Abstract
Background: Fulvestrant produces a clinical benefit rate (CBR) of ∼45% in tamoxifen-resistant, hormone receptor (HR)-positive metastatic breast cancer (MBC) and 32% in aromatase inhibitor (AI)-resistant disease. The farnesyltransferase inhibitor tipifarnib inhibits Ras signaling and has preclinical and clinical activity in endocrine therapy-resistant disease. The objective of this study was to determine the efficacy and safety of tipifarnib–fulvestrant combination in HR-positive MBC.
Patients and methods: Postmenopausal women with no prior chemotherapy for metastatic disease received i.m. fulvestrant 250 mg on day 1 plus oral tipifarnib 300 mg twice daily on days 1–21 every 28 days. The primary end point was CBR.
Results: The CBR was 51.6% [95% confidence interval (CI) 34.0% to 69.2%] in 31 eligible patients and 47.6% (95% CI 26.3% to 69.0%) in 21 patients with AI-resistant disease. A futility analysis indicated that it was unlikely to achieve the prespecified 70% CBR. Tipifarnib dose modification was required in 8 of 33 treated patients (24%).
Conclusions: The target CBR of 70% for the tipifarnib–fulvestrant combination in HR-positive MBC was set too high and was not achieved. The 48% CBR in AI-resistant disease compares favorably with the 32% CBR observed with fulvestrant alone in prior studies and merit further clinical and translational evaluation.
Keywords: farnesyltransferase inhibitor, fulvestrant, metastatic breast cancer, postmenopausal, selective estrogen receptor downregulator, tipifarnib
introduction
Endocrine therapy (ET) is an effective targeted therapy for hormone receptor (HR)-positive metastatic breast cancer (MBC). Patients with HR-positive MBC may benefit from a variety of ETs, including selective estrogen receptor (ER) modulators (e.g. tamoxifen), aromatase inhibitors (AIs, e.g. anastrazole, letrozole, exemestane), and selective ER downregulators (e.g. fulvestrant) [1–3]. Fulvestrant (Faslodex, AstraZeneca Pharmaceuticals LP, Wilmington, DE) binds, inhibits, and degrades the ER and more effectively inhibits the estrogen signaling pathway than either tamoxifen or AIs [4–6]. Fulvestrant has demonstrated clinical efficacy with good tolerability when used as first-, second-, or third-line therapy in postmenopausal women with HR-positive MBC [4–8]. It has activity that is comparable to tamoxifen when used as first-line therapy [6], and it has activity that is comparable to AIs when used as second-line therapy in patients with tamoxifen-resistant disease [5, 9–11]. When used in patients with AI-resistant disease, it has been associated with a clinical benefit rate (CBR) of approximately 30%–35% [12–15]. Studies are ongoing to determine whether a ‘loading dose schedule’ (500 mg every 2 weeks for two doses followed 2 weeks later by a standard dose and schedule) is more effective than a standard dose (250 mg) and schedule (every 4 weeks), including the FINDER I (NCT00305448), FINDER II (NCT00313170), and CONFIRM (NCT00099437) trials. For postmenopausal women with HR-positive MBC, tamoxifen or AIs are typically used as first-line ET, with fulvestrant used after progression on first-line therapy.
Hyperactivation of Ras/MAPK signal transduction pathway has been implicated as a resistance mechanism of ET in breast cancer [16, 17]. Although initially developed for tumors with Ras mutations that result in constitutive activation of the Ras pathway, inhibitors of Ras signaling pathway, such as farnesyltransferase inhibitors (FTIs), are also active in breast cancer cell lines and xenografts that lack Ras mutations [18]. This is an important consideration given that Ras mutations occur only rarely in breast cancer [19]. Tipifarnib produced a CBR of 23% in ET- and/or chemotherapy-resistant MBC in one trial [20]. Preclinical studies have demonstrated that tipifarnib enhances the antitumor effect of tamoxifen in estrogen-dependent breast cancer cell lines and xenograft models, either by overcoming resistance or by preventing/delaying emergence of the resistance phenotype [21–23]. We hypothesized that tipifarnib might enhance the clinical efficacy of fulvestrant by overcoming resistance mechanisms and sought to determine the efficacy and safety of the fulvestrant–tipifarnib combination in postmenopausal women with HR-positive MBC.
