Abstract
Neurodegenerative disorders are marked by extensive neuronal apoptosis and gliosis. Although several apoptosis-inducing agents have been described, understanding of the regulatory mechanisms underlying modes of cell death is incomplete. A major breakthrough in delineation of the mechanism of cell death came from elucidation of the sphingomyelin (SM)-ceramide pathway that has received worldwide attention in recent years. The SM pathway induces apoptosis, differentiation, proliferation, and growth arrest depending upon cell and receptor types, and on downstream targets. Sphingomyelin, a plasma membrane constituent, is abundant in mammalian nervous system, and ceramide, its primary catabolic product released by activation of either neutral or acidic sphingomyelinase, serves as a potential lipid second messenger or mediator molecule modulating diverse cellular signaling pathways. Neutral sphingomyelinase (NSMase) is a key enzyme in the regulated activation of the SM cycle and is particularly sensitive to oxidative stress. In a context of increasing clarification of the mechanisms of neurodegeneration, we thought that it would be useful to review details of recent findings that we and others have made concerning different pro-apoptotic neurotoxins including proinflammatory cytokines, hypoxia-induced SM hydrolysis and ceramide production that induce cell death in human primary neurons and primary oligodendrocytes: redox sensitive events. What has and is emerging is a vista of therapeutically important ceramide regulation affecting a variety of different neurodegenerative and neuroinflammatory disorders.
Keywords: Sphingomyelinases, Ceramide, Signal transduction, Neuronal apoptosis, Oligodendroglial death, Glial activation, Neurodegenerative disorders
Introduction
Neurodegenerative and neuroinflammatory diseases encompass devastating disorders of the central nervous system (CNS) that often strike from age 30 onwards limiting and disabling those in the prime of life. Clinically these diseases are characterized by dementia, disordered movement and gait, ataxia, sleep disorder, and behavioral and psychiatric disturbance [1–3]. Pathologically they are characterized by inflammation, gliosis, axonal degeneration and neuronal apoptosis [1–3] and for multiple sclerosis (MS) demyelination. Environmental factors, viruses, genetic mutation, and activation of non-neuronal cells are predisposing influences [1–3]. In some neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and frontotemporal dementia (FTD), aggregated misfolded proteins contribute to the neural pathogenesis, while in MS, autoimmune mechanisms accompany the demyelination and in HIV-associated dementia (HAD), viral products are crucial for neuronal demise [1–4]. In the context of numerous extrinsic and intrinsic factors affecting neurodegeneration and differing among diseases, a shared biochemical cascade of events playing a role in neuronal damage and attrition assumes great importance for understanding of pathogenesis and potential for intervention. Central to this cascade is increased oxidative stress and activation of an evolutionary conserved signaling pathway: the SM-ceramide pathway [5–8]. Here, we review causes of neuronal and oligodendroglial death during neurodegenerative and related/overlapping neuroinflammatory diseases pertaining to ceramide signaling pathways and highlighting neutral sphingomyelinase (N-SMase) as the major player and a potential target to curtail neurodegeneration.
2. Background
2.1. Historical perspectives
Sphingomyelin (SM), a major class of membrane phospholipids, was first isolated from the brain by Thudichum in 1884 though little was known of its metabolism until recently. SM was originally believed to be synthesized enzymatically by transfer of phosphocholine from cytidine 5′-diphosphate choline (CDP-choline) to the free primary hydroxyl group of ceramide [9]. This pathway has not been subsequently established as the major one, but rather the majority of SM is now known to be synthesized by transfer of phosphocholine from phosphatidylcholine to ceramide via sphingomyelin synthase [10]; an enzyme localized in Golgi and plasma membrane. SMs are amphiphiles abundant in mammalian nervous system that were believed to be inert membrane structural components, but were recognized by Hannun and Bell [11] to be involved in diverse intracellular signaling pathways in a variety of and perhaps all cells [12–14]. The SM pathway is triggered by a diverse range of endogenous and exogenous stimulants including tumor necrosis factor-α(TNF-α), interleukin-1β (IL-1β), FAS ligands (FAS L), anticancer drugs, glucocorticoids, antibody cross-linking, heat shock, ionizing and ultraviolet radiation, serum deprivation, and infection by viruses and bacteria [12,13,15]. A transient hydrolysis of sphingomyelin, by specific phospholipase C enzymes, known as sphingomyelinases, with concomitant generation of ceramide has been observed in response to these apoptosis-inducing stimuli. The ceramide formed serves as a second messenger or modulator of diverse signaling pathways in the cell affecting cell behaviors ranging from proliferation and differentiation to cell cycle arrest and apoptosis [16]. The decision to enter these disparate pathways determining diverse neurobiological outcomes depends on levels and molecular species of ceramide formed, cell types and age, expression of specific receptors, and selective targets coupled to specific signaling pathways.
2.2. Role of ceramide in CNS physiology
2.2.1. Lipid rafts (specialized membrane microdomains)
In the outer plasma membrane leaflet of many cell types, including neurons and glia, microdomains called lipid rafts are found that are implicated in a variety of cellular processes including cytosketal organization, membrane trafficking and signal transduction [17–19]. Ceramide associates with cholesterol and other sphingolipids and mediates lipid raft organization into larger platforms [20,21]. The distribution of lipid rafts over cell membrane depends upon the cell type. In neurons, lipid rafts accumulate on somal and axonal membranes to a greater extent than on somato-dendritic membranes. Lipid rafts are also found in the post-synaptic sites of neurons [22]. In neurons, rafts play an important role in neuronal adhesion, and in the modulation of neuronal ion channels and neurotransmitter receptors. Rafts may modulate neurotransmitter release since the it has essential components of the vesicular exocytic machinery such as syntaxin, synaptosomal-associated protein-25 (SNAP-25) and synaptobrevin [23]. In oligodendrocytes, rafts mediate the interaction between myelin-associated glycoprotein on myelin, and its receptor on neurons [24].
Raft lipid and protein composition differs in cell types. For example, oligodendrocyte raft composition differs from the neuronal raft in containing galactocerebroside (GalC), sulphatide and proteolipid protein. Raft composition also depends on neuronal developmental stage. Aging neurons contain more raft ceramide in association with members of the Src family protein tyrosine kinases, known to be involved in neuronal differentiation [25]. Aberrant organization of sphingomyelin or cholesterol in rafts has been linked to loss of synapse or changes in nerve conduction [26], and depletion of sphingolipid or cholesterol leads to instability of AMPA receptors and to the gradual loss of synapses (both inhibitory and excitatory) and dendritic spines [26].
2.2.2. Differentiation
Galactolipids such as galactocerebroside (GalC) and sulphatide play functional roles in the regulation of oligodendrocyte (OL) differentiation and myelination [27]. OL differentiation is a critical event during brain development culminating in elaboration of the myelin sheath, and impaired myelination leads to serious neurological disorders; mutation or myelin gene deletion has been extensively used to examine this [28,29]. Another approach has been to reversibly inhibit OL progenitor cell differentiation by a specific monoclonal anti-glycolipid antibody that perturbs myelination: following antibody removal, oligodendrocytes re-differentiate [30].
2.2.3. Neuronal development
Ceramide plays an indispensable role in neuronal development and this action is dependent upon neuronal developmental stage, stimulus type, and ceramide species and concentration. In the first stage of neuronal development, ceramide enhances the formation of minor processes from lamellipodia [31]. Decreasing ceramide content by ISP-1, a specific inhibitor of serine palmitoyltransferase, decreased cell survival and provoked aberrant Purkinje cell dendritic differentiation. Addition of cell-permeable ceramide, sphingosine, or sphingomyelin reversed the effects of ISP-1 treatment in Purkinje cells [32]. And, during axonal development, glycosylated ceramide, glucosylceramide, is essential for normal and for accelerated growth to support intracellular transport [31], while inhibition of ceramide synthesis with fumonisin B1 (FB1), or of glucosylceramide synthesis with 2-decanoylamino-3-morpholino-1-propanol (PDMP) produces a shorter axon plexus and fewer axonal branches of hippocampus neurons in culture. These inhibitors have no effect on axonal morphology during the first 2 days in culture, but cause branch retraction by day 3 [33].
The molecular mechanisms by which ceramide accelerates changes in the cytoskeleton and hence process formation is not known. It may be that ceramide alters cytoskeletal organization by stimulating actin stress fiber formation and focal adhesion assembly as a result of tyrosine phosphorylation of p125 focal adhesion kinase (p125FAK) and paxillin [34]. At both of these stages, ceramide at high concentration induces apoptotic cell death [31] while low ceramide concentrations obtained by pharmacological inhibition of sphingomyelinase or employing the acidic sphingomyelinase knockout were protective. Whether ceramide induces axonal development or apoptosis also depends upon the expression status of neurotrophin receptors. Low concentrations of ceramide generated by p75 neurotrophin receptor (p75NTR) activation in cultured hippocampal pyramidal neurons stimulated their growth [35].
2.3. Role of ceramide in CNS pathophysiology
2.3.1. Apoptosis
Apoptosis is physiologically essential for the elimination of damaged or excess cells during development [4], while pathological triggering of unregulated apoptosis leads to the development of several adult neurological disorders including AD, PD, amyotrophic lateral sclerosis (ALS), cerebral ischemia, and probably demyelinating diseases including MS: all of which are characterized by the loss of specific populations of neurons [6] and for MS of oligodendrocytes. Several studies have implicated ceramide in the apoptosis of neuronal and oligodendroglial cells in the neuroinflammatory and neurodegenerative disorders as shown below.
a) Alzheimer’s disease4 (AD)
AD is a major illness of dementia characterized histologically by the presence of amyloid plaques, neurofibrillary tangles, and extensive neuronal apoptosis. Accumulating evidence indicates elevated levels of ceramide at the very earliest clinical stage of the disease, and levels are elevated more than three-fold when compared with age-matched control [36]. Subsequent studies showed that fibrillar Aβ injection in mouse increased ceramide levels in hippocampus and cortex after 7 days following injection [37] and that elevated ceramide levels increased the half-life of BACE1 and promoted Aβ biogenesis. Exogenous C6 ceramide as well as increased levels of endogenous ceramide induced by sphingomyelinase treatment promoted biogenesis of Aβ. C6 ceramide also restored Aβ generation in FB1 treated cells [38].
b) HIV-associated dementia (HAD)
Human immunodeficiency virus type 1 (HIV-1) infection is known to cause CNS disorders, including HAD. Sphingolipid imbalance plays an important role in neuronal dysfunction and death in HAD. Brain tissues and CSF from patients with HAD evidenced increased oxidative stress with abnormal accumulation of sphingomyelin and ceramide [39]. In a separate study, we have shown that HIV-1 coat protein gp120 (glycoprotein 120) induced neuronal apoptosis in the HAD CNS through the CXCR4-NADPH oxidase-superoxide-NSMase-ceramide pathway.
c) Multiple sclerosis (MS)
It is the most common human CNS demyelinating disease, and a disorder in which oxidative stress is proposed to play an important role in oligodendroglial death though molecular mechanisms that couple oxidative stress to the oligodendrocyte losses are poorly understood. Our MS studies have shown that the neutral sphingomyelinase–ceramide pathway is involved in mediating oxidative stress-induced apoptosis and cell death in human primary oligodendrocytes [40]. In a separate study, Lee et al. found that exogenously added bacterial sphingomyelinase exacerbated Aβ-induced oligodendrocyte death via an oxidative mechanism [8].
