has β–D-Glc in addition to D-NeuNAc as terminal sugars (Figure 1). Unexpectedly, significant increases in antigen specific IgM titers were observed when mice were immunized with 0.8 μg of GBS V PS-TT (p <0.01 ∼ 0.05, Figure 5). As expected, GBS V PS specific IgG titer was also increased in MBL null mice compared with that of WT mice using low doses of vaccine (0.8 μg of BS V PS-TT (p <0.005, Figure 5). The increase in IgG was also due to elevated isotypes of IgG1 (data not shown), as was observed in IgG responses against GBS III PS-TT (Table 1). However, the increase in antibody titers was not observed when high dose, 8 μg of BS V PS-TT was used (data not shown), suggesting that the immunization dose was at or above the optimum level at 0.8 μg. These data suggest that lack of MBL results in increased secondary IgG and IgM responses relative to other serotypes of GBS PS vaccine.
Figure 5.
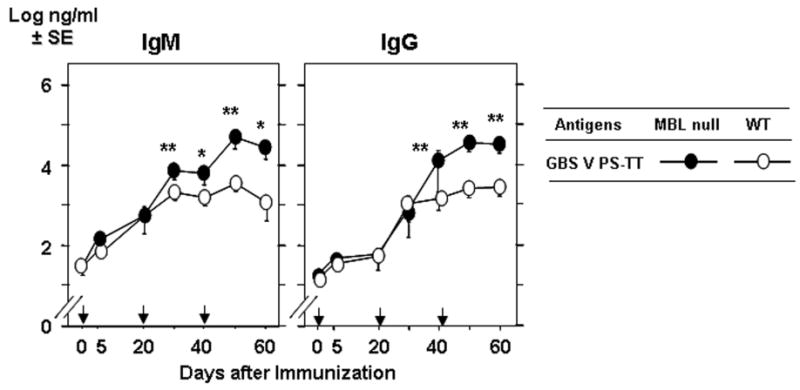
Increased IgM and IgG response to GBS V PS-TT. Experiments were performed as in Figure 2. Five WT and seven MBL null mice were used. *, p < 0.05; **, p < 0.01.
Increased antigen specific IgG response against TD antigen but not PS TI2 antigen in MBL null mice
Finally, we examined whether the antigen specific IgG response was also increased to GBS-PS alone, which is characterized as TI type 2 antigen because of the polysaccharide repeats. MBL null and WT mice were immunized with 8 μg of GBS-PS antigen and GBS-PS specific IgG titers were measured. No IgG response was observed against GBS PS alone, using either type III (Figure 6) or type V (data not shown).
Figure 6.
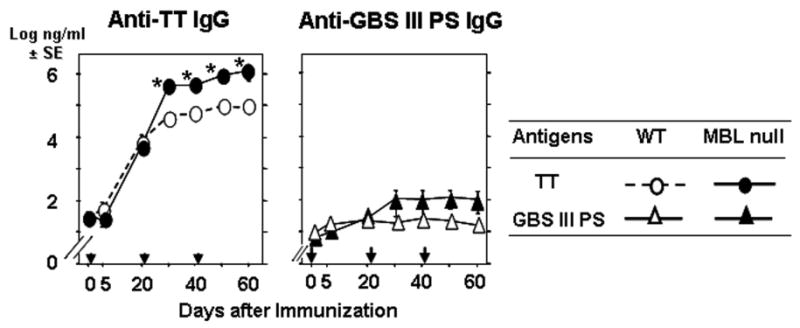
Increased IgG response to TT but not GBS III PS. Experiments were performed as in Figure 2. Five mice in each group were used for TT and seven mice in each group were used for GBS III PS. **, p ≤ 0.01; ***, p ≤ 0.005.
In contrast, antigen specific IgG response was significantly increased against TT alone at 0.8 μg/mouse in MBL null mice compared with WT mice (p<0.0001, Figure 6). The significantly increased IgG response was also observed at low dose, 0.08 μg/mouse (p<0.0001, data not shown). These data suggest that MBL deficient hosts can increase IgG response against TD antigen but not TI2 antigen.
Discussion
It has become clear in recent years that MBL, a serum protein of the innate immune system, has broad roles in host defense such as combating infectious pathogens as well as activity in other non-infectious diseases, including diabetes-related vascular complications and reperfusion injury as we reviewed [7]. An important recent finding is that innate immunity plays a critical role in the regulation of adaptive immunity. In this regard, the present study demonstrates that deficiency in MBL enhances antigen specific IgG production following immunization with GBS III PS-TT and GBS V PS-TT (Figures 2 and 5). The elevation in IgG response is also observed against TT alone although it was not seen with GBS PS alone (Figure 5). These results suggest that MBL deficiency increases T cell-dependent (TD) antigen responses, as TT and TT-conjugated antigens are TD antigen unlike GBS PS, which is a TI2 antigen. These findings demonstrate that antigen specific antibody responses can be upregulated in an MBL deficient host, suggesting that MBL plays an inhibitory role in antibody production.