patients and methods
patient eligibility
Postmenopausal women with histologically or cytologically confirmed HR-positive adenocarcinoma of the breast with metastatic or surgically incurable locally advanced disease were eligible. HR-positive disease was defined as being positive for estrogen and/or progesterone receptors by the local institutional laboratory. Patients were required to have no prior chemotherapy for metastatic disease. The study initially required ET-resistant disease for all patients, which was defined as progression of disease during tamoxifen or AI therapy for metastatic disease or relapse on (or within 6 months of completing) adjuvant tamoxifen or AI therapy. After nine patients had been accrued to the study, eligibility criteria were revised to include a second stratum of postmenopausal women who had no prior ET for MBC (which was an initial exclusion criterion) due to new information indicating the efficacy of fulvestrant in this patient population [6].
Additional key inclusion criteria included at least one measurable lesion by response evaluation criteria in solid tumors (RECIST) [24], age ≥18 years, Eastern Cooperative Oncology Group performance status of zero to two and adequate organ and marrow function (leukocytes ≥3000/μl, absolute neutrophil count ≥1500/μl, platelet count ≥100 000/μl, total bilirubin ≤2.0 mg/dl, aspartate aminotransferase and/or alanine aminotransferase ≤2.5× institutional upper limit of normal, serum creatinine ≤1.5 mg/dl). Patients who had received up to one prior dose of fulvestrant were eligible, but those who had received two or more doses were ineligible. Exclusion criteria included patients with immune deficiency such as HIV, prior chemotherapy for metastatic disease or treatment with any FTI, major surgery or radiation therapy within the last 4 weeks, grade 2 and more peripheral neuropathy, presence of rapidly progressive, life-threatening metastases (which includes patients with >50% hepatic involvement, symptomatic lymphangitic metastases, or uncontrolled brain or leptomeningeal involvement) or uncontrolled comorbidities, and any active gastrointestinal disorder that altered motility or absorption.
The trial was reviewed, approved, and sponsored by the Cancer Therapy Evaluation Program of the National Cancer Institute (ClinicalTrials.gov, identifier NCT00082810). The local institutional review board at each participating institution approved the protocol. All patients gave written, informed consent.
treatment plan
Patients were treated with fulvestrant 250 mg in a single 5-ml i.m. injection every 28 days, which was defined as one cycle. Patients also received tipifarnib (300 mg) twice daily for 21 consecutive days every 28 days. Each tipifarnib dose was taken with food (e.g. snacks, breakfast, and dinner). At each monthly visit, patients underwent a history, physical exam, complete blood count, serum creatinine, electrolytes, liver function tests, and assessment of performance status, adverse events, and drug adherence (using history, a pill diary, and return of unused drug). Treatment was continued without interruption until disease progression, severe or intolerable toxicity, or withdrawal of consent. Concurrent bisphosphonate therapy with an approved bisphosphonate was permitted for patients with bone metastases.
evaluation of response and toxicity
All patients underwent computed tomography (CT) of the chest and abdomen and a bone scan within 4 weeks of registration. Tumor response was assessed every three cycles by CT using RECIST criteria, and bone scans were repeated if the original bone scan was positive or progressive bony metastatic disease was suspected. Toxicity was graded according to the National Cancer Institute Common Terminology for Adverse Events, version 3.0. For patients who experienced grades 3–4 toxicity (or grade 2 neuropathy), the tipifarnib was held until resolution to grades 0–1, then resumed in the same cycle (if before day 21) or next cycle with a one dose level reduction (to 200 mg b.i.d. for the first reduction, 100 mg b.i.d. for the second reduction). Grade 3 neurotoxicity lasting >5 days or grade 4 non-hematological toxicity required permanent discontinuation of tipifarnib. Fulvestrant was not held if tipifarnib was held for toxicity, and no dose reduction of fulvestrant was allowed. Patients who stopped tipifarnib due to severe or intolerable toxicity continued fulvestrant alone until disease progression.