d) Amyotrophic lateral sclerosis (ALS) and Stroke
ALS is a progressive neurodegenerative disease characterized by degeneration of motor neurons in the spinal cord and producing progressive paralysis and death. Abnormal buildup of sphingomyelin, ceramide and cholesterol esters has been observed in ALS, and in the mouse model of ALS (Cu/ZnSOD mutant mice) [7], and this abnormal lipid accumulations occurs in transgenic mice prior to any sign of cell death. Pharmacological blockage of sphingolipid synthesis and ceramide accumulation could suppress neuronal death via various inducers of cell death including oxidative stress [7]. In another study, motor neurons over-expressing the ALS-linked SOD1G93A mutation showed greater susceptibility to the p75NTR-activated apoptotic pathway that is associated with decreased antioxidant defenses and increased neutral sphingomyelinase activation. This apoptotic pathway is critically modulated by nuclear factor erythroid 2-related factor 2 (Nrf2) activity [41]. In cerebral ischemia in vivo, increased ceramide levels have been attributed to down-regulation of glucosylceramide synthase [42–44]. It has also been reported that transient focal cerebral ischemia induces large increases in A-SMase activity, ceramide levels, and production of inflammatory cytokines in wild-type mice, but not in mice lacking A-SMase [42]. The production of inflammatory cytokines is largely abolished in wild type mice by administration of A-SMase inhibitor.
e) Batten disease
Batten disease is a rare and fatal inherited disorder of the CNS that begins in childhood. This neurodegenerative disease is characterized by blindness, seizures, cognitive decline, and early death. Puranam et al [45] have reported an increased brain ceramide level in different Batten disease types.
2.3.2. Aging
Aging is accompanied by a progressive increase of brain ceramide content [5]. During aging, alterations in membrane sphingomyelin-cholesterol levels play a critical role in the susceptibility of neuronal cells to oxidative stress including upregulation of N-SMase activity with a significant brain-region-specific alteration in N-SMase activity [46]. Consequently, membrane fractions isolated from the striatum and hippocampus of aging rats were significantly enriched in N-SMase [46]. Additionally, aging induces a switch in the expression of neurotrophin receptors with differential regulation of the processing of APP in AD. The Trk receptor kinase A (TrkA) reduces whereas p75NTR stimulates β-cleavage of APP; an effect that is suppressed in p75NTR knockout mice. In normal mice this signaling pathway downstream of p75NTR, and ceramide generation can be blocked by both caloric restriction and N-SMase inhibitors [47]. Derangement of sphingomyelin metabolism in combination with oxidative stress causes synaptic dysfunction and neurodegeneration [5]. Increased levels of ceramide during aging may also promote inflammation (Cutler et al., 2004). Furthermore, a direct correlation is seen between intracellular accumulation of ceramide and endocytic abnormalities suggesting that the endosomal/lysosomal abnormalities observed in degenerating neurons during pathological aging, and in age-related neurological disorders may be due to aberrant sphingomyelin metabolism [48]. A chronic increase of intracellular ceramide during aging might inhibit axonal elongation and reduce receptor-mediated internalization of nerve growth factor (NGF) [49]. Elevated ceramide levels also reduce receptor-mediated internalization of lipoprotein-associated cholesterol that is involved in regulation of synaptogenesis [49,50]: an effect that may account for the age-dependent reduction in the plasticity of the synaptic circuitry.
2.4. Transduction of apoptotic signaling by ceramide
2.4.1. Breakdown of mitochondria
A growing body of evidence implicates changes at the mitochondria and release of apoptotic inducing factors early in apoptosis. Mitochondria in cells undergoing apoptosis show enhanced production of oxy-radicals, opening of pores and cytochrome c release. These events are critical to the cell death process because agents such as manganese superoxide dismutase (MnSOD) and cyclosporine A that suppress mitochondrial oxidative stress state and pore formation prevent neuronal death [51]. Mitochondria contain ceramide, derived either from SM during apoptosis or from endoplasmic reticulum (ER) via intimate membrane contact. The numerous indications of links between ceramide elevation and mitochondrial apoptosis include the following. Firstly, in isolated mitochondria, the ceramide level is increased leading to apoptosis in response to CD95/Fas, TNFα, and radiation [52] and, C2-ceramide induces cytochrome c release when added to isolated mitochondria [53] or cultured neural cells [54]. Secondly, bacterial sphingomyelinase that is targeted to mitochondria induces cytochrome c release and apoptosis. Exposure of CGC to cell-permeable C2-ceramide markedly alters mitochondrial integrity with marked decrease in mitochondrial membrane potential associated with a massive release of cytochrome c [55]. The released cytochrome c is then capable of binding to the apoptotic protease activating factor-1 (AFAP-1), and this complex activates the caspase cascade for which there is substantial evidence for implication in ceramide-induced apoptosis [54]. In cortical neurons, ceramide treatment leads to Akt dephosphorylation, mitochondrial depolarization and permeabilization, cytochrome c release and activation of caspase-3 [54]. Bongkrekic acid, an inhibitor of mitochondrial depolarization, inhibits the hallmark manifestations of apoptosis including chromatin condensation and PARP cleavage, thereby significantly reducing ceramide-induced neuronal apoptosis. Furthermore, co-administration of the selective caspase-3 inhibitor z-DEVD-fmk or caspase-9 inhibitor z-LEHD-fmk significantly reduces C2-ceramide-induced death of cultured rat cortical neuronal cells [56]. In contrast, C2-ceramide does not induce release of cytochrome c and caspase cascade in glioma cells, but does activate the Ca2+-activated calpain proteases [57].
Ceramide directly affects the generation of reactive oxygen species (ROS) from mitochondria. Activation of the apoptogenic sphingomyelin-dependent signaling pathway in differentiated neuron-like pheochromocytoma (PC12) cells by cell permeable C2-ceramide produces a transient ROS generation, the site of which has been identified as complex1 of the mitochondrial electron transport chain [58]. Further studies of these rat PC12 cells have revealed that C2-ceramide-induced free radical production is accompanied by increased mitochondrial free calcium concentrations producing swelling and rupture (Gueyffier et al., 1998). The ceramide-induced cell death via increase in the mitochondrial free calcium could be prevented by buffering of mitochondrial calcium with the calcium binding protein calbindinD-28K ectopically expressed in mitochondria [59].
Permeabilization of mitochondrial membrane is controlled by pro-apoptotic members of the Bax/Bcl2 family. C2-ceramide increases the Bax level leading to formation of Bax homodimers, mitochondrial permeabilization and neuronal death [55]. Ceramide-derived glycosphingolipids (gangliosides) induce apoptosis in neurons treated with staurosporine [60], and gangliosides either alone or in concert with pro-apoptotic Bcl2 family members such as t-Bid and Bax affect mitochondrial permeability transition pores releasing cytochrome c. Suppression of GD3 synthase expression by antisense oligodeoxynucleotides inhibits this neuronal apoptosis.
2.4.2. Activation of death-associated kinase (DAP kinase)
Death-associated protein kinase (DAP-kinase), a novel Ca2+/calmodulin-dependent serine/threonine kinase, acts as a positive mediator of apoptosis for several intra- and extracellular stress signals [61]. The apoptotic function of DAP-kinase is thought to be modulated through its several functional domains, including the death domain and a serine-rich C terminus tail site of auto-phosphorylation [62]. By Northern blot and in situ hybridization analysis, DAP- kinase is found to be abundantly expressed in the brain [63]. In developing rat brain, DAP-kinase mRNA is abundant within the proliferative and post-mitotic regions of the cerebral cortex, in the developing hippocampus, and in cerebellar Purkinje cells [63]. The spatial distribution of DAP-kinase expression suggests that it regulates various neuronal functions, including neuron cell death. One example of the possible relationship between DAP-kinase and neuronal cell death via apoptosis and necrosis is indicated by the gradual increase in expression of DAP-kinase mRNA in cerebral cortex at 1–24hr after transient forebrain ischemia. A relationship between ceramide and DAP-kinase activation has been reported in several studies. In PC12 cells, the death-promoting function of DAP-kinase was enhanced in response to ceramide exposure with activity proportional to ceramide concentration [64]. Phosphorylation of serine 308, residing within the calmodulin (CaM) regulatory segment, of DAP-kinase restrained pro-apoptotic functions thereby serving to block this in growing cells [62,64]. Triggering death signals by ceramide removes the restrictions imposed by autophosphorylation of serine 308 suggesting that it becomes a target of regulation when the proapoptotic function of DAPK is active, and ceramide-activated phosphatase may be involved in the dephosphorylation of serine 308. Furthermore, over-expression of wild-type DAP-kinase enhanced the cells’ sensitivity to ceramide treatment [64]. In contrast, a catalytically inactive DAP-kinase mutant rescued the cells from ceramide-induced apoptosis [64]. A study of hippocampus neurons derived from DAP-kinase deficient mice found them significantly less sensitive to ceramide-induced apoptosis and to high concentrations of NGF-generated ceramide [65], while a peptide corresponding to the last 17 amino acids at the C-terminus of DAP-kinase protected the wild type neurons from ceramide-induced death as well as from death induced by addition of exogenous bacterial neutral sphingomyelinase [65]. All the above findings suggest that DAP-kinase is a target of ceramide-induced cell death (Fig. 1). This pathway may not be the only one involved in neuronal death since it has been found that there is not complete resistance to ceramide-induced cell death in DAP kinase −/− neurons [65].
Fig. 1. Schematic representation of ceramide-induced various apoptotic pathways.
Sphingomyelinases cleave sphingomyelin (represented as small circles around the membrane) to ceramide in response to different extracellular insults. Once ceramide is generated, different downstream targets including death-associated protein kinase (DAPK), kinase suppressor of Ras (KSR), protein kinase C (PKC) ζ, Rac, inducible nitric oxide synthase (iNOS), ceramide-activated protein phosphatase (CAPP), and c-Jun N-terminal kinase (JNK) are activated and the release of cytochrome C is augmented. On the other hand, the anti-apoptotic Akt - Bcl2 pathway is down-regulated. All these events eventually lead to apoptosis.
2.4.3. Activation of ceramide-activated protein phosphatase (CAPP)
One proximal target for ceramide is the heterotrimeric cytosolic serine/threonine (class2A) phosphoprotein phosphatase CAPP (Fig. 1) that was found to be activated in vitro [66,67]. This effect is also operative in vivo [67]. Of the three subunits of protein phosphatase, the 2A (PP2A) catalytic subunit was found to contain the putative lipid binding site, and was found to be essential for its activation [68]. The catalytic subunits of PP2A belong to a gene family that includes the catalytic subunits of phosphatase1 (PP-1) and calcineurin (PP-2B). The regulatory subunits modulate the responses of ceramide in vivo either directly by controlling the specificity of the enzyme for different forms of ceramide [66] or controlling the substrate specificity of the enzyme; that is, which substrates are dephosphorylated in response to an increased ceramide level.