In addition, our study also demonstrates that antigen specific IgM increases following immunization with GBS V PS-TT though not GBS III PS-TT (Figure 2 and 5). In a recent report, immunization with live nematode produced a notably low antigen specific IgM response in MBL-A single null mice [21]. Although we did not examine the IgM response in MBL-A null mice that we generated [22], the IgG response against GBS III PS-TT in MBL-A null mice was significantly (p<0.05) elevated on day 21 following the first immunization. The increased IgG response in MBL-A null mice did not last following tertiary immunization, and IgG titers became similar to WT mice (data not shown). The discrepancy in these two studies could be attributed to several factors such as the use of different antigens (live nematodes vs. purified TT-conjugated GBS PS) and immunization route (intravenous vs. intraperitoneal). In any case, these results suggest that MBL is capable of modulating antibody responses, and that there maybe antigen dependency in the regulation of antibody responses.
As to the mechanisms of antigen specific antibody production, clonal expansion of B cells in spleen, that is, involving germinal center B cells, is generally thought to be required. However, B cells in spleen including germinal centers in MBL null mice, were similar to WT mice even after immunization, suggesting that splenic B cells are not involved with the increased IgG production. In contrast, peritoneal B1 cells (B220high/IgMhigh/IgDlow), which are capable of switching to produce IgG, were expanded 3-fold in MBL null mice (Figure 2). However, the elevation with respect to WT mice is only 2-fold increase compared with 1.5-fold increase in B1 cells seen in naïve MBL null mice compared to naïve WT mice that we have previously reported [12]. Consequently, the increase in the number of peritoneal B1 cells does not account for the increased antigen specific IgG response, which was already 4 fold increased in MBL null mice on day 21 following a single vaccination (Figure 1). These data indicate that immunization did not have significant effect resulting in additional expansion or stimulation of B cells in either the spleen or peritoneum, suggesting the increased IgG responses was not simply attributed to increase in numbers of B1 cells.
These data may suggest that existing B cells are being stimulated to produce IgG. Peritoneal B cells have been observed to become hyper productive of IgG1 by stimulation of CD38 in combination with IL-5 [23]. The elevated B1 cells in the MBL null mice may be B1b cells based on our previous work that demonstrated that increased peritoneal B1 cells in MBL null mice were CD5 negative [12]. B1b cells have been reported to have memory independently of T memory cells [24]. However, additional elements of the adaptive immune system that interact with the innate immune system, including regular and memory T cells and antigen presenting cells, need to be further explored.
In recent years, it has become evident that MBL modulates and cooperates with other factors of the innate and adaptive immune systems such as C3 and IgM [10; 25]. C3, in particular has been implicated in B cell receptor signaling, although primarily in the spleen. Indeed, C3 null mice had impaired IgG response to bacteriophage, TD antigen [26] and GBS III PS, a TI2 antigen [17]. However, in our study, C3 deficiency did not have any effect in IgG response against GBS PS-TT vaccines, and C3 null mice responded like WT mice (Figure 4). This discrepancy may be due to (1) Immunization routes: intravenous bacteriophage vs. intraperitoneal GBS-PS vaccine; (2) Antigen form: whole phage vs. purified GBS-PS-TT vaccine; and (3) Different immunizing exposures, in part related to different routes and forms of inoculum [27].
It is intriguing that the increased IgG response in MBL deficient hosts was inhibited by additional C3 deficiency, as demonstrated by the reduced IgG response in MBL/C3 null mice compared with MBL null mice (Figure 4). These data indicate that C3 is required for a complete IgG response in MBL deficient hosts, suggesting that C3 is downstream of MBL in the mechanisms responsible for IgG production.
Our previous research has demonstrated that MBL null mice have increased peritoneal B1b cells [12], a phenomenon also observed in sIgM null mice as reported by Boes et. al. [8], although this latter work revealed that sIgM null mice were impaired in IgG response to TD antigen (nitrophenyl-conjugated keyhole limpet hemocyanin). Contrary to our initial hypotheses, MBL null mice demonstrated an increased IgG response to TD antigens in the work presented here (Figures 2 and 5), raising the possibility that MBL and IgM may use different mechanisms in antibody production, unique from those in complement activation in which MBL and IgM function cooperatively [10; 25]. If MBL and IgM assert different inhibitory effects on the pathways leading to the IgG response, the IgG response in MBL/sIgM double null mice would be higher than MBL single null and sIgM single null. However, the IgG responses were comparable among MBL null, sIgM null and MBL/sIgM double null mice (Figure 4). Thus, our results reveal that MBL and sIgM likely affect the same pathway.