statistical considerations
The primary end point of the study was CBR, which was defined as objective response [complete response plus partial response (PR)] or stable disease (SD) (target lesions neither sufficient shrinkage to qualify for PR (i.e., at least a 30% decrease) nor sufficient increase to qualify for PD (i.e., at least a 20% increase) in the sum of the longest diameter, taking as reference the smallest sum longest diameter since the treatment started, in the absence of any new lesions). For at least 24 weeks. At the time that the protocol was initiated in April 2004, it was anticipated that the study would accrue patients with both tamoxifen-resistant and AI-resistant disease. The reported CBR of fulvestrant monotherapy was 42%–45% in tamoxifen-resistant disease [5, 6] and approximately 30%–35% for AI-resistant disease [13, 14]. The study was initially designed to detect an improvement in CBR from 40% to 60%, assuming an equally mixed population. With an overall alpha of 0.10 (α = 0.10) and a power of 90% (β = 0.10), the regimen would be considered promising if at least 21 of 41 assessable patients achieved clinical benefit. Due to slow accrual and new efficacy data demonstrating first-line fulvestrant produced a CBR of ∼55% in HR-positive MBC patients [6], the study was amended to allow patients who had no prior ET for metastatic disease. The statistical plan was amended to distinguish between a CBR of 50% versus 70% (α = 0.10, β = 0.10), which would require at least 26 of 42 assessable patients to achieve clinical benefit. Secondary end points included the median time to progression (TTP), duration of response (DOR), median overall survival (OS), and toxicity.
All eligible patients were included in the efficacy analysis, and all treated patients were included in the safety analysis. The number and proportion of patients achieving clinical benefit were summarized with the corresponding two-sided 95% CI. TTP and OS were calculated from the date of enrollment to the date of progression or death, respectively. DOR was defined for responders as the time from the onset of first response to disease progression and for nonresponders as zero. Outcomes were censored if an end point was not reached by the time of last follow-up or if a patient was lost to follow-up. Patients who died without documentation of progression were considered to have progressed on the date of their death. TTP and OS were estimated using the Kaplan–Meier method. Univariate analyses of TTP and OS used the log-rank test to examine the effects of baseline clinical factors. All P values were two sided with statistical significance evaluated at the 0.05 alpha level. All analyses were carried out in MedCalc and SAS Version 9.1 (SAS Institute, Inc., Cary, NC).
results
patient characteristics
Thirty-three patients were enrolled from three institutions between March 2004 and August 2006. Two patients were ineligible; one had a performance status of three and elevated liver function tests that exceeded inclusion criteria, whereas the other received prior chemotherapy for metastatic disease. Baseline characteristics of the 31 eligible patients are shown in Table 1.
Table 1.
Baseline clinical characteristics of eligible patients
| Baseline characteristics | n | % |
| Patients eligible/enrolled | 31/33 | 94 |
| Age at enrollment, years | ||
| Mean | 61.0 | |
| Median | 61.0 | |
| Range | 39–77 | |
| Race/ethnicity | ||
| Hispanic | 12 | 39 |
| White | 11 | 36 |
| Black | 6 | 19 |
| Asian | 2 | 6 |
| ECOG performance status | ||
| 0 | 16 | 52 |
| 1 | 14 | 45 |
| 2 | 1 | 3 |
| Sites of metastatic disease | ||
| Nonvisceral only | 9 | 29 |
| Visceral (liver, lung, adrenal) only | 7 | 23 |
| Both | 15 | 48 |
| No. of metastatic sites | ||
| One | 15 | 48 |
| Two | 10 | 32 |
| Three or more | 6 | 19 |
| Prior adjuvant therapy | ||
| Adjuvant ET | 21 | 68 |
| Adjuvant chemotherapy | 15 | 48 |
| Surgery | 22 | 71 |
| Radiation | 16 | 52 |
| Prior metastatic therapya | ||
| Not ET resistant (no prior ET or ET sensitiveb) | 9 | 29 |
| ET resistant | 22 | 71 |
| Tamoxifen | 1 | 5 |
| Aromatase inhibitors | 21 | 95 |
| One prior ET | 12 | 57 |
| Two prior ETs | 7 | 33 |
| Three prior ETs | 2 | 10 |
One hundred percentage is calculated for each subgroup.
