Abstract
Retinoic acid (RA) produced by intestinal dendritic cells (DCs) imprints gut-homing specificity on lymphocytes and enhances Foxp3+ regulatory T-cell differentiation. The expression of aldehyde dehydrogenase (ALDH) 1A in these DCs is essential for the RA production. However, it remains unclear how the steady-state ALDH1A expression is induced under specific pathogen-free (SPF) conditions. Here, we found that bone marrow-derived dendritic cells (BM-DCs) generated with granulocyte-macrophage colony-stimulating factor (GM-CSF) expressed Aldh1a2, an isoform of Aldh1a, but that fms-related tyrosine kinase 3 ligand-generated BM-DCs did not. DCs from mesenteric lymph nodes (MLN) and Peyer's patches (PP) of normal SPF mice expressed ALDH1A2, but not the other known RA-producing enzymes. Employing a flow cytometric method, we detected ALDH activities in 10–30% of PP-DCs and MLN-DCs. They were CD11chighCD4−/lowCD8αintermediateCD11b−/low F4/80low/intermediateCD45RBlowCD86highMHC class IIhighB220−CD103+. Equivalent levels of aldehyde dehydrogenase activity (ALDHact) and ALDH1A2 expression were induced synergistically by GM-CSF and IL-4 in splenic DCs in vitro. In BM-DCs, however, additional signals via Toll-like receptors or RA receptors were required for inducing the equivalent levels. The generated ALDH1A2+ DCs triggered T cells to express gut-homing receptors or Foxp3. GM-CSF receptor-deficient or vitamin A-deficient mice exhibited marked reductions in the ALDHact in intestinal DCs and the T cell number in the intestinal lamina propria, whereas IL-4 receptor-mediated signals were dispensable. GM-CSF+CD11c−F4/80+ cells existed constitutively in the intestinal tissues. The results suggest that GM-CSF and RA itself are pivotal among multiple microenvironment factors that enable intestinal DCs to produce RA.
Keywords: gut, homing, RALDH, regulatory T, Th17
Introduction
The vitamin A metabolite retinoic acid (RA) plays critical roles in gut immunity. We found that RA is produced by dendritic cells (DCs) in the gut-associated lymphoid organs, Peyer's patches (PP) and mesenteric lymph nodes (MLN), and imprints gut-homing specificity on T cells upon antigenic stimulation (1). Indeed, vitamin A-deficient mice exhibited depletion of T cells in the small intestinal tissues (1). RA also imprints B cells with gut-homing specificity and contributes to their IgA production (2). Recently, it was found that RA enhances the transforming growth factor (TGF)-β-dependent differentiation of naive T cells into Foxp3+ regulatory T cells (Treg) (3–10), but suppresses the differentiation of Th17 cells that contribute to inflammation (3, 7, 8, 10), and may contribute to oral tolerance. Therefore, RA appears to be a key molecule that controls lymphocyte trafficking and immune responses. However, it remains poorly understood how intestinal DCs acquire the RA-producing capacity.
The major pathway of RA synthesis depends on two steps (11, 12). The first step from vitamin A (retinol) to retinaldehyde is catalyzed by a subfamily of alcohol dehydrogenases that are expressed in most cells including DCs (1) or by the short-chain dehydrogenase/reductase family (13, 14). The second step from retinaldehyde to RA is catalyzed by aldehyde dehydrogenase (ALDH) 1A [retinal dehydrogenase (RALDH)], a subfamily of ALDH. ALDH1A expression is limited to certain cell types and is thus a key to the RA-producing capacity. MLN-DCs and PP-DCs significantly express Aldh1a or ALDH1A, whereas DCs in the spleen (SPL) or peripheral lymph nodes (PLN) that drain skin barely express it (1). DCs in the lamina propria (LP) of the small intestine also express Aldh1a (15). Here, we obtained a flow cytometric method to estimate aldehyde dehydrogenase activity (ALDHact) in individual DCs by modifying a detection system for hematopoietic stem cells (16) and identified the DC subsets with ALDHact+ in MLN and PP. DCs are likely to start expressing ALDH1A after they immigrated into the gut since DCs are plastic in nature (17) and can be re-programmed by tissue microenvironment to instruct T cells with other homing specificity (18). Thus, the microenvironment of the gut, even without active immunization or pathogenic bacterial infection, might specifically induce ALDH1A expression in DCs. RA itself has been previously implicated to endow swine monocyte-derived DCs with some attributes of gut DCs (19). Here, we found that granulocyte-macrophage colony-stimulating factor (GM-CSF) as well as vitamin A appears to play important roles in the differentiation of ALDH1A-expressing intestinal DCs in vivo. The expression of the major ALDH1A isoform, ALDH1A2 (RALDH2), was synergistically and strongly induced in DCs by GM-CSF and IL-4 in vitro and was further enhanced by Toll-like receptor (TLR) ligands and RA itself, but was only weakly induced by RA alone. The present study also suggests that DCs generated with GM-CSF possess the capacity to produce RA and gradually lose it in culture without GM-CSF.
Materials and methods
Mice
B10.D2 mice were from Japan SLC (Shizuoka, Japan). C57BL/6 and BALB/c mice were from CLEA Japan (Tokyo, Japan). TCR-DO11.10/Rag2−/− (B10.D2 background) and α chain of IL-4 receptor-deficient (IL-4Rα−/−; BALB/c background) mice were from Taconic Farms (Hudson, NY, USA). Common β subunit of GM-CSF/IL-3/IL-5 receptor-deficient (Beta-c−/−; C57BL/6 background) mice were from the Jackson Laboratory (Bar Harbor, ME, USA). Vitamin A-deficient mice were produced as described previously (1) with a modification; control diet contained 5000 IU/kg of retinyl acetate. All animals were maintained in specific pathogen-free (SPF) conditions in our animal facility. All animal experiments were performed according to the protocols approved by the Animal Care and Use Committee of Tokushima Bunri University.
Reagents
Recombinant murine GM-CSF, fms-like tyrosine kinase 3 ligand (Flt3L), IL-23, IFN-α, IFN-β, thymic stromal lymphopoietin (TSLP) and CX3CL1 were from R&D Systems (Minneapolis, MN, USA). Recombinant murine IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IFN-γ, tumor necrosis factor (TNF)-α and human TGF-β1 and TGF-β2 were from Peprotech (Rocky Hill, NJ, USA). Rosiglitazone was from Alexis Biochemicals (Lausanne, Switzerland). TO901317 was from Cayman Chemicals (Ann Arbor, MI, USA). Prostaglandin E2 and leukotriene B4 were from Biomol International (Plymouth Meeting, PA, USA). Soluble sonicated peptidoglycan (sPGN; from Escherichia coli K12), poly(I:C), R837 and immunostimulatory oligodeoxynucleotides containing unmethylated cytosine-phosphate-guanosine (CpG ODN) 1826 were from InvivoGen (San Diego, CA, USA). LPS (from E. coli O55:B5) and all-trans-RA were from Sigma–Aldrich (St Louis, MO, USA). LE540 was a kind gift from Dr Hiroyuki Kagechika (Tokyo Medical and Dental University, Tokyo, Japan). A list of mAbs that were used for cell isolation and staining is provided as Supplementary Table 1 (available at International Immunology Online).
Isolation of DCs and CD11c−F4/80+ cells
PP were treated with complete medium (Dulbecco's modified essential medium supplemented with 100 μM non-essential amino acids, 2 mM L-glutamine, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 25 mM HEPES, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 10% FCS) containing 5 mM EDTA and 1 mM dithiothreitol for 15 min at 37°C to remove intestinal epithelial cells (IECs). PP, MLN, PLN and SPL were digested with 400 Mandl U ml−1 collagenase type I (Sigma–Aldrich) and 30 U ml−1 DNase I (Invitrogen, Carlsbad, CA, USA) in complete medium with continuous stirring for 45–90 min at 37°C. For isolation of DCs, cells were incubated with anti-CD11c microbeads and then immunomagnetically sorted by sequential passage over 2 MACS separation columns (Miltenyi Biotec, Bergisch Gladbach, Germany), resulting in >96% purity. For sorting of DCs into CD103+ and CD103− subsets, cells were incubated with allophycocyanin (APC)-conjugated anti-CD11c and PE-conjugated anti-CD103 mAbs, followed by anti-APC microbeads. For sorting of CD11c−F4/80+ cells, cells were incubated with APC-conjugated anti-CD11c and PE-conjugated F4/80 mAbs, followed by anti-APC and anti-PE microbeads. Cells were first enriched by immunomagnetic cell sorting on a MACS separation column. Positively selected cells were sorted on a FACSAria (BD Biosciences, Franklin Lakes, NJ, USA), to >95% purity.
Isolation of IECs and LP lymphocytes
The jejunum and ileum were dissected and were flushed with HBSS without Ca2+ and Mg2+ to remove fecal contents. The intestinal segments were incubated with HBSS containing 1 mM dithiothreitol for 15 min at 37°C. The cell suspensions were filtered through cell strainers. The recovered cells were washed twice with HBSS and were used as IECs. For isolation of LP lymphocytes, the fragments of small intestine were shaken three times in EDTA and dithiothreitol in HBSS supplemented with 10 mM HEPES, 50 μg ml−1 gentamicin, 2.5 μg ml−1 fungizone, 10% FCS for 20 min at 37°C, cut into smaller pieces and then digested with collagenase and DNase in complete medium for 1 h at 37°C. The cell suspensions were filtered, washed, re-suspended in 40% isotonic Percoll (GE Healthcare, Little Chalfont, UK), layered onto 75% isotonic Percoll and centrifuged at 1000 × g for 20 min at room temperature. LP lymphocytes were collected at the interface of the Percoll gradient.