In addition, the increase in IgG was predominantly IgG1 isotype in all strains of mice (Table 1). However, production of IgG3, which represents a small portion of the total IgG protein, is elevated in sIgM null mice and was reduced by additional MBL deficiency (Table 1). These data suggest that MBL acts downstream of sIgM in IgG3 production. Taken together, these results suggest that: (1) C3 is required for complete IgG response; and (2) MBL and IgM use the same network to produce IgG1; and (3) MBL is required to produce IgG3 at normal physiologic levels. Efficacy of Ig classes and isotypes may be dependent on routes and amount of infection as IgM and IgG3 Ab to Pseudomonas aeruginosa LPS were reportedly most protective against i.p. infection of P. aeruginosa while IgG1 was the most effective in burn infection model [28].
Although many clinical association studies have been performed in correlating MBL deficiency and infection susceptibility no clinical study has been conducted to investigate any effect of MBL deficiency in vaccine responses as far as we searched published literature. Our results, for the first time, suggest that MBL deficiency, which is results of gene polymorphisms in humans, may be beneficial to achieve high antibody titers from certain vaccinations. The first step tot take would be to investigate a relationship between MBL deficiency and vaccination efficiency in humans. If any positive results were observed, then it would be worth while to explore therapeutic applications, including timing and formula to create MBL deficiency in improving vaccination efficiency.
Finally, our findings further support the fact that pattern recognition molecules of the innate immune system have a profound effect on adaptive immunity and suggest that innate immune responses may dictate the outcome of adaptive immune responses. Further investigations are needed to discern the precise mechanisms as to which effector cells and which molecules MBL regulates in the antibody production process. Such detailed understanding of the interaction between innate and adaptive immunity could lead to new approaches to boost immunization efficiency, perhaps, in the case of GBS vaccines, by manipulating MBL levels, and it is possible that these mechanisms might be exploited more broadly for use with other immunizations.
Acknowledgments
The authors thank members of Developmental Immunology at Massachusetts General Hospital for helpful discussion. This work was supported by NIH P01 AI052343.
Abbreviations
- MBL
mannose-binding lectin
- GBS
Group B streptococcus
- MASP
MBL-associated serine protease
- PS
polysaccharide
- TT
tetanus toxoid
- sIgM
soluble IgM
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker CJ, Rench MA, Fernandez M, Paoletti LC, Kasper DL, Edwards MS. Safety and immunogenicity of a bivalent group B streptococcal conjugate vaccine for serotypes II and III. J Infect Dis. 2003;188:66–73. doi: 10.1086/375536. [DOI] [PubMed] [Google Scholar]
- 2.Baker CJ, Paoletti LC, Rench MA, Guttormsen HK, Edwards MS, Kasper DL. Immune response of healthy women to 2 different group B streptococcal type V capsular polysaccharide-protein conjugate vaccines. J Infect Dis. 2004;189:1103–1112. doi: 10.1086/382193. [DOI] [PubMed] [Google Scholar]
- 3.Stein KE. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992;165 1:S49–52. doi: 10.1093/infdis/165-supplement_1-s49. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Ezekowitz RA. The role of the mannose-binding lectin in innate immunity. Clin Infect Dis. 2005;41 7:S440–444. doi: 10.1086/431987. [DOI] [PubMed] [Google Scholar]
- 5.Brown KS, Ryder SD, Irving WL, Sim RB, Hickling TP. Mannan binding lectin and viral hepatitis. Immunology Letters. 2007;108:34–44. doi: 10.1016/j.imlet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Thiel S, Frederiksen PD, Jensenius JC. Clinical manifestations of mannan-binding lectin deficiency. Molecular Immunology. 2006;43:86–96. doi: 10.1016/j.molimm.2005.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi K, Ip WE, Michelow IC, Ezekowitz RA. The mannose-binding lectin: a prototypic pattern recognition molecule. Curr Opin Immunol. 2006;18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 9.Nevens JR, Mallia AK, Wendt MW, Smith PK. Affinity chromatographic purification of immunoglobulin M antibodies utilizing immobilized mannan binding protein. J Chromatogr. 1992;597:247–256. doi: 10.1016/0021-9673(92)80117-d. [DOI] [PubMed] [Google Scholar]
