Abstract
Animals in a variety of taxa discriminate between a greater quantity and a lesser quantity of the same object, an ability that is referred to as relative numerousness judgment. For example, meadow voles can distinguish between areas containing more over-marks by one opposite-sex scent donor and fewer over-marks by another opposite-sex scent donor. Females appear to be able to make better discriminations between more or less over-marks than do males. In that gonadal hormones have been implicated in modulating cognitive function associated with spatial tasks, we tested the hypothesis that high titers of testosterone and estradiol are necessary for male and female voles, respectively, to distinguish between the top- and bottom-scent donors in an area containing mixed over-marks. We gonadectomized voles, giving them either gonadal hormone replacement (testosterone for males and estradiol for females) or no hormone replacement, and tested their spontaneous judgments of distinguishing between the top-and bottom-scent donors in an area containing mixed over-marks; a task involving judgments of relative numerousness Female voles given replacement estradiol performed better than did female voles not given replacement estradiol in determining the top-scent and bottom-scent males in areas containing mixed over-marks. In contrast, males not treated with replacement testosterone performed better than did males treated with testosterone in determining the top-scent and bottom-scent males in areas containing mixed over-marks. Thus, high titers of estradiol and low titers of testosterone are associated with better performance on tasks involving relative numerousness in female and male voles, respectively. The results of this task on relative numerousness judgments are discussed in relation to the effects of gonadal steroid hormone on spatial ability, a closely related cognitive domain, and the social biology of meadow voles.
Introduction
Many animals in a variety of taxa can distinguish between a greater quantity and a lesser quantity of the same object, a capacity that is associated with relative numerousness judgment (Davis and Perusse, 1988; Hauser, 2000; Anderson et al., 2005). Most studies have reported that animals select or spend more time investigating larger amounts of food than smaller amounts of the same food (Beran 2001; Machado and Keen, 2002; Kilian et al., 2003; Uller et al., 2003). Such information can aid animals in determining which food patch they would visit (Beran et al., 2008). However, studies have shown that mosquitofish, rats, and female lions can make quantity judgments beyond foraging and involving social situations and communication between conspecifics (Davis and Bradford, 1986; Davis and Hiestand, 1992; McComb et al., 1994; Argrillo et al., 2007). This would allow individuals to determine the relative quantity of nearby individuals and use it to facilitate or deter interactions with these conspecifics (Kitchen, 2004).
These observations suggest that relative numerousness judgments may be ecologically relevant and impact an individual’s fitness. For example, male meadow voles, Microtus pennsylvanicus, assess the quantities of scent marks from conspecifics surrounding a reproductively active female vole and adjust their sperm investment such that they ejaculate more sperm when they encounter scent marks of a single male than those of multiple males (delBarco-Trillo and Ferkin, 2006). In addition, after exploring an arena that contained a set of over-marks deposited by one individual and a smaller or larger set of over-marks deposited by another individual, male and female meadow voles responded preferentially to the opposite-sex conspecific that was the top-scent donor on more of the over-marks than the opposite-sex conspecific that was the bottom-scent on most of the over-marks (Ferkin et al., 2005). For voles, performance of this task involves both spatial ability and judgments about relative numerousness (Davis and Bradford, 1986; Capaldi and Miller, 1988; Davis and Perusse, 1988; Boysen and Capaldi, 1993). Voles used their spatial ability to identify areas of overlap between two scent marks to discriminate between the top-scent donor and the bottom-scent donor of an over-mark (Ferkin et al., 1999). Voles also used judgments about relative numerousness to distinguish between over-marks deposited by one individual and a smaller or larger set of over-marks deposited by another individual (Ferkin et al., 2005). Consequently, these two cognitive domains may be closely-linked functionally (Ross and Santi, 2000; Gibbs, 2005; Nieder et al., 2006; Cordes et al., 2007; Beran et al., 2008), when voles are investigating areas of mixed over-marks.
Judgments about relative differences in over-marks, however, varied with the sex of the meadow vole. Female meadow voles were better able than male voles in distinguishing between small differences in the relative number of over-marks by the two scent donors (Ferkin et al., 2005). This finding contrasts with those showing that male voles out perform female voles on spatial tasks associated with a water maze (Gaulin et al., 1990; Galea et al., 1995, 1996). A functional explanation for the sex differences in these two cognitive tasks may be associated with the social biology of male and female meadow voles during their spring and summer breeding season. At this time of year, female meadow voles are philopatric, promiscuous and territorial and many males visit and deposit scent marks in their territories (Madison, 1980). Females investigating these over-marks may be able to identify the male that deposited the most over-marks. The top-scent male is likely to be in better condition, of higher relative quality or socially dominant than the bottom-scent male (Johnston et al., 1995; Leonard et al., 2001; Spritzer et al., 2004; Ferkin, 2007). In contrast, during the breeding season, male meadow voles occupy large home ranges that encompass the territories of one or more females. Males may not need to make such discriminations as females do in identifying the most frequent top-scent donor in an area. Male voles mate with nearby females and should not be as interested as females are in the relative social status or quality of their mates (Madison, 1980; Ferkin et al., 2004a, 2004b; 2005). Thus, selection may have favored female voles having a higher capacity than male voles for being able to discriminate smaller differences in the number of over-marks from two different donors in a given area (Ferkin et al., 2005) but weaker spatial ability than male voles (Gaulin and FitzGerald, 1986; Gaulin et al., 1990; Galea et al., 1996). In that meadow voles live in a habitat that contains many runways, nests, and scent marks of numerous conspecifics (Madison, 1980), the manner in which voles negotiate the social cues in their habitat likely involve spatial learning (Gaulin et al., 1990) as well as relative numerousness judgments (Ferkin et al., 2005). Although spatial ability and relative numerousness judgments may be closely linked functionally, these two cognitive domains may differ between the sexes and how gonadal steroids modulate them.
Several studies suggest that sex differences on cognitive tasks associated with spatial memory in animals and humans are moderated by gonadal hormones (Kimura, 1999; Halpern, 2000, Hampson, 2002; Williams, 2002; Baron-Cohen, 2003; Gibbs, 2005). Testosterone generally modulates spatial ability for males. Most male rodents usually perform better on tasks associated with spatial ability if they have higher titers of circulating testosterone (Kritzer et al., 2001; Daniel et al., 2003; Frye et al., 2004; Gibb, 2005, but see Galea et al., 1995). However, it is not clear if performance of females on such tasks is modulated by high or low titers of circulating estradiol; the literature does not provide a clear consensus (Galea et al., 1994, 1995, 2002; Warren and Juraska, 1997; Sandstrom and Williams, 2004; Varga et al., 2002; Bimonte-Nelson et al., 2003; Garza-Meilandt et al., 2006).