Immunoblotting
Rabbit anti-mouse ALDH1A2 antibodies were generated by immunizing the peptide CGGKGLGRKGFFIEP and were affinity purified. Cells solubilized in 1% Triton X-100, 150 mM NaCl, 20 mM Tris–HCl, 1 mM EDTA and 1% protease inhibitor cocktail (Nacalai Tesque, Kyoto, Japan) were centrifugated at 20000 × g for 5 min. Proteins in the supernatants were separated by SDS–PAGE, transferred to nitrocellulose membranes (GE Healthcare), probed with anti-ALDH1A2 antibody and HRP-conjugated goat anti-rabbit IgG antibody (Zymed Laboratories, South San Francisco, CA, USA) and developed by ECL Western Blotting Detection Reagents (GE Healthcare). Densitometric analysis was performed on a LAS-3000 (Fujifilm, Tokyo, Japan). The signal intensity of treated cells was expressed as the fold induction relative to that of untreated cells.
Real-time PCR
Total RNA was isolated from cells using RNeasy Micro Kit, and cDNA was generated using QuantiTect Reverse Transcription Kit (both from Qiagen, Hilden, Germany). cDNA was used as a template for real-time PCR in triplicates with Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and gene-specific primers (Supplementary Table 2 is available at International Immunology Online). PCR and analysis were performed on an Applied Biosystems 7500 Real-time PCR system. The expression of each gene was normalized with Rplp0.
Flow cytometric analysis
Cells were stained with fluorochrome-conjugated mAbs (Supplementary Table 1 is available at International Immunology Online) in the presence of anti-CD16/CD32 mAb. Some cells were stained with mouse E-selectin/human IgG Fc chimera (R&D Systems) and PE-conjugated goat anti-human IgG-Fc antibody (Jackson ImmunoResearch, West Grove, PA, USA). For staining of IgA+ B cells, cells were stained with biotinylated goat anti-mouse IgA antibody (Southern Biotechnology Associates, Inc., Birmingham, AL, USA) and PE-conjugated anti-B220 mAb, followed by APC-conjugated streptavidin (eBioscience, San Diego, CA, USA). To exclude dying cells, 1 ng ml−1 of propidium iodide (Sigma–Aldrich) was added. Intracellular staining of Foxp3 was performed using APC mouse/rat Foxp3 staining sets (eBioscience). For intracellular cytokine staining, cells obtained from cultures or isolated LP lymphocytes were stimulated for 5 h with 50 ng ml−1 phorbol 12-myristate 13-acetate and 750 ng ml−1 ionomycin (both from Calbiochem, Darmstadt, Germany). Monensin (3 μM; Sigma–Aldrich) was added to the cultures during the last 2 h. After surface staining, the cells were fixed and permeabilized with IC Fixation/Permeabilization Buffers (eBioscience), and intracellular cytokine staining was performed according to the manufacturer's protocol. Analysis was performed on a FACSAria, FACSCanto or FACSCalibur flow cytometer (all from BD Biosciences). In some experiments, expression levels were expressed as Δmean fluorescence intensity (MFI) which was calculated as: (MFI of the cells stained with fluorochrome-conjugated antibody) − (MFI of the background staining cells).
Analysis of ALDHact
ALDHact in individual cells was estimated using ALDEFLUOR staining kits (StemCell Technologies, Vancouver, British Columbia, Canada), according to the manufacturer's protocol with modifications. Briefly, cells suspended at 106 cells ml−1 in ALDEFLUOR assay buffer containing activated ALDEFLUOR substrate (150 nM) with or without the ALDH inhibitor diethylaminobenzaldehyde (DEAB; 100 μM; Sigma–Aldrich) were incubated for 30 min at 37°C. For immunophenotyping of ALDEFLUOR-reacted cells, the cells were subsequently stained with PE-, PE-Cy7- or APC-conjugated mAbs in ice-cold ALDEFLUOR assay buffer. ALDEFLUOR-reacted cells were detected using a FACSAria, FACSCanto or FACSCalibur flow cytometer with 488-nm blue laser and standard FITC 530/30 nm bandpass filter. Cell viability was determined by flow cytometry with propidium iodide exclusion, and the viability of DCs was not affected by exposure to ALDEFLUOR.
Immunohistochemical analysis
CD4+ or CD8+ cells were immunostained as previously described using frozen tissue sections and TO-PRO-3 (Molecular Probes, Eugene, OR, USA) for nuclear staining (1). For double immunostaining for GM-CSF and CD11c or F4/80, sections were blocked with 5% normal donkey serum for 1 h at room temperature and incubated with anti-GM-CSF mAb for 48 h at 4°C, followed by Cy3-conjugated anti-rat IgG antibody (Jackson ImmunoResearch) for 1 h at room temperature. The sections were blocked with Avidin D/biotin solutions (Vector Laboratories, Burlingame, CA, USA) and then incubated with biotinylated anti-CD11c or anti-F4/80 mAb at 4°C overnight, followed by FITC-conjugated streptavidin (Jackson ImmunoResearch) for 1 h at room temperature. Immunostained sections were coverslipped with glycerol and observed using a confocal laser scanning microscopy (FV1000, Olympus, Tokyo, Japan).
Culture of DCs
Bone marrow (BM) progenitors were harvested from femurs and tibias of B10.D2 mice and were immunomagnetically sorted by negative selection using PE-conjugated mAbs to B220, CD49b, Gr-1, I-Ad, TER-119 and Thy1.2 followed by anti-PE microbeads. Bone marrow-derived dendritic cells (BM-DCs) were generated with 20 ng ml−1 GM-CSF or 20 ng ml−1 Flt3L for 8 days, as described previously (20, 21). The purity of CD11c+ cells generated from GM-CSF- or Flt3L-supplemented BM cultures routinely exceed 87 or 95%, respectively. For treatment with various reagents, Flt3L-induced BM-DCs (2 × 105 cells) were cultured in 200 μl of complete medium containing indicated reagents in a well of flat-bottomed 96-well plates for 48 h. SPL-DCs were cultured under the same conditions but for 24 h.
DC-T cell co-cultures
Naive CD4+CD62Lhigh T cells were purified from splenocytes as previously described (22). DCs were pulsed with ovalbumin (OVA) peptide P323-339 (1 μM) for 2 h and then co-cultured with naive CD4+ T cells (4 × 104 cells) obtained from TCR-DO11.10/Rag2−/− mice at a ratio of 1:5 (DCs:T cells) in 200 μl of complete medium in a well of round-bottomed 96-well plates for 5 days. For Treg differentiation, DCs (4 × 103 cells) were co-cultured with naive CD4+ T cells (4 × 104 cells) in the presence of OVA peptide P323-339 (1 μM), 5 ng ml−1 TGF-β1 and 100 U ml−1 IL-2 for 3 days. For Th17 differentiation, 20 ng ml−1 IL-6 and 20 ng ml−1 IL-23 were also included in the culture. Treg or Th17 cultures were supplemented with IL-2 and incubated for an additional 2 days. Alternatively, freshly isolated MLN-DCs (1 × 104 cells) obtained from Beta-c−/− or wild-type (wt) mice were co-cultured with naive CD4+ T cells (5 × 104 cells) obtained from wt mice and soluble anti-CD3ε mAb (1 μg ml−1) for 5 days. In some cultures, DEAB was added at a final concentration of 100 μM.
Statistics
Statistical comparisons were performed using the one-way analysis of variance with Tukey–Kramer multiple comparisons test and the two-tailed unpaired Student's t test. Values <0.05 were considered statistically significant.
Results
A subset of CD103+ mature DCs in MLN and PP expresses ALDH1A2
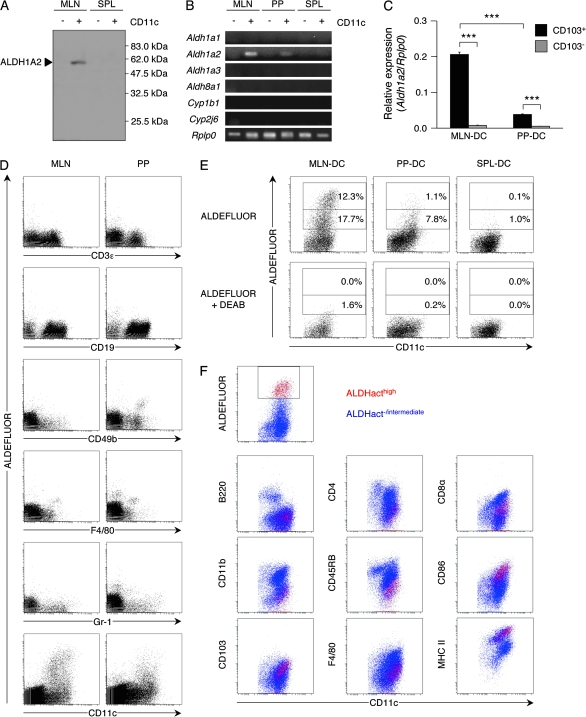
We have previously found that MLN-DCs express Aldh1a2 (1). The expression of ALDH1A2 protein in MLN-DCs was confirmed with specific antibodies (Fig. 1A). PP-DCs also expressed Aldh1a2 but to a much lesser extent than MLN-DCs, whereas CD11c− cells in MLN or PP did not significantly express it (Fig. 1B). Expression of Aldh1a1 (encoding RALDH1), Aldh1a3 (encoding RALDH3), Aldh8a1 (encoding RALDH4), Cyp1b1 or Cyp2j6 (the orthologous gene of rat Cyp2j4) was not significantly detected in DCs or CD11c− cells from MLN, PP or SPL of B10.D2 mice that were kept under SPF conditions (Fig. 1B). CYP1B1 and CYP2J6 are ALDH1A-independent RA-producing enzymes (23, 24). It was reported that CD103+ MLN-DCs expressed Aldh1a2 (4). Similarly, CD103+ PP-DCs but not CD103− PP-DCs expressed Aldh1a2 (Fig. 1C).
Fig. 1.
