Abstract
A role for c-Abl in B cell development and signaling has been suggested by previous work showing that c-Abl-deficient mice have defects in bone marrow B cell development and that c-Abl-deficient B cells are hypoproliferative in response to antigen receptor stimulation. Here we show that in addition to defects in early B cell development, c-Abl-deficient mice have defects in peripheral B cell development, including reduced percentages of peritoneal B-1 cells as well as transitional and marginal zone B cells in the spleen. It has been shown that c-Abl kinase activity increases upon B cell receptor (BCR) stimulation and that one of the targets of tyrosine phosphorylation by c-Abl is CD19. However, the consequences of c-Abl activity on B cell activation and CD19 signaling remain unknown. Here, we show that c-Abl-deficient splenic B cells exhibit reduced calcium flux in response to CD19 cross-linking, consistent with a role for c-Abl in CD19-dependent signaling. Additionally, we show that c-Abl-deficient B cells are defective in their ability to be activated in response to antigen receptor engagement, suggesting a functional role for c-Abl in BCR-dependent activation signaling pathways.
Keywords: co-stimulation, protein kinases, transgenic/knockout mice
Introduction
Developing B cells in the bone marrow must pass through sequential checkpoints to ensure that only B cells expressing a functional B cell receptor (BCR) are released into the periphery. Once these immature or transitional B cells emerge from the bone marrow, they continue to require signals through their antigen receptor for survival as well as for further differentiation into marginal zone (MZ), follicular and B-1 cell lineages. This peripheral differentiation process depends in part on the strength of the signal transmitted through the antigen receptor. Indeed, greater BCR signal strength promotes B-1 cell differentiation, and lower levels of BCR signaling promote MZ and follicular B cell differentiation (1). In the periphery, basal or ‘tonic’ signaling through the BCR continues to provide survival signals to resting mature B cells (2, 3). Functional signaling through the BCR is also required for B cell activation and thus proper antigen responses by B cells. Thus, signaling through the B cell receptor is important for B cell development, maintenance and function.
c-Abl is a non-receptor tyrosine kinase with a variety of known functions, including responses to oxidative stress, DNA damage, growth factor stimulation, cell adhesion and cytoskeletal remodeling (4). The c-Abl gene was first identified as the cellular gene from which the oncogenic v-Abl of Abelson murine leukemia virus (A-MuLV) was derived (5). A-MuLV causes rapid lymphoma in mice and transforms only early B lineage cells both in vivo and in vitro. Another c-Abl-derived oncogene is the chromosomal translocation product breakpoint cluster region–Abelson (BCR–ABL), which causes both chronic myelogenous leukemia as well as some forms of acute lymphoblastic leukemia in humans (6). Thus, early studies with Abl-related genes pointed toward a role for c-Abl in hematopoietic cells. Another early suggestion of a role for c-Abl in the hematopoietic system was the observation that mice homozygous for either the c-Abl null allele (abl2) or the allele encoding a truncated c-Abl protein (ablm1) have a variety of hematopoietic defects, including small thymuses and spleens as well as decreases in numbers of total peripheral lymphocytes and an increased susceptibility to infections (7, 8).
In the years since both the mutant c-Abl mouse lines were made, however, a clearer picture of a role for c-Abl in lymphocytes has been emerging. The defect in developing B cells in c-Abl knockout (KO) mice has been well documented (9, 10). In the absence of wild-type (WT) c-Abl, there is a severe reduction in both the pro-B and pre-B cell populations (9, 10). B cell precursors in c-Abl-deficient mice have also been shown to have an increased rate of apoptosis, as well as possible defects in V(D)J recombination (10, 11).
The role of c-Abl in mature B cells is not as well defined as in developing B cells. Analysis of peripheral lymphocytes in c-Abl null mice demonstrated specific reductions in both B and T cells (8). Although the percentages of B and T cells were found to be nearly normal in ablm1 mice (7), analysis of peripheral B cells revealed defects in the proliferative responses to anti-IgM in liquid culture assays and variable defects in the proliferative response to LPS, but only in soft agar (12). In liquid culture, ablm1 splenic B cells appear to proliferate at control levels in response to LPS (12). Analysis of peripheral B cells in abl2 mice showed a similar response; in liquid culture, these cells proliferate normally in response to LPS but are deficient in the proliferative response to anti-IgM (13). These data suggest that signaling downstream of antigen receptor ligation is impaired in Abl-deficient peripheral B cells. This is consistent with a role for c-Abl in the BCR signaling pathway and is further supported by additional data showing that the kinase activity of c-Abl increases in response to anti-IgM treatment (13).
A few putative targets of c-Abl kinase activity in B cells have been identified, although the functional significance of these phosphorylation events is unknown.
i) First, c-Abl was shown to phosphorylate Y490 of CD19 both in vitro and when co-expressed with CD19 in Bosc23 cells (13). CD19 is a transmembrane molecule expressed on the surface of B cells that acts as a co-receptor to the BCR, modulating the downstream signaling cascades. B cells from CD19-deficient mice have reduced proliferative responses to B cell mitogens and decreased levels of serum Ig, whereas over-expression of CD19 in B cells leads to increased proliferation and serum Ig levels (14). CD19 has nine conserved tyrosines in its cytoplasmic domain that recruit many components of the BCR signaling apparatus upon phosphorylation. Proteins that bind many of these individual phosphotyrosine residues have been identified; however, no binding partner has been identified for the tyrosine residue targeted by c-Abl. Additionally, the response of c-Abl-deficient B cells to CD19 stimulation has not been addressed.
ii) Another substrate of c-Abl that plays an important role in B cell signaling pathways is B cell adaptor for phosphoinositide-3 kinase (PI3K) (BCAP) (15). c-Abl phosphorylates BCAP via a mechanism requiring Abl interactor-1, an adaptor protein that links c-Abl to substrates of its kinase domain. BCAP is also an adaptor molecule and is important for the recruitment of PI3K to the BCR signaling cascade (16). In addition to phosphorylation by c-Abl, BCAP is also phosphorylated by spleen tyrosine kinase and Bruton's tyrosine kinase (Btk), two kinases that play important roles in BCR-mediated signaling. Phosphorylated BCAP is then bound by the p85 subunit of PI3K. In the absence of this phosphorylation, PI3K recruitment to glycolipid-enriched microdomains is reduced, as is the production of the important signaling molecule phosphatidylinositol[3,4,5]triphosphate (16).
iii) Btk is a member of the Tec family of non-receptor tyrosine kinases and is required for normal B cell development. Mutations in btk lead to the diseases X-linked aggamaglobulinemia in humans and X-linked immunodeficiency in mice. c-Abl was found to phosphorylate Y223 on Btk when both proteins are over-expressed in cells (17), but this event has yet to be addressed at the endogenous levels of the proteins.
iv) Despite the well-characterized anti-apoptotic role that c-Abl, v-Abl and BCR–ABL appear to play in developing B cells, less is known about the role of c-Abl in regulating apoptosis in mature B cells. Recent data have suggested a pro-apoptotic role for c-Abl in B cells downstream of the inhibitory Fc receptor, FcγRIIB1 (18). C-Abl was shown to co-immunoprecipitate with and phosphorylate FcγRIIB1 only upon cross-linking of the receptor (18). Deletion of c-Abl in DT40 cells partially abrogated FcγRIIB1-dependent apoptosis, but this response was only completely blocked in cells lacking both c-Abl and the related protein Arg (18).
