Abstract
Immunological adjuvants, such as bacterial LPS, increase the mRNA levels of the IkB-related NF-κB transcriptional transactivator, Bcl-3, in activated T cells. Adjuvants also increase the life expectancy of activated T cells, as does over-expression of Bcl-3, suggesting that Bcl-3 is part of the pathway whereby adjuvants affect T cell lifespans. However, previous reports, confirmed here, show that adjuvants also increase the life expectancies of Bcl-3-deficient T cells, making Bcl-3’s role and effects in adjuvant-induced survival uncertain. To investigate the functions of Bcl-3 further, here we confirm the adjuvant-induced expression of Bcl-3 mRNA and show Bcl-3 induction at the protein level. Bcl-3 was expressed in mice via a transgene driven by the human CD2 promoter. Like other protective events, over-expression of Bcl-3 slows T cell activation very early in T cell responses to antigen, both in vitro and in vivo. This property was intrinsic to the T cells over-expressing the Bcl-3 and did not require Bcl-3 expression by other cells such as antigen-presenting cells.
Keywords: activation, memory, proliferation, T cell
Introduction
Vaccines are important contributors to human and animal health. For many years, it has been appreciated that successful vaccines must contain not only an antigen, a component of the target infectious agent, but also an adjuvant, a material that improves the primary immune response and essential for the creation of good immunological memory (1–5). We and others have shown that one effect of adjuvants is to increase the numbers and survival of antigen-specific memory cells (6, 7).
In efforts to understand how adjuvants affect the creation of memory cells, some years ago we compared gene expression in T cells responding to antigen in the presence or absence of adjuvants, in this case, LPS or a bystander viral infection. We found that a common feature increased Bcl-3 mRNA in the activated T cells that had been stimulated in the presence of an adjuvant (8, 9). Since then, others have confirmed this observation and shown that adjuvants probably raise Bcl-3 levels in T cells via IL-12 produced by the antigen-presenting cells that are the direct targets of the adjuvants themselves (10–14). Levels of Bcl-3 in the cell may be controlled both by gene transcription and also by turnover of the protein itself since a splice variant of the deubiquitinylating enzyme, CYLD, increases the amount of Bcl-3 in the cell and increases cell survival (15).
Bcl-3 is a member of the NF-κB family, originally identified as a component of a chromosomal rearrangement in some B cell lymphomas (16) and is over-expressed in activated human T cells (17). Bcl-3 is thought to act as a transcriptional enhancer for NF-κB p50 dimers, which lack a transactivation domain of their own (18–20) and may also affect transcription via its binding to other proteins such as pirin (21, 22). Bcl-3 may also have effects in the cytoplasm, however, since it is sometimes found in this part of the cell and is affected by plasma membrane proximal tyrosine kinases such as Fyn, Lck and GSK3 (23–25).
To understand more about the effects of Bcl-3 on T cells, we compared the activation and survival of T cells lacking Bcl-3 or over-expressing the gene. Here we show that over-expression of Bcl-3 protects both naive and activated T cells from death. However, surprisingly, adjuvants continue to enhance the survival of activated T cells, even if Bcl-3 is absent, a result that confirms a previous observation by others in a similar system (26). Thus, although Bcl-3 can mimic the effects of adjuvants, it is not essential for their action on T cells. The experiments also followed up on the observation that adjuvants often inhibit T cell proliferation, to some extent. Bcl-3 over-expression has the same effect. The experiments showed that Bcl-3 has this effect very early in the T cell response.
Materials and methods
Mice
C57BL/6J (B6), C57B/6.PLJ (B6.PL), C57Bl/6.SJL (CD45.1) (B6.SJL), OT1 and Rag1−/− mice were purchased from The Jackson Laboratory (West Grove, PA, USA) and intercrossed to derive OT1 mice expressing Thy1.1 or CD45.1 and lacking Rag1. Mouse Bcl-3 (mBcl-3) cDNA was cloned into a plasmid in which expression was driven by the human CD2 promoter (27) and injected into fertilized single-cell C57BL/6 embryos. Mice were screened for presence of the Bcl-3 transgene by PCR of tail DNA. Of four transgenic mice derived from this procedure, one line, named here Bcl-3 Tg, was selected for further study based on their high expression of mBcl-3. Bcl-3−/− mice were the generous gift of Ulrich Siebenlist (28). Mice were housed in the National Jewish Medical and Research Center Animal Care Facility in accordance with institutional guidelines.
Antibodies
Antibodies bound to the fluorochromes FITC, PE or PerCP against TCRβ (H57-H597), CD4 (GK1.5), CD8α (53-6.7), CD25 (PC-61), CD44 (IM7), CD62L (MEL-14), CD69 (H1.2F3), CD127 (SB/199), NK1.1 (PK136), 35Vβ2 (B20.6), Vβ6 (RR4-7), Vβ8 (F23.1) and Thy1.1 (OX-7) were purchased from BD PharMingen (San Diego, CA, USA). Anti-Vβ8 (F23.1), CD45.1 (A20) and Thy1.2 (53-2.1) were purified and coupled to biotin by standard methods. Secondary western blot antibodies, against Igs of various species, chemically coupled to HRP were purchased from Jackson ImmunoResearch. These included donkey anti-mouse Ig–HRP, donkey anti-goat Ig–HRP and goat anti-Armenian hamster Ig–HRP. mAbs against Bcl-3 were prepared as described below.
Preparation of anti-mouse Bcl-3 mAb
The cDNA for mBcl-3 was obtained from pGEM4Z-Bcl-3 (29), given a 6His tag and cloned into baculovirus by standard methods. Bcl-3 was expressed in Hi5 cells and isolated on Ni-NTA beads yielding a protein of ∼64 kD on 10–15% polyacrylamide gradient gels. Armenian hamsters were immunized with 50 μg of the protein in CFA followed by a second and third immunization with 20 μg in incomplete Freund's adjuvant. The hamster with the highest titer of anti-Bcl-3 antibody was rested for 4 weeks, injected with 50 μg soluble Bcl-3 intra-peritoneally and fused 3 days later. Hybridomas were screened for anti-Bcl-3 antibody by ELISA using the baculovirus protein as antigen. Positive hybridomas were grown up, cloned and retested by ELISA and western blot. Of four hybridoma antibodies that detected mBcl-3 by ELISA and western blot, one, Ham150-3.5 (IgG1 lambda), was chosen for further experiments. Ham150-3.5 reacts also with human Bcl-3 on western blots. Anti-Bcl-3 mAbs were purified on protein G sepharose beads.
