Abstract
IL-16, a leukocyte chemoattractant factor (LCF), is involved in the disease process of multiple sclerosis and other autoimmune disorders. However, mechanisms by which this LCF is expressed are poorly understood. The present study underlines the importance of IL-12 p40 homodimer (p402), the so-called biologically inactive molecule, in inducing the expression of IL-16 in primary mouse and human microglia, mouse BV-2 microglial cells, mouse peritoneal macrophages, and RAW264.7 cells. In contrast, IL-12 p70, the bioactive heterodimeric cytokine, was unable to induce the expression of IL-16 in any of these cell types. Similarly IL-12 p402 also induced the activation of IL-16 promoter in microglia. Among various stimuli tested, p402 was the most potent one followed by p40 monomer, IL-16 and IL-23 in inducing the activation of IL-16 promoter in microglial cells. Furthermore, induction of IL-16 mRNA expression by over-expression of p40, but not p35, cDNA and induction of IL-16 expression by p402 in microglia isolated from IL-12p35 (−/−) mice confirm that p40, but not p35, is responsible for the induction of IL-16. Finally, by using primary microglia isolated from IL-12Rβ1 (−/−) and IL-12Rβ2 (−/−) mice, we demonstrate that p402 induces the expression of this LCF via IL-12Rβ1 but not IL-12Rβ2. These results delineate a novel biological function of p402 and raise the possibility that biological function of IL-12 p402 maybe different from IL-12 p70.
Keywords: IL-12 p40 homodimer, IL-12 receptors, IL-16, Microglia, Macrophages, IL-12Rβ1, IL-12Rβ2
1. Introduction
Interleukin-16 (IL-16), also known as a leukocyte chemoattractant factor (LCF), is an immunomodulatory cytokine secreted by various cell types. It has been found that IL-16 promotes lymphocyte migration, induces the expression of proinflammatory molecules and modulates apoptosis (Cruikshank et al., 2000; Guo et al., 2004; Schluesener et al., 1996). Subsequently, the increase in IL-16 expression has been observed in several CNS inflammatory conditions such as experimental autoimmune uveitis (EAU), experimental autoimmune encephalomyelitis (EAE), multiple sclerosis (MS), viral infection, traumatic brain injury, and spinal cord injury (Guo et al., 2004; Mueller et al., 2006; Skundric et al., 2005b, 2006) as well as in peripheral inflammatory diseases such as asthma, rheumatoid arthritis and Crohns disease (Cruikshank et al., 1998; Franz et al., 1998; Keates et al., 2000; Laberge et al., 1997; Seegert et al., 2001). Amelioration of EAE by either inhibition of IL-16 production or IL-16-associated vaccination suggests that this cytokine may play a role in MS and EAE (Mannie and Abbott, 2007; Skundric et al., 2005a). In EAE and MS brains, immunopositive lymphocytes, microglia and macrophages have been found to produce high amount of IL-16 (Guo et al., 2004; Schluesener et al., 1996). Several mitogens and antigens are able to activate the transcription of IL-16 in T-cells. On the other hand, it is not known which factor induces the transcription of IL-16 in microglia and macrophages. Potent stimuli of microglial/macrophage activation such as LPS, IL-1β or IFN-γ fail to induce the expression of IL-16 in either microglia or macrophages (Bannert et al, 1999; Zhao et al, 2004). These results suggest that regulatory mechanisms for the expression of IL-16 are different from that of other cytokines and that understanding such mechanisms is an important area of research.
IL-12 plays a critical role in the early inflammatory response to infection and in the generation of T helper type 1(Th1) cells, which favor cell mediated immunity (Hsieh et al., 1993). It has been found that overproduction of IL-12 can be dangerous to the host as it is involved in the pathogenesis of a number of autoimmune inflammatory diseases (MS, arthritis, insulin dependent diabetes) (Constantinescu et al., 2000; Zipris et al., 1996). IL-12 consists of a heavy chain (p40) and a light chain (p35) linked covalently by disulfide bonds to give rise to a heterodimeric (p70) molecule (Schoenhaut et al., 1992; Wolf et al., 1991). Recently, p40 has been shown to pair with p19 to form a newly discovered cytokine, IL-23. Either p19 or p35 is constitutively expressed in many cell types. However, dendritic cells and macrophages, cells those are able to secrete heterodimeric IL-12 or IL-23, always produce an excess of p40 as homodimer (p402) (Gately et al., 1998). Again, several reports (Constantinescu et al., 2000; Fassbender et al, 1998; Gately et al, 1998) indicate that the level of p40 mRNA in the CNS of patients with MS is much higher than the CNS of control subjects whereas the level of p35 mRNA is about the same or decreases compared to that of controls. Similarly, in mice with EAE, an animal model of MS, expression of p40 mRNA, but not p35 mRNA, increases in brain and spinal cord (Bright et al., 1998). These studies suggest that p40 may have a key function as a homodimer not just as part of the p40:p35 heterodimer forming IL-12 or the p40:p19 heterodimer forming IL-23. However, it was known that p402 was biologically inactive until we demonstrated the induction of nitric oxide synthase (iNOS) and tumor necrosis factor-α (TNF-α) by p402 in microglia and macrophages (Jana et al, 2003; Pahan et al, 2001). Subsequently, it has been found that p402 acts as a chemoattractant for macrophages (Cooper and Khader, 2007) and promotes the migration of dendritic cells (Khader et al, 2006).
Here we delineate a novel function of p402. Interestingly, p402, but not p70, induces the expression of IL-16 in microglia and macrophages. These results further emphasize that p402 is biologically active and suggest that p402 may be considered as a separate cytokine with biological functions distinct from IL-12 p70.
2. Materials and methods
2.1. Reagents
Fetal bovine serum, Hank’s balanced salt solution (HBSS) and Dulbecco’s modified Eagle’s medium (DMEM)/F-12 were from Mediatech, Valley Park, MO, USA. Recombinant mouse IL-12 p70, p402 (the p40 homodimer), TNF-α, IL-1 β, and IFN-γ were obtained from R & D Systems, Minneapolis, MN, USA. Antibodies against IL-16 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). HIV-1 gp120 was obtained from US Biologicals. Poly inosinic:cytidilic acid (poly IC) was purchased from Sigma. IL-12p35 (−/−), IL-12Rβ1(−/−) and IL-12Rβ2(−/−) mice and littermate controls were purchased from Jackson Laboratories.
2.1.1. Isolation of primary mouse microglia
Microglia were isolated from mixed glial cultures according to the procedure of Guilian and Baker (Giulian and Baker, 1986). Animal maintenance and experimental protocols were approved by the Rush University Animal Care Committee. Briefly, mixed glial cells were prepared from 7- to 9-day-old mouse pups. On day 9, the mixed glial cultures were washed three times with DMEM/F-12 and subjected to a shake at 240 rpm for 2 h at 37 °C on a rotary shaker. The floating cells were washed and seeded onto plastic tissue-culture flasks and incubated at 37°C for 1 h. The attached cells were removed by trypsinization and seeded on to new plates for further studies. To monitor purity, cells were immunostained with antibodies against Mac-1 surface Ag (BD Pharmingen), a marker for microglia/macrophages. More than 95% of this preparation was found to be positive for Mac-1. Twenty-four hours after plating, some of these cells showed a well-spread amoeboid morphology and rest exhibited an elongated processed morphology. For the induction of IL-16 expression, cells were stimulated with p70, p402 and other stimuli under serum-free condition. Mouse BV-2 microglial cells (a gift from V. Bocchini of the University of Perugia, Perugia, Italy) were also maintained and induced as indicated above.