MLN-DCs and PP-DCs express Aldh1a2 and possess ALDHact. (A) Expression of ALDH1A2 in CD11c− or CD11c+ cells from MLN or SPL of B10.D2 mice was analyzed by immunoblotting. Data are representative of two independent experiments. (B) Expression of Aldh1a1, Aldh1a2, Aldh1a3, Aldh8a1, Cyp1b1, Cyp2j6 and Rplp0 as a loading control in CD11c− or CD11c+ cells from MLN, PP or SPL was analyzed by RT-PCR. Data are representative of two independent experiments. (C) Expression of Aldh1a2 in CD103+ or CD103− DCs from MLN or PP was analyzed by quantitative real-time PCR. Data are presented as mean ± SEM and are representative of three independent experiments. Statistical significance was determined by the one-way analysis of variance; ***P < 0.001. (D) Cells from MLN or PP were incubated with ALDEFLUOR, co-stained for CD11c and CD3ε, CD19, CD49b, F4/80 or Gr-1 and analyzed by flow cytometry. Pre-gated CD11c− cells are shown in the dot plots except those for CD11c staining. Data are representative of two independent experiments. (E) MLN-DCs, PP-DCs or SPL-DCs were incubated with ALDEFLUOR in the presence (bottom) or absence (top) of the ALDH inhibitor DEAB and stained for CD11c expression. Numbers adjacent to gates indicate percentage of ALDHacthigh cells (upper number) or ALDHactintermediate cells (lower number). Data are representative of three independent experiments. (F) ALDEFLUOR-treated MLN-DCs were co-stained for CD11c and B220, CD4, CD8α, CD11b, CD45RB, CD86, F4/80, MHC class II or CD103 expression. ALDHacthigh and ALDHact−/intermediate cells are shown as red and blue dots, respectively. Data are representative of two independent experiments.
Since ALDH1A constitute a subfamily of ALDH, we measured the relative ALDHact in individual cells by flow cytometry with a fluorescent substrate for ALDH. Among MLN cells, ALDHact was detected almost exclusively in CD11c+ cells (Fig. 1D). The proportion of the cells with high ALDHact (ALDHacthigh) in MLN-DCs was much larger than that in PP-DCs (Fig. 1E). The ALDHact was inhibited by the ALDH inhibitor DEAB. These results collectively suggest that ALDH1A2 is almost solely responsible for producing RA in MLN-DCs and PP-DCs in SPF mice. Furthermore, in MLN, ALDHact was exclusively exhibited by CD11chighCD4−/lowCD8αintermediateCD11b−/lowF4/80low/intermediateCD45RBlowCD86high MHC class IIhighB220−CD103+ DCs (Fig. 1F). We also estimated ALDHact in specific subsets of MLN-DCs divided according to the expression levels of CD8α and CD103. The CD8αintermediateCD103high subset exhibited high ALDH activities, and the CD8α−CD103intermediate and CD8αhigh CD103intermediate subsets exhibited intermediate and low ALDH activities, respectively, whereas the CD103− subsets failed to exhibit the activity (Supplementary Figure 1A is available at International Immunology Online). The Aldh1a2 expression was in good accord with the activity (Supplementary Figure 1B is available at International Immunology Online). The phenotype of ALDHacthigh cells in PP-DCs was similar to that in MLN-DCs (data not shown). SPL-DCs contained few ALDHact+ cells, whereas PLN-DCs consistently contained a small number of ALDHact+ cells, some of which were ALDHacthigh cells (data not shown).
GM-CSF, IL-4 and IL-13 induce ALDH1A2 expression in DCs
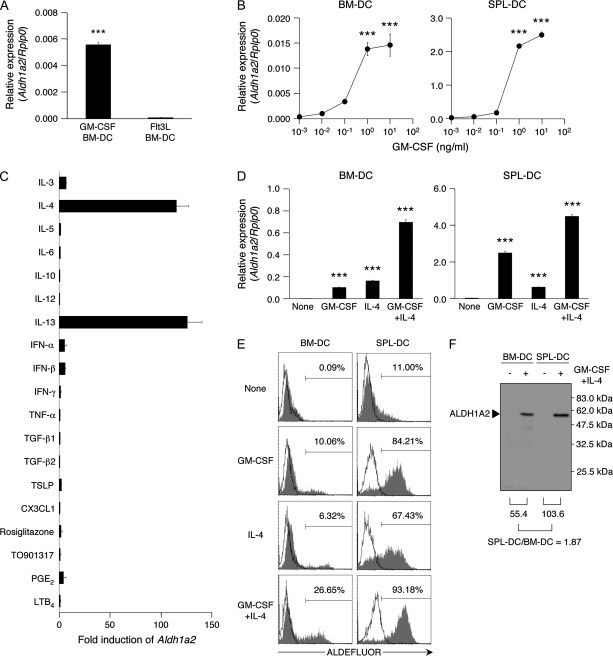
To identify the factors that induce ALDH1A2 expression in DCs, we prepared BM-DCs with GM-CSF or Flt3L. Unexpectedly, we found that GM-CSF-induced BM-DCs expressed Aldh1a2, although an extended culture in new plates without GM-CSF resulted in a gradual loss of the Aldh1a2 expression after a transient recovery in the expression (Supplementary Figure 2 is available at International Immunology Online). The extended culture with or without GM-CSF rapidly triggered the cells to spread and attach to the new culture plates, and it might induce some signals for the transient increase in the expression. On the other hand, Flt3L-induced BM-DCs did not express Aldh1a2 (Fig. 2A). To confirm the ability of GM-CSF to induce Aldh1a2 expression, we cultured Flt3L-induced BM-DCs with GM-CSF for 48 h and found that GM-CSF indeed triggered them to express Aldh1a2 (Fig. 2B). Unless otherwise indicated, we describe Flt3L-induced BM-DCs as simply BM-DCs henceforth. In SPL-DCs, GM-CSF also induced Aldh1a2 expression (Fig. 2B).
Fig. 2.
GM-CSF and IL-4 synergistically induce ALDH1A2 expression in DCs. (A) Expression of Aldh1a2 in GM-CSF-induced or Flt3L-induced BM-DCs was analyzed by quantitative real-time PCR. Data are presented as mean ± SEM and are representative of three independent experiments. Statistical significance was determined by the Student's t test; ***P < 0.001. (B) Expression of Aldh1a2 in Flt3L-induced BM-DCs (left) and SPL-DCs (right) cultured with graded concentrations of GM-CSF was analyzed by quantitative real-time PCR. Data are presented as mean ± SEM and are representative of three independent experiments. Statistical significance was determined by the one-way analysis of variance (ANOVA). ***P < 0.001 versus untreated. (C) Flt3L-induced BM-DCs were cultured with IL-3 (10 ng ml−1), IL-4 (10 ng ml−1), IL-5 (10 ng ml−1), IL-6 (10 ng ml−1), IL-10 (10 ng ml−1), IL-12 (10 ng ml−1), IL-13 (10 ng ml−1), IFN-α (104 U ml−1), IFN-β (104 U ml−1), IFN-γ (10 ng ml−1), TNF-α (10 ng ml−1), TGF-β1 (10 ng ml−1), TGF-β2 (10 ng ml−1), TSLP (10 ng ml−1), CX3CL1 (10 ng ml−1), prostaglandin E2 (PGE2; 10−6 M) or leukotrien B4 (LTB4; 10−7 M). The expression of Aldh1a2 was assessed by semi-quantitative real-time PCR. The mRNA expression of treated cells is expressed as the ‘fold induction’ relative to that of untreated cells. Data are presented as mean ± SEM and are representative of two independent experiments. (D–F) Flt3L-induced BM-DCs (left) or SPL-DCs (right) were cultured with medium alone (none), GM-CSF (10 ng ml−1), IL-4 (10 ng ml−1) or GM-CSF and IL-4 (10 ng ml−1 each). (D) Expression of Aldh1a2 was analyzed by quantitative real-time PCR. Data are presented as mean ± SEM and are representative of more than three independent experiments. Statistical significance was determined by the one-way ANOVA. ***P < 0.001 versus all samples. (E) The cultured DCs were incubated with ALDEFLUOR in the presence (solid lines) or absence (shaded histograms) of DEAB and were analyzed by flow cytometry. Numbers in histogram plots indicate percentage of ALDHact+ cells. Data are representative of more than three independent experiments. (F) Expression of ALDH1A2 in DCs cultured with GM-CSF and IL-4 was analyzed by immunoblotting. Numbers below immunoblot indicate the signal intensity of treated DCs. Data are representative of two independent experiments.
We then examined if other cytokines and factors available in the intestine could also trigger Aldh1a2 expression in BM-DCs. Two days of culture with IL-4 or IL-13 significantly induced Aldh1a2 expression in BM-DCs, but the treatment with other cytokines including IL-3, IL-5, IL-6, IL-10, IL-12, IFN-α, IFN-β, IFN-γ, TNF-α, TGF-β1, TGF-β2, TSLP or CX3CL1 failed to induce or enhance the expression (Fig. 2C).
The antigenic stimulation in the intestine tends to induce Th2-type responses or tolerance with Treg (25). Thus, IL-4, IL-13, IL-10 or TGF-β appears to be more commonly induced in the intestine than in other tissues. The results indicate that IL-4 and IL-13 but neither IL-10 nor TGF-β may contribute to induce the ALDH1A2 expression in intestinal DCs especially upon immune responses. The peroxisome proliferator-activated receptor γ agonist rosiglitazone and the liver X receptor agonist TO901317 were suggested to induce Aldh1a1 or Aldh1a2 expression in some cells or tissues (26, 27). They failed to induce the expression in BM-DCs (Fig. 2C), but weakly induced the Aldh1a2 expression when they were added at an early stage of the BM-DC development (data not shown). IEC-derived eicosanoids modulate some DC functions (28). However, neither prostaglandin E2 nor leukotriene B4 affected the Aldh1a2 expression (Fig. 2C).