SDS–PAGE western blotting
Cell lysates were prepared in the usual manner. Briefly, cells were spun in a microfuge for 5 min at 3500 r.p.m. Cell extracts were made by re-suspending the cell pellet in 1× PBS and adding one-fourth volume of 4× SDS-based loading buffer (NuPage LDS Sample Buffer, Invitrogen, Carlsbad, CA, USA) followed by shearing through 20 guage needle. Alternatively, nuclear and cytoplasmic extracts were made as previously described (30). Briefly, cell pellets were re-suspended in four times pellet volume of hypotonic buffer and incubated on ice for 15 min. Then 1/16th volume of 10% NP-40 detergent was added and the mixture was immediately vortexed for 10 s to lyse cells. Then, lysates were spun at 15 000 × g for 20 min at 4°C in a microcentrifuge, and the cytoplasmic supernatant fraction was mixed with 4× loading buffer and incubated for 5 min at 95°C. The pelleted nuclei were re-suspended in four volumes of high salt extraction buffer, rotated for 15 min at 4°C and then spun at 15 000 × g for 20 min at 4°C in a microcentrifuge. This nuclear fraction supernatant was mixed with 4× SDS-loading buffer and incubated for 5 min at 100°C. Cell lysates were loaded into 12% or 4–12% gradient Bis/Tris acrylamide gels (Invitrogen). These gels were run for 2–3 h at 120 volts. The gels were removed, equilibrated in 15% methanol transfer buffer and then transferred to polyvinyldifluoride membranes using a mini wet transfer apparatus (Invitrogen). The transfer was run at 25 volts for 2 h. The membrane was then removed and washed with Tris-buffered saline with 0.01% Tween-20 detergent (TBST). Western blotting membranes were incubated in a blocking solution of 5% weight to volume of non-fat dry milk in TBST for 1–2 h at 25°C or overnight at 4°C. Antibodies were diluted in blocking solution to an empirically optimized dilution depending on the antibody and allowed to mix for at least 15 min prior to being used. Membranes were then transferred into the primary antibody solution and incubated for 1–2 h at 25°C or overnight at 4°C. Blots were then removed, rinsed once in TBST and then washed in TBST three times for 5–15 minutes per wash. Secondary antibodies were diluted 1:20,000 in TBST and allowed to mix for 30 min prior to use. The membranes were then incubated in secondary antibody for 30–60 min at 25°C and then washed in TBST as described above. The blots were developed with ECL-Plus reagent (Amersham) and exposed on Hyperfilm (Amersham).
T cell purification
To isolate T cells from mice, spleen and lymph nodes (LNs) (inguinal, brachial, axillary, cervical, mesenteric and periaortic) were dissected from the mouse. The organs were crushed in 100-μm cell strainers (Falcon, Franklin Lakes, NJ, USA) with a 5-ml syringe plunger and washed with balanced salt solution (BSS) to isolate single-cell suspensions. The cells were spun and RBCs were removed by lysis with 150 mM ammonium chloride for 30 s, spun and washed one more time with BSS. The cell pellets were re-suspended in 3 ml BSS supplemented with 5% fetal bovine serum (BSS/FBS). Sterile nylon wool columns were pre-equilibrated with BSS/FBS at 37°C. Nylon wool columns were allowed to drain out and then cells were added and allowed to enter the column. Once in the column, the flow was stopped and the column (with cells) was incubated for 20 min at 37°C. After incubation, another 3 ml of BSS/FBS was added and allowed to enter the column. Once again, the flow was stopped and column was incubated for 20 min at 37°C. The non-adherent T cells were then washed out with two column volumes of BSS/5% FBS. This yielded a cell preparation of 85–90% T cells. To increase the purity further, the cells were sorted either with magnetic beads using the MACS System (Miltenyi, Auburn, CA) or by FACS using the MoFLO Cell Sorter (Cytomation, Fort Collins, CO, USA). Magnetic cell sorting was done according to the manufacturer's protocol, using the AutoMACS system. Briefly, 108 cells were incubated with PE anti-Vβ8 antibody for 30 min to 1 h at 4°C. The cells were then washed once with BSS and incubated for 20 min at 4°C with 100 μl anti-PE Miltenyi beads. These were then washed a final time with BSS and brought up in 3-ml volume. This was loaded onto the AutoMACS machine and the ‘Poseld’ program was run, collecting both the positive and negative fractions. Cells thus purified were typically 95% T cells, as determined by flow cytometry. MoFLO sorting was done by incubating cells with the appropriate staining antibodies, usually Alexa488–anti-Vβ8, PE–anti-CD8, cychrome–anti-CD4 and allophycocyanin–anti-MHC II. For staining, cells were brought to 108 ml−1 and incubated with antibodies at 4°C for 60 min in BSS with 20% anti-CD32/64 (2.4G2) supernatant to block Fc receptors. Cells were then washed with BSS, re-suspended in BSS to 4 × 107 cells per milliliters and run on the MoFLO Cell Sorter. Non-MHC II-expressing live cells were gated and the resulting cells were sorted for CD4+Vβ8+ or CD8+Vβ8+ T cells. These cells were collected and analyzed for purity on the FacsCaliber (Becton Dickson, Mountain View, CA, USA) cytometer with generally >97% T cell purity.
T cell culture and activation
T cells isolated as described were cultured at 37°C in 10% CO2 in SMEM medium (Invitrogen) supplemented with 10% FCS (hereafter called CTM). Six-well plates were coated for 24 h at 4°C with anti-CD3 mAb 145-2C11. Plates were then washed with PBS or BSS, and T cells re-suspended in CTM and placed in the incubator for desired length of experiment. CFSE labeling was performed as previously described (31).
Real-time PCR
Total cellular RNA was isolated from cell populations using either Trizol reagent (Invitrogen) or RNAeasy (Qiagen, Valencia, CA, USA) according to manufacturers’ protocols. Typical yields were 1.0 μg 10−6 cells. To make cDNA from mRNA, Superscript II RT (Stratagene, La Jolla, CA, USA) reverse transcriptase was used with random hexamer primers for 1 h at 42°C. Total RNA was used at 2 μg for a final volume of 20 μl. The reaction was stopped by 10 min incubation at 95°C. Real-time quantitative fluorometric PCR was used to quantify mRNA levels in various cell populations. All reactions were performed on the ABI Prism 7700 sequence detection system (Applied Biosystems, Norwalk, CT, USA) according to the manufacturer’s directions. The quantity of the mRNA under analysis was measured using specific primer sets for each message and normalized to β-actin control mRNA. Each set of primers was designed using Primer Express (Applied Biosystems) and included three DNA primers: forward, reverse and a fluorogenic probe. The fluorogenic probes were coupled to the fluorescent molecules FAM on the 5′ end and TAMRA on the 3′ end (Synthegene, Houston, TX, USA). Primers used to detect Bcl-3 were forward primer, 5′-GCGAAGTAGACGTCCATAACAACC-3′; reverse primer, 5′-ACCAAGAGCCGGACCATGT-3′; FAM–TAMRA–fluorogenic probe: 5′-CACCTGGCTGTCATCACCACATTACCA-3′.