The goal of the present study was to determine whether sex differences in the ability to discriminate between two different scent donors in areas containing mixed over-marks may be attributed to testosterone and estradiol. Specifically, we measured whether judgments of relative numerousness were moderated by testosterone in male and moderated by estradiol in female meadow voles. We tested the hypothesis that high titers of testosterone and estradiol are necessary for male and female voles, respectively, to distinguish between the conspecific that provided more top-scent marks as opposed to the conspecific that provided less top-scent marks in a given area. A prediction of this hypothesis is that gonadectomized voles given gonadal hormone replacement (testosterone for males and estradiol for females) display a greater capacity for relative numerousness than do gonadectomized voles not given replacement hormone. The alternative hypothesis is that gonadal steroid hormones do not mediate the behavior. If so, we predict that gonadectomized and gonadectomized voles treated with replacement hormone do not differ in their responses.
General Methods
Animals
Female and male meadow voles were used in the following experiments. In each experiment, we used voles born and raised under long photoperiod (14L: 10D, lights on at 0800 h, CST). This photoperiod simulates a day length typical of the breeding season. Animals were first, second, and third generation offspring of field-caught animals captured in Ohio and Kentucky, USA. All voles used in this experiment were weaned at 21 days of age, housed with littermates until 42 days of age, and then housed singly in clear plastic cages (27 × 16.5 × 12.5 cm). These cages contained wood-chip bedding, cotton nesting material, and ad libitum food (Laboratory Rodent Diet #5008, PMI, Inc., St. Louis, MO, USA) and water. These cages were cleaned once a week and the cotton nesting material was replaced every 2 weeks.
Our experiments (see below) adhered to the Animal Behavior Society Guidelines for the Use of Animals in Research. All procedures involving voles were approved by the Institutional Animal Care and Use Committee of The University of Memphis.
Subjects
All subjects used in these experiments were between 100–120 days of age, sexually mature, but sexually inexperienced. When the subjects were 70–80 days of age 30 male and 30 female voles were selected randomly from a pool of 60 male and 60 female voles. The selected voles were anesthetized with a mixture of ketamine and xylazine (100 mg ketamine hydrochloride/ml and 20 mg xylazine hydrochloride/ml; injection volume of 0.05 ml), and gonadectomized (Leonard et al., 2005; Pierce et al., 2007). During the surgery, 15 males were implanted with an empty Silastic® capsule (BL) (Dow Corning, Midland, MI; od 0.077 in, id 0.058 in), whereas the remaining 15 males were implanted with a capsule containing 15 mm of active length of testosterone, T (Sigma Chemical Co., St Louis, MO). Fifteen females were implanted with an empty capsule (BL), whereas 15 females were implanted with a capsule containing 10 mm of active length of estradiol-17β, E2 (Sigma Chemical Co., St Louis, MO). The circulating concentrations of T were 1.3 ± 0.3 ng/ml for treated males, which is typical of long-photoperiod males, and 0.3 + 0.15 ng/ml for males that received no hormone replacement. These testosterone titers were close to those reported in previous studies (Leonard et al., 2005; Pierce et al., 2007). The circulating titers of E2 for treated females were 259.4 ± 23.6 pg/ml, which is comparable to long-photoperiod females, and 57.4 ± 12.2 pg/ml for females that received no hormone replacement. These estradiol titers were similar to those reported in previous studies (Leonard et al., 2005; Pierce et al., 2007). Procedures for preparing the capsules for implantation, the surgery, and subsequent ELISA hormone analysis were standard, following the methods detailed elsewhere (Leonard et al., 2005; Pierce et al., 2007). The intra-assay coefficient of variation was 5.9% and the inter-assay coefficient of variation was 9.2%.
The resulting surgeries and hormone treatments allowed us to establish two treatment groups of male subjects, GX + BL males (n = 15) and GX + T males (n = 15), and two treatment groups of female subjects, OVX + BL females (n =15) and OVX + E2 females (n = 15). These voles were used as subjects in the behavioral tests 20 days following surgery and implantation. This time interval between surgery and the onset of testing was sufficient to allow for the clearance of residual hormones and the reinstatement of new T titers in the treated males and E2 titers in treated females (Leonard et al., 2005; Pierce et al., 2007).
Scent Donors
Scent donors were an additional 100–150 day-old, sexually experienced, male (n = 40) and female (n = 40) voles that had intact gonads. The male donors were sexually receptive, but had not been paired with a female vole for 30 d before the start of the experiments. The female donors were neither currently pregnant nor lactating, having delivered a litter 30 d prior to be used as scent donors. Female meadow voles are induced ovulators and do not undergo estrous cycles (Milligan 1982; Keller 1985), but will mate within a few hours of being paired with a male conspecific (Meek and Lee, 1993). More importantly, long-photoperiod female voles are attracted to odors produced by male conspecifics and male conspecifics are attracted to the odors of these females (Ferkin et al., 2004a; Ferkin et al., 2004b). To eliminate potential litter effects, we used no more than two individuals from the same litter as either subjects or scent donors. Subjects were not tested with the scent marks of siblings (Ferkin et al., 2005).
Pre-exposure phase-T-shaped Arena
An arena matching this one was used in previous studies of scent marking and over-marking in voles (Ferkin et al., 2004a, 2004b, 2005). The sides of the arena were constructed of opaque green acrylic plastic. Each arm and the stem of the arena were 25 × 13 × 16 cm (length × width × height, respectively). The experimenter could block entry from the stem into the horizontal portion of the arena by placing a solid, opaque partition (made of the same material as the arena) across the stem. A large sheet of white photocopy paper served as the substrate of the arena. The voles were unfamiliar with the arena at the time of testing.
Placing and simulating multiple over-marks
We followed the methods detailed in Ferkin et al. (2005). We created multiple over-marks of two different same-sex conspecifics in a T-shaped arena that simulates a runway that a vole may encounter in the field (Ferkin et al., 2004a, 2004b). Depending on the experiment, we either placed six, seven, or eight evenly spaced, simulated over-marks, each measuring 1.5 cm in diameter on specific locations on the paper substrate on the horizontal portion of the T-shaped arena. Over-marks were placed evenly on the substrate with 5 cm separating contiguous over-marks (Ferkin et al., 2005). Thus, for each experiment we were able to control for both the location and proximity of over-marks to one another.
Over-marks were derived from fecal boli of scent donors; feces scent marks are attractive to opposite-sex conspecifics (Ferkin et al., 2004a, 2005). Over-marks were created by the investigators by rubbing fresh feces from the cage of the first scent donor (bottom-scent vole) on a specified portion of paper substrate in the T-shaped arena and allowing it to dry for 5 minutes, and then rubbing fresh feces from the cage of the second scent donor (top-scent vole) directly on top of it such that it made an “+” shape (Ferkin et al., 2005). Each scent mark was created by rubbing the feces against a plastic template with an opening that was 1.5 cm long and 0.5 cm in width that was placed in the desired location on the substrate. Thus, we were able to control for the size and surface area of each scent mark and over-mark. The template was cleaned thoroughly with a solution of alcohol and warm soapy water and dried between applications.