The Akt pathway for example is down-regulated in NGF-treated PC12 cells by the cell permeable C2-ceramide as a result of enhanced dephosphorylation of Akt1 at residues T308 and S473 [69]. Interestingly, okadaic acid pretreatment rendered NGF-treated cells insensitive to C2-ceramide mediated down-regulation of Akt1 activity, suggesting that this inhibition was a consequence of the activation of dephosphorylation by CAPP. Inactivation of Akt in cortical neurons is followed by dephosphorylation of proapoptotic regulators such as BAD (proapoptotic Bcl-2 family member), forkhead family transcription factors (FKHR), and glycogen synthase kinase 3-beta (GSK-3β) [54]. On the other hand, N-myristylated-Akt1 conferred resistance to apoptosis induced by C2-ceramide in NGF-treated PC12 cells. This differential activity of CAPP towards Akt stems from the CAPP cytosolic location [69]. The downstream target of protein kinase B (PKB/Akt), i.e. Bcl-2, is affected by ceramide-induced dephosphorylation by loss of anti-apoptotic potency, and this was further corroborated by the use of the S70E Bcl-2 mutant, in which a serine 70 was mutated to glutamate that mimicked a phosphorylation charge. The mutant efficiently suppresses ceramide-induced apoptosis [70]. And, the in vitro treatment of Akt with partially purified PP2A phosphatase reduced its intrinsic kinase activity. Prolonged exposure of cultured hippocampal neurons to sphingosine-1-phosphate (S1P) triggered apoptosis through PP2A activation [71]. S1P, a metabolite of ceramide, may either act as is or be converted to ceramide via the combined actions of S1P-phosphatase and ceramide synthase and execute the apoptotic program. S1P-induced apoptosis releases calcium from inositol triphosphate (IP3)-sensitive internal stores and this (alterations in Ca2+ homoeostasis) activates calcineurin [71]. Calcineurin in turn dephosphorylates several cellular substrates, including a repressor for PP2A with resultant PP2A activation. One of the targets for PP2A is the c-Fos-containing AP-1 complex. Dephosphorylation of these c-Fos-containing AP-1 complexes increases it’s binding to, and increased the expression of, death genes containing the TGAGTCA-type enhancer sequences, whose products execute the apoptotic process. Neutralizing antibodies specific for either c-Fos or c-Jun, as well as over-expression of a dominant negative c-Jun mutant, have been reported to be protective against apoptosis whereas enhanced expression of c-Fos leads to apoptosis [72–74]. Another target of CAPP is the retinoblastoma gene (RB) product. Protein phosphatase1 is involved in the ceramide dependent dephosphorylation of the transcriptional regulator RB [75,76], and dephosphorylation of RB and subsequent sequestration by E2F results in cell cycle arrest [76,77].
2.4.4. Activation of stress kinases
Ceramide targets c-Jun N-terminal kinases (JNK) also known as stress-activated protein kinase (SAPK) [78], and this pathway mediates cell death in neurodegenerative diseases [79–82]. There are three JNK genes encoding distinct JNK protein kinases (Davis RJ, 2000). Of these, JNK3 is largely restricted to neurons and JNK1 and JNK2 are ubiquitously expressed [83]. Targeted disruption of the neural-specific jnk3 gene renders neurons resistant to glutamate excitotoxicity [84].
A correlation of increased ceramide level and JNK activation is reported in serum-deprived neuronally differentiated PC-12 cells [85], and JNK activation and nuclear translocation in neurons have been reported in vivo in several different pathological conditions [86]. One potential target of pro-apoptotic signaling by JNK is the transcription factor c-Jun, which is modulated at both transcriptional and post-transcriptional level [87]. An increase in c-Jun phosphorylation was found in neuronal nuclei after ceramide treatment [88], and transient transfections with a dominant negative form of c-Jun partially protected cortical neurons from ceramide-induced apoptosis [88]. Oxidative stress-induced neurodegeneration appears to occur via the JNK pathway and steps of c-jun phosphorylation, mitochondrial cytochrome c release, BAX induction, caspase activation and finally neuronal apoptosis [89]. Protection in the dominant negative form of c-Jun is partial suggesting that other pathways and notably p38 MAPK (mitogen-activated protein kinase) cooperate with JNK in inducing neuronal apoptosis. Support for this includes the finding that treatment of dominant-negative c-Jun-expressing cortical neurons with the pharmacological inhibitor of p38 kinase, SB203580 completely blocks ceramide-induced neuronal death [88]. Furthermore, ceramide generated by binding of NGF to p75NTR induced cell death in hippocampal neurons through JNK activation [90]. In addition, in primary cortical neurons, ceramide inactivated the extracellular signal-regulated kinase (ERK) pathway probably through activation of the ceramide-activated protein kinase (CAPK), the protein kinase cζ (PKCζ) or the CAPP and simultaneously triggered JNK and p38 MAPK cascades inducing neuronal death through activation of caspase3 and enhanced expression of c-jun, c-fos, and p53 [91]. Ceramide-mediated activation of p38 and oligodendrocyte apoptosis was also seen in oligodendrocytes treated with C2-ceramide, and this apoptosis was attenuated by a p38 inhibitor, SB203580, and by expression of a p38α dominant negative mutant [92]. Figure 1 illustrates the signaling mechanisms involved in direct killing of neurons once ceramide is generated in response to different neurotoxic agents and in different neurodegenerative disorders.
2.4.5. Production of neurotoxic molecules from glia
Several neurodegenerative diseases, notably AD, HAD, and PD are characterized by neuronal degeneration and simultaneous activation of glial cells with production of toxic factors that aggravate the neuronal damage. A role for neurotoxic molecules in the pathogenesis of neurodegenerative diseases has been supported by several reports demonstrating elevation of these compounds at the lesion site. Subsequent assay by polymerase chain reaction (PCR) and in situ hybridization revealed increased mRNA [93] expression of IL-1β, TNF-α, LT-α, and iNOS respectively in lesions [93]. It should be noted that some studies have failed to show an augmentation of cytokines and this is reported in MS [94–98]. Discrepancies in reports of these findings may be due to sample handling or temporal variability in collection, methodological variability, short cytokine half-lives, or the like. Furthermore, neurotoxic factors might behave differently in proliferating glia. For instance, in astrocytes of AIDS encephalopathy, the HIV-nef gene product triggered TNF-α-induced ceramide generation and NF-κB activation. On the other hand, blocking AP-1 activation shifted the molecular profile of the cell toward survival and an astrogliosis characteristic of neurodegenerative disorders [99] and consistent with the capability of IL-6 produced from activated astrocytes to induce astrocyte proliferation and activation [100]. Such neurotoxic molecules as inflammatory cytokines, chemokines, excitotoxic molecules, and ROS, elaborated from activated glia increase in the brain and CSF of neurodegenerative disorders, and several of these couple the sphingomyelin-ceramide pathway with its prominent connection to apoptosis (and to other cellular processes as well). Early on, TNF-α released from astrocytes and macrophages was recognized to activate myelin associated sphingomyelinases to generate ceramide and other downstream products with consequent deleterious effects upon membrane integrity [101,102]. This is fully consistent with our own co-culture studies of genetic knockdown of NSMase in human primary neurons using an antisense strategy; finding that NSMase-impotent neurons resist activated glia-mediated cytotoxicity (unpublished data). A potential bidirectional relationship between cytokine balance and ceramide production has also been well documented. For instance, TNF-α, IL-1 and Fas/FasL that are increased in tissue from neurodegenerative disorders potently induce ceramide production, and ceramide in turn stimulates production of IL-2 and IL-6 [103,104] themselves potent inducers of ceramide and mediating neuronal apoptosis or potentiating other neurotoxins [105,106]. For HAD, this hypothesis is supported by the synergistic neurotoxicity between gp120, Tat, and TNF-α at the ceramide level as shown by negation of the toxic effects by inhibition of sphingomyelinase and/or de novo ceramide production [39]. Moreover, the findings that conditioned media from HIV-1 infected monocytes induce neuronal apoptosis by TNF-α and platelet activating factor-mediated induction of ceramide [107] fit this mechanism.
A parallel excitotoxic pathway may well be activated. TNF-α produced by HIV-1-infected macrophages and microglia during HIVD inhibited glutamate uptake by astrocytes leading to an increase in the concentration of extracellular glutamate [108] causing over-activation of glutamate receptors on neurons and neuronal apoptosis by a mechanism involving calcium influx though ionic channels linked to glutamate receptors. Such excito-toxicity may occur in neurodegenerative conditions including stroke, trauma, epileptic seizures, Alzheimer disease and ALS/motor system disorders [109–111]. Perturbations of calcium homeostasis in neurons might induce neuronal death through activation of the redox-sensitive cytosolic calcium dependent PLA2-AA-SMase pathway [112,113]. In addition to cytokines, release of several growth factors including NGF [114] is also associated with gliosis. Although, NGF is critical for differentiation and survival of neurons and for neural plasticity [115,116], it may also promote apoptosis via ceramide depending on the expression levels of neurotrophin receptor p75NTR. Increased NGF levels have been reported in pathological conditions characterized by prominent astrogliosis [117,118]. Further, gliosis is marked by the release of pro-inflammatory mediators such as prostanoids from activated microglia, which is influenced by sphingomyelinase activation and ceramide generation (Akundi et al., 2005). Lipopolysaccharide (LPS)-induced activation of SMase and ceramide formation is considered essential for cyclooxygenase-2 (COX2) expression and prostaglandin E2 (PGE2) synthesis via activation of the p38 MAPK-dependent pathway, and this suggests that ceramide formation in activated microglia could contribute to the amplification of neuroinflammatory events [119].
2.5. Mechanisms of ceramide production in neurodegeneration
2.5.1. Activation of sphingomyelinases
The catabolic pathway for ceramide formation is located either in lysosomes or at the plasma membrane, and is initiated by the action of SMases, sphingomyelin-specific forms of phospholipase C, which hydrolyze the phosphodiester bond of sphingomyelin yielding ceramide and phosphocholine. There are several isoforms of SMases, distinguished by different pH optima, subcellular localization, and cation dependence. A Mg2+-dependent NSMase is found at the plasma membrane [120], and an ASMase is localized in the endosomal-lysosomal compartments [121]. The Mg2+-dependent NSMase is very unstable compared to ASMase [122]. The Mg2+-independent NSMase localized in the cytosol is found exclusively in the myelin sheath [123], while a bile-salt-dependent alkaline SMase is localized in the gastrointestinal tract [124]. NSMase and ASMase are rapidly and transiently activated by diverse exogenous as well as endogenous stimuli. Neutral and acidic SMases appear responsible for stimulus-induced increases of ceramide within a time frame of seconds and minutes compared to de novo ceramide synthesis that accounts for late and sustained ceramide generation [125]. Therefore, these SMase forms are considered to be major pathways for ceramide generation in early signal transduction. In neurons, both SMase types generate ceramide from SM hydrolysis. Analysis of endogenous sphingomyelinase activities reveal that the NSMase is localized in axons while ASMase is concentrated in the neuron cell body [49].
a) NSMase: the predominant form of sphingomyelinase in the brain
NSMase is the major sphingomyelinase found in the brain [126]. Biochemical characterization of this NSMase revealed that it is sensitive to glutathione [127]. Multiple forms of the membrane-bound NSMases have been identified in bovine brain [128]. This SMase is responsible for ceramide generation in the CNS during normal development and particularly in response to neurotrophins [35]. Neurotrophin such as NGF bind to the p75NTR to stimulate growth and development of cultured hippocampal neurons via the ceramide generation by NSMase [35]. The stimulatory effects of NGF on neurite formation and outgrowth are blocked by scyphostatin, a specific NSMase inhibitor. C2-ceramide and exogenously-added SMase release dopamine via this pathway in dopaminergic neurons of the mesencephalon [129]; a possible explanation for which might be ceramide phosphorylation by a calcium-dependent protein kinase located in synaptic vesicles [130] yielding ceramide 1-phosphate. Ceramide phosphorylation could then foster neurotransmitter release through phosphorylation of such synaptic vesicle proteins as synapsin I [131,132].