2.1.2. Isolation of primary human microglia
Primary microglia were isolated from mixed glial cultures according to the procedure of Jana et al (Jana et al., 2007b). Briefly, 13–17-week-old human fetal brains obtained from the Human Embryology Laboratory (University of Washington, Seattle, WA, USA) were dissociated by trituration and trypsinization. Cells were plated on poly-d-lysine precoated 75 cm2 flasks and incubated at 37°C with 5% CO2 in air. On ninth day, the cultures were placed on a rotary shaker at 240 rpm at 37 °C for 2 h to remove microglia. The cell suspensions were placed on uncoated culture plates for 30 min followed by removal of nonadherent cells by washing. Adherent cells were cultured in DMEM/F-12 containing 10% FBS. More than 98% of these cells stained for microglial marker CD11b (Jana et al., 2007b).
2.1.3. Isolation of mouse peritoneal macrophages
Macrophages were isolated by peritoneal lavage from mice with sterile RPMI1640 medium containing 1% FBS and antibiotic-antimycotic mixture (Sigma) as described earlier (Jana et al., 2003; Pahan et al., 2001). Cells were washed three times with the same media at 4 °C and were maintained at 37 °C in a humidified incubator containing 5% CO2 in air. Cells were plated in culture dishes in RPMI 1640 medium containing 1% FBS, l-glutamine and antibiotic-antimycotic mixture. After 1 h, nonadherent cells were removed by washing, and adherent cells were ∼95% pure according to immuno-logical and morphological criteria.
2.1.4. Immunostaining of IL-16
Immunostaining was performed as described earlier (Jana and Pahan, 2005, 2007; Jana et al., 2007b). Briefly, coverslips containing 200–300 cells/mm2 were fixed with 4% paraformaldehyde for 15 min, followed by treatment with cold ethanol (−20 °C) for 5 min and two rinses in PBS. Samples were blocked with 3% BSA in PBS containing Tween 20 (PBST) for 30 min and incubated in PBST con-taining 1% BSA and rabbit anti-IL-16 (1:50). After three washes in PBST (15 min each), slides were further incubated with Cy5 andCy2 (Jackson ImmunoResearch, West Grove, PA, USA). For negative controls, a set of culture slides was incubated under similar conditions without the primary antibodies. The samples were mounted and observed under a Bio-Rad (Hercules, CA, USA) MRC1024ES confocal laser-scanning microscope.
2.1.5. Semi-quantitative RT-PCR analysis
The expression of different proinflammatory molecules was analyzed by semi-quantitative RT-PCR using a RT-PCR kit from BD Clontech as described earlier (Jana et al., 2007a; Jana and Pahan, 2005). Briefly, total RNA was isolated from stimulated or unstimulated cells by using the Qiagen mini kit followed by digestion with DNase to remove contaminating genomic DNA. Briefly, 1 µg of DNase-digested RNA was reverse transcribed using oligo(dT)12–18 as primer and MMLV reverse transcriptase (BD Clontech) in a 20-µl reaction mixture. The resulting cDNA was appropriately diluted, and diluted cDNA was amplified using Titanium TaqDNA polymerase and the following primers. The following primers were used to amplify mouse proinflammatory molecules: iNOS (497 bp): sense: 5′-CCC TTC CGA AGT TTC TGG CAG CAG C-3′, antisense: 5′-GGC TGT CAG AGC CTC GTG GCT TTG G-3′; TNF-α (354 bp): sense: 5′-TTC TGT CTA CTG AAC TTC GGG GTG ATC GGT CC-3′, antisense: 5′-GTA TGA GAT AGC AAA TCG GCT GAC GGT GTG GG-3′; IL-16 (302 bp): sense: 5′-AGG ACC CAG TCC TGC GAG ACA A-3′; antisense: 5′-TTC CTG TCC TCG GTT TCC CC-3′; GAPDH (276 bp): sense: 5′-GGT GAA GGT CGG TGT GAA CG-3′, antisense: 5′-TTG GCT CCA CCC TTC AAG TG-3′.
On the other hand, following primers were used to amplify human IL-16.
IL-16 (347 bp): sense: 5′-ATG CCT GAC CTC AAC TCC TC-3′
Antisense: 5-CTC CTG ATG ACA ATC GTG AC-3′; GAPDH: sense: 5′- GGT GAA GGT CGG SGT CAA CG-3′; antisense: 5′-GTG AAG ACG CCA GTG GAC TC-3′
Amplified products were electrophoresed on 1.8% agarose gels and visualized by ethidium bromide staining. GAPDH was used to ascertain that an equivalent amount of cDNA was synthesized from different samples. The relative expression of cytokines or IL-16 (cytokines or IL-16/GAPDH) was measured after scanning the bands with a Fluor Chem 8800 Imaging System (Alpha Innotech).
2.1.6. Real-time PCR analysis
Real-time PCR analysis was performed using the ABI-Prism7700 sequence detection system (Applied Biosystems) as described earlier (Jana et al, 2007a; Jana and Pahan, 2005) using forward and reverse primers and FAM-labeled probes (Applied Biosystems). The mRNA expression of IL-16 and cytokines was normalized to the level of GAPDH mRNA. Data were processed by the ABI Sequence Detection System 1.6 software and analyzed by ANOVA.
2.1.7. Western blot analysis
Culture supernatants were concentrated using 5-kDa (Millipore microconYM 5) protein separation filters followed electrophoresis in 4–12% gradient gels, transfer onto a nitrocellulose membrane and immunoreaction with antibodies against mouse IL-16. Immunoblots were visualized by infra-red fluorophore-tagged secondary Ab (Molecular Probes) in an Odyssey infrared scanner as described earlier (Saha et al, 2007)
2.1.8. Construction of mouse IL-16 promoter-driven luciferase construct
Mouse genomic DNA isolated from mouse BV-2 microglial cells were used as template during PCR. The 5′flanking sequence of mouse IL-16 (−1237/+83) gene was isolated by PCR. Primers were designed from gene bank sequences.
IL-16: sense: 5′-acgcgt GAA GCA TCA CGT GAA TGG GGG CCC TG-3′; antisense: 5′-agatct GAA GTC TGC GTC GTG GCT GCA GGT-3′
The sense primer was tagged with Mlu1 restriction enzyme while the antisense primer was tagged with Bgl II. The PCR was performed using Advantage-2 PCR kit (Clontech) according to the manufacturer’s instruction. The resulting fragments were gel purified and ligated into the PGEM-TEasy vector (Promega). These fragments were further subcloned into the PGL-3 Enhancer vector after digestion with corresponding restriction enzymes and verification by sequencing in the automated sequencer of the University of Nebraska at Lincoln Biotechnology Center.
2.1.9. Assay of IL-16 promoter-driven reporter activity
Cells plated at 50–60% confluence in 12-well plates were cotransfected with 0.25 µg of pIL-16-Luc and 25 ng of pRL-TK (a plasmid encoding Renilla luciferase, used as transfection efficiency control; Promega) using Lipofectamine Plus (Invitrogen). After 24 h of transfection, cells were stimulated with different stimuli under serum free condition for 6 h. Firefly and Renilla luciferase activities were analyzed in cell extracts using the Dual Luciferase kit (Promega) in a TD-20/20 Luminometer (Turner Designs) as described earlier (Jana et al., 2005; Pahan et al., 2002). Relative luciferase activity of cell extracts was typically represented as the ratio of firefly luciferase value:Renilla luciferase value × 10−3.
2.1.10. Expression of mouse p35 and p40 cDNA in BV-2 microglial cells
Cells at 50–60% confluence were transfected with different amounts of p35 and p40 cDNA expression construct (Pahan et al., 2001) by LipofectAMINE Plus (Invitrogen) following the manufacturers protocol (Jana et al., 2001; Pahan et al., 2001, 2002). Twenty-four hours after transfection, cells were incubated with serum-free media. After 6 h of incubation, cells were harvested and RNA was analyzed by semi-quantitative and real time PCR.