We chose to use feces in this series of experiments to eliminate our handling of the scent donors. However, anogenital area scents and urine may also be used as sources of scents for over-marks (Ferkin et al., 2004a, 2004b). We used black ink to trace the outline of the feces marks of the first scent donor (bottom-scent vole), and red ink to trace the outline of the second scent donor (top-scent vole). Previous tests indicated that the presence or color of the ink used in tracing does not affect the behavior of voles investigating over-marks (Ferkin et al., 2005).
Exposure to multiple over-marks
Each subject was tested once for each of the five experimental conditions (see below) for a total of 5 tests with a unique combination of over-marks. The order of tests was randomized. Five days elapsed between successive tests with the same subject. Each subject was exposed to areas containing the scent marks of a unique pair of odor donors in each experimental condition.
All pairs of donors were similar in weight (within 5 g), and unfamiliar and unrelated to each other and to the subjects. Within a trial, all donors were same-sex conspecifics and opposite in sex to the subjects. We randomly assigned the donors that provided more (donor A) as opposed to those that provides less top-scent marks (donor B).
Subjects were placed into the stem of the T-shaped arena, behind an opaque divider 10 min after the last over-mark was placed in the horizontal section of the maze (Ferkin et al., 2005). After the subject was placed in the stem of the T-shaped arena for two minutes, the divider was raised and the subject was allowed 10 min to explore the arena. All the subjects used in this study explored the entire horizontal portion of the arena.
The experiment contained 5 testing conditions (1–5). In conditions 1–4 male and female subjects (hormone-treated and blank-treated voles) were placed in an arena that contained seven over-marks (conditions 1–4) in which donor A provided more over-marks than donor B. Subjects were exposed to 7 over-marks by donor A and zero over-marks by donor B in condition 1, 6 over-marks by donor A and 1 over-mark by donor B in condition 2, 5 over-marks by donor A and 2 by donor B in condition 3, and 4 over-marks by donor A and 3 by donor B in condition 4. In condition 5, subjects were placed in an arena containing 4 over-marks from donor A and 4 by donor B.
Post Exposure Odor Preference Test
The testing procedure was identical to that described in Ferkin et al., (2005). Immediately after exploring the T-shaped arena, subjects were returned to their home cage. Fifteen min after returning to their home cage, each subject underwent a single 5-min preference test; this test has been used previously in voles (Ferkin et al., 2005). The voles were presented with a clean glass microscope slide (2.5 × 7.6 cm) that contained the fecal scent marks from the two donors, A and B.
Fresh fecal scent marks were obtained for each trial from donor A and donor B. We collected scent marks by rubbing a fresh fecal bolus against a clean glass microscope slide against it for 3–5 seconds. The slides were used within 5 min of being prepared. The experimenter wore disposable latex gloves to minimize human scent transfer while handling all slides.
Each slide was divided into three equal sections (each 2.5 cm long). One end section contained a scent mark from donor A. The other end section contained a scent mark from donor B; donors A and B were the same donors that that subject was exposed to during the exposure phase. The middle section contained no stimulus odor. We randomly placed the stimulus odors on the left or right side of the slide. The glass slide was suspended by a wire hook and a clasp 1 cm above the substrate in the subject’s home cage, against the wall opposite the animal’s nest.
The test began 5 s after the slide was placed in the subject’s home cage. During each 5-min trial, we recorded continuously the time each subject investigated the two scented sections of the slide, and the middle section of the slide. The experimenter was not aware of which animal provided more over-marks. Criteria for investigation of a mark were that: 1) a vole was obviously licking or sniffing a stimulus odor or its nose was within approximately 1 cm of one end of the slide, 2) the vole investigated the two scented areas on the slide, and 3) the vole spent more time (cumulative) investigating the two scented areas of the slide than the clean middle section (Ferkin et al., 2005). Each slide was used in only one trial and discarded.
Statistics
For each subject we calculated the amount of time it spent investigating the mark of the donor who had provided more (donor A) as opposed to less top-scent marks (donor B) for that trial. For each experimental condition (1–5), we calculated the time voles spent investigating the top-scent marks divided by the time investigating the top-scent and the bottom-scent marks. This value was arcsine square-root transformed, allowing us a continuous variable that was comparable across groups. We used a two-way (mixed) analysis of variance on ratios with a hormone treatment factor at 4 levels and an experimental condition factor at five levels as the repeated measure. We used post hoc multiple pairwise tests (Holm-Sidak method) to determine if significant differences existed in the amount of time subjects spent investigating the odor of the two scent donors in that experimental condition (see below). Statistical differences were accepted at p < 0.05. The actual investigation times are indicated on the graphs (see below).
Results
Experimental Condition 1
All male and female subjects investigated both scent marks in this and subsequent conditions. In addition, there was little variability in the overall investigation time across all of the conditions.