Patient #23 had complete remission to prior ET and a relapse interval of 2 years.
ECOG, Eastern Cooperative Oncology Group; ET, endocrine therapy.
clinical benefit rate
Of 31 eligible patients, 16 patients (51.6%; 95% CI 34.0% to 69.2%) met the definition for clinical benefit, including 11 patients (35.5%) with PR and five patients (16.1%) with SD for at least 24 weeks. When performing a futility analysis after accrual of 31 of the planned 42 eligible patients, we determined that it was unlikely that we would observe clinical benefit in 26 of 42 eligible patients required in order to reach the 70% CBR benchmark stipulated a priori in the statistical plan. Based upon this futility analysis, we elected not to reopen the trial to accrual.
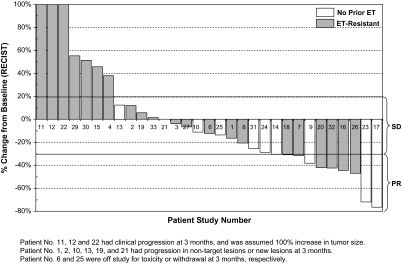
Among those 11 patients who achieved PR, five received no prior ET, two received one prior ET, three received two prior ETs, and one received three prior ETs. Among those five patients who achieved SD for at least 24 weeks, one received no prior ET, three received one prior ET, and one received two prior ETs. Ten of 16 patients who exhibited clinical benefit achieved best clinical response at 3 months (n = 5; two PR and three SD) and 6 months (n = 5, three PR and two SD); the remaining six patients achieved their best response after 6 months, including PRs at 9 (n = 3), 15 (n = 1), 18 (n = 1), and 30 (n = 1) months, respectively. The reduction in target lesions by RECIST between 6 months and baseline for all patients who received the fulvestrant–tipifarnib combination is shown in Figure 1.
Figure 1.
Waterfall plot of magnitude of tumor response in targeted lessions after 6-month treatment of fulvestrant and tipifarnib in all eligible patients (N=31).
response duration, TTP and OS
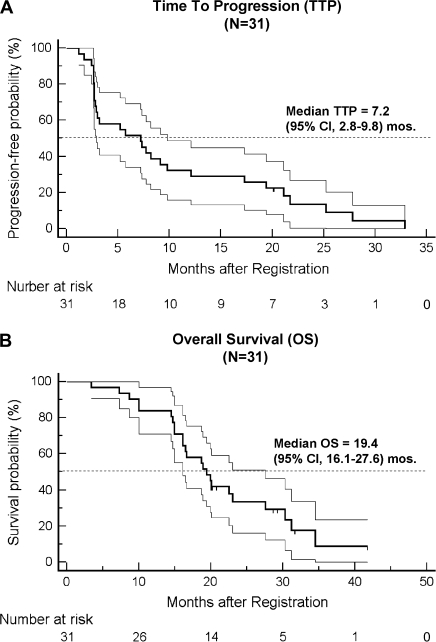
At the median follow-up of ∼20 months, the median DOR in the 16 responding patients was 16.0 months (95% CI 5.2–19.4 months). For the entire patient population of 31 eligible patients, median TTP was 7.2 months (95% CI 2.8–9.8 months; Figure 2A), and median OS was 19.4 months (95% CI 16.1–27.6 months; Figure 2B).