3. Results
3.1. IL-12 p40 homodimer (p402), but not IL-12 p70, induces the expression of IL-16 in BV-2 microglial cells
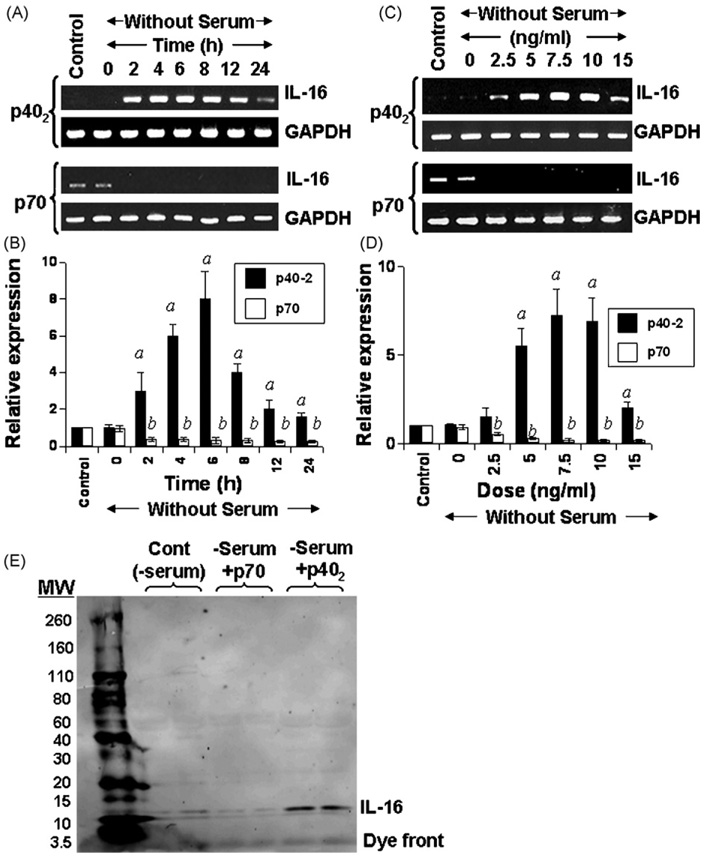
Molecules or agents that stimulate/induce the expression of IL-16, an important T cell chemoattractant factor (TCF), in microglia/macrophages are not known. Earlier we have demonstrated that p402 and IL-12p70 are capable of inducing the expression of nitric oxide synthase (iNOS) and TNF-α in microglia and macrophages (Jana et al., 2003; Pahan et al., 2001). To determine whether p402 and p70 play any role in the induction of IL-16 in microglia, we treated the mouse BV-2 microglial cells with 10 ng/ml p402 and p70 for different time period. Serum withdrawal alone did not modulate the expression of IL-16 (Fig. 1A–D). It is clearly evident from Fig. 1A that p402 markedly induced the mRNA expression of IL-16 in microglial cells at different h of incubation. Although the mRNA level of IL-16 increased significantly within 2 h of stimulation, the maximum increase was observed at 6 h of challenge followed by a gradual decrease at further h of stimulation (Fig. 1A). On the other hand, p70 was unable to induce the expression of IL-16 at different h of stimulation (Fig. 1A). In fact, p70 treatment led to marginal inhibition of IL-16 mRNA expression compared to untreated cells (Fig. 1A). Real-time PCR results of Fig. 1B also confirm that p402, but not p70, induced the expression of IL-16 mRNA in BV-2 microglial cells at different h of stimulation exhibiting the maximum induction at 6 h.
Fig. 1.
Time- and dose-dependent induction of IL-16 expression by IL-12 p40 homodimer (p402) and p70 in mouse BV-2 microglial cells. Cells were stimulated with p402(10 ng/ml) and p70 (10 ng/ml) under serum-free conditions. At different time point of stimulation, total RNA was analyzed for the expression of IL-16 by semi-quantitative RT-PCR (A) and quantitative real time PCR (B). Cells were stimulated with different concentration of p402 and p70 under serum free condition. After 6 h of stimulation, the mRNA expression of IL-16 was monitored by semi-quantitative RT-PCR (C) and quantitative real time PCR (D). Results are means ± S.D. of three different experiments. ap < 0.001 vs control for p402; bp < 0.001 vs control for p70. (E) Cells (5 × 105) were stimulated with p402 (10 ng/ml) and p70 (10 ng/ml) under serum-free conditions. After 24 h, supernatants were collected, concentrated, loaded onto SDS–PAGE, and immunoblotted with antibodies against IL-16. Two separate wells from each treatment group have been processed and shown. Results represent three independent experiments.
Dose-dependent studies in Fig. 1C and D also show that p402, but not p70, induced the expression of IL-16 mRNA in BV-2 microglial cells. Although the induction was observed at a concentration of 2.5 ng/ml p402, the maximum increase was recorded at 7.5 or 10 ng/ml p402 (Fig. 1C and D). On the other hand, the expression of IL-16 mRNA decreased at a concentration of 15 ng/ml p402 (Fig. 1C and D). Similar to that observed during time-dependent studies (Fig. 1A and B), p70, at different doses tested, inhibited the expression of IL-16 mRNA (Fig. 1C and D).
An ELISA system is not available to monitor the level of mouse IL-16 in serum or supernatants. Therefore, to understand if p402 induced the release of IL-16 protein, BV-2 microglial cells were stimulated with p402 followed by western blot of supernatants using antibodies against IL-16. Consistent to the effect of p402 and p70 on the mRNA expression of IL-16, p402, but not p70, increased the production of IL-16 from microglial cells (Fig. 1E).