GM-CSF and IL-4 synergistically induce ALDH1A2 expression in DCs
We also found that the combination of GM-CSF and IL-4 synergistically induced Aldh1a2 expression and ALDHact in BM-DCs and more efficiently in SPL-DCs and PLN-DCs (Fig. 2D and E and data not shown). We confirmed the production of ALDH1A2 protein in BM-DCs and SPL-DCs treated with the combination of GM-CSF and IL-4 (Fig. 2F). On the other hand, the combination or each cytokine failed to induce the expression of Aldh1a1, Aldh1a3 or Aldh8a1 in BM-DCs or SPL-DCs (data not shown).
TLR ligands enhance ALDH1A2 expression in BM-DCs
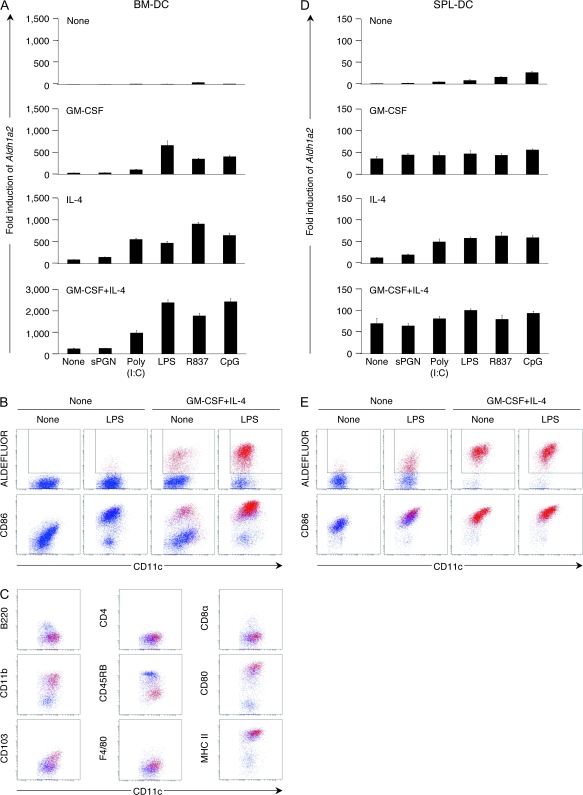
In germ-free mice, intestinal T cells do not increase after birth (29), indicating that commensal bacteria affect the development or immigration of gut mucosal T cells. DCs in the intestine appear to have chances to capture components of commensal bacteria even under normal steady-state conditions (30). TLRs and nucleotide-binding oligomerization domain proteins (Nod1 and Nod2) are key pattern recognition receptors that bind to conserved molecular patterns unique to prokaryotes and mediate innate host defense in the intestinal mucosa (31, 32). All the TLR ligands we tested, but not the Nod ligand sPGN, significantly enhanced GM-CSF/IL-4-induced Aldh1a2 expression (Fig. 3A) and the number of ALDHact+ (Fig. 3B and data not shown) in BM-DCs. TLR ligands up-regulated the CD86 expression (Fig. 3B). Accordingly, ALDHact+ DCs in MLN were CD86high (Fig. 1F). BM-DCs treated with GM-CSF, IL-4 and a TLR ligand for 48 h exhibited high ALDH activities comparable to those in MLN-DCs in normal mice (Figs 1E and 3B). They were B220−, CD45RBlow, CD80high and MHC class IIhigh, indicating that they were mature non-plasmacytoid DCs. The phenotype of the ALDHact+ BM-DCs corresponds to that of the ALDHact+ MLN-DCs except for the CD8α and CD11b expressions (Figs 1F and 3C). These cells were F4/80−, indicating that they were DCs but not macrophages. The CD103 expression was low in BM-DCs, but was up-regulated upon treatment with GM-CSF, IL-4 and LPS (Fig. 3C).
Fig. 3.
TLR-mediated maturation signals enhance Aldh1a2 expression and ALDHact in BM-DCs. Flt3L-induced BM-DCs (A–C) or SPL-DCs (D and E) were cultured with medium alone (none), sPGN (10 μg ml−1), poly(I:C) (1 μg ml−1), LPS (1 μg ml−1), R837 (1 μg ml−1) or CpG ODN 1826 (CpG; 1 μM) in the presence or absence of GM-CSF and/or IL-4 (10 ng ml−1 each). (A and D) Expression of Aldh1a2 was assessed by semi-quantitative real-time PCR. The mRNA expression of treated cells is expressed as the ‘fold induction’ relative to that of untreated cells (cultured with medium alone without GM-CSF or IL-4). (B and E) DCs were cultured with medium alone or LPS in the presence or absence of GM-CSF and IL-4, incubated with ALDEFLUOR and co-stained for CD11c and CD86 expression. (C) BM-DCs were cultured with LPS, GM-CSF and IL-4, incubated with ALDEFLUOR and co-stained for CD11c and B220, CD4, CD8α, CD11b, CD45RB, CD80, CD103, F4/80 or MHC class II expression. ALDHact+ cells are shown as red dots. All data are representative of two independent experiments.
In SPL-DCs, TLR ligands enhanced the IL-4-induced Aldh1a2 expression, but failed to enhance the GM-CSF-induced expression (Fig. 3D). GM-CSF with IL-4 induced high levels of ALDHact in SPL-DCs, and no further enhancement was observed with additional TLR stimulation (Fig. 3E). Similar results were obtained with PLN-DCs (data not shown).
DCs treated with GM-CSF and IL-4 and/or a TLR ligand induce gut-homing receptor expression on T cells and Treg differentiation
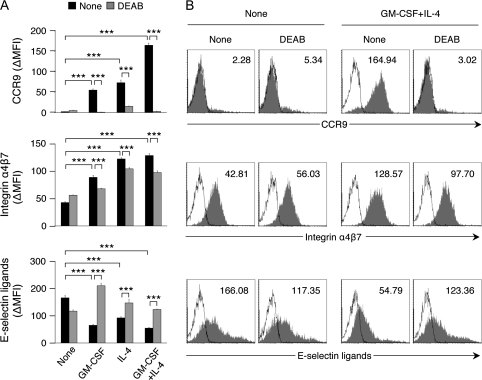
We examined if the generated ALDH1A2+ DCs could trigger T cells to express the gut-homing receptors, the integrin α4β7 and the chemokine receptor CCR9 (33, 34). BM-DCs treated with GM-CSF and/or IL-4 together with LPS induced CCR9 expression, enhanced α4β7 expression and suppressed the expression of the skin-homing receptors, E-selectin ligands, on T cells upon antigenic stimulation (Fig. 4). SPL-DCs treated with both GM-CSF and IL-4 did so even without the LPS treatment (Supplementary Figure 3 is available at International Immunology Online). The induction of CCR9 expression was almost completely inhibited by DEAB, indicating that de novo synthesis of RA was essential for the expression. The α4β7 expression was partly suppressed by DEAB. Unlike CCR9 expression, α4β7 expression can be induced to some extent on T cells in vitro even without RA (1, 18). DEAB restored the suppressed expression of E-selectin ligands (Fig. 4 and Supplementary Figure 3 is available at International Immunology Online). BM-DCs treated with other cytokines including IL-12 and TGF-β failed to induce gut-homing receptors on T cells (data not shown). These results suggest that Aldh1a2+ and ALDHact+ DCs induced with GM-CSF and IL-4 and/or a TLR ligand can induce or enhance gut-homing receptors on T cells.
Fig. 4.
DCs treated with GM-CSF or IL-4 and/or LPS induce the expression of gut-homing receptors on CD4+ T cells. Flt3L-induced BM-DCs were treated with medium alone (none), GM-CSF (10 ng ml−1), IL-4 (10 ng ml−1) or GM-CSF and IL-4 (10 ng ml−1 each). To promote full maturation of BM-DCs, LPS (1 μg ml−1) was added to their cultures. The treated DCs were pulsed with OVA peptide P323-339 (1 μM) and co-cultured with naive DO11.10 CD4+ T cells at a ratio of 1:5 in the presence or absence of DEAB (100 μM). On day 5 of culture, cells were stained for CCR9 (top), α4β7 (middle) and E-selectin ligands (bottom) and analyzed by flow cytometry. (A) The expression levels are presented as ΔMFI. Data are presented as mean ± SEM and are representative of more than three independent experiments. Statistical significance was determined by the one-way analysis of variance; ***P < 0.001. (B) Histogram plots of each staining of T cells co-cultured with untreated or GM-CSF + IL-4-treated DCs are shown. Shaded histograms and solid lines indicate specific staining and isotype control, respectively. Numbers in histogram plots indicate ΔMFI.
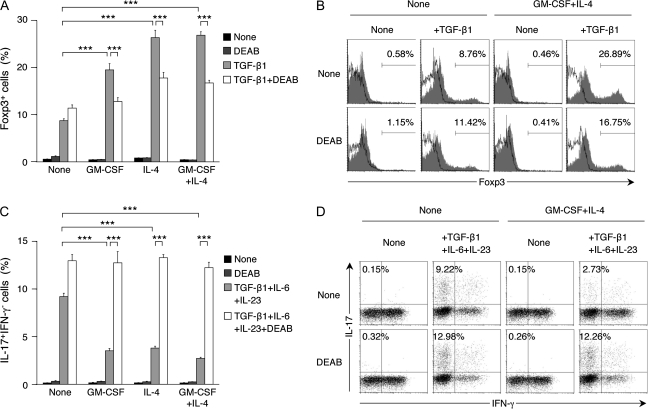
We also examined if ALDH1A2+ DCs generated in vitro could promote the TGF-β1-dependent differentiation of naive T cells to Foxp3+ T cells and the TGF-β1/IL-6/IL-23-dependent differentiation to Th17 cells. SPL-DCs treated with GM-CSF and/or IL-4 could more efficiently induce Foxp3+ T cells (Fig. 5A and B) and less efficiently induce Th17 cells (Fig. 5C and D) than control SPL-DCs. In the presence of DEAB, however, these effects were largely canceled, indicating that de novo synthesis of RA by DCs affected the T-cell differentiation.