Figure 2.
Kaplan–Meier plots of (A) time to progression (TTP) and (B) overall survival (OS) in all eligible patients. The ranges of 95% confidence interval for the median TTP or OS are included in the graphs.
clinical efficacy by prior ET
We carried out an unplanned, post hoc analysis of CBR for the fulvestrant–tipifarnib combination according to prior ET. Of the nine patients who were either ET naive (n = 8) or had ET-sensitive disease (n = 1), five had PR and one had SD, yielding a CBR of 66.7% (95% CI 35.9% to 97.5%). Of 22 patients with ET-resistant disease (of whom 21 had AI-resistant disease), six had PR and four had SD for at least 24 weeks, yielding a CBR of 45.5% (95% CI 24.6% to 66.3%). Among the 21 patients with AI-resistant disease, 10 patients exhibited clinical benefit (CBR 47.6%, 95% CI 26.3% to 69.0%).
treatment administered and adverse effects
All treated patients (n = 33) who received at least one dose of tipifarnib were included in the safety analysis. A total of 342 cycles of therapy were administered (median 7 cycles/patient, range 1–36 cycles). As of 31 March 2008, two patients (nos. 31 and 32) were still receiving therapy after 21 and 22 cycles, respectively. Adverse events are summarized in Table 2. The most common grades 2–4 adverse events occurring in at least 5% of patients included nausea (21%), diarrhea (18%), vomiting (15%), neutropenia (15%), anemia (12%), neuropathy (9%), rash (9%), fatigue (9%), dyspnea (9%), elevated serum creatinine (9%), thrombosis (6%), anxiety (6%), hyperglycemia (6%), hypocalcemia (6%), and anorexia (6%). The majority of patients discontinued treatment for progressive disease (n = 24, 77.4%). Other reasons for discontinuing therapy included toxicity in three patients (10%) and withdrawal of consent in two patients (6.5%). Eight patients (25.8%) required dose reduction of tipifarnib, of whom six (37.5%) achieved clinical benefit. Reasons for tipifarnib dose reduction included grade 2 or greater nausea (n = 2), diarrhea (n = 1), tremor (n = 1), neutropenia (n = 1), elevated creatinine (n = 1), fatigue (n = 1), and confusion (n = 1).
Table 2.
Adverse events (n = 33)
| Adverse event (n = 33) | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Any Grade |
|||||
| No. of patients | % | No. of patients | % | No. of patients | % | No. of patients | % | No. of patients | % | |
| Hematologic | ||||||||||
| Neutropenia | 0 | 0 | 1 | 3 | 2 | 6 | 2 | 6 | 5 | 15 |
| Anemia | 6 | 18 | 2 | 6 | 2 | 6 | 0 | 0 | 10 | 30 |
| Infection | 1 | 3 | 2 | 6 | 1 | 3 | 0 | 0 | 4 | 12 |
| Gastrointestinal | ||||||||||
| Nausea | 8 | 24 | 4 | 12 | 3 | 9 | 0 | 0 | 15 | 45 |
| Vomiting | 1 | 3 | 3 | 9 | 2 | 6 | 0 | 0 | 6 | 18 |
| Diarrhea | 6 | 18 | 5 | 15 | 1 | 3 | 0 | 0 | 12 | 36 |
| Constitutional | ||||||||||
| Anorexia | 5 | 15 | 1 | 3 | 1 | 3 | 0 | 0 | 7 | 21 |
| Fatigue | 10 | 30 | 2 | 6 | 1 | 3 | 0 | 0 | 13 | 39 |
| Fever | 3 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 9 |
| Cardiovascular | ||||||||||
| Thrombosis | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 3 | 2 | 6 |
| Myocarditis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 3 |
| Pericardial effusion | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 3 |
| Cardiac ischemia | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 3 |
| Metabolic | ||||||||||
| Hyperglycemia | 4 | 12 | 1 | 3 | 1 | 3 | 0 | 0 | 6 | 18 |
| Hypocalcemia | 1 | 3 | 0 | 0 | 2 | 6 | 0 | 0 | 3 | 9 |
| Hypokalemia | 1 | 3 | 0 | 0 | 1 | 3 | 0 | 0 | 2 | 6 |
| Neurologic | ||||||||||
| Insomnia | 4 | 12 | 0 | 0 | 1 | 3 | 0 | 0 | 5 | 15 |
| Neuropathy | 4 | 12 | 3 | 9 | 0 | 0 | 0 | 0 | 7 | 21 |