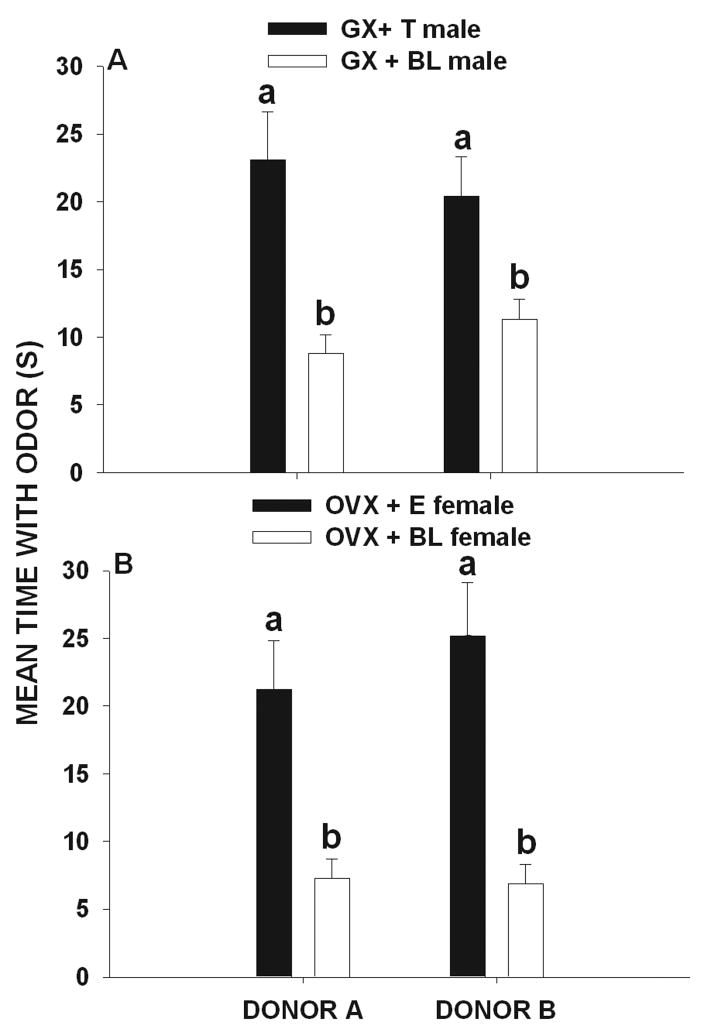
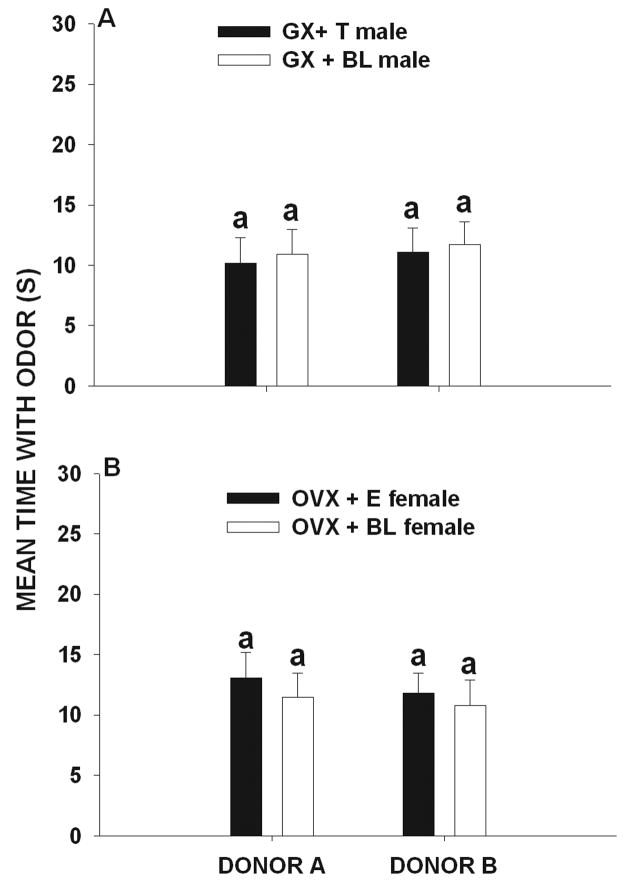
The experimental test condition (F 4, 299 = 1804.92, p < 0.001) and hormone treatment (F 3, 299 = 211.65, p < 0.001) affected the amount of time investigating that subjects spent investigating the scent mark of donor A and the scent marks of donor B. There was, however, a significant interaction effect between the main effects (F 12, 299 =194.48, p < 0.001). To investigate this interaction, we conducted subsequent separate one-way ANOVA’s for repeated measures for each experimental condition. In experimental treatment 1 (A= 7 over-marks and B= 0 over-marks), significant differences in the amounts of time that male (F 3, 59 = 72.33, p < 0.001) and female (F 3, 59 = 51.8, p < 0.001) voles investigated the scent mark of donor A and that of donor B. Post hoc comparisons showed that GX + T and GX + BL males spent more time investigating the marks of donor A than those of donor B (p < 0.05, Holm-Sidak method; Fig. 1a). Likewise, OVX + E2 and OVX + BL females spent more time investigating the marks of donor A than those of donor B (p < 0.05, Fig. 1b).
Figure 1.
Mean (± SEM) amount of time (seconds) that (A) gonadectomized + testosterone-treated (GX + T) male and (gonadectomized + blank-treated male (GX + BL) voles and (B) ovariectomized + estradiol-treated (OVX + E) female and ovariectomized + blank-treated (OVX + BL) female voles exposed to 7 over-marks by donor A and to 0 over-marks by donor B later spent investigating the odor of these two scent donors. Histograms capped with different letters are statistically different at p < 0.05.
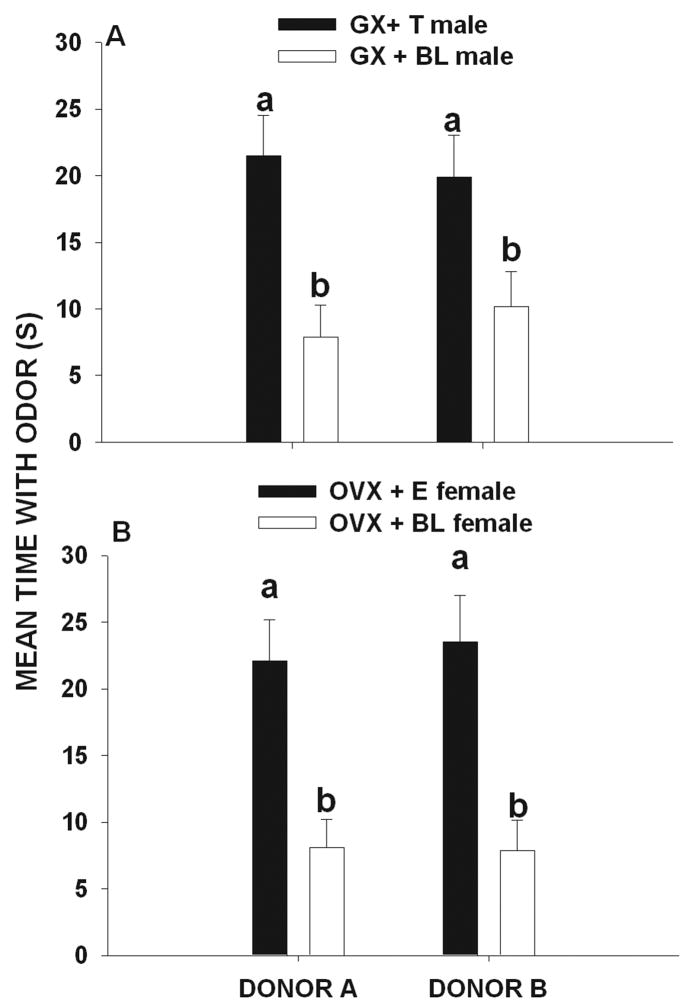
In experimental condition 2 (A=6 over-marks and B= 1 over-mark) significant differences existed in the amounts of time that male (F 3, 59 = 33.7, p < 0.001) and female (F 3, 59 = 30.2, p < 0.001) voles investigated the scent marks of donor A and B. Post hoc comparisons showed that GX + T and GX + BL males spent more time investigating the marks of donor A than those of donor B (p < 0.05, Fig. 2a). Likewise, OVX + E2 and OVX + BL females spent more time investigating the marks of donor A than those of donor B (p < 0.05, Fig. 2b).
Figure 2.