To better understand the role of endogenous c-Abl activity in peripheral B cell differentiation and function, we analyzed transitional and mature B cell populations in mice homozygous for the abl2 null allele. We found decreased percentages of peritoneal B-1 cells as well as early transitional B cell populations in the spleen. We also observed variable reductions in the percentages of MZ B cell populations. Additionally, splenic B cells derived from these animals were defective in their response to BCR stimulation, as indicated by reduced calcium flux and reduced surface up-regulation of activation markers. We also show that c-Abl-deficient B cells have defects in CD19-dependent signaling as monitored by reduced calcium flux in response to CD19 ligation alone. Although c-Abl has been suggested to play a role in BCAP-dependent signaling, we failed to observe a decrease in c-Rel protein levels or altered nuclear factor-κB (NF-κB) DNA binding in c-Abl −/− B cells as was observed in BCAP-deficient B cells (19).
We conclude that c-Abl is important for peripheral B cell differentiation into transitional, MZ and B-1 cells as well as for peripheral B cell receptor and co-receptor signaling. This suggests that in addition to its known role in developing B cells, c-Abl is also important for peripheral B cell differentiation and function.
Materials and methods
Mice
Mice carrying the c-Abl null allele (ablm2) were maintained in either a pure 129/SvEv background or a mixed 129/SvEv and C57BL/6 background. Mice were bred as heterozygotes to obtain WT, heterozygous and homozygous c-Abl mutants. Six- to eight-week old mice were used for all experiments except where otherwise specified. Mice were housed and maintained in accordance with institutional guidelines defined by the Columbia University Institutional Animal Care and Use Committee.
FACS
Spleens were removed and passed through a cell strainer [Becton Dickinson (BD), Franklin Lakes, NJ, USA] to yield a single-cell suspension. RBCs were lysed in RBC lysis buffer (0.16 M ammonium chloride, 0.01 M potassium bicarbonate, 0.05 mM EDTA, pH 8.0) on ice. The remaining cells were washed in FACS Stain Buffer with fetal bovine serum (FBS) (BD) and incubated in the presence of Fc Block (BD) and then stained with the appropriate antibodies for 30 min on ice. Cells were then washed in Stain Buffer and analyzed on a LSRII flow cytometer (BD). The following antibodies were obtained from BD: FITC anti-CD21, FITC anti-IgD, PE anti-CD23, PE anti-CD25, PE anti-CD86, PE anti-I-Ab, PE anti-Mac-1, PE anti-CD95, PECy7 anti-B220, allophycocyanin (APC) anti-IgM and APC anti-CD5. APC anti-CD23 was purchased from Invitrogen (Carlsbad, CA, USA) and peanut agglutinin-FITC was purchased from Vector Labs (Burlingame, CA, USA). FACS data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OH, USA). Dead cells were excluded from analysis by forward and side-scatter gating.
In vitro stimulation of splenocytes
Splenocytes were prepared as single-cell suspensions and RBCs were lysed as described above. Cells were counted and plated at 1 × 106 cells per milliliter in Roswell Park Memorial Institute media (RPMI), 5% FBS and 27.5 μM β-mercaptoethanol. Cells were treated with either 50 μg ml−1 goat anti-IgM F(ab′)2 (ICN Pharmaceuticals, Aurora, CO, USA) or 10 μg ml−1 LPS (Sigma–Aldrich, St Louis, MO, USA) for the indicated times. Cells were harvested, washed in PBS and stained for FACS analysis as described above.
ELISA
Plates (Nunc Immuno plate, Thermo Fisher Scientific, Roskilde, Denmark) were coated with capture antibody (BD, anti-IgM/anti-IgG1/anti-IgG2a/anti-IgG3) 1 h at 37°C, washed with PBS + 0.1% Tween-20 (PBST) and blocked with PBS + 1% BSA for 30 min at room temperature. Serum from peripheral blood as well as purified isotype standards (BD, IgM/IgG1/IgG2a/IgG3) was applied to the plate and incubated overnight at 4°C. Plates were washed in PBST and biotinylated detection antibodies were added (BD, anti-IgM/anti-IgG1/anti-IgG2a/anti-IgG3) for 1 h at room temperature. Plates were washed in PBST and streptavidin–HRP (BD) was added for 30 min at room temperature. Plates were washed and bound HRP was detected with ABTS peroxidase substrate kit (Vector Labs). Plates were read at 405 nm in a plate reader (Beckman Coulter AD 200, Fullerton, CA, USA).
Electrophoretic mobility shift assay
Nuclear fractions were isolated from cells using a protocol based on previously published method (20) and incubated with a radiolabeled NF-κB probe (5′CCGGCTGGGGATTCCCCATCTCGGTAC3′) previously described (21). Samples were run on a 5% acrylamide gel in 0.5× Tris–borate–EDTA, dried and exposed on a phosphoimager screen.
B cell purification
Single-cell suspensions of total splenocytes were prepared as for FACS and then washed in column buffer (PBS + 0.5% BSA). Cells were counted and incubated with MACS anti-CD43 microbeads (Miltenyi Biotec, Auburn, NY, USA) for 15 min and then applied to a MACS LS Separation Column (Miltenyi Biotec). B cells were isolated by negative selection by collecting the column flow through.