3.2. IL-12p402, but not IL-12p70, induces the expression of IL-16 in primary mouse microglia
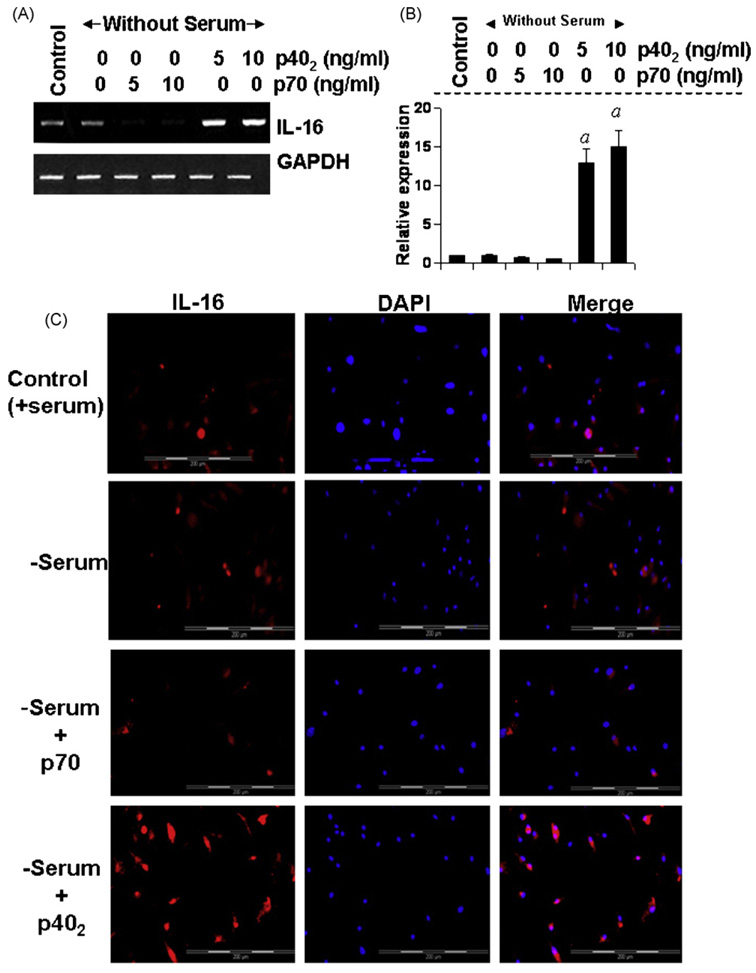
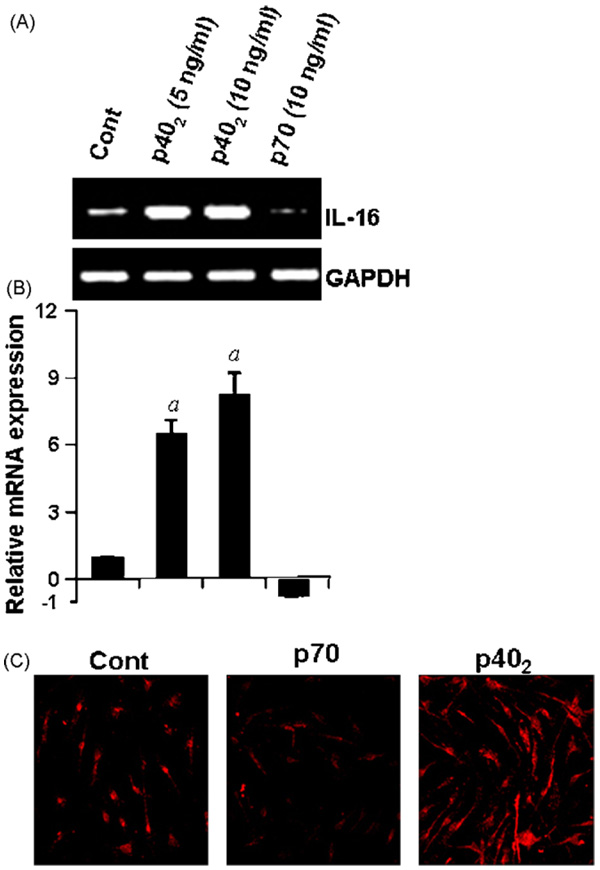
Next, to investigate whether p402 induces the expression of IL-16 in primary cells, primary microglia isolated from 7 to 9-day-old mouse pups, were stimulated with different concentrations of p402 and p70 for 6h and the expression of IL-16 was analyzed. Semi-quantitative RT-PCR results in Fig. 2A and quantitative real-time PCR results in Fig. 2B clearly show that p402, at different doses tested, markedly increased the mRNA expression of IL-16 whereas p70 inhibited the mRNA expression of IL-16 as compared to control. Immunofluorescence analysis in Fig. 2C also shows that p402 markedly increased the expression of IL-16 protein in primary microglia. On the other hand, p70 suppressed the level of IL-16 protein in microglia compared to untreated controls (Fig. 2B). Serum withdrawal alone was unable to modulate the expression of IL-16 at the level of either mRNA (Fig. 2A and B) or protein (Fig. 2C). These results show that p402, but not p70, is capable of inducing the expression of IL-16 in primary mouse microglia.
Fig. 2.
Effect of p402 and p70 on the expression of IL-16 in primary mouse microglia. Microglia isolated from 7 to 9-day-old mouse pups were stimulated with different concentrations of p402 and p70 under serum-free condition. After 6 h of stimulation, the mRNA expression of IL-16 was monitored by semi-quantitative RT-PCR (A) and real-time PCR (B). ap < 0.001 vs control. After 18 h of stimulation, the expression of IL-16 protein was monitored by immunofluorescence (C). DAPI was used to visualize nucleus. Results represent three independent experiments.
3.3. IL-12p402, but not IL-12p70, induces the expression of IL-16 in primary human microglia
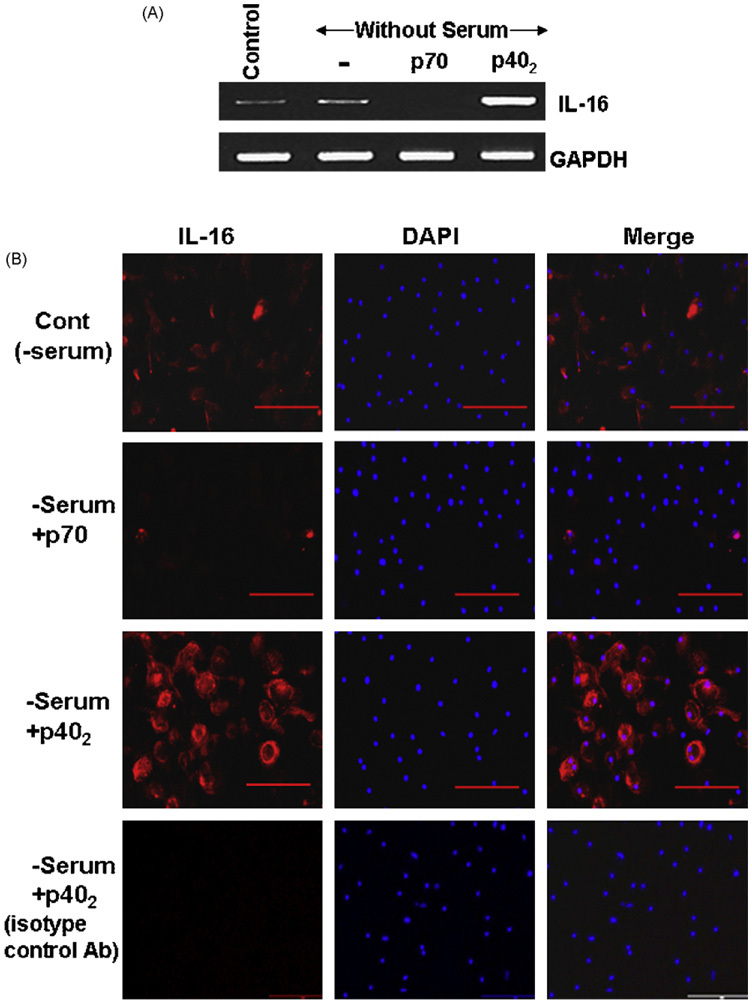
Results from mouse cells are not often replicated in the human system. Therefore, next we examined the effect of p402 and p70 on the expression of IL-16 in primary human microglia. Microglia isolated from 13 to 17-week-old human fetal brains were stimulated with 10 ng/ml p402 and p70 separately. As evident from semi-quantitative RT-PCR results in Fig. 3A, p70 suppressed while p402 increased the expression of IL-16 mRNA in human fetal microglia. Again serum withdrawal had no effect on mRNA expression of IL-16 (Fig. 3A). Next we examined the protein level of IL-16 by immunofluorescence analysis. Similar to that found in mouse microglia, Fig. 3B showed stimulation of IL-16 protein expression by p402 and inhibition of IL-16 protein expression by p70 in human microglia. Immunofluorescence staining with an isotype control antibody (bottom panel) shows that the experiment was specific and that p402 induced the expression of IL-16 protein in human microglia (Fig. 3B).
Fig. 3.
Effect of p402 and p70 on the expression of IL-16 in primary human microglia. Microglia isolated from 13 to 17-week-old human fetal brains were stimulated with 10 ng/ml p402 and p70 separately under the serum-free condition. (A) After 6 h of stimulation, the mRNA expression of IL-16 was monitored by semi-quantitative RT-PCR. (B) After 18 h of stimulation, the expression of IL-16 protein was monitored by immunofluorescence using antibodies against mouse IL-16. Bottom panel represents labeling of p402-stimulated cells with isotype control antibodies. DAPI was used to visualize nucleus. Results represent three independent experiments.