Mean (± SEM) amount of time (seconds) that (A) gonadectomized + testosterone-treated (GX + T) male and (gonadectomized + blank-treated male (GX + BL) voles and (B) ovariectomized + estradiol-treated (OVX + E) female and ovariectomized + blank-treated (OVX + BL) female voles exposed to 6 over-marks by donor A and to 1 over-marks by donor B later spent investigating the odor of these two scent donors. Histograms capped with different letters are statistically different at p < 0.05.
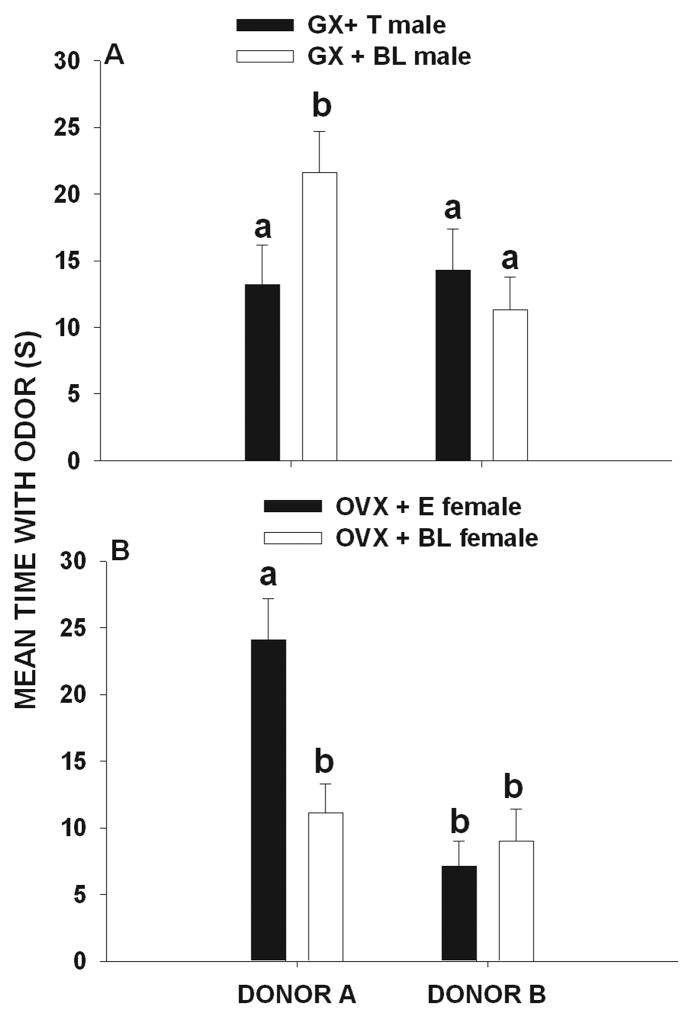
In experimental Condition 3 (A = 5 over-marks and B= 2 over-marks) significant differences were found in the amounts of time that male (F 3, 59 = 24.4, p < 0.001) and female (F 3, 59 = 21.3, p < 0.001) voles investigated the scent marks of donor A and B. Post hoc comparisons showed that GX + BL males spent more time investigating the marks of donor A than those of donor B (p < 0.05, Fig. 3a). However, GX + T males spent similar amounts of time investigating the marks of donor A and those of donor B (p > 0.05, Fig 3 a). In contrast, OVX + E2 females spent more time investigating the marks of donor A than those of donor B (p < 0.05, Fig 3b) but OVX + BL females spent similar amounts of time investigating the marks of donor A and those of donor B (p > 0.05; Fig. 3b).
Figure 3.
Mean (± SEM) amount of time (seconds) that (A) gonadectomized + testosterone-treated (GX + T) male and (gonadectomized + blank-treated male (GX + BL) voles and (B) ovariectomized + estradiol-treated (OVX + E) female and ovariectomized + blank-treated (OVX + BL) female voles exposed to 5 over-marks by donor A and to 2 over-marks by donor B later spent investigating the odor of these two scent donors. Histograms capped with different letters are statistically different at p < 0.05.
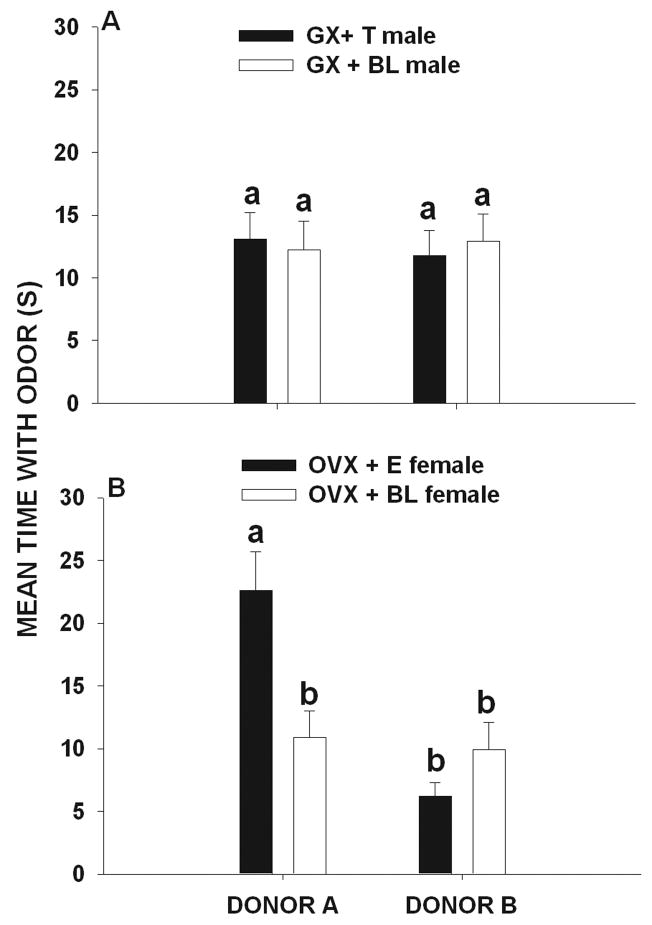
In experimental condition 4 (A = 4 over-marks and B= 3 over-marks) significant differences were found in the amount of time that female (F 3, 59 = 27.5, p < 0.001) but not male (F 3, 59 = 1.09, p = 0.36) voles investigated the scent marks of donor A and B. Post hoc comparisons showed that OVX + E2 females (p < 0.05, Fig 4b) but not OVX + BL females spent more time investigating the marks of donor A than those of donor B (p > 0.05, Fig 4b). GX + BL males and GX + T males spent similar amounts of time investigating the marks of donor A and donor B (both comparisons, p > 0.05, Fig. 4a).
Figure 4.
Mean (± SEM) amount of time (seconds) that (A) gonadectomized + testosterone-treated (GX + T) male and (gonadectomized + blank-treated male (GX + BL) voles and (B) ovariectomized + estradiol-treated (OVX + E) female and ovariectomized + blank-treated (OVX + BL) female voles exposed to 4 over-marks by donor A and to 3 over-marks by donor B later spent investigating the odor of these two scent donors. Histograms capped with different letters are statistically different at p < 0.05.