| Anxiety | 1 | 3 | 1 | 3 | 1 | 3 | 0 | 0 | 3 | 9 |
| Agitation | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 3 |
| Ataxia | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 3 |
| Pulmonary | ||||||||||
| Dyspnea | 2 | 6 | 2 | 6 | 1 | 3 | 0 | 0 | 5 | 15 |
| Pneumonitis | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 3 |
| Renal | ||||||||||
| Creatinine | 0 | 0 | 2 | 6 | 1 | 3 | 0 | 0 | 3 | 9 |
| Skin | ||||||||||
| Rash | 0 | 0 | 2 | 6 | 1 | 3 | 0 | 0 | 3 | 9 |
discussion
We carried out a phase II trial of the FTI tipifarnib in combination with the selective ER downregulator fulvestrant in 31 eligible postmenopausal women with HR-positive MBC. Eligible patients had not received prior chemotherapy for MBC, of whom about two-thirds had disease that was resistant to AI therapy and one-third received no ET for metastatic disease. The primary end point was CBR, an accepted end point that is commonly used in trials evaluating ET for MBC. We elected to perform the futility analysis before completion of our study because the results of a randomized phase II trial revealed no benefit for tipifarnib when added to the AI letrozole in tamoxifen-resistant disease [25]. The study included 120 patients who were randomized in a 2 : 1 fashion to receive letrozole (2.5 mg daily) in combination with either tipifarnib (n = 80) or a placebo (n = 40). The dose and schedule of tipifarnib was 300 mg b.i.d. for 21 of 28 days, same as in our study. The CBR was 49% (95% CI 37% to 61%; including 30% PR and 19% SD for at least 24 weeks) in the letrozole–tipifarnib arm and 62% (95% CI 45% to 77%; including 38% PR and 23% SD for at least 24 weeks) in the letrozole–placebo arm [26]. There was no significant difference in response duration, time to disease progression, or survival. Based upon the results of this study, and the fact that the futility analysis of our trial determined that we could not achieve a prespecified 70% CBR, we elected not to resume accrual.
Although our trial failed to meet its primary end point, and tipifarnib does not appear to enhance the efficacy of letrozole, continued evaluation of the fulvestrant–tipifarnib combination in AI-resistant disease may be warranted for several reasons. First, fulvestrant is a more effective inhibitor of ER signaling than a nonsteroidal AI such as letrozole since the former blocks ligand–receptor interaction and degrades the ER, whereas the latter only reduces the amount of ligand from binding to the ER. Secondly, breast tumor cells that have become resistant to AI therapy may be more dependent on alternative growth signals, such as Ras signaling, than those cells that are resistant to tamoxifen. Long-term estrogen deprivation by an AI results in sustained activation of the ERK/MAP kinase and the PI3 kinase/mTOR pathways that are sensitive to the action of FTIs [16]. In contrast, there is no sustained activation of ERK/MAPK in tamoxifen-resistant MCF7 cells [16]. Thirdly, we observed clinical benefit in 10 of 21 patients (48%) with AI-resistant disease, which is considerably higher than the 32% CBR rate for fulvestrant alone in AI-resistant disease observed in a large trial [15]. In a post hoc analysis, an improvement in CBR from 32% to 60% (α = 0.10, β = 0.10) that we initially sought would require at least 10 of 21 patients having clinical benefit using Simon's minimax two-stage design, a benchmark that was achieved in the AI-resistant patients in our study. Further preclinical and clinical studies are needed to identify optimal strategies to incorporate tipifarnib in the treatment of HR-positive breast cancer.