Different cellular effects may reflect cellular concentrations. For example, the response of hippocampal neurons to NGF changes in mature neurons that switch from acceleration of axonal outgrowth to apoptotic cell death as levels of p75NTR expression and ceramide generation increase [90]. The NSMase inhibitor, scyphostatin, inhibits NGF-induced ceramide generation and neuronal death, while neurons derived from wild type or ASMase deficient mice are equally susceptible to NGF-induced cell death. Thus, in these neuronal cultures, NSMase rather ASMase is largely responsible for ceramide generation in response to trophic factors. In contrast, an excitotoxic cell death pathway in hippocampal neurons is via ASMase activation suggesting that different pathways generate different ceramide pools in the same neurons in response to different stimuli [42]. The potential players in the regulation of NSMase activation include FAN (factor associated with NSMase activation), arachidonic acid (AA), soluble phospholipase-A2 (PLA2), and intracellular oxidation that is sensitive to glutathione; the prominent negative regulator of NSMase [133–136]. No disease is associated with NSMase deficiency, and therefore signaling pathways associated with NSMase are less well characterized than those involving ASMase.
b) Activation of NSMase by virotoxins
We first reported that NSMase activation plays a major role in neuronal apoptosis and cell death in HAD [137]. Viral proteins such as gp120, induce apoptosis of human primary neurons by coupling with CXCR4 (CXC chemokine receptor 4) receptor in neurons to induce NADPH oxidase-mediated production of superoxide radical in neurons (Fig. 2), NSMase activation and production of ceramide without affect upon ASMase activity [137]. The tight coupling of NADPH oxidase-mediated superoxide production to NSMase activation in gp120-treated neurons was corroborated by antisense knock-down of p22phox, a NADPH oxidase subunit, and by use of the antioxidants diphenyliodonium(DPI) or N-acetylcysteine (NAC). Superoxide radicals exogenously produced in human primary neurons by hypoxanthine and xanthine oxidase also markedly activated NSMase, but not ASMase, in response to HIV-1 gp120 [137]. Similarly, the viral protein Tat induced neuronal apoptosis and cell death via NSMase activation.
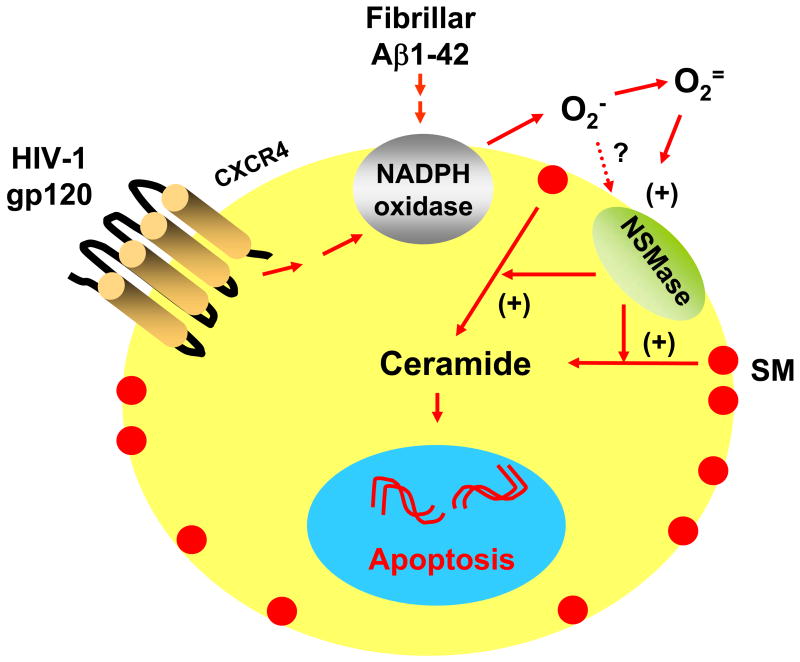
Fig. 2. Model showing the possible involvement of NSMase-ceramide pathway in neuronal apoptosis and cell death under different neurodegenerative conditions.
Fibrillar Aβ1–42 peptides induce neuronal apoptosis through the NADPH oxidase-superoxide-hydrogen peroxide-NSMase-ceramide pathway. On the other hand, HIV-1 gp120 requires CXCR4 to activate the same apoptotic pathway in neurons.
c) Activation of NSMase by fibrillar Aβ
In a separate study [138], we found that fibrillar Aβ1–42 peptides induce neuronal apoptosis through the NADPH oxidase-superoxide-hydrogen peroxide-NSMase-ceramide pathway (Fig. 2). Also superoxide radicals exogenously generated by hypoxanthine and xanthine oxidase induced the activation of NSMase, but not ASMase, activity, through a catalase-sensitive pathway. Moreover, antisense knockdown of p22phox, a subunit of NADPH oxidase, rescued Aβ1–42-induced neuronal apoptosis and cell death [138]. Lee et al have found that addition of bacterial sphingomyelinase (mimicking cellular NSMase activity) exacerbated Aβ-induced oligodendrocyte death, while NSMase inhibition by 3-O-methyl-sphingomyelin or by gene knockdown using antisense oligonucleotides attenuated Aβ-induced OLG death. Glutathione (GSH) precursors inhibited Aβ-induced activation of NSMase and prevented oligodendrocyte death, whereas GSH depletors augmented NSMase activity and Aβ-induced death. These suggest that Aβ induces oligodendrocyte death by activating the NSMase-ceramide cascade via an oxidative mechanism [8].
d) Activation of NSMase in oligodendrocytes
Cytokines
As in many other cell types, the proinflammatory cytokines TNFα and IL-1β induced the degradation of SM to ceramide via NSMase in rat primary oligodendrocytes [139]. Interestingly, pretreatment of rat primary oligodendrocytes with N-acetylcysteine, an antioxidant and efficient thiol source for glutathione, prevented cytokine-induced decrease in GSH and degradation of sphingomyelin to ceramide [139].
Reactive oxygen species (ROS)
In addition to proinflammatory cytokines, ROS are directly involved in degradation of SM to ceramide via NSMase in human primary oligodendrocytes. Our findings of NSMase activation and ceramide production in human primary oligodendrocytes showed that various ROS producing molecules or ROS itself, such as, superoxide radical produced by hypoxanthine and xanthine oxidase, hydrogen peroxide, aminotriazole that is capable of inhibiting catalase and increasing the intracellular level of H2O2, or reduced glutathione-depleting diamide all induced this NSMase activation and ceramide production [40]. Interestingly we found that antisense knockdown of neutral, but not acidic, sphingomyelinase ablated oxidative stress-induced apoptosis and cell death in human primary oligodendrocytes [40]. How NSMase is activated by ROS remains an open question. We speculate that there is either post-translational modification of NSMase by ROS thereby influencing the subcellular localization of NSMase, activation of one of the several protein kinases, inhibition of tyrosine phosphatase or receptor-mediated activation of NSMase. Alternatively, ROS might trigger the generation of second messengers that in turn activate NSMase.
2.5.2. Inhibition of ceramidase
The intracellular level of ceramide can be modulated by regulation of ceramide degradation; i.e., regulation of the hydrolysis of ceramide catalyzed by ceramidases. Three types of ceramidases are distinguished on the basis of pH optima, substrate specificity and subcellular localization [140–143]. Deficiency of lysosomal acid ceramidase leads to a rare form of inborn lipid storage disorder called Farber’s disease. The severity of the disease and its rate of progression correlate with the ceramide accumulation, and importantly, ceramidase inhibition by N-oleylethylamine prevents neuronal dysfunction and apoptosis [93].
3. Neurons and oligodendrocytes are more vulnerable than astrocytes and microglia to N-SMase activation!
Both neurons and glia show increased NSMase activation during neurodegenerative diseases but only neurons and oligodendrocytes succumb to apoptosis and cell death. The reasons for this differential fate are summarized below.
3.1. Low levels of antioxidant enzymes
Cells prevent oxidative damage by utilizing several in situ antioxidant systems that negate oxidative stress by eliminating ROS. These include such antioxidant enzymes as superoxide dismutase (SOD), glutathione peroxidase, and catalase. The SOD system converts O2•− to H2O2 that in turn is detoxified by catalase and glutathione peroxidase [144,145]. Currently, there are three different known SOD isoforms. One is cytosolic utilizing Cu and Zn as its prosthetic groups and is designated as Cu/Zn-SOD. SOD3 is found in extracellular spaces, and SOD2 uses manganese at its active site, is strictly localized in the mitochondrial matrix, and is designated as MnSOD [146].
Neurons and oligodendrocyte are more prone to oxidative stress-induced damage via NSMase because their levels of catalase and glutathione peroxidase are low [145,147–150]. On the other hand, astrocytes contain higher GSH levels as a means to combat oxidative stress [151,152]. In addition to GSH, intracellular oxidative stress signals for MnSOD gene induction in astroglia [153]. Therefore, we hypothesize that neurons that have limited inflammatory processing capacity are incapable of inducing MnSOD in an oxidative stress milieu and succumb while astrocytes and microglia respond with proliferative and/or inflammatory activation [154].
3.2. Differences in NF-κB activation
As discussed above, astrocytes have higher constitutive levels of MnSOD than neurons and this level increases during gp120 treatment [155]. The disparity in MnSOD levels stems from the fact that astrocytes have a greater level of NF-κB p65 subunit than neurons thereby allowing them to generate more MnSOD in response to gp120 treatment. Because activation of NF-κB is necessary for transcription of the MnSOD gene, over-expression of p65 in neurons increases the MnSOD level and elicited neuronal resistance to gp120 [155] while p65 antisense, p65 siRNA, or a specific inhibitor, NBD peptide, leads to reduced MnSOD and enhanced vulnerability of astrocytes to gp120 treatment [155].
Pahan et al [156] have shown earlier that ceramide induces the expression of MnSOD in rat astrocytes, and this is consistent with ceramide’s capability to induce activation of NF-κB [157]; a step important for the expression of MnSOD [155]. Although this study did not test the neuronal expression of MnSOD, the lower level of NF-κB p65 in neurons than astrocytes may prevent ceramide induction of MnSOD expression in neurons to save these cells under neurodegenerative conditions. Consistent with this hypothesis, our recent studies demonstrate that gp120 is toxic to neurons, but not astrocytes [155], and that gp120 kills neurons via ceramide production [137].