Western blots
Cell lysates were subject to separation by SDS–PAGE and transferred to nitrocellulose membranes. Membranes were blocked in Tris-buffered saline (TBS) + 5% BSA and probed with the following primary antibodies: rabbit anti-c-Rel (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-p65 (Santa Cruz Biotechnology) and mouse anti-actin (Sigma–Aldrich). Membranes were washed in TBST (TBS + 0.1% Tween 20) and incubated with a fluorescent secondary antibody followed by detection on an Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
Calcium flux analysis
Following RBC lysis, total splenocytes were loaded with Indo-1 AM Ester calcium indicator (Invitrogen) for 1 h at 37°C in PBS. Cells were washed in PBS and re-suspended in FACS Stain Buffer (BD). Cells were then stained with FACS antibodies as described above, washed and re-suspended in RPMI/5% FBS. Cells were warmed to 37°C prior to Ca2+ recording on an LSRII flow cytometer. For each sample, baseline measurements were recorded for 1 min prior to the addition of 100 μg ml−1 anti-IgM F(ab′)2 (ICN Pharmaceuticals), 2 μM ionomycin (Sigma–Aldrich), 1 μg ml−1 biotin anti-CD19 (BD), 1 μg ml−1 biotin-Rat IgG2a or 20 μg ml−1 streptavidin (Sigma–Aldrich). For negative controls, cells were treated with 8 mM ethylene glycol tetraacetic acid (EGTA) 1 h prior to stimulation. Data were analyzed with FlowJo software.
Results
Splenic B cell populations
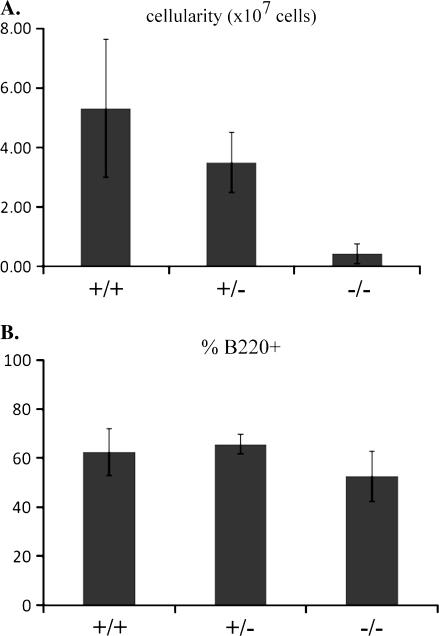
In the absence of c-Abl, developing B cells exhibit a reduction in the percentages of pro-B and pre-B cells and an increase in their apoptotic index (9–11). c-Abl KO mice also display decreases in numbers of peripheral lymphocytes (on average a 30% decrease in the number of lymphocytes per microiliter) (8) and a reduction in the cellularity of both the thymus (8) and spleen (Fig. 1A), but little is known about which peripheral B cell populations are most affected. In order to better define the role of c-Abl in peripheral B cell development, we analyzed splenic B cell populations in WT and c-Abl KO mice by flow cytometry. We observed a similar percentage of total B220+ cells as compared with controls in the spleens of the mutant animals (Fig. 1B) within the overall reduction in cellularity.
Fig. 1.
c-Abl KO mice have reduced splenic cellularity. (A) Total spleen cellularity of WT (+/+), heterozygous (+/−) and homozygous mutant (−/−) spleens with error bars showing standard deviation. Spleens were passed through a cell strainer following RBC lysis and leukocytes were counted in a hemacytometer. (B) Percentages of total B220+ splenocytes in WT (+/+), heterozygous (+/−) and KO (−/−) c-Abl mice with error bars showing standard deviation. (A and B) Five animals were counted for each genotype.
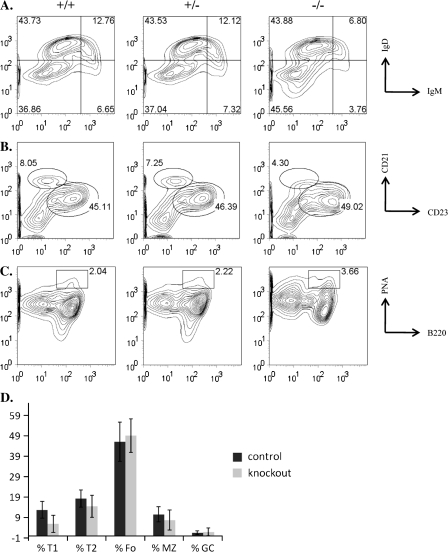
We next assessed the various sub-populations of B cells within the spleen. We observed a ∼50% decrease in the percentages of transitional 1 (T1) (IgMhi IgDlo) transitional B cell populations in the KO animals relative to controls (Fig. 2A and D). We observed a more variable reduction in the percentages of T2 (IgMhi IgDhi) transitional B cell populations (Fig. 2A and D) and MZ (CD23lo CD21hi) B cells in the KO animals (Fig. 2B and D). Although the KO animals commonly had reduced percentages of these populations relative to controls, the difference was seen less consistently than that observed in the T1 populations. The percentages of follicular (CD23hi CD21lo) and germinal center (B220+ peanut agglutininhi) B cells were similar between control and mutant animals (Fig. 2B, C and D). The general lymphopenia and reduced cellularity of the spleens from c-Abl-deficient mice suggests a role for c-Abl in the development of all lymphocytes. However, these data further indicate a specific role for c-Abl in the development of B cells and, in particular, transitional and possibly MZ B cells.
Fig. 2.
Splenic B cell populations. (A) Gated on B220+ cells, stained for T1 (IgMhi and IgDlo), T2 (IgMhi and IgDhi) and mature (IgMlo and IgDhi) B cells. (B) Gated on B220+ cells, stained for follicular (CD23hi and CD21lo) and MZ (CD23lo and CD21hi) B cells. (C) Stained for germinal center (B220+ and peanut agglutininhi) B cells. (D) Average percentages of T1, T2, follicular (Fo), MZ and germinal center (GC) B cells with error bars showing standard deviation. At least five animals were analyzed for each experiment.