Results
Adjuvants inhibit the death of antigen-activated T cells and increase T cell levels of Bcl-3 mRNA and protein
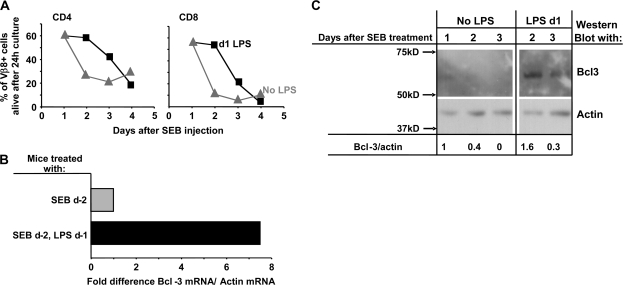
We and others have previously shown that adjuvants inhibit the rapid deaths in vivo and in vitro of T cells responding to antigen (6–8, 32). An example of this phenomenon is shown in Fig. 1(A). In this experiment, T cells bearing Vβ8 were activated with the Vβ8-specific superantigen (Sag), staphylococcal enterotoxin B (SEB), in vivo. One group of animals received the adjuvant, LPS, 1 day later. One–four days after the SEB administration, spleen cells were isolated from the animals, cultured alone for 24 h and the percentage of Vβ8+ CD4 and CD8 T cells then evaluated. T cells isolated from animals 1 day after SEB injection survived well in culture. However, T cells isolated at a later time died rapidly. Activated T cell death was inhibited if the mice had been given LPS 1–2 days previously, but the inhibitory effects of adjuvant were no longer apparent 4 days after SEB/3 days after LPS administration.
Fig. 1.
Bcl-3 mRNA and protein are increased in T cells activated in the presence of an adjuvant. Vβ8 T cells were isolated from mice given SEB with or without LPS 1 day later. (A) T cells were purified from the mice at various times after SEB/LPS injection and cultured without added factors. The percentage of Vβ8+ CD4 and CD8 cells that were dead was measured by flow cytometry. Results shown are representative of four independent experiments. (B) Vβ8+ T cells were isolated 48 h after mice were given SEB with or without LPS 24 h later. Real-time PCR was used to measure levels of Bcl-3 and actin mRNA. Results shown are typical of four independent experiments. (C) Mice were immunized with SEB with or without LPS 1 day later. Vβ8+ T cells were isolated from the mice on various days after SEB administration and western blotted for their content of Bcl-3 and actin. Each lane contained lysates from 2 × 105 cells.
The effects of adjuvant are correlated with induction of the Bcl-3 gene (8, 9, 13, 14). Bcl-3 mRNA is readily measurable; however, in the past, it has been difficult to measure Bcl-3 protein. To deal with this problem, we produced histidine-tagged Bcl-3 protein in insect cells, used the protein to immunize Armenian hamsters and produced a hybridoma-secreting mAb specific for Bcl-3. The specificity of the antibody was established by its ability to western blot a protein of molecular weight ∼63 kD (Fig. 1C) and by the fact that it did not react with proteins in T cells of Bcl-3−/− mice (Fig. 3C).
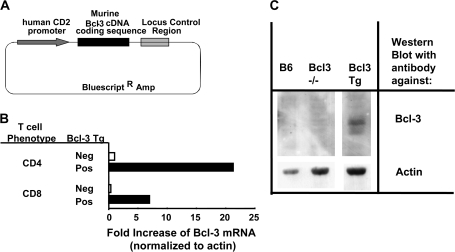
Fig. 3.
Production of a transgenic mouse line over-expressing Bcl-3 in T cells. (A) Structure of a plasmid coding for mBcl-3 driven by the human CD2 promoter. The plasmid was injected into the fertilized eggs of C57BL/6 mice. (B) CD4+ and CD8+ T cells were isolated from the spleens and LNs of normal B6 and Bcl-3 Tg mice. RNA was purified from the cells and real-time PCR used to measure the amount of mBcl-3 mRNA by comparison with actin in the cells. (C) T cells were purified from naive B6, Bcl-3 knockout or Bcl-3 Tg mice. Whole-cell lysates were western blotted for Bcl-3 protein (5 × 106 cells per lane) or for actin (3.5 × 106 cells per lane). Results shown are typical of two independent experiments.
As shown in Fig. 1(B) and as previously reported (8, 9, 13), antigen-activated T cells from animals given LPS contain considerably more Bcl-3 mRNA, measured by real-time PCR, than do T cells activated without adjuvant. This increase is reflected in an increase in the amount of Bcl-3 protein, with T cells 2 days after Sag activation containing ∼4 times more Bcl-3 protein if they had been exposed to LPS than those activated without adjuvant. Even 3 days after antigen plus LPS, the activated cells contained some Bcl-3 protein, although such protein was not apparent in cells activated without LPS (Fig. 1C).
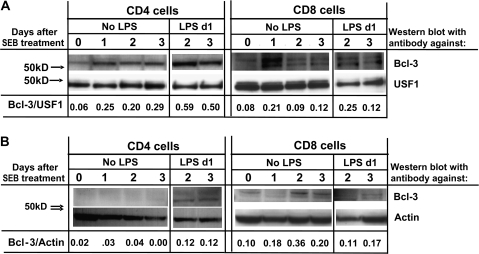
Bcl-3 has been reported to be usually located in the nucleus, with some protein found in the cytoplasm. To find the location of the induced Bcl-3 in T cells, cells were activated in vivo with SEB, with or without LPS 1 day later, and purified at various times thereafter by high-speed sorting. Nuclear and cytoplasmic fractions of the cells were prepared, run on SDS–PAGE and blotted with anti-Bcl-3 and anti-USF1 (a nuclear marker) or anti-actin (Fig. 2). In the absence of adjuvant, the amounts of Bcl-3 in the nuclei of CD4 T cells increased 1 day after activation and remained elevated to the same extent at days 2 and 3. Bcl-3 was barely detectable in the cytoplasms of the same cells. If the CD4 cells were isolated from animals given SEB and LPS, however, the amounts of Bcl-3 were further increased. Similar results were observed for CD8 T cells, with nuclear levels of Bcl-3 elevated 2 and 3 days after activation if the cells had also been exposed to LPS. Interestingly, two forms of Bcl-3 (33) were apparent, particularly in the CD8 T cells.
Fig. 2.