3.3. Iron content in oligodendrocytes
Iron, an essential compound in brain tissue, is a vital cofactor in many biological pathways including myelin production in oligodendrocytes [158]. Inadequate iron levels impair myelination and lead to mental retardation [159]. Histochemical stains for iron indicate that oligodendrocytes are the predominant brain cell type staining for iron [160–162]. This differential staining arises from the fact that oligodendrocytes have a relatively high metabolic rate and an associated enzyme activity that is iron dependent [150,163]. In addition, ferritin, the major protein involved in iron storage, is abundant in oligodendrocytes [158,164]. This high iron content in oligodendrocytes generates highly toxic hydroxyl radicals by reacting with peroxides through the non-enzymatic Fenton reaction that affects many cellular macromolecules including DNA, proteins and lipids [165]. On the other hand, astrocytes contain little ferritin suggesting that they are en route trafficking of iron to other brain cells [166,167]. They also express ceruloplasmin that functions as a ferroxidase converting ferrous iron to ferric form thereby allowing iron transport out of the cell [166]. In addition, astrocytes express lower levels of transferrin receptors [166]. Fully differentiated microglia resemble astrocytes in rarely containing histologically detectable levels of iron, transferrin, ferritin or other iron-related protein [168]. From these studies one could speculate that the low levels of iron in astrocytes and microglia bias them to a cellular anti-oxidative machinery.
3.4. High level of unsaturated fatty acids
Myelin sheaths of oligodendrocytes contain polyunsaturated fatty acids (PUFA) that react with peroxides and hydroxyl radicals triggering thereby a cascade of oxidative damage and cell death probably via NSMase activation [40,145]. Furthermore, PUFA enhances iron uptake by modulating iron transporters, and accelerating apoptotic death [169,170]. Similar to oligodendrocytes, neurons have a high content of easily oxidizable polyunsaturated membrane lipids [171]. Docosahexaenoic acid (DHA), a major PUFA in the central nervous system, is used continuously for the biogenesis and maintenance of neuronal membrane and is a target for lipid peroxidation [171].
4. Conclusion and therapeutic perspectives
Neuronal and oligodendrocyte death is a common denominator of many human neurological disorders, and therefore, understanding mechanisms involved in rescuing and sustaining neurons and oligodendrocytes within and near their nervous system lesions is of high priority in developing effective therapies. This is especially important for biochemical ‘final cellular molecular pathways’. Over the past fifteen years, the key role and novel regulatory importance of sphingolipid metabolites has opened new vistas warranting the current vigorous and rigorous investigation of bioactive lyso-spingolipids for roles in neurodegenerative/inflammatory pathogenesis in diseases as diverse as AD, HAD, ALS, and MS. Our findings and those of other investigators indicate that activation of the neutral sphingomyelinase -ceramide pathway via oxidative stress mechanisms plays a cardinal role in apoptosis of neurons and oligodendrocytes. It follows then that pharmacological inhibitors of neutral sphingomyelinase and/or upstream NADPH oxidase represent a novel way to halt the neurodegeneration that is a prominent feature of several prevalent, different and often devastating neurological diseases.
Acknowledgments
This study was supported by grants from Alzheimer’s Association (IIRG-07-58684), NIH (NS39940 and NS48923) and Michael J. Fox Foundation for Parkinson’s Research to Dr. Pahan and grants from NIH (NS 51666) and the National Multiple Sclerosis Society (NMSS G3473) to Dr. Hogan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kolson DL, Gonzalez-Scarano F. Hiv and hiv dementia. J Clin Invest. 2000;106:11–13. doi: 10.1172/JCI10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–1980. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- 3.Morales A, Lee H, Goni FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis. 2007;12:923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 4.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 5.Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariga T, Jarvis WD, Yu RK. Role of sphingolipid-mediated cell death in neurodegenerative diseases. J Lipid Res. 1998;39:1–16. [PubMed] [Google Scholar]
- 7.Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 2002;52:448–457. doi: 10.1002/ana.10312. [DOI] [PubMed] [Google Scholar]
- 8.Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, Chen S, Hsu CY. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol. 2004;164:123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sribney M, Kennedy EP. The enzymatic synthesis of sphingomyelin. J Biol Chem. 1958;233:1315–1322. [PubMed] [Google Scholar]
- 10.Voelker DR, Kennedy EP. Cellular and enzymic synthesis of sphingomyelin. Biochemistry. 1982;21:2753–2759. doi: 10.1021/bi00540a027. [DOI] [PubMed] [Google Scholar]
- 11.Hannun YA, Bell RM. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989;243:500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- 12.Kolesnick R, Golde DW. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 13.Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- 14.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Kolesnick R. Signaling through the sphingomyelin pathway. Endocrinology. 1995;136:4157–4160. doi: 10.1210/endo.136.10.7664631. [DOI] [PubMed] [Google Scholar]
- 16.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 17.Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM., Jr Lipid rafts in neuronal signaling and function. Trends Neurosci. 2002;25:412–417. doi: 10.1016/s0166-2236(02)02215-4. [DOI] [PubMed] [Google Scholar]
- 18.Paratcha G, Ibanez CF. Lipid rafts and the control of neurotrophic factor signaling in the nervous system: Variations on a theme. Curr Opin Neurobiol. 2002;12:542–549. doi: 10.1016/s0959-4388(02)00363-x. [DOI] [PubMed] [Google Scholar]
- 19.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 20.Venkataraman K, Futerman AH. Ceramide as a second messenger: Sticky solutions to sticky problems. Trends Cell Biol. 2000;10:408–412. doi: 10.1016/s0962-8924(00)01830-4. [DOI] [PubMed] [Google Scholar]
- 21.van Blitterswijk WJ, van der Luit AH, Veldman RJ, Verheij M, Borst J. Ceramide: Second messenger or modulator of membrane structure and dynamics? Biochem J. 2003;369:199–211. doi: 10.1042/BJ20021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki T. Lipid rafts at postsynaptic sites: Distribution, function and linkage to postsynaptic density. Neurosci Res. 2002;44:1–9. doi: 10.1016/s0168-0102(02)00080-9. [DOI] [PubMed] [Google Scholar]
- 23.Chamberlain LH, Burgoyne RD, Gould GW. Snare proteins are highly enriched in lipid rafts in pc12 cells: Implications for the spatial control of exocytosis. Proc Natl Acad Sci U S A. 2001;98:5619–5624. doi: 10.1073/pnas.091502398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinson M, Rausch O, Maycox PR, Prinjha RK, Chapman D, Morrow R, Harper AJ, Dingwall C, Walsh FS, Burbidge SA, Riddell DR. Lipid rafts mediate the interaction between myelin-associated glycoprotein (mag) on myelin and mag-receptors on neurons. Mol Cell Neurosci. 2003;22:344–352. doi: 10.1016/s1044-7431(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 25.Prinetti A, Chigorno V, Prioni S, Loberto N, Marano N, Tettamanti G, Sonnino S. Changes in the lipid turnover, composition, and organization, as sphingolipid-enriched membrane domains, in rat cerebellar granule cells developing in vitro. J Biol Chem. 2001;276:21136–21145. doi: 10.1074/jbc.M010666200. [DOI] [PubMed] [Google Scholar]
- 26.Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface ampa receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bansal R, Gard AL, Pfeiffer SE. Stimulation of oligodendrocyte differentiation in culture by growth in the presence of a monoclonal antibody to sulfated glycolipid. J Neurosci Res. 1988;21:260–267. doi: 10.1002/jnr.490210218. [DOI] [PubMed] [Google Scholar]
- 28.Greenfield S, Brostoff S, Hogan E. Evidence for defective incorporation of proteins in myelin of the quaking mutant mouse. Brain Res. 1977;120:507–515. doi: 10.1016/0006-8993(77)90403-6. [DOI] [PubMed] [Google Scholar]
- 29.Trapp BD, Nave KA. Multiple sclerosis: An immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 30.Bansal R, Pfeiffer SE. Reversible inhibition of oligodendrocyte progenitor differentiation by a monoclonal antibody against surface galactolipids. Proc Natl Acad Sci U S A. 1989;86:6181–6185. doi: 10.1073/pnas.86.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarz A, Futerman AH. Distinct roles for ceramide and glucosylceramide at different stages of neuronal growth. J Neurosci. 1997;17:2929–2938. doi: 10.1523/JNEUROSCI.17-09-02929.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuya S, Mitoma J, Makino A, Hirabayashi Y. Ceramide and its interconvertible metabolite sphingosine function as indispensable lipid factors involved in survival and dendritic differentiation of cerebellar purkinje cells. J Neurochem. 1998;71:366–377. doi: 10.1046/j.1471-4159.1998.71010366.x. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz A, Rapaport E, Hirschberg K, Futerman AH. A regulatory role for sphingolipids in neuronal growth. Inhibition of sphingolipid synthesis and degradation have opposite effects on axonal branching. J Biol Chem. 1995;270:10990–10998. doi: 10.1074/jbc.270.18.10990. [DOI] [PubMed] [Google Scholar]
- 34.Seufferlein T, Rozengurt E. Sphingosine induces p125fak and paxillin tyrosine phosphorylation, actin stress fiber formation, and focal contact assembly in swiss 3t3 cells. J Biol Chem. 1994;269:27610–27617. [PubMed] [Google Scholar]
- 35.Brann AB, Scott R, Neuberger Y, Abulafia D, Boldin S, Fainzilber M, Futerman AH. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J Neurosci. 1999;19:8199–8206. doi: 10.1523/JNEUROSCI.19-19-08199.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han X, D MH, McKeel DW, Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early alzheimer’s disease: Potential role in disease pathogenesis. J Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- 37.Alessenko AV, Bugrova AE, Dudnik LB. Connection of lipid peroxide oxidation with the sphingomyelin pathway in the development of alzheimer’s disease. Biochem Soc Trans. 2004;32:144–146. doi: 10.1042/bst0320144. [DOI] [PubMed] [Google Scholar]