Peritoneal B-1 cell populations
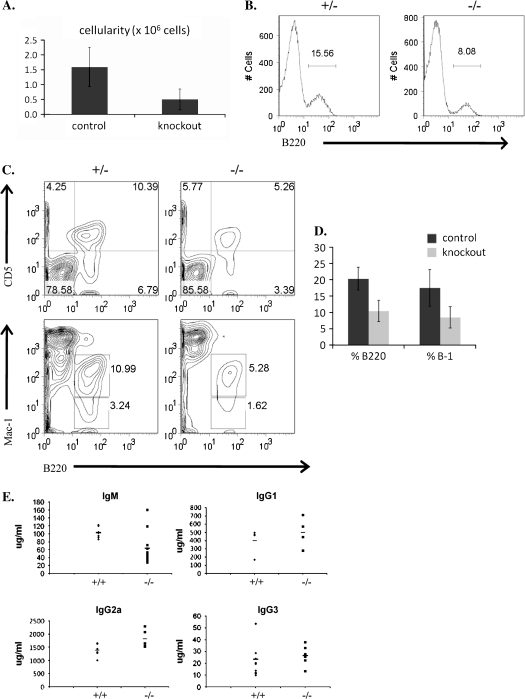
The development of B-1 cells has been shown to be dependent on strong BCR signaling levels and as a result deficiencies in any of a large number of BCR-related signaling molecules [e.g. CD19, Btk, BCAP, B cell linker protein BLNK) and phospholipase C gamma] result in a decrease in the B-1 cell population. To assess whether or not c-Abl also contributes to B-1 cell development, we investigated the status of the peritoneal B-1 cell population in c-Abl-deficient mice. The c-Abl KO animals exhibited a reduced total cellularity of the peritoneal cavity (Fig. 3A); however, we found a specific ∼50% decrease in the percentage of total B cells (Fig. 3B and D) as well as a decrease in the percentage of total B-1 (B220+ and Mac-1+) and B-1a (B220+ and CD5+) cells in the peritoneum (Fig. 3C and D). Because B-1 cells are an important source of serum IgM, we analyzed the levels of serum Ig in c-Abl-deficient animals by ELISA and found a pronounced reduction in serum IgM levels relative to WT (Fig. 3E). The serum concentrations of other Ig isotypes were not significantly different from controls (Fig. 3E). These data suggest that like other known signaling molecules such as CD19, c-Abl is also important for the generation of peritoneal B-1 cells.
Fig. 3.
Peritoneal cavity B cells. (A) Total cellularity of peritoneal cavity of control (WT and heterozygous) and KO animals with error bars showing standard deviation. (B) Percentage of total peritoneal B220+ cells. (C) Percentage of B-1 cells (B220+, CD5+ or B220+ Mac-1+). (D) Average percentage of B220+ and B-1 cells in control and KO animals with error bars showing standard deviation. (E) Serum Ig levels (μg ml−1) of WT (+/+) and KO (−/−) animals measured by ELISA. (A–E) At least five animals were analyzed for each experiment.
Normal c-Rel and p65/RelA expression levels in c-Abl −/− B cells
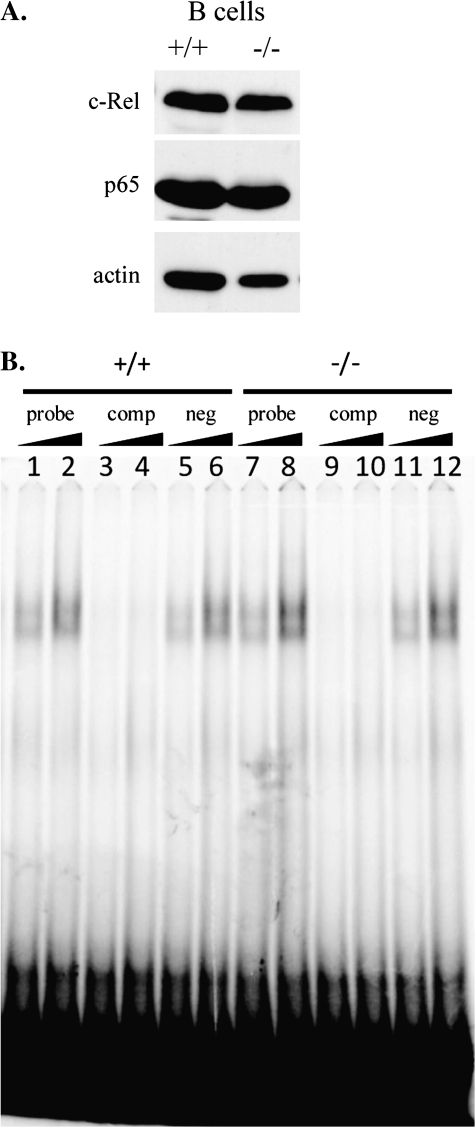
It has been previously demonstrated that BCAP-deficient splenic and mature B cells have reduced expression levels of the NF-κB family member c-Rel and altered NF-κB DNA-binding activity by electrophoretic mobility shift assay (EMSA) (19). NF-κB family members are important for multiple signaling pathways in many cell types; however, in lymphocytes, NF-κB signaling is particularly important for development and survival (22). Given that BCAP is a substrate of c-Abl kinase activity and that c-Abl KO mice have specific defects in B cell development and survival (9, 11, 23), we investigated the possibility that c-Abl KO B cells might have reduced levels of NF-κB protein or altered DNA-binding activity similar to BCAP-deficient B cells.
We isolated splenic B cells from mutant and control animals and analyzed them for expression levels of c-Rel and p65/RelA. The levels of both c-Rel and p65 were similar between WT and mutant B cells (Fig. 4A). To analyze NF-κB DNA-binding activity in c-Abl KO B cells, we fractionated purified splenic B cells and assayed the nuclear fraction for NF-κB DNA-binding activity by EMSA. Dilution of WT and mutant nuclear extracts revealed similar levels of NF-κB-binding activity (Fig. 4B, lanes 1, 2, 7 and 8). As expected, the shifted band disappeared upon competition with cold probe (Fig. 4B, lanes 3, 4, 9 and 10), but not with addition of cold, scrambled probe (Fig. 4B, lanes 5, 6, 11 and 12). Analysis of unstimulated BCAP-deficient splenic B cells revealed a novel, slower migrating, mobility complex by EMSA (19), but this was not detected in c-Abl-deficient cells. The mobility complexes detected in unstimulated cells were similar between WT and KO cells (Fig. 4B). We conclude that c-Abl is not critical for c-Rel or p65 expression or for NF-κB DNA binding in splenic B cells.
Fig. 4.
c-Abl KO B cells have WT levels of NF-κB subunits and DNA-binding activity. (A) Western blot analysis of splenic B cell lysates. Membranes were probed with antibodies against c-Rel, p65 and actin. (B) NF-κB DNA-binding activity was assayed by EMSA. B cells from WT (+/+) or mutant (−/−) spleens were fractionated and increasing amounts of nuclear lysates were incubated with 33P-labeled NF-κB-specific probe (probe), unlabeled probe + labeled probe (comp) or unlabeled scrambled probe + labeled probe (neg).
Defective activation of c-Abl null B cells in response to antigen receptor ligation
Ligation of the antigen receptor on mature B cells leads to the induction of signaling cascades required for cell activation and appropriate responses to antigens. Follicular and MZ B cells respond differently to BCR stimulation, in accordance with their differing roles in immune responses. MZ B cells rapidly proliferate and differentiate into antibody-secreting plasma cells in response to antigen recognition (24). Follicular B cells, on the other hand, generally respond more slowly and interact with T cells to generate a higher affinity response (24). One of the hallmarks of activated follicular B cells is the up-regulation of certain cell surface molecules important for interactions with T cells, including the co-stimulatory molecules CD80 and CD86 as well as MHC class II molecules required for antigen presentation to T cells and the high-affinity chain of the IL-2 receptor (CD25).