Fig. 5.
DCs treated with GM-CSF and/or IL-4 enhance the differentiation of Foxp3+ T cells, but suppress the differentiation of Th17 cells. (A and B) SPL-DCs were treated with medium alone (none), GM-CSF (10 ng ml−1), IL-4 (10 ng ml−1) or GM-CSF and IL-4 (10 ng ml−1 each) and co-cultured with naive DO11.10 CD4+ T cells at a ratio of 1:10 in the presence of OVA peptide P323-339 (1 μM) and IL-2 (100 U ml−1) with or without TGF-β1 (5 ng ml−1). DEAB (100 μM) was added to some culture wells. On day 5 of culture, cells were stained for intracellular Foxp3 and analyzed by flow cytometry. (A) The graph shows the mean ± SEM of percentage of Foxp3+ T cells generated in each culture condition. (B) Histogram plots of T cells co-cultured with untreated or GM-CSF + IL-4-treated DCs are shown. Shaded histograms and solid lines indicate specific staining and isotype control, respectively. The number shown in each panel indicates the percentage of Foxp3+ cells. (C and D) The treated DCs were co-cultured with naive DO11.10 CD4+ T cells as described in (A and B), but IL-6 and IL-23 (20 ng ml−1 each) were also included in the culture. On day 5 of culture, cells were re-stimulated for 5 h with phorbol 12-myristate 13-acetate and ionomycin and stained for intracellular IL-17 and IFN-γ. (C) The graph shows the mean ± SEM of percentage of IL-17+IFN-γ− cells generated in each culture condition. (D) Dot plots of T cells co-cultured with untreated or GM-CSF + IL-4-treated DCs are shown. The number shown in each panel indicates the percentage of IL-17+IFN-γ− cells. All data are representative of two independent experiments. Statistical significance was determined by the one-way analysis of variance; ***P < 0.001.
GM-CSF is a major physiological inducer of the ALDH1A2 expression in intestinal DCs
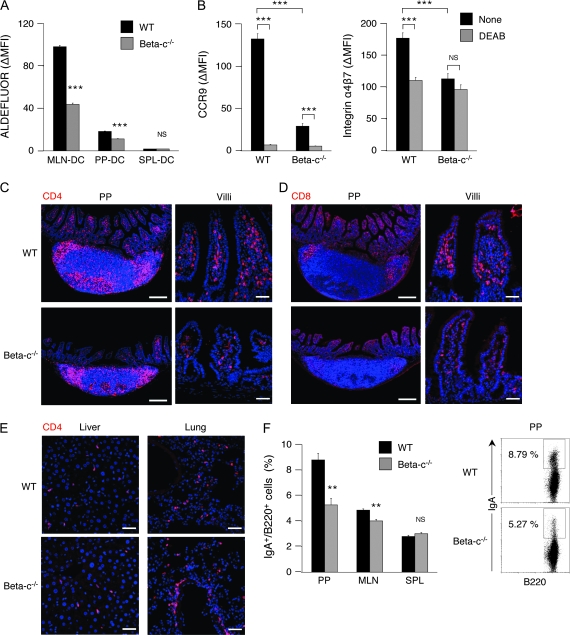
To examine if GM-CSF plays a physiological role in inducing the ALDH1A2 expression in intestinal DCs, we analyzed GM-CSF receptor-deficient mice, which were deficient of the common β subunit (Beta-c) of GM-CSF/IL-3/IL-5 receptor (35). These mice show normal DC development defined by myeloid- and lymphoid-related DC markers, except for a decrease in the DC numbers in their lymph nodes (LN) (36). We found, however, that the mean ALDHact in MLN-DCs from Beta-c−/− mice was markedly lower than that from wt mice (Fig. 6A). The ALDHact in PP-DCs was also affected by Beta-c deficiency. Accordingly, MLN-DCs from Beta-c−/− mice less efficiently induced the expression of CCR9 and α4β7 than those from wt mice (Fig. 6B). Furthermore, the number of CD4+ cells in the small intestinal LP in Beta-c−/− mice was markedly lower than that in wt mice (Fig. 6C). The number of CD8+ cells in LP and the intra-epithelial spaces was also reduced in Beta-c−/− mice (Fig. 6D). The distributions of CD4+ cells and CD8+ cells in PP of Beta-c−/− mice were similar to those of wt mice, although the sizes of PP of Beta-c−/− mice were smaller than those of wt mice (Fig. 6C and D). No difference was observed in the distribution of CD4+ cells in the liver of these mice (Fig. 6E, left). In the lung of Beta-c−/− mice, however, the number of CD4+ cells was significantly higher than that of wt mice (Fig. 6E, right), probably due to the alveolar proteinosis that is commonly developed in these mice (35, 37–39). We have previously shown that vitamin A deficiency affects IgA+ cell frequencies in PP and MLN (2). Similarly, IgA+ cell frequencies in PP and MLN were significantly reduced in Beta-c−/− mice (Fig. 6F). As only GM-CSF but not the other Beta-c-sharing cytokines, IL-3 or IL-5, could induce ALDH1A2 expression in DCs (Fig. 2), these results suggest that GM-CSF is a major physiological inducer of the ALDH1A2 expression in intestinal DCs in SPF mice under steady-state condition.
Fig. 6.
GM-CSF receptor-mediated signals are critical for MLN-DCs to acquire ALDHact and the capacity to imprint gut-homing specificity on T cells. (A) Cells from MLN, PP or SPL of wt or Beta-c−/− mice were incubated with ALDEFLUOR and stained for CD11c expression. Values indicate ΔMFI of ALDEFLUOR in CD11c+ cells. Data are presented as mean ± SEM (four mice in each genotype). Statistical significance was determined by the Student's t test. ***P < 0.001 versus wt mice. (B) MLN-DCs were isolated from Beta-c−/− or wt mice and co-cultured with naive CD4+ T cells from wt mice at a ratio of 1:5 in the presence of soluble anti-CD3ε mAb (1 μg ml−1). DEAB (100 μM) was added to some wells. On day 5 of culture, the expression levels of CCR9 (left) and α4β7 (right) on T cells were analyzed by flow cytometry. Data are presented as mean ± SEM and are representative of two independent experiments. Statistical significance was determined by the one-way analysis of variance; ***P < 0.001. (C–E) Immunohistochemical analysis was performed on CD4+ cells or CD8+ cells in PP, villi of small intestine, liver or lung from wt or Beta-c−/− mice. Frozen sections were stained for CD4 (C and E, red) or CD8 (D, red). Cell nuclei were visualized with TO-PRO-3 (blue). Data are representative of two independent experiments. Scale bars, 200 μm (PP); 50 μm (villi, liver and lung). (F) Cells from PP, MLN or SPL of wt or Beta-c−/− mice were stained for B220 and IgA. The graph shows the mean ± SEM (four mice in each genotype) of percentage of IgA+ cells in B220+ cells. Statistical significance was determined by the Student's t test. **P < 0.01 versus wt mice. Dot plots of pre-gated B220+ cells from PP are shown. Numbers adjacent to gates indicate percentage of IgA+ cells.
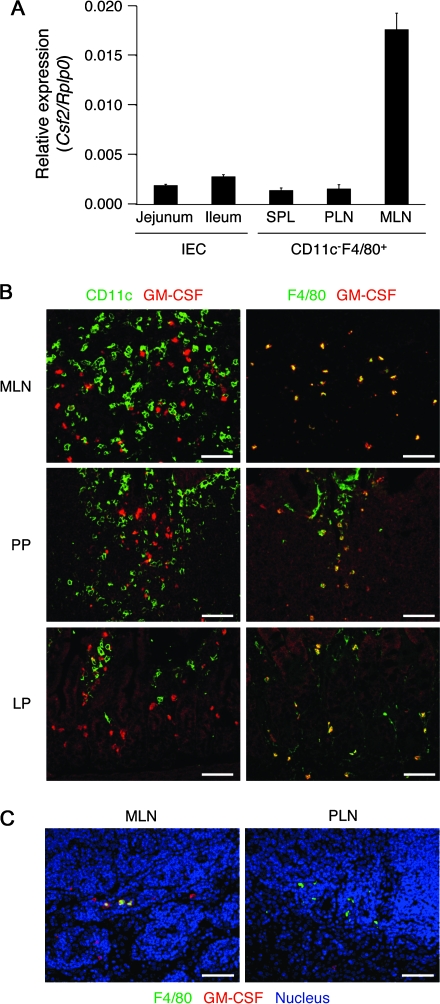
Colonic epithelial cells produce GM-CSF upon exposure to certain stimuli including pathogenic bacteria (40). However, the small intestinal IECs in normal SPF mice did not significantly express Csf2 mRNA (encoding GM-CSF) (Fig. 7A). On the other hand, CD11c−F4/80+ cells in MLN but not those in SPL or PLN expressed Csf2 (Fig. 7A). Indeed, GM-CSF+ cells were found outside of the follicles in MLN and PP and in the intestinal LP (Fig. 7B). Many of the GM-CSF+ cells were CD11c−F4/80+ (Fig. 7B and 7C, left), whereas, such cells were not found in the brachial LN (Fig. 7C, right). The results suggest that GM-CSF is constitutively produced by macrophages or granulocytes but not DCs in the intestinal tissues.
Fig. 7.
GM-CSF+CD11c−F4/80+ cells constitutively exist in MLN, PP and the intestinal LP. (A) IECs were isolated from the jejunum and ileum of B10.D2 mice. CD11c−F4/80+ cells were isolated from SPL, PLN and MLN of the same mice. The mRNA expression of Csf2 was assessed by quantitative real-time PCR. Data are presented as mean ± SEM and are representative of two independent experiments. (B) Frozen sections of MLN, PP or the intestinal LP were co-stained for GM-CSF (red) and CD11c (left, green) or F4/80 (right, green). (C) Frozen sections of MLN or PLN (brachial LN) were stained for GM-CSF (red) and F4/80 (green). Cell nuclei were visualized with TO-PRO-3 (blue). Data are representative of two independent experiments. Scale bars, 50 μm.