- 38.Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- 39.Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in hiv-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- 40.Jana A, Pahan K. Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: Implications for multiple sclerosis. J Neuroimmune Pharmacol. 2007;2:184–193. doi: 10.1007/s11481-007-9066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pehar M, Vargas MR, Robinson KM, Cassina P, Diaz-Amarilla PJ, Hagen TM, Radi R, Barbeito L, Beckman JS. Mitochondrial superoxide production and nuclear factor erythroid 2-related factor 2 activation in p75 neurotrophin receptor-induced motor neuron apoptosis. J Neurosci. 2007;27:7777–7785. doi: 10.1523/JNEUROSCI.0823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu ZF, Nikolova-Karakashian M, Zhou D, Cheng G, Schuchman EH, Mattson MP. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production, and neuronal apoptosis. J Mol Neurosci. 2000;15:85–97. doi: 10.1385/JMN:15:2:85. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi K, Ginis I, Nishioka R, Klimanis D, Barone FC, White RF, Chen Y, Hallenbeck JM. Glucosylceramide synthase activity and ceramide levels are modulated during cerebral ischemia after ischemic preconditioning. J Cereb Blood Flow Metab. 2004;24:623–627. doi: 10.1097/01.WCB.0000119990.06999.A9. [DOI] [PubMed] [Google Scholar]
- 44.Ohtani R, Tomimoto H, Kondo T, Wakita H, Akiguchi I, Shibasaki H, Okazaki T. Upregulation of ceramide and its regulating mechanism in a rat model of chronic cerebral ischemia. Brain Res. 2004;1023:31–40. doi: 10.1016/j.brainres.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Puranam K, Qian WH, Nikbakht K, Venable M, Obeid L, Hannun Y, Boustany RM. Upregulation of bcl-2 and elevation of ceramide in batten disease. Neuropediatrics. 1997;28:37–41. doi: 10.1055/s-2007-973664. [DOI] [PubMed] [Google Scholar]
- 46.Denisova NA, Cantuti-Castelvetri I, Hassan WN, Paulson KE, Joseph JA. Role of membrane lipids in regulation of vulnerability to oxidative stress in pc12 cells: Implication for aging. Free Radic Biol Med. 2001;30:671–678. doi: 10.1016/s0891-5849(00)00513-x. [DOI] [PubMed] [Google Scholar]
- 47.Costantini C, Weindruch R, Della Valle G, Puglielli L. A trka-to-p75ntr molecular switch activates amyloid beta-peptide generation during aging. Biochem J. 2005;391:59–67. doi: 10.1042/BJ20050700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ditaranto-Desimone K, Saito M, Tekirian TL, Berg M, Dubowchik G, Soreghan B, Thomas S, Marks N, Yang AJ. Neuronal endosomal/lysosomal membrane destabilization activates caspases and induces abnormal accumulation of the lipid secondary messenger ceramide. Brain Res Bull. 2003;59:523–531. doi: 10.1016/s0361-9230(02)00948-6. [DOI] [PubMed] [Google Scholar]
- 49.de Chaves EP, Bussiere M, MacInnis B, Vance DE, Campenot RB, Vance JE. Ceramide inhibits axonal growth and nerve growth factor uptake without compromising the viability of sympathetic neurons. J Biol Chem. 2001;276:36207–36214. doi: 10.1074/jbc.M104282200. [DOI] [PubMed] [Google Scholar]
- 50.Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. Cns synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto S, Friberg H, Ferrand-Drake M, Wieloch T. Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1999;19:736–741. doi: 10.1097/00004647-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 53.Ghafourifar P, Klein SD, Schucht O, Schenk U, Pruschy M, Rocha S, Richter C. Ceramide induces cytochrome c release from isolated mitochondria. Importance of mitochondrial redox state. J Biol Chem. 1999;274:6080–6084. doi: 10.1074/jbc.274.10.6080. [DOI] [PubMed] [Google Scholar]
- 54.Stoica BA, Movsesyan VA, Lea PMt, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of akt, bad, fkhr, gsk-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003;22:365–382. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 55.Falluel-Morel A, Aubert N, Vaudry D, Basille M, Fontaine M, Fournier A, Vaudry H, Gonzalez BJ. Opposite regulation of the mitochondrial apoptotic pathway by c2-ceramide and pacap through a map-kinase-dependent mechanism in cerebellar granule cells. J Neurochem. 2004;91:1231–1243. doi: 10.1111/j.1471-4159.2004.02810.x. [DOI] [PubMed] [Google Scholar]
- 56.Movsesyan VA, Yakovlev AG, Dabaghyan EA, Stoica BA, Faden AI. Ceramide induces neuronal apoptosis through the caspase-9/caspase-3 pathway. Biochem Biophys Res Commun. 2002;299:201–207. doi: 10.1016/s0006-291x(02)02593-7. [DOI] [PubMed] [Google Scholar]
- 57.Poppe M, Reimertz C, Munstermann G, Kogel D, Prehn JH. Ceramide-induced apoptosis of d283 medulloblastoma cells requires mitochondrial respiratory chain activity but occurs independently of caspases and is not sensitive to bcl-xl overexpression. J Neurochem. 2002;82:482–494. doi: 10.1046/j.1471-4159.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- 58.France-Lanord V, Brugg B, Michel PP, Agid Y, Ruberg M. Mitochondrial free radical signal in ceramide-dependent apoptosis: A putative mechanism for neuronal death in parkinson’s disease. J Neurochem. 1997;69:1612–1621. doi: 10.1046/j.1471-4159.1997.69041612.x. [DOI] [PubMed] [Google Scholar]
- 59.Darios F, Lambeng N, Troadec JD, Michel PP, Ruberg M. Ceramide increases mitochondrial free calcium levels via caspase 8 and bid: Role in initiation of cell death. J Neurochem. 2003;84:643–654. doi: 10.1046/j.1471-4159.2003.01590.x. [DOI] [PubMed] [Google Scholar]
- 60.Melchiorri D, Martini F, Lococo E, Gradini R, Barletta E, De Maria R, Caricasole A, Nicoletti F, Lenti L. An early increase in the disialoganglioside gd3 contributes to the development of neuronal apoptosis in culture. Cell Death Differ. 2002;9:609–615. doi: 10.1038/sj.cdd.4401020. [DOI] [PubMed] [Google Scholar]
- 61.Feinstein E, Kimchi A, Wallach D, Boldin M, Varfolomeev E. The death domain: A module shared by proteins with diverse cellular functions. Trends Biochem Sci. 1995;20:342–344. doi: 10.1016/s0968-0004(00)89070-2. [DOI] [PubMed] [Google Scholar]
- 62.Shohat G, Spivak-Kroizman T, Cohen O, Bialik S, Shani G, Berrisi H, Eisenstein M, Kimchi A. The pro-apoptotic function of death-associated protein kinase is controlled by a unique inhibitory autophosphorylation-based mechanism. J Biol Chem. 2001;276:47460–47467. doi: 10.1074/jbc.M105133200. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto M, Takahashi H, Nakamura T, Hioki T, Nagayama S, Ooashi N, Sun X, Ishii T, Kudo Y, Nakajima-Iijima S, Kimchi A, Uchino S. Developmental changes in distribution of death-associated protein kinase mrnas. J Neurosci Res. 1999;58:674–683. doi: 10.1002/(sici)1097-4547(19991201)58:5<674::aid-jnr8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto M, Hioki T, Ishii T, Nakajima-Iijima S, Uchino S. Dap kinase activity is critical for c(2)-ceramide-induced apoptosis in pc12 cells. Eur J Biochem. 2002;269:139–147. doi: 10.1046/j.0014-2956.2002.00029.x. [DOI] [PubMed] [Google Scholar]
- 65.Pelled D, Raveh T, Riebeling C, Fridkin M, Berissi H, Futerman AH, Kimchi A. Death-associated protein (dap) kinase plays a central role in ceramide-induced apoptosis in cultured hippocampal neurons. J Biol Chem. 2002;277:1957–1961. doi: 10.1074/jbc.M104677200. [DOI] [PubMed] [Google Scholar]
- 66.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2a. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- 67.Dobrowsky RT, Hannun YA. Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem. 1992;267:5048–5051. [PubMed] [Google Scholar]
- 68.Law B, Rossie S. The dimeric and catalytic subunit forms of protein phosphatase 2a from rat brain are stimulated by c2-ceramide. J Biol Chem. 1995;270:12808–12813. doi: 10.1074/jbc.270.21.12808. [DOI] [PubMed] [Google Scholar]
- 69.Salinas M, Lopez-Valdaliso R, Martin D, Alvarez A, Cuadrado A. Inhibition of pkb/akt1 by c2-ceramide involves activation of ceramide-activated protein phosphatase in pc12 cells. Mol Cell Neurosci. 2000;15:156–169. doi: 10.1006/mcne.1999.0813. [DOI] [PubMed] [Google Scholar]
- 70.Ruvolo PP, Deng X, Ito T, Carr BK, May WS. Ceramide induces bcl2 dephosphorylation via a mechanism involving mitochondrial pp2a. J Biol Chem. 1999;274:20296–20300. doi: 10.1074/jbc.274.29.20296. [DOI] [PubMed] [Google Scholar]
- 71.Moore AN, Kampfl AW, Zhao X, Hayes RL, Dash PK. Sphingosine-1-phosphate induces apoptosis of cultured hippocampal neurons that requires protein phosphatases and activator protein-1 complexes. Neuroscience. 1999;94:405–415. doi: 10.1016/s0306-4522(99)00288-2. [DOI] [PubMed] [Google Scholar]
- 72.Ham J, Babij C, Whitfield J, Pfarr CM, Lallemand D, Yaniv M, Rubin LL. A c-jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 73.Estus S, Zaks WJ, Freeman RS, Gruda M, Bravo R, Johnson EM., Jr Altered gene expression in neurons during programmed cell death: Identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994;127:1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of erk and jnk-p38 map kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 75.Plummer G, Perreault KR, Holmes CF, Posse De Chaves EI. Activation of serine/threonine protein phosphatase-1 is required for ceramide-induced survival of sympathetic neurons. Biochem J. 2005;385:685–693. doi: 10.1042/BJ20040929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferguson KL, Slack RS. The rb pathway in neurogenesis. Neuroreport. 2001;12:A55–62. doi: 10.1097/00001756-200107030-00001. [DOI] [PubMed] [Google Scholar]
- 77.Dbaibo GS, Pushkareva MY, Jayadev S, Schwarz JK, Horowitz JM, Obeid LM, Hannun YA. Retinoblastoma gene product as a downstream target for a ceramide-dependent pathway of growth arrest. Proc Natl Acad Sci U S A. 1995;92:1347–1351. doi: 10.1073/pnas.92.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manev H, Cagnoli CM. Ceramide-mediated and isoquinolinesulfonamide-sensitive pathways of neuronal death: Anything in common? Neurochem Int. 1997;31:203–206. doi: 10.1016/s0197-0186(96)00149-0. [DOI] [PubMed] [Google Scholar]
- 79.Cassarino DS, Halvorsen EM, Swerdlow RH, Abramova NN, Parker WD, Jr, Sturgill TW, Bennett JP., Jr Interaction among mitochondria, mitogen-activated protein kinases, and nuclear factor-kappab in cellular models of parkinson’s disease. J Neurochem. 2000;74:1384–1392. doi: 10.1046/j.1471-4159.2000.0741384.x. [DOI] [PubMed] [Google Scholar]