Administration of LPS in vivo increases the level of Bcl-3 protein expressed in activated T cells. Vβ8 T cells were activated in vivo as in Fig. 1(C) and activated CD4 and CD8 cells were purified by high-speed cell sorting. Nuclear (A) and cytoplasmic (B) fractions were prepared and western blotted for Bcl-3 and USF1 (A) and Bcl-3 and actin (B). Each lane contains lysates from 5 × 105 cells. Numbers represent the ratio of intensity of the Bcl-3 versus USF1 or actin bands.
Thus, exposure to adjuvant increases both the mRNA and the protein levels of Bcl-3 in activated T cells.
Production of Bcl-3 transgenic mice and expression of Bcl-3 in their T cells
To study the effects of Bcl-3 on T cells, we decided to produce Bcl-3 transgenic mice. Preliminary experiments using the proximal or distal Lck promoter failed and no increase in Bcl-3 mRNA or protein was detected in the T cells of mice transgenic for Bcl-3 driven by these promoters was detected (data not shown). However, the human CD2 promoter was more successful. A transgene was constructed in which mBcl-3 was driven by the human CD2 promoter (Fig. 3A). Plasmid coding for this construct was injected into fertilized B6 eggs and the mice produced screened by PCR of tail DNA for the presence of the transgene. A single line was established which over-expressed Bcl-3 protein in T cells at detectable levels (Bcl-3 Tg). Flow cytometry experiments showed that the thymuses, spleens and LNs of the Bcl-3 Tg mice had normal contents of thymocyte, T cell and B cell populations. There was no evidence that over-expression of Bcl-3 inhibited self-tolerance, induced by mouse mammary tumor virus Sags (34) (data not shown).
Naive CD4 and CD8 T cells from Bcl-3 Tg mice expressed 7- to 20-fold more Bcl-3 mRNA than naive T cells from normal B6 mice did (Fig. 3B). Likewise, naive T cells from Bcl-3 Tg mice expressed more Bcl-3 protein than B6 mice did (Fig. 3C).
Bcl-3 over-expression protects activated T cells from death, but is not required for the protective effects of adjuvant
To measure the effects of Bcl-3 over-expression on activated T cell survival, B6, Bcl-3 Tg and Bcl-3−/− mice were given SEB. One day later, groups of each type of mice were given LPS, and one day after this, the animals were sacrificed, T cells were isolated from their LN and spleen and cultured for 24 h. At this point, flow cytometry was used to evaluate the percentages of activated Vβ8+ T cells from each type of mouse that were alive.
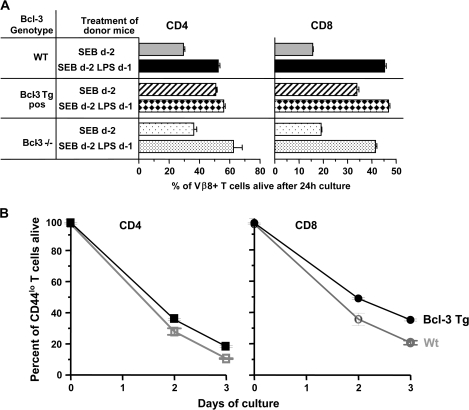
As expected, most of the CD4 and CD8 Vβ8 T cells from B6 mice were dead. Fewer of these cells died if they had come from B6 mice given both SEB and LPS (Fig. 4A). Activated T cells from Bcl-3 Tg mice given just SEB died less readily than similar cells from B6 mice. However, prior administration of LPS protected the cells from death still further. SEB-activated T cells from Bcl-3−/− animals also died at about the same rates as their counterparts from B6 animals. As noted by others (26), LPS prevented the deaths of Bcl-3−/− activated T cells just as effectively as it prevented the deaths of wild-type B6 cells (Fig. 4A). Likewise, over-expression of Bcl-3, as previously reported (8–14), protected the activated T cells from death. This is probably not due to the effects of Bcl-3 transgenic expression during development of the cells, since similar pro-survival effects of Bcl-3 have been described when the protein is expressed in transient transduction experiments (8).
Fig. 4.
Resting and activated T cells over-expressing Bcl-3 are protected from death, but Bcl-3 is not required for adjuvant inhibition of T cell death. (A) Wild-type (Wt) B6, Bcl-3 Tg or Bcl-3−/− mice were injected with SEB with or without LPS, 1 day later. Two days after administration of SEB, T cells were isolated from the mice and cultured. The percentage of activated Vβ8+ T cells alive 24 h later was evaluated by flow cytometry. Results shown are the means and standard errors of triplicate cultures and are representative of three independent experiments. (B) Spleen cells were isolated from unmanipulated Wt B6 or Bcl-3 Tg mice. The cells were cultured and CD4 CD44lo and CD8 CD44lo cells assessed for viability at various times thereafter. Results shown are the means and standard errors of triplicate cultures and are representative of at least five similar independent experiments in which survival of total unstimulated CD4 or CD8 T cells was evaluated.
Somewhat surprisingly, LPS improved the already quite good survival of the activated Bcl-3 Tg cells (Fig. 4A). This could have been either due to increased expression of endogenous Bcl-3 induced by the LPS (as illustrated in Fig. 2) or due to some other Bcl-3-independent effect of the LPS. The latter idea is supported by the fact that LPS administration improved the survival of activated T cells that lacked Bcl-3 (Fig. 4A) (26). Thus, although Bcl-3 is protective and Bcl-3 is induced by LPS, the protective effects of LPS do not require Bcl-3, and some pathway in addition to that downstream of Bcl-3 may be involved in the effects of adjuvants on T cell survival. The experiments described here do not reveal whether or not the protective effects of adjuvants on normal cells depend mainly on the Bcl-3 pathway or on the other route.
Bcl-3 over-expression prolongs the survival in vitro of naive T cells
Bcl-3 protein is barely detectable in naive T cells from normal animals (Figs 1C and 2); however, the protein is evident in naive T cells from the Bcl-3 Tg mice (Fig. 3C). To find out if this expression affected the survival of the cells, we isolated spleen cells from normal B6 and Bcl-3 Tg mice and cultured the cells. At zero time and at various times thereafter, the survival of naive (defined by expression of low levels of CD44) CD4 and CD8 T cells was evaluated. The results are shown in Fig. 4(B). Both CD4 and CD8 naive T cells from Bcl-3 Tg mice survived better in vitro than cells from B6 animals did. Thus, over-expression of Bcl-3 protects not only activated but also naive T cells from death.