It has been previously demonstrated that c-Abl is phosphorylated and its kinase activity increases following anti-IgM stimulation (13). Additionally, it has been shown that c-Abl-deficient B cells are hypoproliferative in response to anti-IgM (13). However, a role for c-Abl in other aspects of BCR signal-induced activation has yet to be established. Indeed, the signaling pathways leading to proliferation and activation or maturation of B cells are not entirely overlapping (25).
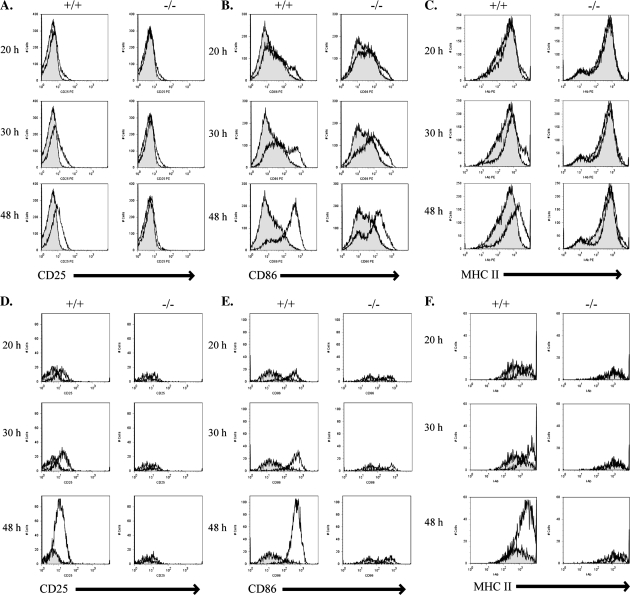
To address the question of whether c-Abl is required for B cell activation, we cultured total splenocytes in vitro and stimulated them with either anti-IgM or LPS for 20, 30 and 48 h and monitored the up-regulation of activation markers. Splenic B cells from WT mice were readily activated by antigen receptor ligation by anti-IgM. Activation was detectable by 20 h, and by 48 h, there was a clear increase in the surface expression level of CD25, CD86 and MHC II on WT B cells (Fig. 5A–C). In contrast, splenic B cells from c-Abl KO animals were defective in their ability to up-regulate CD25, CD86 and MHC II in response to anti-IgM. There was almost no detectable increase in CD25 or MHC II surface expression in mutant B cells even by 48 h (Fig. 5A and C). Mutant B cells were partially able to up-regulate CD86, however, with delayed kinetics and to a lesser overall extent (Fig. 5B).
Fig. 5.
In vitro stimulation of antigen receptor signaling in splenic B cells. (A–F) Equal numbers of primary splenocytes from WT (+/+) or c-Abl homozygous KO (−/−) animals were cultured and left either untreated or treated with 50 μg ml−1 anti-IgM F(ab′)2 in vitro for 20, 30 or 48 h and monitored for surface up-regulation of CD25 (A and D), CD86 (B and E) or MHC II (C and F) by FACS analysis. Histograms represent stimulated cells (line) overlaid on unstimulated cells (filled in). (A–C) Gated on total B220+ cells. (D–F) Gated on B220+ and CD23+ cells.
Although the similar percentages of follicular B cells in WT and c-Abl KO mice (Fig. 2C) suggest that c-Abl signaling is not as important for the specific development of this B cell subset, we wanted to investigate the possible contribution of c-Abl to antigen receptor-dependent activation of these cells. Surface CD23 expression levels are used to phenotypically identify follicular as well as later (T2) transitional B cells, and so we specifically analyzed the activation of B220+CD23+ B cells. In WT B220+CD23+ B cells, there was a dramatic increase in the number of cells that up-regulated surface expression of CD25, CD86 and MHC II in response to anti-IgM stimulation (Fig. 5D–F). In contrast, c-Abl-deficient B220+CD23+ B cells showed limited up-regulation of surface CD25, CD86 and MHC II in response to anti-IgM stimulation and little to no increase in the number of activated cells with increasing stimulation time as was seen in WT cells (Fig. 5D–F). These data suggest that in addition to a hypoproliferative response to anti-IgM stimulation, c-Abl-deficient B cells have defects in BCR-induced activation.
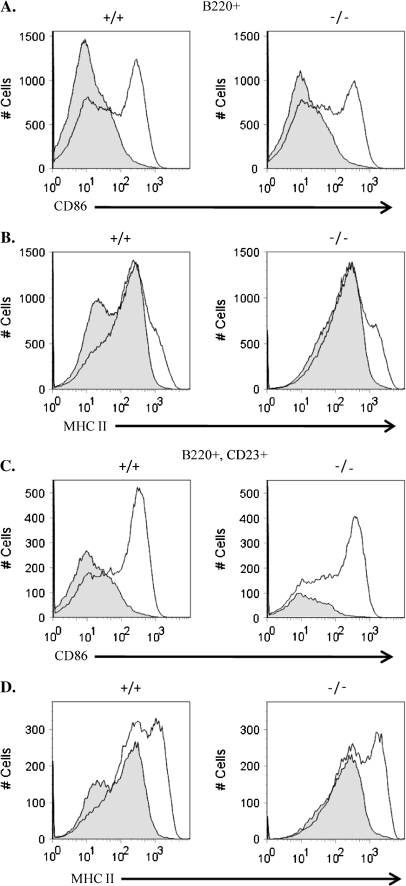
To determine if c-Abl-deficient B cells were defective in other activation pathways, we assayed their response to LPS. LPS activates B cells through engagement of Toll-like receptor 4 and also induces up-regulation of co-stimulatory molecules and MHC II. We cultured total splenocytes from WT and KO animals and treated them in vitro with LPS for 48 h (Fig. 6). Both WT and KO B cells up-regulated CD86 and MHC II to a similar extent in response to LPS (Fig. 6A and B). In addition, the CD23+ B cell subset also displayed a similar response to LPS stimulation as compared with WT cells (Fig. 6C and D). These data suggest that although c-Abl KO B cells are defective in their activation in response to anti-IgM, they have a grossly normal activation response to LPS treatment, suggesting that they are not defective in activation per se, but rather specifically defective in antigen receptor-dependent activation.
Fig. 6.