In experimental condition 5 (A = 4 over-mark and B= 4 over-marks) no significant differences were found in the amount of time that female (F 3, 59 = 0.86, p =0.46) and male (F 3, 59 = 0.52, p = 0.67) voles investigated the scent marks of donor A and B (Fig. 5a, b). This result indicated that male and female voles, independent of gonadal hormone condition, spent similar amounts of time investigating the mark of donor A and that of donor B.
Figure 5.
Mean (± SEM) amount of time (seconds) that (A) gonadectomized + testosterone-treated (GX + T) male and (gonadectomized + blank-treated male (GX + BL) voles and (B) ovariectomized + estradiol-treated (OVX + E) female and ovariectomized + blank-treated (OVX + BL) female voles exposed to 4 over-marks by donor A and to 4 over-marks by donor B later spent investigating the odor of these two scent donors. Histograms capped with same letters are not statistically different at p > 0.05.
Discussion
We determined whether judgments of relative numerousness were modulated by gonadal steroid hormones in meadow voles. Specifically, we tested the hypothesis that high titers of testosterone and estradiol are necessary for male and female voles, respectively, to distinguish between who had provided more as opposed to less top marks in areas containing mixed over-marks. The data indicated that male voles with low titers of testosterone performed better on this task of relative numerousness than did males with high titers of testosterone. Both gonadectomized males not given testosterone replacement and those given testosterone replacement and exposed to areas containing 7 over-marks by donor A and 0 over-mark by donor B and 6 over-marks by donor A and 1 over-mark by donor B, later behaved as if they could discriminate between the mark of donor A, the top-scent female, and the mark of donor B, the bottom-scent female. Interestingly, gonadectomized male voles not given testosterone replacement performed better than those given testosterone replacement in distinguishing the scent donor that deposited the most over-marks in an area. Male voles not treated with testosterone and exposed to an area containing 5 over-marks by donor A and 2 over-marks by donor B, later behaved as if they could discriminate between the mark of the top-scent female and the mark of the bottom-scent female. Thus, high titers of testosterone were not necessary for male meadow voles to perform better on this task of relative numerousness judgments. High titers of testosterone, however, are needed by male rats and mice to performed better on tasks associated with spatial ability, a cognitive domain similar to judgments of quantity (Ross and Santi, 2000; Gibbs, 2005; Nieder et al., 2006; Cordes et al., 2007; Beran et al., 2008), and likely involved in the discrimination of top- and bottom-scent marks in an over-mark. In tasks associated with spatial ability, male mice and rats with high titers of testosterone performed better than males with low titers of testosterone (Kritzer et al., 2001; Williams, 2002; Daniel et al., 2003; Frye et al., 2004; Gibb, 2005). It is interesting to note, however, that male meadow voles with high testosterone and low testosterone performed similarly in spatial learning tasks in a Morris water maze (Galea et al., 1995). Thus, it is possible that the performance of males involving tasks of judgments of relative quantity and spatial ability are under different neural and endocrine control and less dependent on testosterone than they are among males in other species of rodents. In this case for voles, the selective response to the mark of donors that provided more rather than less over-marks would be based solely relative numerousness judgments. It is also possible that such discriminations represent a cognitive domain that incorporates pattern recognition, labeling (Davis and Bradford, 1986; Davis and Perusse, 1988) and some degree of numerical competence (Capaldi and Miller, 1988; Boysen and Capaldi, 1993).
Unlike male voles, estradiol was necessary for female voles to make discriminations in determining who had provided more as opposed to less top marks in areas containing mixed over-marks. Ovariectomized female voles treated with estradiol and exposed to areas containing 5 over-marks and 4 over-marks by donor A and 2 and 3 over-marks by donor B, respectively, later behaved as if they could discriminate between the marks of the top-scent male and those of the bottom-scent male. However, ovariectomized females not treated with estradiol behaved as if they could not distinguish between the marks of the top- and the bottom-scent male. When we compare the role of estradiol on tasks involving spatial ability and relative numerousness in female rodents, we cannot find agreement in their proximate control. Some studies suggest that on days of low estradiol females perform as well as males on spatial tasks, but more poorly on days of high estradiol (Galea et al., 1995, 1996, 2002; Warren and Juraska, 1997; Bimonte-Nelson et al., 2003). When estradiol titers are high, as they are during the breeding season, female voles perform poorly relative to males on spatial tasks (Galea et al., 1995, 1996). However, other studies suggest that high titers of estradiol are needed to improve performance on tasks associated with spatial ability and place learning in female rodents (Hlinak, 1993; Packard, 1998; Sandstrom and Williams, 2004; Garza-Meilandt et al., 2006; Zurkovsky et al., 2007). Finally, some studies suggest that estradiol has little or no effect on spatial ability and sequence processing in female rodents (Ross and Santi, 2000; Varga et al., 2002).
Our results indicate that high titers of estradiol for female voles and low titers of testosterone for male voles are necessary for them to make better discriminations of relative numerousness. We do not know whether the high or low titers of testosterone and estradiol are directly affecting cognitive processes in voles by their direct action on the neural substrates controlling the perception of over-marks, whether these hormones are affecting cognition indirectly by affecting the vole’s attention, or whether low and high titers of testosterone and estradiol are affecting sensory mechanisms and olfactory processing in voles (Williams, 2002). Differences in the role of estradiol and testosterone in modulating cognitive tasks in females may be attributed to the particular tasks involved, the dosage and delivery of the hormones, the path that these hormones travel in the brain and differences in the neural substrates that govern the responses of animals (Williams et al., 1990; Williams and Meck, 1991; Garza-Meilandt et al., 2006; Nieder et al., 2006; Zurkovsky et al., 2007; Quinlan et al., 2008). It is also possible that olfactory acuity of female and male voles is sexually dimorphic as shown in other species. For example, Wesson et al. (2006) show that sensitivity in discriminating between similar urinary odorants is sexually dimorphic, favoring wild type male mice than female mice, and that this affect is affected by sex and gonadal hormones.