We also analyzed mice deficient of the IL-4 receptor α chain (41). IL-4Rα is a common component shared by IL-4 and IL-13 receptors. ALDH activities in the intestinal DCs from IL-4Rα-deficient mice were comparable to or rather higher than those from wt mice (Supplementary Figure 4 is available at International Immunology Online). The results indicate that the IL-4 receptor-mediated signals are dispensable for the ALDH1A2 expression in intestinal DCs.
Vitamin A is essential for the Csf2 expression in non-DCs in MLN
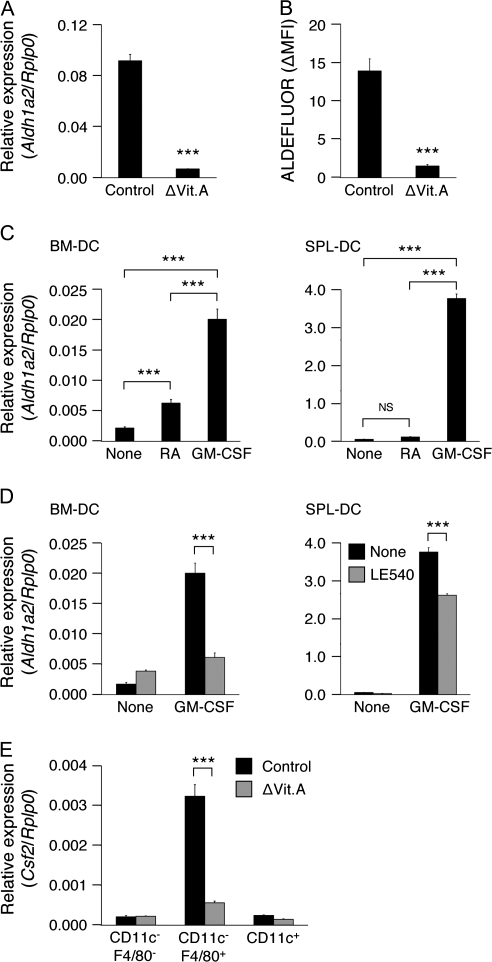
Swine monocyte-derived DCs pretreated with 1 μM RA instructed lymphocytes to enhance the expression of α4, β7 and Ccr9 (19). Accordingly, Aldh1a2 expression and ALDHact in MLN-DCs from vitamin A-deficient mice were dramatically lower than those from control mice (Fig. 8A and B), suggesting that vitamin A is essential for ALDH1A2 expression in MLN-DCs. All-trans-RA even at 1 μM weakly or hardly induced Aldh1a2 expression in BM-DCs or SPL-DCs, respectively (Fig. 8C), but moderately enhanced GM-CSF and/or IL-4-induced Aldh1a2 expression in BM-DCs (data not shown). 9-cis-RA exerted similar effects (data not shown). Accordingly, in the presence of the retinoic acid receptor (RAR) pan-antagonist LE540 (42), the GM-CSF-induced expression of Aldh1a2 was reduced by 70 and 30% in BM-DCs and SPL-DCs, respectively (Fig. 8D). There was a possibility that SPL-DCs prestored RA in vivo despite their inability to produce RA by themselves, and thus required less RA than BM-DCs in vitro. However, the effects of RA or LE540 on SPL-DCs from vitamin A-deficient mice were similar to those on SPL-DCs from control mice (data not shown), suggesting that the possibility is unlikely. These results indicate that RA-mediated signals contribute to the GM-CSF-induced ALDH1A2 expression in BM-DCs but to a much lesser extent in SPL-DCs.
Fig. 8.
Vitamin A is essential for GM-CSF-induced Aldh1a2 expression in DCs and Csf2 expression in CD11c−F4/80+ cells in MLN. (A) Expression of Aldh1a2 in MLN-DCs from control or vitamin A-deficient (ΔVit.A) mice was analyzed by quantitative real-time PCR. Data are presented as mean ± SEM and are representative of two independent experiments. Statistical significance was determined by the Student's t test; ***P < 0.001. (B) Cells from MLN of control or vitamin A-deficient mice were incubated with ALDEFLUOR and stained for CD11c expression. Values indicate ΔMFI of ALDEFLUOR in CD11c+ cells. Data are presented as mean ± SEM (four mice in each group). Statistical significance was determined by the Student's t test; ***P < 0.001. (C) Expression of Aldh1a2 in Flt3L-induced BM-DCs (left) or SPL-DCs (right) cultured with medium alone (none), all-trans-RA (1 μM) or GM-CSF (10 ng ml−1) was analyzed by quantitative real-time PCR. Data are presented as mean ± SEM and are representative of two independent experiments. Statistical significance was determined by the one-way analysis of variance (ANOVA); ***P < 0.001. (D) Expression of Aldh1a2 expression in Flt3L-induced BM-DCs (left) or SPL-DCs (right) cultured with GM-CSF (10 ng ml−1) in the presence or absence of LE540 (1 μM) was analyzed by quantitative real-time PCR. Data are presented as mean ± SEM and are representative of two independent experiments. Statistical significance was determined by the one-way ANOVA; ***P < 0.001. (E) Expression of Csf2 in CD11c+, CD11c−F4/80+ or CD11c−F4/80− cells from MLN of control or vitamin A-deficient mice was analyzed by quantitative real-time PCR. Data are presented as mean ± SEM and are representative of two independent experiments. Statistical significance was determined by the one-way ANOVA. ***P < 0.001 versus control mice.
We also found that the Csf2 expression in CD11c−F4/80+ cells in MLN was markedly reduced in vitamin A-deficient mice (Fig. 8E), suggesting that vitamin A is essential for the GM-CSF production in MLN.
Discussion
RA production by intestinal DCs appears to be critical for gut immunity and tolerance. However, it has remained unsolved which DC subsets are responsible for the RA production and how they acquire their RA-producing capacity. Here, we identified CD11chighCD4−/lowCD8αintermediateCD11b−/lowF4/80low/intermediateCD45RBlowCD86high MHC class IIhighB220−CD103+ cells as the DCs expressing the major RA-synthesizing enzyme, ALDH1A2, in MLN and PP of normal SPF mice. Especially, CD8αintermediateCD103high MLN-DCs highly expressed Aldh1a2 (Supplementary Figure 1 is available at International Immunology Online). MLN-DCs are derived from three sources: the blood, LP and PP (43). A subset of the LP-derived DCs was CD11chighCD8αintermediateCD11blowαLlowβ7high (44). Since the Aldh1a2+ and ALDHact+ DCs are a minor DC population in PP in normal SPF mice (Fig. 1), ALDH1A2+ DCs in MLN may be derived largely from LP and slightly from PP. In LP, at least two cell populations, CD103+CD11b−/low DCs and CD11b+F4/80+ macrophages, appear to be potential RA producers or their precursors (6, 9), and these DCs but not macrophages may migrate into MLN. Alternatively, ALDH1A2+ DCs generated in PP might quickly migrate into MLN, or ALDH1A2 expression might be also induced in DCs after migrating into MLN. Recently, it has been shown that a subset of LP-DCs with a macrophage-like character (CD11chighCD11bhighCD86highCD103+F4/80moderate) also expresses Aldh1a2 (15).
GM-CSF appears to play a physiological role to induce the ALDH1A2 expression in intestinal DCs. GM-CSF+ cells were detected in the intestinal tissues of normal SPF mice, and many of them were CD11c−F4/80+. Macrophages (9) or eosinophil-like cells (44) may contribute to the GM-CSF production in the intestinal tissues. IECs did not produce GM-CSF without exposure to pathogenic bacteria, but may produce it with these stimuli. GM-CSF is also constitutively expressed in BM. However, fresh CD11c+ BM cells expressed very low levels of Aldh1a2 (data not shown). CD11c+ BM cells may be too immature to express high levels of ALDH1A2 upon exposure to GM-CSF. Alternatively, collaborative factors including RA, IL-4 and TLR ligands may not be enough in BM. GM-CSF is also required for maintaining ALDH1A2 expression since GM-CSF-induced Aldh1a2 expression in BM-DCs decreased when the culture was extended in the absence of GM-CSF (Supplementary Figure 2 is available at International Immunology Online). Peripheral blood monocyte-derived DCs cultured in GM-CSF and IL-4 are likely to express ALDH1A2 as well, but may lose it with an extended culture without GM-CSF. DCs generated with GM-CSF and IL-4 are widely used for research or therapeutic purposes. Our results suggest that these cells express ALDH1A2 immediately after the generation.
Beta-c deficiency caused marked reductions in the ALDHact in MLN-DCs and the T cell number in LP. Beta-c is a common component of receptors of GM-CSF, IL-3 and IL-5, but only GM-CSF induced ALDH1A2 expression in wt DCs. In IL-5 receptor-deficient mice, there were normal numbers of IL-4-, IL-5- or IL-6-producing cells in LP (45), implying that IL-5 signals may not be essential for the T-cell distribution in LP. IL-3 signals can be transduced through an alternative IL-3 receptor β in the absence of Beta-c in mice (39). Therefore, the above-mentioned reductions in Beta-c-deficient mice are likely to be due to the lack of GM-CSF-dependent signals. However, the reductions were not as severe as those observed in vitamin A-deficient mice (1). The GM-CSF-dependent signals might be partly compensated by IL-4, IL-13, TLR ligands or other unknown stimuli. Accordingly, it has been recently shown that IL-4 enhanced the Aldh1a2 expression in MLN-DCs and that T cells stimulated with MLN-DCs from IL-4Rα-deficient mice exhibited lower CCR9 expression than T cells stimulated with wt MLN-DCs (46). Although IL-4/IL-13-dependent signals were dispensable for the ALDH1A2 expression in DCs, they might be produced and contribute to the expression, especially during immune responses in the presence of RA and TLR-mediated signals in the gut.