In vitro stimulation of Toll-like receptor 4 signaling in splenic B cells. Primary splenocytes from WT (+/+) or c-Abl homozygous KO (−/−) animals were cultured and left either untreated or treated with 10 μg ml−1 LPS in vitro for 48 h and monitored for surface up-regulation of CD86 (A and C) or MHC II (B and D) by FACS analysis. Histograms represent stimulated cells (line) overlaid on unstimulated cells (filled in).
c-Abl-deficient B cells exhibit reduced Ca2+ flux in response to antigen receptor or CD19 stimulation
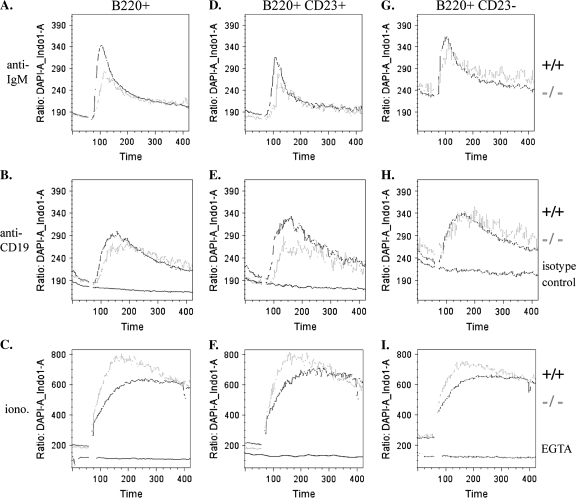
An early result of signaling through the B cell receptor is the mobilization of intracellular calcium. In addition to BCR stimulation, cross-linking of the CD19 co-receptor alone has also been shown to elicit calcium mobilization (26). To assess the competence of the early signaling events following either antigen receptor or CD19 ligation, we measured intracellular calcium release in response to stimulation with anti-IgM, anti-CD19 or the calcium ionophore ionomycin. Total splenocytes were loaded with Indo-1 AM dye and stained for B cell markers. Data were recorded for 1 min prior to stimulation to establish a baseline and then for 6 min post-stimulation. In response to stimulation with anti-IgM F(ab′)2, B220+ cells from c-Abl KO mice displayed a reduced calcium response as compared with WT cells (Fig. 7A). For CD19 stimulation, biotinylated anti-CD19 was added to cells on ice prior to recording, and the anti-CD19 antibodies were cross-linked with streptavidin at 1 min. Cells from KO mice had a mildly reduced calcium response to CD19 cross-linking as compared with WT (Fig. 7B). As a negative control for all CD19 stimulation experiments, WT cells were treated with a biotinylated isotype control antibody and cross-linked with streptavidin which elicited no detectable calcium response (Fig. 7B, E and H).
Fig. 7.
Calcium flux analysis. Splenocytes were stimulated in vitro with F(ab′)2 anti-IgM (A, D and G), biotin anti-CD19 or biotin isotype control antibody followed by streptavidin at 1 min (B, E and H) or ionomycin with or without pre-treatment with EGTA (C, F and I). Baselines were recorded for 60 s, and then samples were treated with anti-IgM, streptavidin or ionomycin at 1 min. The biotin anti-CD19 and biotin isotype control antibodies were pre-bound on ice. EGTA was added 1 h prior to recording.
Within the CD23+ B cell population, c-Abl-deficient cells exhibited a greater defect in their response to CD19 ligation (Fig. 7E), as well as a defect in response to anti-IgM (Fig. 7D). In CD23− B cells, there was a slight reduction in the response to anti-IgM in KO cells (Fig. 7G), but in contrast to CD23+ B cells, the response to anti-CD19 stimulation was nearly identical to WT cells (Fig. 7H). As a positive control, the response to ionomycin in all B cell subsets examined was robust in both WT and c-Abl KO cells (Fig. 7C, F and I). As a negative control, WT cells pre-treated with EGTA did not respond to ionomycin (Fig. 7C, F and I).
These data suggest that c-Abl-deficient CD23+ and CD23− splenic B cells have similarly modest defects in calcium responses to antigen receptor ligation but that the CD23+ subset is particularly defective in the calcium response to CD19 ligation.
Discussion
Mice deficient in c-Abl have profound defects in BM B cell development (7, 9), but the development and function of peripheral B cells in these animals has not seriously been well defined. It has been demonstrated that the proliferative response of Abl-deficient B cells to BCR stimulation is reduced (13) and the CD19 co-receptor has been identified as a target of c-Abl kinase activity (13), along with BCAP and Btk (15, 17), but the functional relevance of these interactions is unknown. Signals originating from the BCR and CD19 surface molecules and the intermediates through which these signals are transduced are important for B cell development in the BM and periphery, as well as the functional responses to antigen carried out by mature B cells. Thus, defects in BCR signaling can result in many defects in B cell development and activation. Splenic B cells from c-Abl KO mice were defective in their ability to up-regulate surface expression of CD86, MHC II and CD25 upon in vitro stimulation with anti-IgM. These data, along with the calcium flux analysis upon anti-IgM stimulation, point to a functional role for c-Abl in BCR-mediated B cell activation. Additionally, c-Abl-deficient B cells were hyporesponsive to CD19 ligation as determined by calcium flux responses, pointing to a functional role for c-Abl downstream of CD19-dependent signaling as well.
Throughout B cell development in both the bone marrow and periphery, the vast majority of B cells undergo apoptosis as a result of the mechanisms of either positive or negative selection. The absence of c-Abl in B cells has been shown to lead to enhanced apoptosis (11, 23), suggesting that in B cells (in contrast to other cell types, such as fibroblasts) the normal role of c-Abl is primarily anti-apoptotic. This may explain why the defect in B cell populations in c-Abl-deficient mice appears to be most severe in developing B cells in both the bone marrow and transitional B cells in the spleen; these are populations that are particularly sensitive to apoptosis. It has also been proposed that MZ B cells develop directly from T1 B cells (27), which also might explain the selective loss of MZ B cells observed in c-Abl KO mice. In mature B cells, BCR engagement predominantly leads to cell activation, rather than apoptosis. In these cells, we observe a defect in the ability to respond to BCR engagement in the absence of c-Abl, but not a decrease in the overall percentages of mature cells.