Our results are consistent with the conjecture that the capacity for relative numerousness may vary seasonally, with females performing better than males during the breeding season but not during the non-breeding season. Seasonal differences were also found in the spatial ability of male and female voles. Male voles perform better on spatial tasks during the spring and summer breeding season and perform poorer on such tasks during the fall and winter non-breeding season; the reverse seasonal pattern was reported in female performance (Galea et al., 1996). Better performance on spatial tasks in complex mazes by male as opposed to female meadow voles has been attributed differences between the sexes in the size of their home ranges, presumably to find mates (Gaulin and FitzGerald 1986; Gaulin et al., 1990). Interestingly, male meadow voles occupy relatively large home ranges during both the breeding and non-breeding season (Madison 1980). If the performance of males on these tasks are associated with finding a mate, this hypothesis does not explain why male reproductively active and reproductively quiescent male voles performing similarly in spatial tasks involving a Morris water maze (Galea et al. 1995) and why reproductively quiescent males performed better than reproductively active males on relative numerousness judgments (this study). However, male and female white-footed mice and rats housed in long photoperiod perform better than those housed in short-photoperiod in spatial tasks (Pyter et al., 2005; Macdonald et al., 2007). Our results may be explained by the seasonal differences that exist in the reproductive and social behaviors of meadow voles, which are induced by seasonal shifts in gonadal steroid concentrations (Ferkin and Zucker, 1991; Leonard et al., 2005; Pierce et al., 2007). High titers of estradiol may be necessary for female voles to establish and defend territories, reduce their space use, attract males, distinguish between males that are socially dominant or of higher quality, the top-scent male, from those males that are socially subordinate or of lower quality, and presumably the bottom-scent male (Leonard et al. 2001; Johnston et al., 1995; Spritzer et al., 2004). High titers of testosterone may be associated with male voles visiting the territories of many females and distinguish between sexually receptive females and females that are not sexually receptive (Leonard et al., 2001; Ferkin et al., 2004, a, b). Such males may be less motivated or gain little benefit by making such discriminations of the top- and bottom-scent females as wandering males could mate with both female scent donors (Ferkin et al., 2004a, 2004b). However, during the fall and winter female voles form communal nests and most are reproductively quiescent (Madison, 1980). Females may have little to gain by distinguishing between the marks of the top- and those of the bottom-scent males. However, wandering male voles may be motivated or benefit from discriminating between the scent marks of reproductively active females, presumably the top-scent female, and those of reproductively quiescent females, the bottom-scent female (Leonard et al., 2001; Ferkin et al., 2004a, 2004b).
Acknowledgments
We thank Amy Combs, Amy Whalen, and Hong Li for technical assistance and Javier delBarco-Trillo, Stan Franklin, and Lara LaDage for reading earlier drafts of this manuscript. Funding was provided by the NSF UMEB program at The University of Memphis, NSF grant IOB -0444553G 16954-01 and NIH grant HD0 49525-01 to MHF. This research adhered to the Animal Behavior Society Guidelines for the Use of Animals in Research. All procedures involving voles were approved by the Institutional Animal Care and Use Committee of The University of Memphis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson US, Stoinski TS, Bloomsith MA, Marr MJ, Smith AD, Maple TL. Relative numerousness judgment and summation in young and old Western lowland gorillas. J Comp Psych. 2005;119:285–295. doi: 10.1037/0735-7036.119.3.285. [DOI] [PubMed] [Google Scholar]
- Argrillo C, Dadda M, Bisazza A. Quantity discrimination in female mosquitofish. Anim Cogn. 2007;10:63–70. doi: 10.1007/s10071-006-0036-5. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The essential difference: The truth about the male and female brain. Basic Books; New York: 2003. [Google Scholar]
- Beran MJ. Summation and numerousness judgments of sequentially presented sets of items by chimpanzees (Pan troglodytes) J Comp Psych. 2001;115:181–191. doi: 10.1037/0735-7036.115.2.181. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA, Harris EH. Perception of food amounts by chimapanzees based on the number, size, contour length and visibility of items. Anim Behav. 2008;75:1793–1802. doi: 10.1016/j.anbehav.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biomonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm AC. Ovarian hormones and cognition in the aged female rat: 1. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci. 2003;117:1395–1406. doi: 10.1037/0735-7044.117.6.1395. [DOI] [PubMed] [Google Scholar]
- Capaldi EJ, Miller DJ. Counting in rats: Its functional significance and the independent cognitive processes that constitute it. J Exper Psych: Anim Behav Proc. 1988;14:3–17. [Google Scholar]
- Cordes S, Williams CL, Meck WH. Common representations of abstract quantities. Current Directions in Psychological Science. 2007;16:156–161. [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scolopamine and mecamylamine. Pyschopharm. 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- Davis H, Bradford SA. Counting behavior by rats in a simulated natural environment. Ethology. 1986;73:265–280. [Google Scholar]
- Davis H, Hiestand L. Rats counting rats: The use of conspecifics as discriminative stimuli. Bulletin of the Psychonomic Soc. 1992;30:356–358. [Google Scholar]
- Davis H, Perusse R. Numerical competence in animals: definitional issues, current evidence, and a new research agenda. Behav Brain Sci. 1988;11:561–615. [Google Scholar]
- delBarco-Trillo J, Ferkin MH. Male meadow voles respond differently to risk and intensity of sperm competition. Behav Ecol. 2006;17:581–585. [Google Scholar]
- Ferkin MH. Effects of previous interactions and sex on over-marking in meadow voles. Behaviour. 2007;144:1297–1313. [Google Scholar]
- Ferkin MH, Zucker I. Seasonal control of odour preferences of meadow voles (Microtus pennsylvanicus) by photoperiod and ovarian hormones. J Reprod Fert. 1991;92:433–441. doi: 10.1530/jrf.0.0920433. [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Dunsavage J, Johnston RE. What kind of information do meadow voles, Microtus pennsylvanicus, use to distinguish between the top and bottom scent of an over-mark? J Comp Psychol. 1999;113:43–51. [Google Scholar]
- Ferkin MH, Lee DN, Leonard ST. The reproductive state of female voles affects their scent marking behavior and the responses of male conspecifics to such marks. Ethology. 2004a;110:257–272. [Google Scholar]
- Ferkin MH, Li HZ, Leonard ST. Meadow voles and prairie voles differ in the percentage of conspecific marks that they over-mark. Acta Ethol. 2004b;7:1–7. [Google Scholar]