Bcl-3 over-expression inhibits T cell expansion in response to antigen in vivo
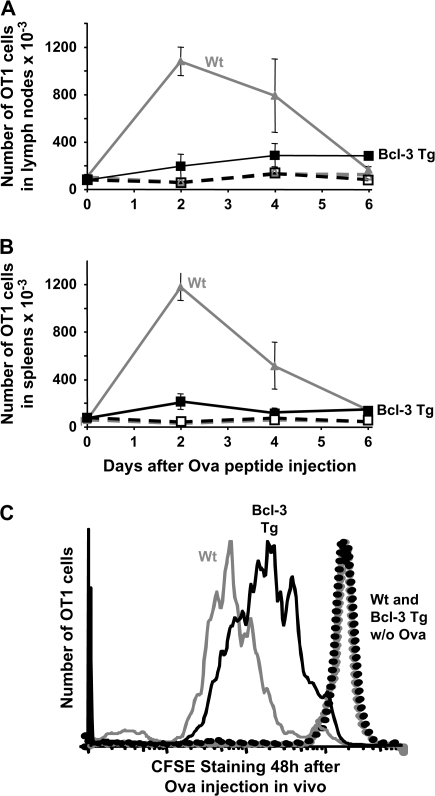
We and others have noticed that, in spite of the fact that adjuvants inhibit the deaths of activated T cells, the initial T cell expansions in response to antigen in vivo are less profound if adjuvants are also present (7). Adjuvants both reduce the initial rate and delay the peak of expansion of such cells. We tested whether Bcl-3 over-expression had similar effects by immunizing Bcl-3 Tg and control mice with SEB and following the numbers of Vβ8+ T cells in vivo over time. There was an indication that the response was slowed in the Bcl-3 Tg animals, but the results did not achieve significant differences from controls. Therefore, to do the experiment in a more definitive way and to eliminate possible effects of expression of the CD2-driven transgene in cells other than T cells, we crossed the Bcl-3 Tg mice with OT1 animals to produce Bcl-3 Tg, OT1+ cells. To allow efficient discrimination of these T cells from normal OT1 cells, the Bcl-3 Tg+, OT1+ mice were crossed additionally with B6.PL (Thy1.1+) animals to give rise to Bcl-3 Tg+, OT1 Tg+ Thy1.1+ mice. OT1+ RAG−/− mice expressing CD45.1 were produced by intercrossing with B6.SJL animals. Spleen cells from both these animals were mixed and transferred into normal B6 (Thy1.2+ CD45.2+) mice. One day later, the animals were given ovalbumin (OVA) peptide, the peptide antigen for the OT1 TCR, and at various times thereafter the numbers of OT1 TCR+ T cells of various origins measured, using antibodies to congenic markers and variable regions.
The OT1+ T cells expanded well in vivo in response to this challenge and fell back to baseline by day 6. By contrast, the Bcl-3 Tg, OT1 T cells barely expanded at all (Fig. 5A and B) but nevertheless maintained their expanded numbers, such as they were, between days 2 and 6 of the response, suggesting that the cells that had expanded might have been protected from death. These results, obtained for the two types of cells responding in the same mice, showed that the effects of the Bcl-3 Tg were intrinsic to the T cell expressing the Tg and were not caused by effects of the Tg on bystander cells. The result was not due to competition between the wild-type and Bcl-3 Tg OT1 cells, since similar results were found when CSFE-labeled cells were injected into separate animals (data not shown).
Fig. 5.
Over-expression of Bcl-3 slows T cell responses to antigen. (A) Spleen cells were isolated from mice expressing the OT1 TCR, RAG, the Bcl-3 Tg and Thy1.1 (Bcl-3 Tg) and from mice expressing the OT1 TCR, deficient in RAG and expressing CD45.1 (Wt). Analysis showed that the percentage of OT1+ cells in each population was the same (data not shown). Normal recipients were injected intravenously with 2.5 × 106 of an equal mixture of each type of spleen cell. One day later, the mice were immunized with 10 μg OVA peptide (solid lines) or untreated (dotted lines). At various times thereafter, LN (A) and spleen (B) cells were harvested and analyzed for their content of OT1 cells by congenic marker staining. Results shown are the means and standard errors of three identically treated mice and are representative of three independent experiments. (C) Spleen cells from OT1+ Bcl-3 Tg+ Thy1.1+ (Bcl-3 Tg) and OT1+ Bcl-3Tg Tg− Thy1.2+ (Wt) mice were stained with CFSE and transferred as described in (A) and (B). One day later, recipients were given 10 μg OVA peptide or unimmunized (dotted lines) and 2 days later the mice were sacrificed and the transferred cells analyzed for dilution of their CFSE label.
This result could have been caused by reduced proliferation and then reduced death of the Bcl-3 Tg T cells, a result suggested by the protective effects of the Bcl-3 Tg in vitro (Fig. 4A and B). On the other hand, Bcl-3 over-expression could have been killing T cells as they began to respond to antigen, protecting them only after the peak of the response had passed. To find out which of these explanations were correct, we checked the initial rates of proliferation of the cells by repeating the previous experiment, using this time OT1+ and Bcl-3 Tg+, OT1+ T cells that had been labeled with the cell cycle monitor, CFSE. The two types of T cells were labeled with the dye, transferred to B6 mice, challenged with OVA peptide or not challenged and harvested 2 days later. Cells in mice that had not received OVA peptide did not divide. However, analysis of the CFSE labeling of the transferred cells showed that, in the same animals, the OT1 T cells lacking the Bcl-3 Tg had divided more rapidly than their Bcl-3 Tg+ counterparts (Fig. 5C). Thus, the Bcl-3 Tg inhibited T cell expansion in response to antigen in vivo. These effects were intrinsic to the T cell involved.
Bcl-3 over-expression inhibits T cell activation in vitro
The in vivo experiment described above showed that the OT1+ Bcl-3 Tg T cells divided much less rapidly in response to antigen than the OT1+ RAG− T cells did. This result was most probably due to over-expression of Bcl-3; however, the two sets of cells were also distinguished by the fact that the Bcl-3 Tg cells were not deficient in RAG; therefore, some of them might have expressed a second TCR, a receptor that does not bind the OVA peptide antigen. This could have caused slower proliferation of the cells. We think that this latter explanation is unlikely, since only some of the cells would be expressing second TCR and the effect on proliferation of the Bcl-3 Tg cells applies to the whole population (Fig. 5C). However, to check this possibility directly, we decided to evaluate the response of Bcl-3 Tg and control T cells in vitro to anti-CD3. This experiment also gave an opportunity to find out if the defect in the Bcl-3 Tg cells was manifested in vitro as well as in vivo and also whether or not it occurred very early in the response, at the time of CD69 expression, for example.