- 80.Chun HS, Gibson GE, DeGiorgio LA, Zhang H, Kidd VJ, Son JH. Dopaminergic cell death induced by mpp(+), oxidant and specific neurotoxicants shares the common molecular mechanism. J Neurochem. 2001;76:1010–1021. doi: 10.1046/j.1471-4159.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 81.Harper SJ, LoGrasso P. Signalling for survival and death in neurones: The role of stress-activated kinases, jnk and p38. Cell Signal. 2001;13:299–310. doi: 10.1016/s0898-6568(01)00148-6. [DOI] [PubMed] [Google Scholar]
- 82.Saporito MS, Thomas BA, Scott RW. Mptp activates c-jun nh(2)-terminal kinase (jnk) and its upstream regulatory kinase mkk4 in nigrostriatal neurons in vivo. J Neurochem. 2000;75:1200–1208. doi: 10.1046/j.1471-4159.2000.0751200.x. [DOI] [PubMed] [Google Scholar]
- 83.Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, Bao J, Banasiak KJ, Haddad GG, Flavell RA, Davis RJ, Rakic P. A critical role of neural-specific jnk3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100:15184–15189. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ, Rakic P, Flavell RA. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 85.Soeda S, Tsuji Y, Ochiai T, Mishima K, Iwasaki K, Fujiwara M, Yokomatsu T, Murano T, Shibuya S, Shimeno H. Inhibition of sphingomyelinase activity helps to prevent neuron death caused by ischemic stress. Neurochem Int. 2004;45:619–626. doi: 10.1016/j.neuint.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 86.Borsello T, Forloni G. Jnk signalling: A possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–1886. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- 87.Davis RJ. Signal transduction by the jnk group of map kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 88.Willaime-Morawek S, Brami-Cherrier K, Mariani J, Caboche J, Brugg B. C-jun n-terminal kinases/c-jun and p38 pathways cooperate in ceramide-induced neuronal apoptosis. Neuroscience. 2003;119:387–397. doi: 10.1016/s0306-4522(02)00996-x. [DOI] [PubMed] [Google Scholar]
- 89.Choi HJ, Lee SY, Cho Y, Hwang O. Jnk activation by tetrahydrobiopterin: Implication for parkinson’s disease. J Neurosci Res. 2004;75:715–721. doi: 10.1002/jnr.20012. [DOI] [PubMed] [Google Scholar]
- 90.Brann AB, Tcherpakov M, Williams IM, Futerman AH, Fainzilber M. Nerve growth factor-induced p75-mediated death of cultured hippocampal neurons is age-dependent and transduced through ceramide generated by neutral sphingomyelinase. J Biol Chem. 2002;277:9812–9818. doi: 10.1074/jbc.M109862200. [DOI] [PubMed] [Google Scholar]
- 91.Willaime S, Vanhoutte P, Caboche J, Lemaigre-Dubreuil Y, Mariani J, Brugg B. Ceramide-induced apoptosis in cortical neurons is mediated by an increase in p38 phosphorylation and not by the decrease in erk phosphorylation. Eur J Neurosci. 2001;13:2037–2046. doi: 10.1046/j.0953-816x.2001.01581.x. [DOI] [PubMed] [Google Scholar]
- 92.Hida H, Nagano S, Takeda M, Soliven B. Regulation of mitogen-activated protein kinases by sphingolipid products in oligodendrocytes. J Neurosci. 1999;19:7458–7467. doi: 10.1523/JNEUROSCI.19-17-07458.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levade T, Moser HW, Fensom AH, Harzer K, Moser AB, Salvayre R. Neurodegenerative course in ceramidase deficiency (farber disease) correlates with the residual lysosomal ceramide turnover in cultured living patient cells. J Neurol Sci. 1995;134:108–114. doi: 10.1016/0022-510x(95)00231-0. [DOI] [PubMed] [Google Scholar]
- 94.Maimone D, Gregory S, Arnason BG, Reder AT. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. J Neuroimmunol. 1991;32:67–74. doi: 10.1016/0165-5728(91)90073-g. [DOI] [PubMed] [Google Scholar]
- 95.Trotter JL, Collins KG, van der Veen RC. Serum cytokine levels in chronic progressive multiple sclerosis: Interleukin-2 levels parallel tumor necrosis factor-alpha levels. J Neuroimmunol. 1991;33:29–36. doi: 10.1016/0165-5728(91)90031-2. [DOI] [PubMed] [Google Scholar]
- 96.Tsukada N, Miyagi K, Matsuda M, Yanagisawa N, Yone K. Tumor necrosis factor and interleukin-1 in the csf and sera of patients with multiple sclerosis. J Neurol Sci. 1991;104:230–234. doi: 10.1016/0022-510x(91)90315-x. [DOI] [PubMed] [Google Scholar]
- 97.Peter JB, Boctor FN, Tourtellotte WW. Serum and csf levels of il-2, sil-2r, tnf-alpha, and il-1 beta in chronic progressive multiple sclerosis: Expected lack of clinical utility. Neurology. 1991;41:121–123. doi: 10.1212/wnl.41.1.121. [DOI] [PubMed] [Google Scholar]
- 98.Gallo P, Piccinno MG, Krzalic L, Tavolato B. Tumor necrosis factor alpha (tnf alpha) and neurological diseases. Failure in detecting tnf alpha in the cerebrospinal fluid from patients with multiple sclerosis, aids dementia complex, and brain tumours. J Neuroimmunol. 1989;23:41–44. doi: 10.1016/0165-5728(89)90071-4. [DOI] [PubMed] [Google Scholar]
- 99.Richard A, Robichaud G, Lapointe R, Bourgoin S, Darveau A, Poulin L. Interference of hiv-1 nef in the sphingomyelin transduction pathway activated by tumour necrosis factor-alpha in human glial cells. Aids. 1997;11:F1–7. doi: 10.1097/00002030-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 100.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 101.Chakraborty G, Ziemba S, Drivas A, Ledeen RW. Myelin contains neutral sphingomyelinase activity that is stimulated by tumor necrosis factor-alpha. J Neurosci Res. 1997;50:466–476. doi: 10.1002/(SICI)1097-4547(19971101)50:3<466::AID-JNR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 102.Ledeen RW, Chakraborty G. Cytokines, signal transduction, and inflammatory demyelination: Review and hypothesis. Neurochem Res. 1998;23:277–289. doi: 10.1023/a:1022493013904. [DOI] [PubMed] [Google Scholar]
- 103.Scurlock B, Dawson G. Differential responses of oligodendrocytes to tumor necrosis factor and other pro-apoptotic agents: Role of ceramide in apoptosis. J Neurosci Res. 1999;55:514–522. doi: 10.1002/(SICI)1097-4547(19990215)55:4<514::AID-JNR11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 104.Sortino MA, Condorelli F, Vancheri C, Canonico PL. Tumor necrosis factor-alpha induces apoptosis in immortalized hypothalamic neurons: Involvement of ceramide-generating pathways. Endocrinology. 1999;140:4841–4849. doi: 10.1210/endo.140.10.7062. [DOI] [PubMed] [Google Scholar]
- 105.Mayne M, Bratanich AC, Chen P, Rana F, Nath A, Power C. Hiv-1 tat molecular diversity and induction of tnf-alpha: Implications for hiv-induced neurological disease. Neuroimmunomodulation. 1998;5:184–192. doi: 10.1159/000026336. [DOI] [PubMed] [Google Scholar]
- 106.Jeohn GH, Kong LY, Wilson B, Hudson P, Hong JS. Synergistic neurotoxic effects of combined treatments with cytokines in murine primary mixed neuron/glia cultures. J Neuroimmunol. 1998;85:1–10. doi: 10.1016/s0165-5728(97)00204-x. [DOI] [PubMed] [Google Scholar]
- 107.Perry SW, Hamilton JA, Tjoelker LW, Dbaibo G, Dzenko KA, Epstein LG, Hannun Y, Whittaker JS, Dewhurst S, Gelbard HA. Platelet-activating factor receptor activation. An initiator step in hiv-1 neuropathogenesis. J Biol Chem. 1998;273:17660–17664. doi: 10.1074/jbc.273.28.17660. [DOI] [PubMed] [Google Scholar]
- 108.Fine SM, Angel RA, Perry SW, Epstein LG, Rothstein JD, Dewhurst S, Gelbard HA. Tumor necrosis factor alpha inhibits glutamate uptake by primary human astrocytes. Implications for pathogenesis of hiv-1 dementia. J Biol Chem. 1996;271:15303–15306. doi: 10.1074/jbc.271.26.15303. [DOI] [PubMed] [Google Scholar]
- 109.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 110.Wong PC, Rothstein JD, Price DL. The genetic and molecular mechanisms of motor neuron disease. Curr Opin Neurobiol. 1998;8:791–799. doi: 10.1016/s0959-4388(98)80123-2. [DOI] [PubMed] [Google Scholar]
- 111.Samuels A. Excitatory amino acids in neurologic disorders. N Engl J Med. 1994;331:274–275. doi: 10.1056/NEJM199407283310414. [DOI] [PubMed] [Google Scholar]
- 112.Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 113.Malaplate-Armand C, Florent-Bechard S, Youssef I, Koziel V, Sponne I, Kriem B, Leininger-Muller B, Olivier JL, Oster T, Pillot T. Soluble oligomers of amyloid-beta peptide induce neuronal apoptosis by activating a cpla(2)-dependent sphingomyelinase-ceramide pathway. Neurobiol Dis. 2006 doi: 10.1016/j.nbd.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 114.Eddleston M, Mucke L. Molecular profile of reactive astrocytes--implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 116.Snider WD. Functions of the neurotrophins during nervous system development: What the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 117.Crutcher KA, Scott SA, Liang S, Everson WV, Weingartner J. Detection of ngf-like activity in human brain tissue: Increased levels in alzheimer’s disease. J Neurosci. 1993;13:2540–2550. doi: 10.1523/JNEUROSCI.13-06-02540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fahnestock M, Scott SA, Jette N, Weingartner JA, Crutcher KA. Nerve growth factor mrna and protein levels measured in the same tissue from normal and alzheimer’s disease parietal cortex. Brain Res Mol Brain Res. 1996;42:175–178. doi: 10.1016/s0169-328x(96)00193-3. [DOI] [PubMed] [Google Scholar]
- 119.Akundi RS, Candelario-Jalil E, Hess S, Hull M, Lieb K, Gebicke-Haerter PJ, Fiebich BL. Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia. 2005;51:199–208. doi: 10.1002/glia.20198. [DOI] [PubMed] [Google Scholar]
- 120.Hostetler KY, Yazaki PJ. The subcellular localization of neutral sphingomyelinase in rat liver. J Lipid Res. 1979;20:456–463. [PubMed] [Google Scholar]
- 121.Ferlinz K, Hurwitz R, Vielhaber G, Suzuki K, Sandhoff K. Occurrence of two molecular forms of human acid sphingomyelinase. Biochem J. 1994;301(Pt 3):855–862. doi: 10.1042/bj3010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gatt S. Magnesium-dependent sphingomyelinase. Biochem Biophys Res Commun. 1976;68:235–241. doi: 10.1016/0006-291x(76)90034-6. [DOI] [PubMed] [Google Scholar]
- 123.Yamaguchi S, Suzuki K. A novel magnesium-independent neutral sphingomyelinase associated with rat central nervous system meylin. J Biol Chem. 1978;253:4090–4092. [PubMed] [Google Scholar]
- 124.Duan RD, Nyberg L, Nilsson A. Alkaline sphingomyelinase activity in rat gastrointestinal tract: Distribution and characteristics. Biochim Biophys Acta. 1995;1259:49–55. doi: 10.1016/0005-2760(95)00137-2. [DOI] [PubMed] [Google Scholar]