In conclusion, this phase II study suggests that fulvestrant–tipifarnib combination may warrant further evaluation in postmenopausal MBC patients who have developed resistance to AI therapy. Should these studies be pursued, a loading dose of fulvestrant should be used to produce more rapid saturation and inhibition of ER signaling, and a randomized, double-blind, placebo-controlled trial design should be used in order to provide a greater level of confidence in the ultimate result than could be afforded by a single-arm trial. In addition, intermittent dosing of tipifarnib might also be used to allow tolerable, higher dose administration to achieve best clinical outcome [27].
funding
N01-CM-62204 to the New York Cancer Consortium (JAS) from the National Institutes of Health.
Acknowledgments
Initial protocol was developed under the mentorship of faculty at the 2003 Fifty FECS/AACR/ASCO Workshop on ‘Methods in Clinical Cancer Research’, Flims, Switzerland (TL). This study was approved and sponsored by the Cancer Therapy Evaluation Program of the National Cancer Institute (ClinicalTrials.gov, identifier NCT00082810, P6205). Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, 1–5 June 2007.
References
- 1.Muss HB. Endocrine therapy for advanced breast cancer: a review. Breast Cancer Res Treat. 1992;21:15–26. doi: 10.1007/BF01811960. [DOI] [PubMed] [Google Scholar]
- 2.Hortobagyi GN. Treatment of breast cancer. N Engl J Med. 1998;339:974–984. doi: 10.1056/NEJM199810013391407. [DOI] [PubMed] [Google Scholar]
- 3.Pritchard KI. Endocrine therapy for breast cancer. Oncology (Williston Park) 2000;14:483–492. discussion 493, 497–488. [PubMed] [Google Scholar]
- 4.Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 5.Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20:3386–3395. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 6.Howell A, Robertson JF, Abram P, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol. 2004;22:1605–1613. doi: 10.1200/JCO.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 7.Gradishar WJ, Sahmoud T. Current and future perspectives on fulvestrant. Clin Breast Cancer. 2005;6(Suppl 1):S23–S29. doi: 10.3816/cbc.2005.s.011. [DOI] [PubMed] [Google Scholar]
- 8.Robertson JF, Come SE, Jones SE, et al. Endocrine treatment options for advanced breast cancer—the role of fulvestrant. Eur J Cancer. 2005;41:346–356. doi: 10.1016/j.ejca.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Howell A, Pippen J, Elledge RM, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma: a prospectively planned combined survival analysis of two multicenter trials. Cancer. 2005;104:236–239. doi: 10.1002/cncr.21163. [DOI] [PubMed] [Google Scholar]
- 10.Robertson JF, Osborne CK, Howell A, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women: a prospective combined analysis of two multicenter trials. Cancer. 2003;98:229–238. doi: 10.1002/cncr.11468. [DOI] [PubMed] [Google Scholar]
- 11.Mauriac L, Pippen JE, Quaresma Albano J, et al. Fulvestrant (Faslodex) versus anastrozole for the second-line treatment of advanced breast cancer in subgroups of postmenopausal women with visceral and non-visceral metastases: combined results from two multicentre trials. Eur J Cancer. 2003;39:1228–1233. doi: 10.1016/s0959-8049(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 12.Dodwell D, Vergote I. A comparison of fulvestrant and the third-generation aromatase inhibitors in the second-line treatment of postmenopausal women with advanced breast cancer. Cancer Treat Rev. 2005;31:274–282. doi: 10.1016/j.ctrv.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Ingle JN, Suman VJ, Rowland KM, et al. Fulvestrant in women with advanced breast cancer after progression on prior aromatase inhibitor therapy: North Central Cancer Treatment Group Trial N0032. J Clin Oncol. 2006;24:1052–1056. doi: 10.1200/JCO.2005.04.1053. [DOI] [PubMed] [Google Scholar]