3.4. IL-12p402, but not IL-12p70, induces the expression of IL-16 in mouse macrophages
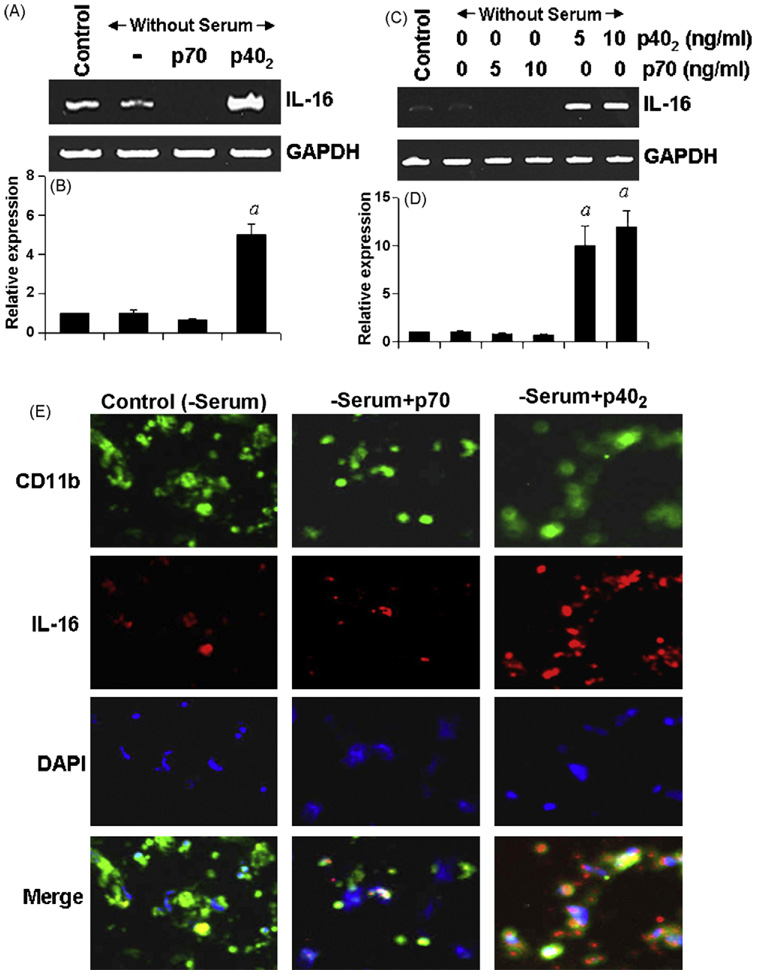
Results described above clearly indicate that p402 induces the expression of IL-16 mRNA and protein in CNS microglia. In addition to controlling CNS inflammatory disease, IL-16 is also known to be involved in the development of several peripheral inflammatory and autoimmune disorders. Peripheral macrophages are also an important producer of IL-16. Therefore, we investigated if p402 was also capable of increasing the expression of IL-16 in macrophages. Mouse peritoneal macrophages and RAW 264.7 cells were treated with p402 and p70 for 6 h under serum-free condition and the expression of IL-16 mRNA was analyzed by semi-quantitative RT-PCR and real-time PCR. Similar to that observed in microglia, the mRNA expression of IL-16 was stimulated by p402 but inhibited by p70 in mouse peritoneal macrophages (Fig. 4A and B) and RAW264.7 cells (Fig. 4C and D). Serum withdrawal had no effect on the expression of IL-16 in both cell types (Fig. 4). Immunofluorescence analysis also demonstrates an increase in IL-16 protein expression by p402 and a decrease in IL-16 protein expression by p70 in mouse peritoneal macrophages (Fig. 4E).
Fig. 4.
Effect of p402 and p70 on the expression of IL-16 in mouse primary macrophages and RAW cells. Mouse peritoneal macrophages were stimulated with 10 ng/ml p402 and p70 separately under the serum-free condition. After 6 h of stimulation, the mRNA expression of IL-16 was monitored by semi-quantitative RT-PCR (A) and quantitative real-time PCR (B). ap < 0.001 vs control. RAW cells were stimulated with different concentrations of p402 and p70 under the serum-free condition. After 6 h of stimulation, the mRNA expression of IL-16 was monitored by semi-quantitative RT-PCR (C) and quantitative real-time PCR (D). ap < 0.001 vs control. After 18 h of stimulation by 10 ng/ml p402 and p70, the expression of IL-16 protein was monitored by immunofluorescence in peritoneal macrophages (E). DAPI was used to visualize nucleus. Results represent three independent experiments.
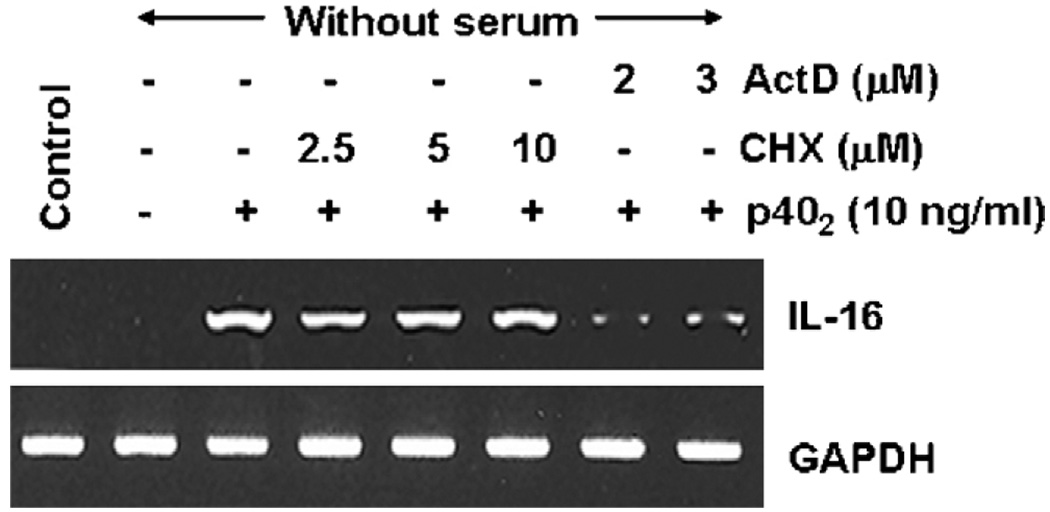
3.5. Does p402-induced expression of IL-16 in microglial cells depend on new protein synthesis?
To understand if new protein synthesis was required for the expression of IL-16 after p402 stimulation, BV-2 microglial cells pretreated with different concentrations of cycloheximide, a protein synthesis inhibitor, were challenged by p402. It is clearly evident from Fig. 5 that cycloheximide at a dose of even 10 µM was unable to suppress the mRNA expression of IL-16 in p402-stimulated microglial cells suggesting that p402 does not require any new protein synthesis for the induction of IL-16 in microglial cells. On the other hand, as expected, actinomycin D, an inhibitor of transcription, markedly inhibited the mRNA expression of IL-16 (Fig. 5).
Fig. 5.
Effect of cycloheximide and actinomycin D on p402-induced expression of IL-16 in mouse BV-2 microglial cells. Cells preincubated with different concentrations of cycloheximide (CHX) and actinomycin D (ActD) for 30 min were stimulated with p402 (10 ng/ml) under serum-free conditions. After 6 h of stimulation, the mRNA expression of IL-16 was monitored by semi-quantitative RT-PCR. Results represent three independent experiments.