- 125.Wiegmann K, Schutze S, Machleidt T, Witte D, Kronke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994;78:1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 126.Liu B, Hassler DF, Smith GK, Weaver K, Hannun YA. Purification and characterization of a membrane bound neutral ph optimum magnesium-dependent and phosphatidylserine-stimulated sphingomyelinase from rat brain. J Biol Chem. 1998;273:34472–34479. doi: 10.1074/jbc.273.51.34472. [DOI] [PubMed] [Google Scholar]
- 127.Bernardo K, Krut O, Wiegmann K, Kreder D, Micheli M, Schafer R, Sickman A, Schmidt WE, Schroder JM, Meyer HE, Sandhoff K, Kronke M. Purification and characterization of a magnesium-dependent neutral sphingomyelinase from bovine brain. J Biol Chem. 2000;275:7641–7647. doi: 10.1074/jbc.275.11.7641. [DOI] [PubMed] [Google Scholar]
- 128.Jung SY, Suh JH, Park HJ, Jung KM, Kim MY, Na DS, Kim DK. Identification of multiple forms of membrane-associated neutral sphingomyelinase in bovine brain. J Neurochem. 2000;75:1004–1014. doi: 10.1046/j.1471-4159.2000.0751004.x. [DOI] [PubMed] [Google Scholar]
- 129.Blochl A, Sirrenberg C. Neurotrophins stimulate the release of dopamine from rat mesencephalic neurons via trk and p75lntr receptors. J Biol Chem. 1996;271:21100–21107. doi: 10.1074/jbc.271.35.21100. [DOI] [PubMed] [Google Scholar]
- 130.Bajjalieh SM, Martin TF, Floor E. Synaptic vesicle ceramide kinase. A calcium-stimulated lipid kinase that co-purifies with brain synaptic vesicles. J Biol Chem. 1989;264:14354–14360. [PubMed] [Google Scholar]
- 131.Chao MV. Ceramide: A potential second messenger in the nervous system. Mol Cell Neurosci. 1995;6:91–96. doi: 10.1006/mcne.1995.1009. [DOI] [PubMed] [Google Scholar]
- 132.Knipper M, Leung LS, Zhao D, Rylett RJ. Short-term modulation of glutamatergic synapses in adult rat hippocampus by ngf. Neuroreport. 1994;5:2433–2436. doi: 10.1097/00001756-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 133.Jayadev S, Linardic CM, Hannun YA. Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to tumor necrosis factor alpha. J Biol Chem. 1994;269:5757–5763. [PubMed] [Google Scholar]
- 134.Jayadev S, Hayter HL, Andrieu N, Gamard CJ, Liu B, Balu R, Hayakawa M, Ito F, Hannun YA. Phospholipase a2 is necessary for tumor necrosis factor alpha-induced ceramide generation in l929 cells. J Biol Chem. 1997;272:17196–17203. doi: 10.1074/jbc.272.27.17196. [DOI] [PubMed] [Google Scholar]
- 135.Liu B, Hannun YA. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J Biol Chem. 1997;272:16281–16287. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- 136.Yoshimura S, Banno Y, Nakashima S, Hayashi K, Yamakawa H, Sawada M, Sakai N, Nozawa Y. Inhibition of neutral sphingomyelinase activation and ceramide formation by glutathione in hypoxic pc12 cell death. J Neurochem. 1999;73:675–683. doi: 10.1046/j.1471-4159.1999.0730675.x. [DOI] [PubMed] [Google Scholar]
- 137.Jana A, Pahan K. Human immunodeficiency virus type 1 gp120 induces apoptosis in human primary neurons through redox-regulated activation of neutral sphingomyelinase. J Neurosci. 2004;24:9531–9540. doi: 10.1523/JNEUROSCI.3085-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jana A, Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via nadph oxidase-mediated activation of neutral sphingomyelinase. Implications for alzheimer’s disease. J Biol Chem. 2004;279:51451–51459. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Singh I, Pahan K, Khan M, Singh AK. Cytokine-mediated induction of ceramide production is redox-sensitive. Implications to proinflammatory cytokine-mediated apoptosis in demyelinating diseases. J Biol Chem. 1998;273:20354–20362. doi: 10.1074/jbc.273.32.20354. [DOI] [PubMed] [Google Scholar]
- 140.Mao C, Xu R, Szulc ZM, Bielawska A, Galadari SH, Obeid LM. Cloning and characterization of a novel human alkaline ceramidase. A mammalian enzyme that hydrolyzes phytoceramide. J Biol Chem. 2001;276:26577–26588. doi: 10.1074/jbc.M102818200. [DOI] [PubMed] [Google Scholar]
- 141.Mitsutake S, Tani M, Okino N, Mori K, Ichinose S, Omori A, Iida H, Nakamura T, Ito M. Purification, characterization, molecular cloning, and subcellular distribution of neutral ceramidase of rat kidney. J Biol Chem. 2001;276:26249–26259. doi: 10.1074/jbc.M102233200. [DOI] [PubMed] [Google Scholar]
- 142.Koch J, Gartner S, Li CM, Quintern LE, Bernardo K, Levran O, Schnabel D, Desnick RJ, Schuchman EH, Sandhoff K. Molecular cloning and characterization of a full-length complementary DNA encoding human acid ceramidase. Identification of the first molecular lesion causing farber disease. J Biol Chem. 1996;271:33110–33115. doi: 10.1074/jbc.271.51.33110. [DOI] [PubMed] [Google Scholar]
- 143.El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. Molecular cloning and characterization of a human mitochondrial ceramidase. J Biol Chem. 2000;275:21508–21513. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- 144.Klein JA, Ackerman SL. Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest. 2003;111:785–793. doi: 10.1172/JCI18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 146.Culotta VC, Yang M, O’Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta. 2006;1763:747–758. doi: 10.1016/j.bbamcr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mo JQ, Hom DG, Andersen JK. Decreases in protective enzymes correlates with increased oxidative damage in the aging mouse brain. Mech Ageing Dev. 1995;81:73–82. doi: 10.1016/0047-6374(95)01586-o. [DOI] [PubMed] [Google Scholar]
- 148.Baek BS, Kwon HJ, Lee KH, Yoo MA, Kim KW, Ikeno Y, Yu BP, Chung HY. Regional difference of ros generation, lipid peroxidation, and antioxidant enzyme activity in rat brain and their dietary modulation. Arch Pharm Res. 1999;22:361–366. doi: 10.1007/BF02979058. [DOI] [PubMed] [Google Scholar]
- 149.Thorburne SK, Juurlink BH. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem. 1996;67:1014–1022. doi: 10.1046/j.1471-4159.1996.67031014.x. [DOI] [PubMed] [Google Scholar]
- 150.Juurlink BH, Thorburne SK, Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22:371–378. doi: 10.1002/(sici)1098-1136(199804)22:4<371::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 151.Juurlink BH, Schultke E, Hertz L. Glutathione release and catabolism during energy substrate restriction in astrocytes. Brain Res. 1996;710:229–233. doi: 10.1016/0006-8993(95)01358-x. [DOI] [PubMed] [Google Scholar]
- 152.Chatterjee S, Noack H, Possel H, Keilhoff G, Wolf G. Glutathione levels in primary glial cultures: Monochlorobimane provides evidence of cell type-specific distribution. Glia. 1999;27:152–161. [PubMed] [Google Scholar]
- 153.Frankel D, Mehindate K, Schipper HM. Role of heme oxygenase-1 in the regulation of manganese superoxide dismutase gene expression in oxidatively-challenged astroglia. J Cell Physiol. 2000;185:80–86. doi: 10.1002/1097-4652(200010)185:1<80::AID-JCP7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 154.Fox RM, Jones JE, Atterwill CK. Gliotoxicity in brain reaggregate cultures caused by oxidants and excitatory amino acids can be prevented by alpha-tocopherol and mk-801. Neurotoxicology. 1996;17:705–710. [PubMed] [Google Scholar]
- 155.Saha RN, Pahan K. Differential regulation of mn-superoxide dismutase in neurons and astroglia by hiv-1 gp120: Implications for hiv-associated dementia. Free Radic Biol Med. 2007;42:1866–1878. doi: 10.1016/j.freeradbiomed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pahan K, Dobashi K, Ghosh B, Singh I. Induction of the manganese superoxide dismutase gene by sphingomyelinase and ceramide. J Neurochem. 1999;73:513–520. doi: 10.1046/j.1471-4159.1999.0730513.x. [DOI] [PubMed] [Google Scholar]
- 157.Manna SK, Sah NK, Aggarwal BB. Protein tyrosine kinase p56lck is required for ceramide-induced but not tumor necrosis factor-induced activation of nf-kappa b, ap-1, jnk, and apoptosis. J Biol Chem. 2000;275:13297–13306. doi: 10.1074/jbc.275.18.13297. [DOI] [PubMed] [Google Scholar]
- 158.Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 159.Beard J. Recent evidence from human and animal studies regarding iron status and infant development. J Nutr. 2007;137:524S–530S. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- 160.Zhang X, Haaf M, Todorich B, Grosstephan E, Schieremberg H, Surguladze N, Connor JR. Cytokine toxicity to oligodendrocyte precursors is mediated by iron. Glia. 2005;52:199–208. doi: 10.1002/glia.20235. [DOI] [PubMed] [Google Scholar]
- 161.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 162.LeVine SM. Oligodendrocytes and myelin sheaths in normal, quaking and shiverer brains are enriched in iron. J Neurosci Res. 1991;29:413–419. doi: 10.1002/jnr.490290317. [DOI] [PubMed] [Google Scholar]
- 163.Beard JL, Wiesinger JA, Connor JR. Pre- and postweaning iron deficiency alters myelination in sprague-dawley rats. Dev Neurosci. 2003;25:308–315. doi: 10.1159/000073507. [DOI] [PubMed] [Google Scholar]
- 164.Bartzokis G. Age-related myelin breakdown: A developmental model of cognitive decline and alzheimer’s disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- 165.Ferriero DM. Oxidant mechanisms in neonatal hypoxia-ischemia. Dev Neurosci. 2001;23:198–202. doi: 10.1159/000046143. [DOI] [PubMed] [Google Scholar]
- 166.Gaasch JA, Lockman PR, Geldenhuys WJ, Allen DD, Van der Schyf CJ. Brain iron toxicity: Differential responses of astrocytes, neurons, and endothelial cells. Neurochem Res. 2007;32:1196–1208. doi: 10.1007/s11064-007-9290-4. [DOI] [PubMed] [Google Scholar]
- 167.Dringen R, Bishop GM, Koeppe M, Dang TN, Robinson SR. The pivotal role of astrocytes in the metabolism of iron in the brain. Neurochem Res. 2007;32:1884–1890. doi: 10.1007/s11064-007-9375-0. [DOI] [PubMed] [Google Scholar]
- 168.Moos T, Rosengren Nielsen T, Skjorringe T, Morgan EH. Iron trafficking inside the brain. J Neurochem. 2007;103:1730–1740. doi: 10.1111/j.1471-4159.2007.04976.x. [DOI] [PubMed] [Google Scholar]
- 169.Schonfeld E, Yasharel I, Yavin E, Brand A. Docosahexaenoic acid enhances iron uptake by modulating iron transporters and accelerates apoptotic death in pc12 cells. Neurochem Res. 2007;32:1673–1684. doi: 10.1007/s11064-007-9378-x. [DOI] [PubMed] [Google Scholar]
- 170.Brand A, Schonfeld E, Isharel I, Yavin E. Docosahexaenoic acid-dependent iron accumulation in oligodendroglia cells protects from hydrogen peroxide-induced damage. J Neurochem. 2008;105:1325–1335. doi: 10.1111/j.1471-4159.2008.05234.x. [DOI] [PubMed] [Google Scholar]
- 171.Bazan NG. Synaptic lipid signaling: Significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]