CD19 was shown to be a target of phosphorylation by c-Abl (13), but the functional relevance of this interaction was previously unknown. Interestingly, the decrease in transitional, MZ and peritoneal B-1 cells in c-Abl-deficient mice is similar to that seen in CD19-deficient mice (14, 28), supporting a functional role for c-Abl in CD19-dependent signaling pathways. Of the nine conserved tyrosines in the cytoplasmic tail of CD19, eight have been evaluated in mice using transgenic expression of mutant CD19 constructs in a CD19-deficient background (29). In this system, it was revealed that Y482 and Y513 were essential for CD19 function with respect to B cell development and responses to antigen, including development of B-1 and MZ B cells (29). The only conserved tyrosine not evaluated in this study was Y490, the residue previously shown to be targeted by c-Abl (13).
Tyrosine 490 lies between and in close proximity to Y482 and Y513 of CD19, binding sites for PI3K and the Src family kinase Lyn. It has been suggested that c-Abl may play a role in modulating the function of Y482 and Y513 via phosphorylation of Y490 and perhaps recruitment of Src homology 2 domain-containing proteins (13). Mutation of Y482 and Y513 had no effect on the calcium response in response to either anti-IgM alone or anti-CD19 alone, but B cells bearing these CD19 mutations did not display enhanced calcium release in response to the combination of anti-IgM and anti-CD19 treatment as is normally observed (29). These data suggest that these residues are involved in CD19-dependent calcium responses at some level, although this is in contrast to the data observed in c-Abl-deficient cells which display a reduced calcium response to cross-linking of CD19 alone or surface IgM alone.
It is possible that c-Abl might be involved in another aspect of CD19 signaling. Indeed, many proteins are recruited to the CD19 signaling complex including Btk, another putative target of c-Abl (17). It has been demonstrated that intracellular calcium responses downstream of CD19 ligation are partially dependent on Btk (30), raising the possibility that c-Abl plays a role in the CD19-dependent activation of Btk.
During B cell development, B-1 cells are selected for by high BCR signaling levels. In WT mice, BCR signal strength is determined by the dosage of antigen as well as the density of BCRs on the cell surface. In addition to alterations in antigen dosage and BCR density, signal strength can be manipulated in KO and/or transgenic mice by the removal, addition or mutation of the downstream BCR signaling components. Indeed, mice deficient in any of several important components of the BCR signal transduction cascade, including CD19, Btk, BCAP, P85α, BLNK and others, have reductions in peritoneal B-1 cells. The reduction in peritoneal B-1 cells observed in c-Abl KO mice suggests that, like these other important signaling molecules, c-Abl contributes to the high BCR signaling levels required for the development of B-1 cells.
There are similarities between the B cell phenotypes of BCAP-deficient mice and the B cell phenotype observed in c-Abl-deficient mice. In particular, both BCAP and c-Abl are important for B-1 cell development. These similarities, in addition to the identification of BCAP as a putative substrate of c-Abl, suggest a possible role for c-Abl in BCAP signaling pathways. However, there is not a complete overlap between these different KO models. Although BCAP has been shown to be a target of c-Abl kinase activity, we failed to detect reductions in c-Rel protein levels and altered NF-κB DNA-binding complexes in c-Abl-deficient B cells as was observed in BCAP-deficient B cells. The phosphorylation of BCAP by c-Abl has not yet been evaluated at the level of endogenous proteins (15), and thus, BCAP may not be an important target at physiological levels of c-Abl.
Peripheral B cell maturation leads to various changes in cell surface phenotype. The CD23/FcεRII surface molecule first appears on the surface of B cells as they progress from the T1 to T2 stage and remains on in mature, follicular B cells and a proportion of germinal center B cells (31). Our data suggest that while the total pool of splenic B cells in c-Abl-deficient mice are hyporesponsive to both BCR- and CD19-dependent activation, the CD23+ subset of c-Abl-deficient B cells appears to be most affected. Thus, we postulate that c-Abl would be important for the functional responses of mature B cells to antigen. Although the splenic B cell subsets differ in abundance between WT and KO mice, the majority of B cells in the spleen are mature, follicular cells, which are present in similar percentages in both mutant and control samples. However, because different B cell subsets respond differently to antigen receptor and co-receptor stimulation, it cannot be ruled out that this has some effect on the results of the stimulation assays, particularly when examining the total B220+ cell population.
The perinatal lethality of the vast majority of c-Abl-deficient mice combined with the reduced numbers of B cells has precluded detailed biochemical analysis of signal transduction pathways as well as in vivo analyses of antigen responses in B cells. However, the generation of B cell-specific KO animals could help address some unanswered questions by providing a more plentiful source of c-Abl-deficient B cells for study as well as the animal longevity required for in vivo immune response studies. It would be interesting to monitor the tyrosine phosphorylation status of important molecules such as Btk and BCAP, as putative targets of c-Abl kinase activity, in response to antigen receptor and CD19 co-receptor stimulation. Additionally, it would be interesting to analyze the immediate consequences of tyrosine phosphorylation of CD19 by c-Abl and whether it is relevant to the recruitment of additional signaling factors.
c-Abl is ubiquitously expressed and involved in multiple signaling pathways in a variety of cell types. Importantly, c-Abl has been shown to play a role in T cell signaling (32–34), a function that may very well impact the B cell phenotype of KO animals. Further studies with c-Abl conditional KO animals will likely help shed more light on these issues.
Funding
National Institutes of Health Grant (P01 CA 023767); Public Health Services grant (T32 AI 7525) to R.A.L.
Acknowledgments
Stephen P. Goff is an Investigator of the Howard Hughes Medical Institute. We would like to thank Kristie Gordon for flow cytometry technical assistance.