Myeloid differentiation primary response gene 88 (MyD88) is a central adaptor shared by almost all TLRs (47, 48), but the numbers of intra-epithelial lymphocytes and LP lymphocytes are comparable between wt and MyD88−/− intestines (49). It remains to be solved if TLR-mediated signals are essential for the ALDH1A2 expression since TIR domain containing adaptor-inducing IFN-β-dependent pathways could be contributing to the signals in these mice. Nonetheless, any combinations of two from GM-CSF, IL-4 and a TLR ligand synergistically induced both Aldh1a2 expression and ALDHact in BM-DCs. Three of them further strongly induced the expression and activity. Their ALDHact was comparable to that found in ALDHacthigh MLN-DCs. Accordingly, it was recently reported that the Aldh1a2 expression in CD11chighCD11bhigh LP-DCs was enhanced upon TLR5-mediated stimulation (15). Since ALDH1A2+ MLN-DCs were mature CD86high DCs, maturation of DCs is likely to be required for inducing the high levels of ALDH1A2 expression. Indeed, TLR-mediated signals also induced maturation of BM-DCs in the presence of GM-CSF and IL-4 (Fig. 3A and B). On the other hand, most SPL-DCs became CD86+ cells after 24 h of culture even without TLR-mediated stimulation (Fig. 3E). Accordingly, neither TLR-mediated signals nor IL-4 were required for inducing high levels of ALDHact in SPL-DCs in the presence of GM-CSF. Indeed, depending on the ALDHact, SPL-DCs treated with GM-CSF and/or IL-4 without LPS significantly induced CCR9 expression on T cells (Supplementary Figure 2 is available at International Immunology Online). However, the increase in α4β7 expression on T cells was moderate even when SPL-DCs were pretreated with both GM-CSF and IL-4. The pretreated SPL-DCs might also provide a factor or signal that interfered with the RA-induced α4β7 expression. Nonetheless, DEAB partly but significantly suppressed the α4β7 expression. The pretreated SPL-DCs also enhanced the differentiation of naive CD4+ T cells to Foxp3+ T cells but suppressed that to Th17, and these effects were canceled by DEAB (Fig. 5). Accordingly, in vivo administration of GM-CSF has been shown to induce anti-inflammatory DCs and Tregs and suppress autoimmune disorders, such as experimental autoimmune thyroiditis, type I diabetes and myasthenia gravis (50–53). The GM-CSF administration also decreased the severity of Crohn's diseases (54). These beneficial effects of GM-CSF might involve the ALDH1A-dependent mechanism. Interestingly, GM-CSF is also known as an inflammatory cytokine. It has recently been reported that GM-CSF was critical for IL-6 and IL-23 responses by DCs and thus critical for generation of Th17 cells in vivo (55). Frequencies of IL-17-producing cells in the small intestinal LP of Beta-c−/− mice were indeed lower than those of wt mice (Supplementary Figure 5 is available at International Immunology Online), as found in immunized GM-CSF−/− mice. Various factors during antigen priming might affect the preferential development of Th17 or Treg. TGF-β is another example with dual functions for inducing the development of Th17 and Treg. RA itself might also have the dual functions depending on its concentration. A low concentration of RA was suggested to be required for Th17 development, although high concentrations of RA suppressed it (15). After the Th17 development, Th17 cells may produce GM-CSF, which may then contribute to a feedback suppression of Th17 development by enhancing ALDH1A2 expression and RA production in DCs.
DEAB only partly inhibited the α4β7 expression induced by wt MLN-DCs as well, and the residual expression was equivalent to the α4β7 expression induced by Beta-c−/− MLN-DCs in the presence or absence of DEAB (Fig. 6B, right), suggesting that there are RA-dependent and RA-independent pathways to induce α4β7. On the other hand, the CCR9 expression induced by wt MLN-DCs or GM-CSF/IL-4-treated BM-DCs or SPL-DCs was almost completely inhibited by DEAB [Fig. 4A, top and Fig. 6B, left and Supplementary Figure 3, left (available at International Immunology Online], indicating that de novo synthesis of RA in these DCs was essential for the CCR9 expression and that there was little prestored RA pool, if any, in these DCs. Alternatively, the expression of CCR9 might require higher concentrations of RA than that of α4β7 and a low concentration of preformed RA in the DCs might only affect α4β7 expression.
Since IECs strongly express ALDH1A1 (RALDH1), they may produce RA from dietary vitamin A or blood-derived retinol (1, 56) and may provide it to adjacent DCs. Hammerschmidt et al. (57) have recently suggested that a small number of stromal cells in MLN expressed Aldh1a1, Aldh1a2 and Aldh1a3 and might contribute to imprinting gut-homing specificity on T cells in the presence of DCs . Although RA by itself only poorly induced Aldh1a2 expression in DCs and moderately enhanced the GM-CSF and/or IL-4-induced expression of Aldh1a2 in BM-DCs in our culture systems, the RAR antagonist LE540 significantly suppressed the GM-CSF-induced expression of Aldh1a2 in BM-DCs (Fig. 7C and D), suggesting that the GM-CSF-induced expression of ALDH1A2 may largely depend on an autocrine loop of RA generated from serum retinol in the culture. The stromal cells in MLN and IECs might provide the initial RA to DCs to acquire the GM-CSF responsiveness for the expression of ALDH1A2 in MLN and/or PP in vivo. Within the 3-kb region upstream of the murine Aldh1a2 gene, there are multiple signal transducers and activators of transcription (STAT)-binding motifs and putative RAR-binding motifs, which may contribute to the synergistic effect by GM-CSF and RA. GM-CSF and RA synergistically induce several other genes in myeloid cells, involving Janus kinase (JAK) 2 activation (58). Present results suggest that vitamin A is essential for both ALDH1A2 expression in MLN-DCs and GM-CSF expression in F4/80+ non-DCs in MLN. It has been reported that RA stimulates BM stromal cells to produce GM-CSF (59). However, it remains to be solved if RA is involved in the induction of CD11c−GM-CSF+ cells or their migration to MLN.
Our previous experiments suggested that some PP-DCs expressed ALDH1A1 in B10.D2 mice that were maintained in a clean conventional animal facility (1). In the same strain of mice kept in an SPF animal facility, however, Aldh1a1 expression was not detected in PP-DCs. We detected low levels of Aldh1a1 expression in SPL-DCs from BALB/c mice but not in those from B10.D2 mice (data not shown). Animal strains and/or the environment might affect ALDH1A1 expression in DCs. Since GM-CSF, IL-4, TLR ligands or RA did not induce ALDH1A1 expression in DCs, ALDH1A1 and ALDH1A2 differ in their induction mechanisms.
The present results collectively indicate that multiple factors in the intestinal microenvironment affect the expression of ALDH1A2 in DCs. Although it is still possible that yet-to-be-discovered factors might also contribute to the expression, GM-CSF appears to play a major role, and TLR ligands and RA may play supporting roles (Fig. 9). In addition, IL-4 and/or IL-13 that can be produced during immune responses in the gut may also contribute to the ALDH1A2 expression. The results may also suggest a possibility that local immune responses involving the production of GM-CSF and/or IL-4 or exposure to TLR ligands might affect ALDH1A2 expression in local DCs even in non-intestinal tissues, although the lack of RA or unknown inhibitory factors might limit the expression. RA regulates not only the homing specificity of lymphocytes but also the differentiation of Treg and Th17 and is thus likely to control gut immunity and oral tolerance. Therefore, the induction of ALDH1A2 expression in intestinal DCs is critical for the regulation of immune and inflammatory responses, and it might be a new target for drug discovery.
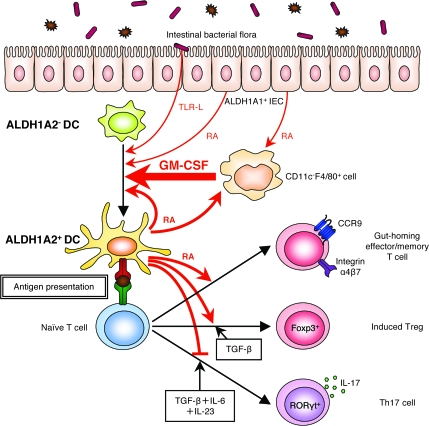
Fig. 9.
Proposed model for the mechanism of GM-CSF-mediated induction of RA-producing DCs in the intestinal tissues. GM-CSF plays a critical role to induce the ALDH1A2 expression in DCs. In the intestinal tissues, CD11c−F4/80+ cells (i.e. macrophages or granulocytes) produce GM-CSF, which induces the ALDH1A2 expression in DCs. DC maturation signals induced by intestinal stimuli including TLR ligands from intestinal bacterial flora enhance the ALDH1A2 expression. The ALDH1A2+ DCs produce RA and imprint gut-homing specificity on T cells upon antigenic stimulation. These DCs also promote the differentiation of naive T cells to Foxp3+ Treg cells in the presence of TGF-β. RA is required not only for the GM-CSF-induced expression of ALDH1A2 in DCs but also for the GM-CSF production by CD11c−F4/80+ cells or their proper migration. Thus, ALDH1A2+ DCs as well as ALDH1A1+ IECs may provide RA to DCs and CD11c−F4/80+ cells and enhance the positive feedback loop for RA production in the intestine.
Supplementary data
Supplementary Tables 1 and 2 and Figures 1–5 are available at International Immunology Online.
Funding
Ministry of Education, Culture, Sports, Science and Technology of Japan (17047052, 20060029); Japan Society for the Promotion of Science (17390147, 19659154, 19780107); Naito Foundation; Uehara Memorial Foundation; Long-range Research Initiative of Japan Chemical Industry Association.