3.6. Effect of different proinflammatory molecules on the activation of IL-16 promoter in BV-2 microglial cells
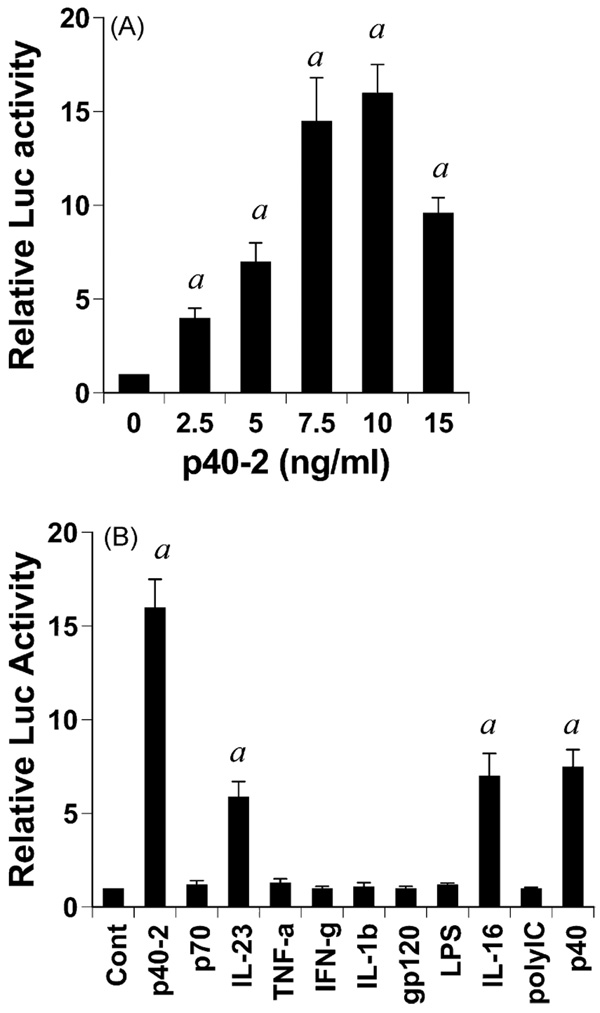
To understand the mechanism of p402-mediated stimulation of IL-16 mRNA, we examined the effect of p402 on the activation of IL-16 promoter in BV-2 microglial cells. Cells were transfected with the reporter plasmid PGL3-1320 containing IL-16 promoter fragment (−1237 to +83) relative to the transcription start site. As evident from Fig. 6A, p402 dose-dependently increased IL-16 promoter-driven luciferase activity in microglial cells. Although p402 induced the activation of IL-16 promoter significantly at a concentration of 2.5 ng/ml, the activation of IL-16 promoter was maximum at a dose of 7.5 or 10ng/ml p402 and decreased at higher concentrations (Fig. 6A). Next we examined the effect of other proinflammatory stimuli on the activation of IL-16 promoter. Surprisingly, bacterial LPS, the prototype inducer of inflammatory reactions, did not induce IL-16 promoter-driven luciferase activity in microglial cells (Fig. 6B). Similarly, most common proinflammatory cytokines such as, TNF-α, IL-1 β and IFN-γ were also ineffective in inducing the activation of IL-16 promoter (Fig. 6B). Double-stranded RNA (dsRNA), the active component of viral infection, is known to trigger many inflammatory processes. Similarly, HIV-1 coat protein gp120 (glycoprotein 120) also induces inflammatory reactions in many cell types including glial cells. However, these virotoxins (dsRNA and gp120) also remained ineffective in activating IL-16 promoter in microglia (Fig. 6B). Out of all these proinflammatory stimuli tested, only p402, IL-23, p40 monomer (p40), and IL-16 itself were capable of inducing IL-16 promoter-driven luciferase activity (Fig. 6B). Incidentally, p402 has been found to be the strongest inducer of IL-16 promoter activation in microglia (Fig. 6B).
Fig. 6.
Effect of different proinflammatory molecules on IL-16 promoter-driven luciferase activity in mouse BV-2 microglial cells. Cells plated at 50–60% confluence in twelve-well plates were transfected with 0.25 µg pIL-16-Luc and 25 ng pRL-TK (Renilla luciferase control) by Lipofectamine Plus (Invitrogen) as described Section 2. Twenty-four hours after transfection, cells were stimulated with different concentrations of p402 (A) or different stimuli (B) for 6 h under serum free condition. Firefly and Renilla luciferase activities were determined by Dual Luciferase Kit (Promega) following the manufacturer’s protocol. Data are mean ± S.D. of three separate experiments. ap < 0.001 vs control. Concentrations of different stimuli are as follows: p402, 10 ng/ml; LPS, 1 µg/ml; p70, 10 ng/ml; IL-23, 10 ng/ml; TNF-α, 20 ng/ml; IFN-γ, 12.5 mU/ml; gp120, 200 pg/ml; IL-16, 10 ng/ml; poly IC, 100 µg/ml; IL-1β, 10 ng/ml; p40, 10 ng/ml.
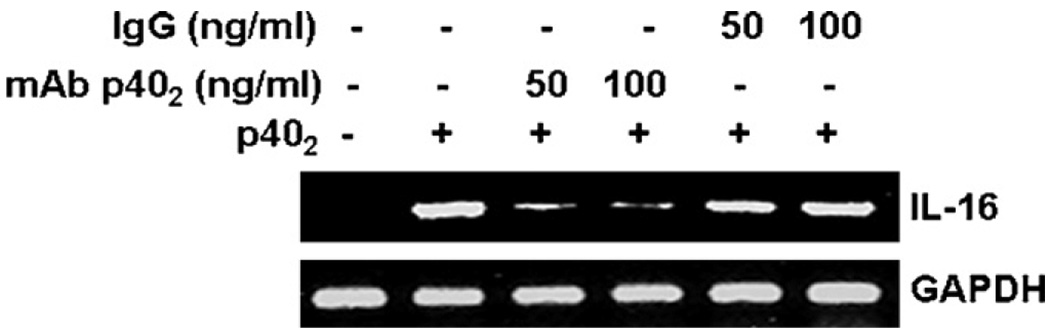
Because p40 monomer was also capable of inducing the activation of IL-16 promoter, we examined if IL-16-inducing activity of p402 was due to a minor presence of p40. Recently, we have generated functional blocking monoclonal antibodies (mAbs) against mouse p402 and p40 in hamster (Dasgupta et al., 2008). We used mAb a3-1d to neutralize p402 (Dasgupta et al., 2008). Fig. 7 shows that mAbs against p402, but not normal hamster IgG, suppressed p402-induced expression of IL-16 in BV-2 microglial cells. Although we cannot rule out the possibility that commercial p402 may have a minor amount of p40 as a contaminant, our results that p402 is a much stronger inducer of IL-16 than p40 and that mAbs against p402 abrogate the IL-16-inducing activity of p402 clearly suggest p402 as an inducer of IL-16 in microglia.
Fig. 7.
Effect of functional blocking monoclonal antibodies (a3-1d) against p402 on p402-induced expression of IL-16 in mouse BV-2 microglial cells. Cells preincubated with different concentrations of either mAb a3-1d or normal hamster IgG for 30 min were stimulated with p402 (10 ng/ml)under serum-free conditions. After 6 h of stimulation, the mRNA expression of IL-16 was monitored by semi-quantitative RT-PCR. Results represent three independent experiments.