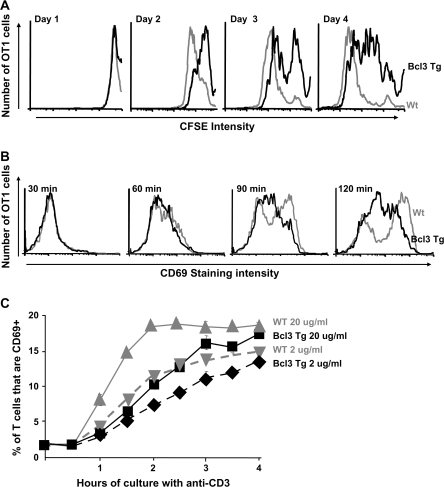
Spleen cells from B6 and Bcl-3 Tg mice were labeled with CFSE and cultured on anti-CD3-coated plates for various lengths of time. The T cells responded by dividing and thus diluting their levels of CFSE. The Bcl-3 Tg T cells responded less rapidly than the B6 T cell did, however. Even on day 4 of culture, some of the Bcl-3 Tg T cells had barely gone through more than two or three rounds of proliferation, whereas virtually all the B6 T cells had divided more than six times and completely lost detectable levels of their CFSE label by this time (Fig. 6A). T cells from B6 and Bcl-3 Tg T cells express equivalent levels of TCR (data not shown); therefore, the defect in proliferation of Bcl-3 Tg T cells is not due to lack of surface TCR. Also, the defect in Bcl-3 Tg T cells is measurable in vitro.
Fig. 6.
Over-expression of Bcl-3 inhibits an early event in T cell activation. (A) T cells from OT1+ Bcl-3 Tg+ Thy1.1+ mice (solid lines) and from OT1+ Bcl-3 Tg− Thy1.2 mice (gray lines) were labeled with CFSE, mixed in equal numbers and cultured on plates coated with 20 mg ml−1 anti-CD3. At various times thereafter, CFSE dilution of the T cells was evaluated. (B) T cells from OT1+ Bcl-3 Tg+ Thy1.1+ mice (solid lines) and from OT1+ Bcl-3 Tg− Thy1.2 mice (gray lines) were mixed in equal numbers and cultured on plates coated with 20 μg ml−1 anti-CD3. At various times thereafter, CD69 expression by the cells was evaluated. The results for this and a similar experiment in which the anti-CD3 was used at 2 μg ml−1 are summarized in (C). Results shown are representative of three independent experiments.
To find out how quickly the effects of Bcl-3 over-expression were manifested, we checked induction of one of the earliest markers expressed on T cells when they begin activation, CD69. The OT1+ and Bcl-3 Tg+, OT1+ T cells used in previous experiments were mixed together and cultured on plates coated with anti-CD3 antibody. CD69 became apparent on the OT1 cells beginning ∼60 min after the start of culture and increasing in amounts thereafter (Fig. 6B). The Bcl-3 Tg T cells responded by increasing their surface levels of CD69 too, but less rapidly. Similar results were obtained with other doses of anti-CD3 antibody (Fig. 6C) and also when the two types of cells were plated separately (data not shown). Thus, Bcl-3 over-expression reduces the rate of T cell activation, even at very early stages of the response.
Discussion
Adjuvants affect immune responses via many processes, with several outcomes. Among these is the fact that adjuvants increase the likelihood that activated T cells will convert to memory T cells, thus increasing the pool of memory cells available to defend its host against second infections by the same organism.
We and others have previously shown that one effect of adjuvants is to increase expression in cells of the gene for the IkB-related protein, Bcl-3, a phenomenon that is demonstrated in this paper in terms of Bcl-3 protein. In previous publications and here, it has been shown that Bcl-3 over-expression protects activated T cells from death (8–10, 13). Therefore, the idea that adjuvants act to promote T cell survival via induction of Bcl-3 is a natural conclusion to draw. However, adjuvants affect T cell survival in ways that do not necessarily involve this protein (35). Thus, the finding reported here and elsewhere (Chilton and Mitchell, 2006), that adjuvants can increase the rates of survival of T cells lacking Bcl-3, is perhaps not unexpected. This result may mean that Bcl-3 is not a crucial factor in wild-type T cells and that the adjuvant-induced expression and survival effects are merely correlative. On the other hand, it may be that Bcl-3 participates in the creation of memory T cells in wild-type cells but that in Bcl-3-deficient cells, some other process has been called into play to substitute for the absent protein. Such compensatory effects have been observed in many other systems and may be especially evident for phenomena involving cell survival, where, of course, cells expressing alternative means of survival will be well selected.
Besides affecting activated T cells, Bcl-3 over-expression increased the survival of naive T cells. This perhaps unexpected result is in line with the effects of other rescuing agents. For example, over-expression of Bcl-2 protects both resting and activated T cells from death. In vivo, the life expectancy of both kinds of cells is prolonged by IL-2-related cytokines(36–39) and such factors are thought to affect lymphocyte survival via their induction of anti-apoptotic proteins like Bcl-2 (40, 41). Although different cytokines affect naive and activated T cells, this may reflect differential expression of the receptors for the cytokines, rather than dependence on different intracellular signaling pathways. Nevertheless, gene array analyses have shown that over-expression of Bcl-3 does not change the levels of mRNA for Bcl-2-related proteins (data not shown). Therefore, if the Bcl-3 and IL-2 family pathways for rescuing T cells intersect, Bcl-3 cannot be affecting Bcl-2 family expression.
It is not clear how Bcl-3 affects T cell survival. Bcl-3 is unique among most IkB members in containing a transactivation domain capable of activating transcription for NF-κB members and many groups have shown that Bcl-3 can bind and regulate p50 and p52 homodimers (42–44). This suggests a transcriptional regulation of NF-κB targets as a potential mechanism of action of the Bcl-3 protein. The notion is supported by two other sets of results. First, Bcl-3 and the transactivation-containing NF-κB protein, p65, act redundantly with each other in supporting the survival of thymus medullary epithelial cells (45). Second, additional work has shown that Bcl-3 can control access of the signaling protein, Bcl-10, to the nucleus (46).
On the other hand, Bcl-3 may have a function that is restricted to the cytoplasm. Previous reports have also suggested that only a small portion of the ankryn repeat domain of Bcl-3, independent of the transactivation domain, is required for its ability to prevent apoptosis of activated T cells (47), suggesting a non-transcriptional mechanism of action. Here we show that adjuvants increase the level of both nuclear and cytoplasmic Bcl-3. In this context, it is interesting to note that preliminary gel shift experiments, not shown here, have not revealed any clear changes in NF-κB binding to DNA in Bcl-3 transgenic versus normal T cells, so perhaps a cytoplasmic function is involved in the effects of the protein.