- 10.McMullen ME, Hart ML, Walsh MC, Buras J, Takahashi K, Stahl GL. Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology. 2006;211:759–766. doi: 10.1016/j.imbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Chan RK, Ibrahim SI, Takahashi K, Kwon E, McCormack M, Ezekowitz A, Carroll MC, Moore FD, Jr, Austen WG., Jr The Differing Roles of the Classical and Mannose-Binding Lectin Complement Pathways in the Events following Skeletal Muscle Ischemia-Reperfusion. J Immunol. 2006;177:8080–8085. doi: 10.4049/jimmunol.177.11.8080. [DOI] [PubMed] [Google Scholar]
- 12.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–3226. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 13.Shi L, Takahashi K, Dundee J, Shahroor-Karni S, Thiel S, Jensenius JC, Gad F, Hamblin MR, Sastry KN, Ezekowitz RA. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199:1379–1390. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi K, Shi L, Gowda LD, Ezekowitz RA. Relative roles of complement factor 3 and mannose-binding lectin in host defense against infection. Infect Immun. 2005;73:8188–8193. doi: 10.1128/IAI.73.12.8188-8193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wessels MR, Paoletti LC, Kasper DL, DiFabio JL, Michon F, Holme K, Jennings HJ. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Invest. 1990;86:1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wessels MR, Paoletti LC, Pinel J, Kasper DL. Immunogenicity and protective activity in animals of a type V group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Infect Dis. 1995;171:879–884. doi: 10.1093/infdis/171.4.879. [DOI] [PubMed] [Google Scholar]
- 17.Pozdnyakova O, Guttormsen HK, Lalani FN, Carroll MC, Kasper DL. Impaired antibody response to group B streptococcal type III capsular polysaccharide in C3- and complement receptor 2-deficient mice. J Immunol. 2003;170:84–90. doi: 10.4049/jimmunol.170.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 19.Mori S, Sakamoto A, Yamashita K, Fujimura L, Arima M, Hatano M, Miyazaki M, Tokuhisa T. Effect of c-fos overexpression on development and proliferation of peritoneal B cells. Int Immunol. 2004;16:1477–1486. doi: 10.1093/intimm/dxh149. [DOI] [PubMed] [Google Scholar]
- 20.Carroll MC. CD21/CD35 in B cell activation. Semin Immunol. 1998;10:279–86. doi: 10.1006/smim.1998.0120. [DOI] [PubMed] [Google Scholar]
- 21.Carter T, Sumiya M, Reilly K, Ahmed R, Sobieszczuk P, Summerfield JA, Lawrence RA. Mannose-binding lectin A-deficient mice have abrogated antigen-specific IgM responses and increased susceptibility to a nematode infection. J Immunol. 2007;178:5116–5123. doi: 10.4049/jimmunol.178.8.5116. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K, Gordon J, Liu H, Sastry KN, Epstein JE, Motwani M, Laursen I, Thiel S, Jensenius JC, Carroll M, Ezekowitz RA. Lack of mannose-binding lectin-A enhances survival in a mouse model of acute septic peritonitis. Microbes Infect. 2002;4:773–784. doi: 10.1016/s1286-4579(02)01597-6. [DOI] [PubMed] [Google Scholar]
- 23.Mizoguchi C, Uehara S, Akira S, Takatsu K. IL-5 induces IgG1 isotype switch recombination in mouse CD38-activated sIgD-positive B lymphocytes. J Immunol. 1999;162:2812–2819. [PubMed] [Google Scholar]
- 24.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, Jensenius JC, Ezekowitz RA, Moore FD, Carroll MC. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol. 2006;177:4727–4734. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- 26.Fischer MB, Ma M, Goerg S, Zhou X, Xia J, Finco O, Han S, Kelsoe G, Howard RG, Rothstein TL, Kremmer E, Rosen FS, Carroll MC. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J Immunol. 1996;157:549–556. [PubMed] [Google Scholar]
- 27.Lee Y, Haas KM, Gor DO, Ding X, Karp DR, Greenspan NS, Poe JC, Tedder TF. Complement component C3d-antigen complexes can either augment or inhibit B lymphocyte activation and humoral immunity in mice depending on the degree of CD21/CD19 complex engagement. J Immunol. 2005;175:8011–8023. doi: 10.4049/jimmunol.175.12.8011. [DOI] [PubMed] [Google Scholar]
- 28.Pollack M, Koles NL, Preston MJ, Brown BJ, Pier GB. Functional properties of isotype-switched immunoglobulin M (IgM) and IgG monoclonal antibodies to Pseudomonas aeruginosa lipopolysaccharide. Infect Immun. 1995;63:4481–4488. doi: 10.1128/iai.63.11.4481-4488.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