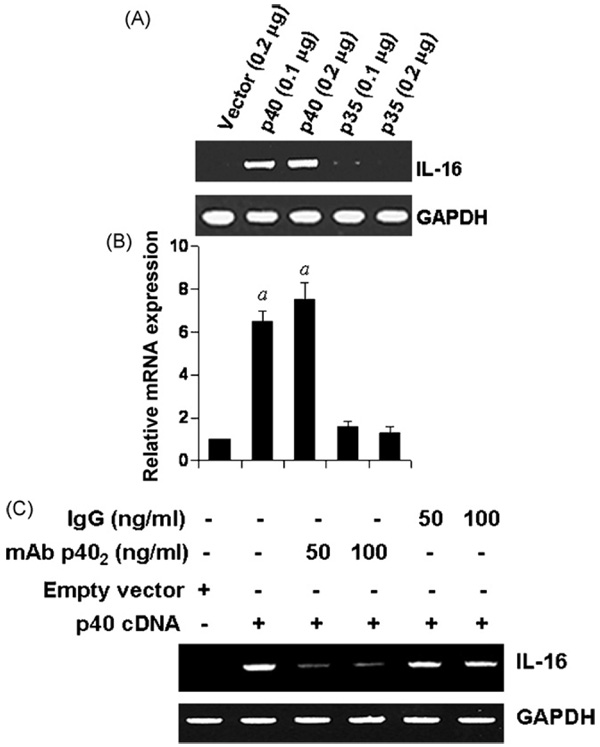
3.7. Expression of mouse p40 cDNA induces the expression of IL-16 in BV-2 microglial cells
We used recombinant mouse p402 for the stimulation of microglia and macrophages. We have also demonstrated that p40 is not responsible for the IL-16-inducing activity of p402 (Fig. 7). However, it is possible that any other unknown contaminant in p402 preparation is actually inducing the expression of IL-16. To investigate the validity of this possibility, we examined the effect of transient expression of mouse p40 and p35 cDNA (Pahan et al., 2001) on the expression of IL-16 in BV-2 microglial cells. As evident from semi-quantitative RT-PCR analysis in Fig. 8A and quantitative real-time PCR analysis in Fig. 8B, expression of p40 cDNA but neither p35 nor an empty vector induced the expression of IL-16 mRNA. After over-expression of p40, microglial cells could produce p402, p40, p40:p35 (IL-12), and p40:p19 (IL-23) suggesting that these cytokines together, but not p402 alone, might increase the expression of IL-16. To examine the validity of this possibility, we used functional blocking mAbs against p402 (Dasgupta et al., 2008). The mAb a3-1d, but not normal hamster IgG, markedly suppressed p402-induced expression of IL-16 in p40 cDNA-transfected microglial cells (Fig. 8C). These results suggest that the expression of IL-16 in p40 cDNA-transfected cells is primarily due to p402.
Fig. 8.
Expression of p40, but not p35, cDNA induces the expression of IL-16 in BV2 microglial cells: Microglial cells plated in 12-well plates were transfected with different amounts of either p40 or p35 cDNA by LipofectAMINE Plus (Invitrogen). Empty vector (pCIneo mammalian expression vector from Promega) was used as control. After 24 h of transfection, cells were incubated in serum-free media. After 6 h, the mRNA expression of IL-16 was monitored by semi-quantitative RT-PCR (A) and quantitative real-time PCR (B). ap < 0.001 vs control. C) After 24 h of transfection, cells were incubated in serum-free media in the presence or absence of different concentrations of either p402 mAb a3-1d or normal hamster IgG. After 6 h, the mRNA expression of IL-16 was monitored by semi-quantitative RT-PCR. Results represent three independent experiments.
3.8. IL-12 p402 induces the expression of IL-16 in mouse primary microglia independent of p35
It is known that biologically active p70 is comprised of 35kDa (p35) and 40 kDa (p40) subunits (Gately et al., 1998). Therefore, one may envision that p40 of p402 may interact with p35 of microglia and the resulting molecule is involved in the induction of IL-16. To sign off/on this possibility, we examined the effect of mouse p402 on the induction of IL-16 in primary microglia isolated from p35 (−/−) mice. Cells were treated with p402 and p70 for 6h and mRNA expression was analyzed both by semi-quantitative RT-PCR and real-time PCR. As expected, similar to that observed in wild type microglia, p70 suppressed the expression of IL-16 mRNA (Fig. 9A and B) and protein (Fig. 9C) expression in p35 (−/−) microglia. Increase in IL-16 mRNA (Fig. 7A and B) and protein (Fig. 9C) expression by p402 in p35 (−/−) microglia suggest that p402 does not need any co-operation from p35 for the induction of IL-16 in microglia.
Fig. 9.
Effect of p402 and p70 on the expression of IL-16 in p35 (−/−) microglia. Microglia isolated from wild type and p35 (−/−) mice were stimulated with different concentrations of p402 under serum-free condition. After 6 h of stimulation, the mRNA expression of IL-16 was monitored by RT-PCR (A) and real time PCR (B). After 18 h of stimulation, cells were immunostained with antibodies against IL-16 (C). Results are means ± S.D. of three different experiments. ap < 0.001 vs control.
3.9. IL-12 p402 induces the expression of IL-16 via IL-12Rβ1, but not IL-12Rβ2
Next we investigated mechanisms by which p402 induces the expression of IL-16 in microglia. Previous studies have demonstrated that p402 binds to IL-12Rβ1 (Trinchieri et al., 2003) whereas p70 interacts with both IL-12Rβ1 and IL-12Rβ2 inT-cells (Gately et al., 1998), and microglia/macrophages express both IL-12Rβ1 and IL-12Rβ2(Cua et al., 2003). However, it is not known if p70 and p402 are utilizing any of these two receptors to induce the expression of IL-16 in microglia.
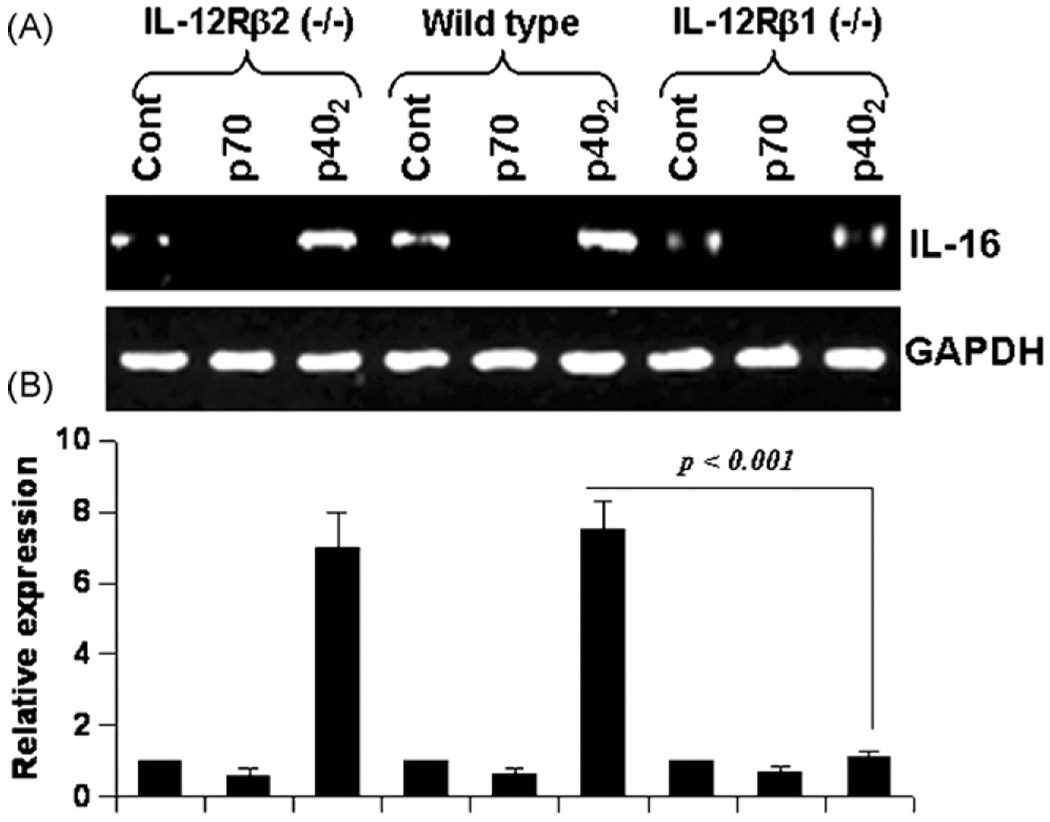
Therefore, primary microglia isolated from wild type, IL-12Rβ1 (−/−) and IL-12Rβ2(−/−) mice were challenged with p70 and p402. We monitored IL-16 mRNA expression by semi-quantitative RT-PCR and quantitative real-time PCR. As expected, p402, but not p70, induced the expression of IL-16 mRNA (Fig. 10A and B) in microglia isolated from wild type mice. However, p402 was unable to induce the expression of IL-16 mRNA (Fig. 8A and B) in microglia isolated from IL-12Rβ1(−/−) mice. In contrast, p402 induced the expression of IL-16 mRNA (Fig. 10A and B) in microglia isolated from IL-12Rβ2 (−/−) mice. These results clearly suggest that p402 requires IL-12Rβ1, but not IL-12Rβ2, for the expression of IL-16. On the other hand, p70 was unable to induce the expression of IL-16 in microglia isolated from wild type, IL-12Rβ1 (−/−) and IL-12Rβ2 (−/−) mice (Fig. 10A and B).