Glossary
Abbreviations
- A-MuLV
Abelson murine leukemia virus
- APC
allophycocyanin
- BCAP
B cell adaptor for PI3K
- BCR
B cell receptor
- BCR–ABL
breakpoint cluster region–Abelson
- BLNK
B cell linker protein
- Btk
Bruton's tyrosine kinase
- EGTA
ethylene glycol tetraacetic acid
- EMSA
electrophoretic mobility shift assay
- FBS
fetal bovine serum
- KO
knockout
- MZ
marginal zone
- NF-κB
nuclear factor-κB
- PBST
PBS + 0.1% Tween-20
- PI3K
phosphoinositide-3 kinase
- RPMI
Roswell Park Memorial Institute media
- T1/T2
transitional 1 or 2
- TBS
Tris-buffered saline
- V(D)J
variable (diversity) joining
- WT
wild type
References
- 1.Casola S, Otipoby KL, Alimzhanov M, et al. B cell receptor signal strength determines B cell fate. Nat. Immunol. 2004;5:317. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 2.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 4.Van Etten RA. Cycling, stressed-out and nervous: cellular functions of c-Abl. Trends Cell Biol. 1999;9:179. doi: 10.1016/s0962-8924(99)01549-4. [DOI] [PubMed] [Google Scholar]
- 5.Goff SP, Gilboa E, Witte ON, Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980;22:777. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986;233:212. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- 7.Schwartzberg PL, Stall AM, Hardin JD, et al. Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell. 1991;65:1165. doi: 10.1016/0092-8674(91)90012-n. [DOI] [PubMed] [Google Scholar]
- 8.Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 9.Hardin JD, Boast S, Schwartzberg PL, et al. Bone marrow B lymphocyte development in c-abl-deficient mice. Cell. Immunol. 1995;165:44. doi: 10.1006/cimm.1995.1185. [DOI] [PubMed] [Google Scholar]
- 10.Lam QL, Lo CK, Zheng BJ, et al. Impaired V(D)J recombination and increased apoptosis among B cell precursors in the bone marrow of c-Abl-deficient mice. Int. Immunol. 2007;19:267. doi: 10.1093/intimm/dxl143. [DOI] [PubMed] [Google Scholar]
- 11.Lu L, Osmond DG. Apoptosis and its modulation during B lymphopoiesis in mouse bone marrow. Immunol. Rev. 2000;175:158. [PubMed] [Google Scholar]
- 12.Hardin JD, Boast S, Schwartzberg PL, et al. Abnormal peripheral lymphocyte function in c-abl mutant mice. Cell. Immunol. 1996;172:100. doi: 10.1006/cimm.1996.0220. [DOI] [PubMed] [Google Scholar]
- 13.Zipfel PA, Grove M, Blackburn K, Fujimoto M, Tedder TF, Pendergast AM. The c-Abl tyrosine kinase is regulated downstream of the B cell antigen receptor and interacts with CD19. J. Immunol. 2000;165:6872. doi: 10.4049/jimmunol.165.12.6872. [DOI] [PubMed] [Google Scholar]
- 14.Engel P, Zhou LJ, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 15.Maruoka M, Suzuki J, Kawata S, et al. Identification of B cell adaptor for PI3-kinase (BCAP) as an Abl interactor 1-regulated substrate of Abl kinases. FEBS Lett. 2005;579:2986. doi: 10.1016/j.febslet.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 16.Okada T, Maeda A, Iwamatsu A, Gotoh K, Kurosaki T. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity. 2000;13:817. doi: 10.1016/s1074-7613(00)00079-0. [DOI] [PubMed] [Google Scholar]
- 17.Backesjo CM, Vargas L, Superti-Furga G, Smith CI. Phosphorylation of Bruton's tyrosine kinase by c-Abl. Biochem. Biophys. Res. Commun. 2002;299:510. doi: 10.1016/s0006-291x(02)02643-8. [DOI] [PubMed] [Google Scholar]
- 18.Tzeng SJ, Bolland S, Inabe K, Kurosaki T, Pierce SK. The B cell inhibitory Fc receptor triggers apoptosis by a novel c-Abl family kinase-dependent pathway. J. Biol. Chem. 2005;280:35247. doi: 10.1074/jbc.M505308200. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki T, Kurosaki T. Contribution of BCAP to maintenance of mature B cells through c-Rel. Nat. Immunol. 2003;4:780. doi: 10.1038/ni949. [DOI] [PubMed] [Google Scholar]
- 20.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauxion F, Jamieson C, Yoshida M, Arai K, Sen R. Comparison of constitutive and inducible transcriptional enhancement mediated by kappa B-related sequences: modulation of activity in B cells by human T-cell leukemia virus type I tax gene. Proc. Natl Acad. Sci. USA. 1991;88:2141. doi: 10.1073/pnas.88.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-kappaB. Nat. Rev. Immunol. 2005;5:435. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- 23.Dorsch M, Goff SP. Increased sensitivity to apoptotic stimuli in c-abl-deficient progenitor B-cell lines. Proc. Natl Acad. Sci. USA. 1996;93:13131. doi: 10.1073/pnas.93.23.13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas MD, Srivastava B, Allman D. Regulation of peripheral B cell maturation. Cell. Immunol. 2006;239:92. doi: 10.1016/j.cellimm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Jumaa H, Hendriks RW, Reth M. B cell signaling and tumorigenesis. Annu. Rev. Immunol. 2005;23:415. doi: 10.1146/annurev.immunol.23.021704.115606. [DOI] [PubMed] [Google Scholar]
- 26.Pezzutto A, Dorken B, Rabinovitch PS, Ledbetter JA, Moldenhauer G, Clark EA. CD19 monoclonal antibody HD37 inhibits anti-immunoglobulin-induced B cell activation and proliferation. J. Immunol. 1987;138:2793. [PubMed] [Google Scholar]
- 27.Debnath I, Roundy KM, Weis JJ, Weis JH. Defining in vivo transcription factor complexes of the murine CD21 and CD23 genes. J. Immunol. 2007;178:7139. doi: 10.4049/jimmunol.178.11.7139. [DOI] [PubMed] [Google Scholar]
- 28.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12:39. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Brooks SR, Li X, Anzelon AN, Rickert RC, Carter RH. The physiologic role of CD19 cytoplasmic tyrosines. Immunity. 2002;17:501. doi: 10.1016/s1074-7613(02)00426-0. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto M, Poe JC, Satterthwaite AB, Wahl MI, Witte ON, Tedder TF. Complementary roles for CD19 and Bruton's tyrosine kinase in B lymphocyte signal transduction. J. Immunol. 2002;168:5465. doi: 10.4049/jimmunol.168.11.5465. [DOI] [PubMed] [Google Scholar]
- 31.Shinall SM, Gonzalez-Fernandez M, Noelle RJ, Waldschmidt TJ. Identification of murine germinal center B cell subsets defined by the expression of surface isotypes and differentiation antigens. J. Immunol. 2000;164:5729. doi: 10.4049/jimmunol.164.11.5729. [DOI] [PubMed] [Google Scholar]
- 32.Gu JJ, Zhang N, He YW, Koleske AJ, Pendergast AM. Defective T cell development and function in the absence of Abelson kinases. J. Immunol. 2007;179:7334. doi: 10.4049/jimmunol.179.11.7334. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Comiskey EO, Dupree RS, Li S, Koleske AJ, Burkhardt JK. The c-Abl tyrosine kinase regulates actin remodeling at the immune synapse. Blood. 2008;112:111. doi: 10.1182/blood-2007-10-118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zipfel PA, Zhang W, Quiroz M, Pendergast AM. Requirement for Abl kinases in T cell receptor signaling. Curr. Biol. 2004;14:1222. doi: 10.1016/j.cub.2004.07.021. [DOI] [PubMed] [Google Scholar]