- 14.Perey L, Paridaens R, Hawle H, et al. Clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer and primary or acquired resistance to aromatase inhibitors: final results of phase II Swiss Group for Clinical Cancer Research Trial (SAKK 21/00) Ann Oncol. 2007;18:64–90. doi: 10.1093/annonc/mdl341. [DOI] [PubMed] [Google Scholar]
- 15.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–1670. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 16.Yue W, Fan P, Wang J, et al. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol. 2007;106:102–110. doi: 10.1016/j.jsbmb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berstein LM, Zheng H, Yue W, et al. New approaches to the understanding of tamoxifen action and resistance. Endocr Relat Cancer. 2003;10:267–277. doi: 10.1677/erc.0.0100267. [DOI] [PubMed] [Google Scholar]
- 18.Kelland LR, Smith V, Valenti M, et al. Preclinical antitumor activity and pharmacodynamic studies with the farnesyl protein transferase inhibitor R115777 in human breast cancer. Clin Cancer Res. 2001;7:3544–3550. [PubMed] [Google Scholar]
- 19.Li T, Sparano JA. Inhibiting Ras signaling in the therapy of breast cancer. Clin Breast Cancer. 2003;3:405–416. doi: 10.3816/CBC.2003.n.005. discussion 417–420. [DOI] [PubMed] [Google Scholar]
- 20.Johnston SR, Hickish T, Ellis P, et al. Phase II study of the efficacy and tolerability of two dosing regimens of the farnesyl transferase inhibitor, R115777, in advanced breast cancer. J Clin Oncol. 2003;21:2492–2499. doi: 10.1200/JCO.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 21.Fan M, Yan PS, Hartman-Frey C, et al. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Res. 2006;66:11954–11966. doi: 10.1158/0008-5472.CAN-06-1666. [DOI] [PubMed] [Google Scholar]
- 22.Johnston SR, Head J, Pancholi S, et al. Integration of signal transduction inhibitors with endocrine therapy: an approach to overcoming hormone resistance in breast cancer. Clin Cancer Res. 2003;9:524S–532S. [PubMed] [Google Scholar]
- 23.Martin LA, Head JE, Pancholi S, et al. The farnesyltransferase inhibitor R115777 (tipifarnib) in combination with tamoxifen acts synergistically to inhibit MCF-7 breast cancer cell proliferation and cell cycle progression in vitro and in vivo. Mol Cancer Ther. 2007;6:2458–2467. doi: 10.1158/1535-7163.MCT-06-0452. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Johnston SR, Semiglazov VF, Manikhas GM, et al. A phase II, randomized, blinded study of the farnesyltransferase inhibitor tipifarnib combined with letrozole in the treatment of advanced breast cancer after antiestrogen therapy. doi: 10.1007/s10549-007-9726-1. San Antonio Breast Cancer Symposium: 2005; (Abstr 5087) [DOI] [PubMed] [Google Scholar]
- 26.Johnston SR, Semiglazov VF, Manikhas GM, et al. A phase II, randomized, blinded study of the farnesyltransferase inhibitor tipifarnib combined with letrozole in the treatment of advanced breast cancer after antiestrogen therapy. Breast Cancer Res Treat. 2008;110:327–335. doi: 10.1007/s10549-007-9726-1. [DOI] [PubMed] [Google Scholar]
- 27.Lara PN, Jr, Law LY, Wright JJ, et al. Intermittent dosing of the farnesyl transferase inhibitor tipifarnib (R115777) in advanced malignant solid tumors: a phase I California Cancer Consortium Trial. Anticancer Drugs. 2005;16:317–321. doi: 10.1097/00001813-200503000-00011. [DOI] [PubMed] [Google Scholar]