- Ferkin MH, Pierce AA, Sealand RO, delBarco-Trillo J. Meadow voles, Microtus pennsylvanicus, can distinguish more over-marks from fewer over-marks. Anim Cogn. 2005;8:82–89. doi: 10.1007/s10071-004-0244-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL, Selige AM, Wawrzycki JM. 5α-reduced androgens may have actions in the hippocampus to enhance cognitive performance of male rats. Psychoneuroendo. 2004;29:1019–1027. doi: 10.1016/j.psyneuen.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Kaveliers M, Ossenkopp K–P, Innes D, Hargreaves EL. Sexually dimorphic spatial learning varies seasonally in populations of deer mice. Brain Research. 1994;635:18–26. doi: 10.1016/0006-8993(94)91419-2. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Kaveliers M, Ossenkopp K-P, Hampson E. Gonadal hormone levels and spatial learning performance in the Morris water maze in male and female meadow voles, Microtus pennsylvanicus. Horm Behav. 1995;29:106–125. doi: 10.1006/hbeh.1995.1008. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Kaveliers M, Ossenkopp K-P. Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. J Exper Biol. 1996;199:195–200. doi: 10.1242/jeb.199.1.195. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Omerod BK, Sampath S, Kostaras X, Wilike DM, Phelps MT. Spatial memory and hippocampal size across pregnancy in rats. Horm Behav. 2000;37:86–95. doi: 10.1006/hbeh.1999.1560. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Lee TTY, Kostaras X, Sidhu JA, Barr AM. High levels of estradiol impair spatial performance in the Morris water maze and increase “depressive-like” behaviors in the female meadow vole. Physiol Behav. 2002;77:217–225. doi: 10.1016/s0031-9384(02)00849-1. [DOI] [PubMed] [Google Scholar]
- Garza-Meilandt A, Cantu RE, Clairborne BJ. Estradiol’s effects on learning and neuronal morphology vary with route of administration. Behav Neurosci. 2006;120:905–916. doi: 10.1037/0735-7044.120.4.905. [DOI] [PubMed] [Google Scholar]
- Gaulin SJ, FitzGerald RW. Sex differences in spatial ability: an evolutionary hypothesis and test. Amer Nat. 1986;127:74–88. [Google Scholar]
- Gaulin SJ, FitzGerald RW, Wartell MS. Sex differences in spatial ability and activity in two vole species (Microtus ochrogaster and M. pennsylvanicus) J Comp Psych. 1990;104:83–93. doi: 10.1037/0735-7036.104.1.88. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MD. What do animals think about numbers? Amer Sci. 2000;88:144–151. [Google Scholar]
- Halpern D. Sex differences in cognitive ability. 3. Earlbaum; New Jersey: 2000. [Google Scholar]
- Hampson E. Sex differences in human brain and cognition: the influence of sex steroids in early and adult life. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. 2. MIT Press; Massachusetts: 2002. pp. 579–628. [Google Scholar]
- Hlinak Z. Social recognition in ovariectomized and estradiol-treated female rats. Horm Behav. 1993;27:158–168. doi: 10.1006/hbeh.1993.1012. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Munver R, Tung C. Scent counter marks: selective memory for the top scent by golden hamsters. Anim Behav. 1995;49:1435–1442. [Google Scholar]
- Keller BL. Reproductive patterns. In: Tamarin RH, editor. Biology of New World Microtus. 8. American Society of Mammalogists, Special Publication; Kansas: 1985. pp. 725–778. [Google Scholar]
- Kilian A, Yana S, von Fersen L, Gunturkun O. A bottlenose dolphin discriminates visual stimuli differing in numerosity. Learn Behav. 2003;31:133–142. doi: 10.3758/bf03195976. [DOI] [PubMed] [Google Scholar]
- Kimura D. Sex and cognition. MIT Press; Masssachusetts: 1999. [Google Scholar]
- Kitchen DM. Alpha male black howler monkeys responses to loud calls: effects of numeric odds, male comparison behavior and reproductive investment. Anim Behav. 2004;67:125–139. [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Leonard ST, Ferkin MH, Johnson MM. The response of meadow voles to an over-mark in which the two donors differ in gonadal hormone status. Anim Behav. 2001;62:1171–1177. [Google Scholar]
- Leonard ST, Naderi R, Stokes K, Ferkin MH. The role of prolactin and testosterone in mediating seasonal differences in the self-grooming behavior of male meadow voles, Microtus pennsylvanicus. Physiol Behav. 2005;85:461–468. doi: 10.1016/j.physbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Cheng R-K, Williams CL, Meck WH. Combined organizational effects of short and long photoperiods on spatial and temporal memory in rats. Behav Processes. 2007;74:226–233. doi: 10.1016/j.beproc.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Machado A, Keen R. Relative numerosity discrimination in the pigeon: further tests of the linear-exponential-ratio model. Behav Processes. 2003;57:131–148. doi: 10.1016/s0376-6357(02)00010-4. [DOI] [PubMed] [Google Scholar]
- Madison DM. An integrated view of the social biology of meadow voles, Microtus pennsylvanicus. The Biologist. 1980;62:20–33. [Google Scholar]
- McComb K, Packer C, Pusey A. Roaring and numerical assessment in contests between groups of female lions, Panthera leo. Anim Behav. 1994;47:379–387. [Google Scholar]
- Meek LR, Lee TM. Prediction of fertility by mating latency and photoperiod in nulliparous and primiparous meadow voles (Microtus pennsylvanicus) J Reprod Fert. 1993;97:353–357. doi: 10.1530/jrf.0.0970353. [DOI] [PubMed] [Google Scholar]
- Milligan SR. Induced ovulation in mammals. Oxford Reviews of Reproduction. 1982;4:1–46. [Google Scholar]
- Nieder A, Diester I, Tudusciuc O. Temporal and spatial enumeration processes in the primate parietal cortex. Science. 2006;313:1431–1435. doi: 10.1126/science.1130308. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;39:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Pierce AA, Iwueke I, Ferkin MH. Estradiol titers, food deprivation and sexual behavior in meadow voles. Physiol Behav. 2007;90:353–361. doi: 10.1016/j.physbeh.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Reader BF, Nelson RJ. Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscus leucopus) J Neurosci. 2005;25:4521–4526. doi: 10.1523/JNEUROSCI.0795-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan MG, Hussain D, Brake WG. Use of cognitive strategies in rats: the role of estradiol and its interactions with dopamine. Horm Behav. 2008;53:185–191. doi: 10.1016/j.yhbeh.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Ross L, d Santi A. The effects of estrogen on temporal and numerical processing in ovariectomized female rats. Psychobiol. 2000;28:394–405. [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:126–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Meikle DB, Solomon NG. The relationship between dominance rank and spatial ability among meadow voles (Microtus pennsylvanicus) J Comp Psych. 2004;118:332–339. doi: 10.1037/0735-7036.118.3.332. [DOI] [PubMed] [Google Scholar]
- Uller C, Jaegar R, Guidry G, Martin C. Salamanders (Plethodon cinerus) go for more: rudiments of number in an amphibian. Anim Cogn. 2003;6:105–112. doi: 10.1007/s10071-003-0167-x. [DOI] [PubMed] [Google Scholar]
- Varga H, Nerheth H, Toth T, Kis Z, Farkas T, Toldi J. Weak if any effect of estrogen on spatial memory in rats. Acta Biologica Szegediensis. 2002;48:13–16. [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Keller M, Douhard Q, Baum MJ, Bakker J. Enhanced urinary odor discrimination in female aromatase knockout (ArKO) mice. Horm Behav. 2006;49:580–586. doi: 10.1016/j.yhbeh.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Barnett AM, Meck WH. Organizational effects of gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci. 1990;104:84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendo. 1991;16:155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- Williams CL. Hormones and cognition in nonhuman animals. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. 2. MIT Press; Massachusetts: 2002. pp. 527–577. [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neurosci. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]