Supplementary Material
Acknowledgments
We thank Shin-ichi Kamijo for advice for animal care, Hiroyuki Kagechika for LE540, Yoko Tominaga and Miwako Oda for technical and secretarial assistance.
Funding to pay the Open Access publication charges for this article was provided by JST, CREST.
Glossary
Abbreviations
- ALDH
aldehyde dehydrogenase
- ALDHact
aldehyde dehydrogenase activity
- APC
allophycocyanin
- BM-DC
bone marrow-derived dendritic cell
- DC
dendritic cell
- DEAB
diethylaminobenzaldehyde
- Flt3L
fms-like tyrosine kinase 3 ligand
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IEC
intestinal epithelial cell
- LN
lymph nodes
- LP
lamina propria
- MFI
mean fluorescence intensity
- MLN
mesenteric lymph nodes
- OVA
ovalbumin
- PLN
peripheral lymph nodes
- PP
Peyer's patches
- RA
retinoic acid
- RALDH
retinal dehydrogenase
- RAR
retinoic acid receptor
- SPF
specific pathogen free
- sPGN
soluble sonicated peptidoglycan
- SPL
spleen
- TGF
transforming growth factor
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TSLP
thymic stromal lymphopoietin
- Treg
regulatory T cell
- wt
wild-type
References
- 1.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 3.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 4.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007;204:1765. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-α favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur. J. Immunol. 2007;37:2396. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 8.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J. Immunol. 2007;179:3724. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 9.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 2007;8:1086. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 10.Elias KM, Laurence A, Davidson TS, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim. Biophys. Acta. 1999;1440:139. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 12.Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur. J. Biochem. 2000;267:4315. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 13.Gallego O, Belyaeva OV, Porté S, et al. Comparative functional analysis of human medium-chain dehydrogenases, short-chain dehydrogenases/reductases and aldo-keto reductases with retinoids. Biochem. J. 2006;399:101. doi: 10.1042/BJ20051988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lidén M, Eriksson U. Understanding retinol metabolism: structure and function of retinol dehydrogenases. J. Biol. Chem. 2006;281:13001. doi: 10.1074/jbc.R500027200. [DOI] [PubMed] [Google Scholar]
- 15.Uematsu S, Fujimoto K, Jang MH, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 2008;9:769. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 16.Hess DA, Meyerrose TE, Wirthlin L, et al. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 17.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002;20:621. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 18.Dudda JC, Lembo A, Bachtanian E, et al. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur. J. Immunol. 2005;35:1056. doi: 10.1002/eji.200425817. [DOI] [PubMed] [Google Scholar]
- 19.Saurer L, McCullough KC, Summerfield A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J. Immunol. 2007;179:3504. doi: 10.4049/jimmunol.179.6.3504. [DOI] [PubMed] [Google Scholar]
- 20.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenna HJ, Stocking KL, Miller RE, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489. [PubMed] [Google Scholar]
- 22.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int. Immunol. 2003;15:1017. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 23.Chambers D, Wilson L, Maden M, Lumsden A. RALDH-independent generation of retinoic acid during vertebrate embryogenesis by CYP1B1. Development. 2007;134:1369. doi: 10.1242/dev.02815. [DOI] [PubMed] [Google Scholar]
- 24.Zhang QY, Raner G, Ding X, Dunbar D, Coon MJ, Kaminsky LS. Characterization of the cytochrome P450 CYP2J4: expression in rat small intestine and role in retinoic acid biotransformation from retinal. Arch. Biochem. Biophys. 1998;353:257. doi: 10.1006/abbi.1998.0654. [DOI] [PubMed] [Google Scholar]
- 25.Gonnella PA, Chen Y, Inobe J, Komagata Y, Quartulli M, Weiner HL. In situ immune response in gut-associated lymphoid tissue (GALT) following oral antigen in TCR-transgenic mice. J. Immunol. 1998;160:4708. [PubMed] [Google Scholar]
- 26.Szatmari I, Pap A, Ruhl R, et al. PPARγ controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J. Exp. Med. 2006;203:2351. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huq MD, Tsai NP, Gupta P, Wei LN. Regulation of retinal dehydrogenases and retinoic acid synthesis by cholesterol metabolites. EMBO J. 2006;25:3203. doi: 10.1038/sj.emboj.7601181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harizi H, Gualde N. The impact of eicosanoids on the crosstalk between innate and adaptive immunity: the key roles of dendritic cells. Tissue Antigens. 2005;65:507. doi: 10.1111/j.1399-0039.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 29.Williams AM, Probert CS, Stepankova R, Tlaskalova-Hogenova H, Phillips A, Bland PW. Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse. Immunology. 2006;119:470. doi: 10.1111/j.1365-2567.2006.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 31.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J. Immunol. 2005;174:4453. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- 32.Sanderson IR, Walker WA. TLRs in the Gut I. The role of TLRs/Nods in intestinal development and homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G6. doi: 10.1152/ajpgi.00275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo. J. Immunol. 1994;152:3282. [PubMed] [Google Scholar]
- 34.Svensson M, Marsal J, Ericsson A, et al. CCL25 mediates the localization of recently activated CD8αβ+ lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 2002;110:1113. doi: 10.1172/JCI15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robb L, Drinkwater CC, Metcalf D, et al. Hematopoietic and lung abnormalities in mice with a null mutation of the common β subunit of the receptors for granulocyte-macrophage colony-stimulating factor and interleukins 3 and 5. Proc. Natl Acad. Sci. USA. 1995;92:9565. doi: 10.1073/pnas.92.21.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vremec D, Lieschke GJ, Dunn AR, Robb L, Metcalf D, Shortman K. The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur. J. Immunol. 1997;27:40. doi: 10.1002/eji.1830270107. [DOI] [PubMed] [Google Scholar]
- 37.Dranoff G, Crawford AD, Sadelain M, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 38.Stanley E, Lieschke GJ, Grail D, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc. Natl Acad. Sci. USA. 1994;91:5592. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishinakamura R, Nakayama N, Hirabayashi Y, et al. Mice deficient for the IL-3/GM-CSF/IL-5 βc receptor exhibit lung pathology and impaired immune response, while βIL3 receptor-deficient mice are normal. Immunity. 1995;2:211. doi: 10.1016/1074-7613(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 40.Jung HC, Eckmann L, Yang SK, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Invest. 1995;95:55. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noben-Trauth N, Shultz LD, Brombacher F, Urban JF, Jr, Gu H, Paul WE. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc. Natl Acad. Sci. USA. 1997;94:10838. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umemiya H, Fukasawa H, Ebisawa M, et al. Regulation of retinoidal actions by diazepinylbenzoic acids. Retinoid synergists which activate the RXR-RAR heterodimers. J. Med. Chem. 1997;40:4222. doi: 10.1021/jm9704309. [DOI] [PubMed] [Google Scholar]
- 43.Iwasaki A. Mucosal dendritic cells. Annu. Rev. Immunol. 2007;25:381. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 44.Jang MH, Sougawa N, Tanaka T, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J. Immunol. 2006;176:803. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 45.Nikawa T, Ikemoto M, Kano M, et al. Impaired vitamin A-mediated mucosal IgA response in IL-5 receptor-knockout mice. Biochem. Biophys. Res. Commun. 2001;285:546. doi: 10.1006/bbrc.2001.5138. [DOI] [PubMed] [Google Scholar]
- 46.Elgueta R, Sepulveda FE, Vilches F, et al. Imprinting of CCR9 on CD4 T cells requires IL-4 signaling on mesenteric lymph node dendritic cells. J. Immunol. 2008;180:6501. doi: 10.4049/jimmunol.180.10.6501. [DOI] [PubMed] [Google Scholar]
- 47.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 48.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007;7:353. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 49.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Vasu C, Dogan RN, Holterman MJ, Prabhakar BS. Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J. Immunol. 2003;170:5511. doi: 10.4049/jimmunol.170.11.5511. [DOI] [PubMed] [Google Scholar]
- 51.Gangi E, Vasu C, Cheatem D, Prabhakar BS. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J. Immunol. 2005;174:7006. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 52.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulatingfactor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J. Immunol. 2007;179:3638. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 53.Sheng JR, Li LC, Ganesh BB, Prabhakar BS, Meriggioli MN. Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia gravis. Clin. Immunol. 2008;128:172. doi: 10.1016/j.clim.2008.03.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korzenik JR, Dieckgraefe BK, Valentine JF, Hausman DF, Gilbert MJ. Sargramostim for active Crohn's disease. N. Engl. J. Med. 2005;352:2193. doi: 10.1056/NEJMoa041109. [DOI] [PubMed] [Google Scholar]
- 55.Sonderegger I, Iezzi G, Maier R, Schmitz N, Kurrer M, Kopf M. GM-CSF mediates autoimmunity by enhancing IL-6-dependent Th17 cell development and survival. J. Exp. Med. 2008;205:2281. doi: 10.1084/jem.20071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frota-Ruchon A, Marcinkiewicz M, Bhat PV. Localization of retinal dehydrogenase type 1 in the stomach and intestine. Cell Tissue Res. 2000;302:397. doi: 10.1007/s004410000281. [DOI] [PubMed] [Google Scholar]
- 57.Hammerschmidt SI, Ahrendt M, Bode U, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J. Exp. Med. 2008;205:2483. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimizu T, Esaki L, Mizuno H, Takeda K. Granulocyte macrophage colony-stimulating factor enhances retinoic acid-induced gene expression. J. Leukoc. Biol. 2006;80:889. doi: 10.1189/jlb.0905502. [DOI] [PubMed] [Google Scholar]
- 59.Nakajima H, Kizaki M, Sonoda A, Mori S, Harigaya K, Ikeda Y. Retinoids (all-trans and 9-cis retinoic acid) stimulate production of macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor by human bone marrow stromal cells. Blood. 1994;84:4107. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.