Most interestingly, here we show that over-expression of Bcl-3 not only protects T cells from death but also dramatically slows their rates of activation, very early in their responses. The effect is intrinsic to the T cells themselves and is not mediated by bystander effects on other cells such as antigen-presenting cells. This result supports the idea that Bcl-3 itself is involved in the normal effects of adjuvants on activated T cells since they, like Bcl-3, both protect the cells from death and slow their activation. A number of papers have shown that interference with death processes is often accompanied by slowed T cell activation. For example, T cells lacking the death receptor, Fas, or part of its signaling cascade proliferate less well (48–50). Likewise, over-expression of the anti-apoptotic proteins, Bcl-2 or Bcl-xl, or under-expression of the pro-apoptotic factor, Bim, delays division of T cells [(51–53), M. Wang and P. Marrack, unpublished observation]. A recent paper showed that T cells lacking the executioner proteins, Bax and Bak, fail to die upon activation and also enter cell cycle slowly. The inhibition of activation was shown to be due to failure to release Ca++ from intracellular stores (54). However, preliminary experiments show that Ca++ release is normal after TCR engagement in Bcl-3 over-expressing cells. Thus, reduced Ca++ fluxes are probably not the cause of the slowed activation of Bcl-3 Tg T cells.
Disclosures
The authors have no financial conflicts of interest with the work described here.
Funding
National Institutes of Health Grants (AI-18785, P01 AI-22295, CA-046934).
Acknowledgments
The authors thank Dean Becker for producing the Bcl-3 transgenic mice, Anthony Desbien for his help in analyzing data and Tibor Vass for his help in breeding the animals.
Glossary
Abbreviations
- BSS
balanced salt solution
- FBS
fetal bovine serum
- LN
lymph node
- mBcl-3
mouse Bcl-3
- OVA
ovalbumin
- Sag
superantigen
- SEB
staphylococcal enterotoxin B
- TBST
Tris-buffered saline with 0.01% Tween-20 detergent
References
- 1.Dresser DW. Effectiveness of lipid and lipidophilic substances as adjuvants. Nature. 1961;191:1169. doi: 10.1038/1911169a0. [DOI] [PubMed] [Google Scholar]
- 2.Dresser DW. The role of T cells and adjuvant in the immune response of mice to foreign erythrocytes. Eur. J. Immunol. 1972;2:50. doi: 10.1002/eji.1830020111. [DOI] [PubMed] [Google Scholar]
- 3.Chiller JM, Weigle WO. Termination of tolerance to human gamma globulin in mice by antigen and bacterial lipopolysaccharide (endotoxin) J. Exp. Med. 1973;137:740. doi: 10.1084/jem.137.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beverley PC. Immunology of vaccination. Br. Med. Bull. 2002;62:15. doi: 10.1093/bmb/62.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Sprent J, Surh CD. T cell memory. Annu. Rev. Immunol. 2002;20:551. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 6.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J. Immunol. 1997;159:591. [PubMed] [Google Scholar]
- 7.Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2:261. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell TC, Hildeman D, Kedl RM, et al. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat. Immunol. 2001;2:397. doi: 10.1038/87692. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell TC, Teague TK, Hildeman DA, et al. Stronger correlation of bcl-3 than bcl-2, bcl-xL, costimulation, or antioxidants with adjuvant-induced T cell survival. Ann. N. Y. Acad. Sci. 2002;975:114. doi: 10.1111/j.1749-6632.2002.tb05946.x. [DOI] [PubMed] [Google Scholar]
- 10.Bauer A, Villunger A, Labi V, et al. The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells. Proc. Natl Acad. Sci. USA. 2006;103:10979. doi: 10.1073/pnas.0603625103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Eppolito C, Odunsi K, Shrikant PA. IL-12-programmed long-term CD8+ T cell responses require STAT4. J. Immunol. 2006;177:7618. doi: 10.4049/jimmunol.177.11.7618. [DOI] [PubMed] [Google Scholar]
- 12.Salem ML, Kadima AN, Cole DJ, Gillanders WE. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J. Immunother. 2005 doi: 10.1097/01.cji.0000156828.75196.0d. 28:220. [DOI] [PubMed] [Google Scholar]
- 13.Valenzuela JO, Hammerbeck CD, Mescher MF. Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T cells. J. Immunol. 2005;174:600. doi: 10.4049/jimmunol.174.2.600. [DOI] [PubMed] [Google Scholar]
- 14.Rangelova S, Kirschnek S, Strasser A, Hacker G. FADD and the NF-kappaB family member Bcl-3 regulate complementary pathways to control T-cell survival and proliferation. Immunology. 2008;125:549. doi: 10.1111/j.1365-2567.2008.02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovelmeyer N, Wunderlich FT, Massoumi R, et al. Regulation of B cell homeostasis and activation by the tumor suppressor gene CYLD. J. Exp. Med. 2007;204:2615. doi: 10.1084/jem.20070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crossen PE. Cytogenetic and molecular changes in chronic B-cell leukemia. Cancer Genet. Cytogenet. 1989;43:143. doi: 10.1016/0165-4608(89)90027-7. [DOI] [PubMed] [Google Scholar]
- 17.Ohno H, Takimoto G, McKeithan TW. The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell. 1990;60:991. doi: 10.1016/0092-8674(90)90347-h. [DOI] [PubMed] [Google Scholar]
- 18.Franzoso G, Bours V, Azarenko V, et al. The oncoprotein Bcl-3 can facilitate NF-kappa B-mediated transactivation by removing inhibiting p50 homodimers from select kappa B sites. EMBO J. 1993;12:3893. doi: 10.1002/j.1460-2075.1993.tb06067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brasier AR, Lu M, Hai T, Lu Y, Boldogh I. NF-kappa B-inducible BCL-3 expression is an autoregulatory loop controlling nuclear p50/NF-kappa B1 residence. J. Biol. Chem. 2001;276:32080. doi: 10.1074/jbc.M102949200. [DOI] [PubMed] [Google Scholar]
- 20.Grundstrom S, Anderson P, Scheipers P, Sundstedt A. Bcl-3 and NFkappaB p50-p50 homodimers act as transcriptional repressors in tolerant CD4+ T cells. J. Biol. Chem. 2004;279:8460. doi: 10.1074/jbc.M312398200. [DOI] [PubMed] [Google Scholar]
- 21.Dechend R, Hirano F, Lehmann K, et al. The Bcl-3 oncoprotein acts as a bridging factor between NF-kappaB/Rel and nuclear co-regulators. Oncogene. 1999;18:3316. doi: 10.1038/sj.onc.1202717. [DOI] [PubMed] [Google Scholar]
- 22.Pang H, Bartlam M, Zeng Q, et al. Crystal structure of human pirin: an iron-binding nuclear protein and transcription cofactor. J. Biol. Chem. 2004;279:1491. doi: 10.1074/jbc.M310022200. [DOI] [PubMed] [Google Scholar]
- 23.Weyrich AS, Dixon DA, Pabla R, et al. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc. Natl Acad. Sci. USA. 1998;95:5556. doi: 10.1073/pnas.95.10.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Ramakrishnan A, Kim KE, Rabson AB. Regulation of Bcl-3 through interaction with the Lck tyrosine kinase. Biochem. Biophys. Res. Commun. 2005;335:865. doi: 10.1016/j.bbrc.2005.07.162. [DOI] [PubMed] [Google Scholar]
- 25.Viatour P, Merville MP, Bours V, Chariot A. Protein phosphorylation as a key mechanism for the regulation of BCL-3 activity. Cell Cycle. 2004;3:1498. doi: 10.4161/cc.3.12.1328. [DOI] [PubMed] [Google Scholar]
- 26.Chilton PM, Mitchell TC. CD8 T cells require Bcl-3 for maximal gamma interferon production upon secondary exposure to antigen. Infect. Immun. 2006;74:4180. doi: 10.1128/IAI.01749-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhumabekov T, Corbella P, Tolaini M, Kioussis D. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J. Immunol. Methods. 1995;185:133. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
- 28.Franzoso G, Carlson L, Scharton-Kersten T, et al. Critical roles for the Bcl-3 oncoprotein in T cell-mediated immunity, splenic microarchitecture, and germinal center reactions. Immunity. 1997;6:479. doi: 10.1016/s1074-7613(00)80291-5. [DOI] [PubMed] [Google Scholar]
- 29.Bhatia K, Huppi K, McKeithan T, Siwarski D, Mushinski JF, Magrath I. Mouse bcl-3: cDNA structure, mapping and stage-dependent expression in B lymphocytes. Oncogene. 1991;6:1569. [PubMed] [Google Scholar]
- 30.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bender J, Mitchell T, Kappler J, Marrack P. CD4+ T cell division in irradiated mice requires peptides distinct from those responsible for thymic selection. J. Exp. Med. 1999;190:367. doi: 10.1084/jem.190.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J. Immunol. 2004;172:6065. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bundy DL, McKeithan TW. Diverse effects of BCL3 phosphorylation on its modulation of NF-kappaB p52 homodimer binding to DNA. J. Biol. Chem. 1997;272:33132. doi: 10.1074/jbc.272.52.33132. [DOI] [PubMed] [Google Scholar]
- 34.Kappler JW, Staerz U, White J, Marrack PC. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988;332:35. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Park SM, Wang Y, et al. Serine protease inhibitor 6 protects cytotoxic T cells from self-inflicted injury by ensuring the integrity of cytotoxic granules. Immunity. 2006;24:451. doi: 10.1016/j.immuni.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Vella A, Teague TK, Ihle J, Kappler J, Marrack P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J. Exp. Med. 1997;186:325. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boursalian TE, Bottomly K. Survival of naive CD4 T cells: roles of restricting versus selecting MHC class II and cytokine milieu. J. Immunol. 1999;162:3795. [PubMed] [Google Scholar]
- 38.Akbar AN, Borthwick NJ, Wickremasinghe RG, et al. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur. J. Immunol. 1996;26:294. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 39.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc. Natl Acad. Sci. USA. 1998;95:3810. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boise LH, Minn AJ, Noel PJ, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 41.Miyazaki T, Liu ZJ, Kawahara A, et al. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 42.Bours V, Franzoso G, Azarenko V, et al. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell. 1993;72:729. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- 43.Fujita T, Nolan GP, Liou HC, Scott ML, Baltimore D. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-kappa B p50 homodimers. Genes Dev. 1993;7:1354. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- 44.Nolan GP, Fujita T, Bhatia K, et al. The bcl-3 proto-oncogene encodes a nuclear I kappa B-like molecule that preferentially interacts with NF-kappa B p50 and p52 in a phosphorylation-dependent manner. Mol. Cell. Biol. 1993;13:3557. doi: 10.1128/mcb.13.6.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Wang H, Claudio E, Brown K, Siebenlist U. A role for the IkappaB family member Bcl-3 in the control of central immunologic tolerance. Immunity. 2007;27:438. doi: 10.1016/j.immuni.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeh PY, Kuo SH, Yeh KH, et al. A pathway for tumor necrosis factor-alpha-induced Bcl10 nuclear translocation. Bcl10 is up-regulated by NF-kappaB and phosphorylated by Akt1 and then complexes with Bcl3 to enter the nucleus. J. Biol. Chem. 2006;281:167. doi: 10.1074/jbc.M511014200. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell TC, Thompson BS, Trent JO, Casella CR. A short domain within Bcl-3 is responsible for its lymphocyte survival activity. Ann. N. Y. Acad. Sci. 2002;975:132. doi: 10.1111/j.1749-6632.2002.tb05947.x. [DOI] [PubMed] [Google Scholar]
- 48.Chau H, Wong V, Chen NJ, et al. Cellular FLICE-inhibitory protein is required for T cell survival and cycling. J. Exp. Med. 2005;202:405. doi: 10.1084/jem.20050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Falk M, Ussat S, Reiling N, Wesch D, Kabelitz D, Adam-Klages S. Caspase inhibition blocks human T cell proliferation by suppressing appropriate regulation of IL-2, CD25, and cell cycle-associated proteins. J. Immunol. 2004;173:5077. doi: 10.4049/jimmunol.173.8.5077. [DOI] [PubMed] [Google Scholar]
- 50.Walsh CM, Wen BG, Chinnaiyan AM, O'Rourke K, Dixit VM, Hedrick SM. A role for FADD in T cell activation and development. Immunity. 1998;8:439. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 51.Huang DC, O'Reilly LA, Strasser A, Cory S. The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J. 1997;16:4628. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linette GP, Li Y, Roth K, Korsmeyer SJ. Cross talk between cell death and cell cycle progression: BCL-2 regulates NFAT-mediated activation. Proc. Natl Acad. Sci. USA. 1996;93:9545. doi: 10.1073/pnas.93.18.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazel S, Burtrum D, Petrie HT. Regulation of cell division cycle progression by bcl-2 expression: a potential mechanism for inhibition of programmed cell death. J. Exp. Med. 1996;183:2219. doi: 10.1084/jem.183.5.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones RG, Bui T, White C, et al. The proapoptotic factors Bax and Bak regulate T cell proliferation through control of endoplasmic reticulum Ca(2+) homeostasis. Immunity. 2007;27:268. doi: 10.1016/j.immuni.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]