Fig. 10.
Effect of p402 and p70 on the expression of IL-16 in primary microglia isolated from wild type, IL-12Rβ1 (−/−) and IL-12Rβ2 (−/−) mice: Microglia isolated from B6.129 wild-type, IL-12 Rβ1 (−/−) and IL-12Rβ2 (−/−) mice were stimulated with 10 ng/ml of p402 and p70 under serum free condition. After 6 h of stimulation, the mRNA expression of IL-16 was monitored by semi-quantitative RT-PCR (A) and quantitative real-time PCR (B). Results are means ± S.D. of three different experiments.
4. Discussion
Being a leukocyte chemoattractant factor, IL-16 may contribute to the trafficking of CD4+ve cells to the CNS under autoimmune and neuroinflammatory conditions (Schluesener et al., 1996; Schwab et al., 2001a). Recently Guo et al. (2004) have shown that IL-16 was highly expressed in microglia during both early and late phases of the experimental allergic encephalomyelitis (EAE). This study suggests that IL-16 (+ve) microglia are among the earliest cells to respond to neuroinflammation. IL-16 expression is cell specific and is regulated at multiple levels. Furthermore, several reports indicate that only activated microglia in the CNS express IL-16 mRNA and protein (Engel et al., 2000; Schwab et al., 2001a,b; Yoshida et al., 2001). Though mitogens and antigens can activate transcription of IL-16 in T-cells, but the stimulus that induces the expression of IL-16 in macrophages/microglia has not been defined. Therefore, identifying stimuli that trigger the expression of IL-16 in microglia and understanding underlying mechanisms for the regulation of microglial IL-16 are important areas of investigation.
Although p40 is a component of the bioactive cytokines IL-12 and IL-23, p402 (the p40 homodimer) is not considered as an intrinsically functional cytokine. Earlier we have demonstrated that both p402, the so-called inactive molecule, and IL-12p70, the biologically active cytokine, are capable of inducing the expression of nitric oxide synthase (iNOS) and TNF-α in microglia and macrophages (Jana et al., 2003; Pahan et al., 2001). Several lines of evidence presented in this study clearly support the conclusion that p402, but not p70, induces the expression of IL-16 in microglia and macrophages. This conclusion was based on the following observations. First, p402 induced the expression of IL-16 mRNA and protein in human and mouse microglia and mouse macrophages. On the other hand, p70 treatment led to an inhibition of IL-16 expression as compared to control. Second, p402 markedly induced the activation of IL-16 promoter as monitored by IL-16 promoter-driven luciferase activity. On the other hand, common inflammatory stimuli (IL-12 p70, bacterial LPS, TNF-α, IL-1 β, IFN-γ, double-stranded RNA, and HIV-1 gp120) were unable to induce the activation of IL-16 promoter. Only three other stimuli (p40 monomer, IL-23 and IL-16 itself) were capable of stimulating the activation of IL-16 promoter, although at a potency weaker than p402. Third, the expression of mouse p40, but not mouse p35, cDNA induced the expression of IL-16 in microglial cells. Furthermore, a functional blocking mAb against p402 suppressed the expression of IL-16 in p40 cDNA-transfected microglial cells. These results suggest that the p40, but not the p35, subunit of IL-12 is involved in the induction of IL-16 and that p402 is primarily involved in this process. Fourth, p402 was capable of inducing the expression of IL-16 in microglia isolated from p35 (−/−) mice suggesting that p402 does not need any involvement from p35 for the induction of IL-16 in microglia.
Yoshida et al. (2001) have shown that IL-4, IL-9, IL-16, and TNF-α alone do not exhibit any significant effect on the expression of IL-16 mRNA or IL-16 protein in bronchial epithelial cell line. However, this same cell line produces IL-16 after synergistic stimulation by either (IL-4 + IL-16) or (IL-9 + IL-16). It appears that only a cytokine combination involving IL-16 as a partner would be able to induce the expression of IL-16 in bronchial epithelial cells. Similarly, we have found that IL-16 alone is also capable of inducing the activation of IL-16 promoter in microglia. However, in addition to IL-16, we have identified three other stimuli (p402, IL-23 and p40 monomer) for the activation of IL-16 promoter in microglia and most importantly, p402 has emerged out as the most potent stimulus for IL-16 in our study.
Infiltration of inflammatory cells into the CNS is an important event in the pathogenesis of neuroinflammatory disorders including MS and EAE. Although biological functions of IL-16 have not been clearly defined, its chemotactic activity seems to be the most prominent one and it does not require any earlier activation of target cells. Therefore, IL-16 may act as an initial chemokine for the recruitment of leukocytes/mononuclear cells for the development of local inflammation. One recent report shows that p402, but not IL-12, results in macrophage chemotaxis that is independent of IL-12 and mediated through the IL-12Rα1 (Russell et al., 2003). Although the article did not demonstrate any mechanism behind the chemotactic activity of p402, our results suggest that p402 may facilitate the recruitment of leukocytes/mononuclear cells into the CNS via increasing the production of IL-16 in microglia and macrophages. In addition to having chemotactic activity, according to Zhang et al. (2007), IL-16 also primes CD4+/CD14+ monocytes and mature macrophages for the secretion of IL-1 β, IL-6, IL-15 and TNF-α (Zhang et al., 2007). Therefore, p402 may also amplify the secretion of proinflammatory molecules from monocytes and macrophages via IL-16 production.
Intracellular signaling events that lead to the expression of IL-16 have been poorly defined. IL-12 p402 has been shown to antago nize bioactive IL-12 p70 by binding to the IL-12 receptor complex (Gately et al., 1998). The high affinity IL-12 receptor is composed of a low affinity IL-12Rβ1 combined with a low affinity IL-12Rβ2. It appears that p402 binds to IL-12Rβ1 rather than IL-12Rβ2 whereas bioactive IL-12 binds both the receptors on T cells with high affinity (Gately et al., 1998). Therefore, it is possible that p402 may engage IL-12Rs for the expression of IL-16 in microglia. Here we present evidence that p402 induces the expression of IL-16 via IL-12Rβ1 but not IL-12Rβ2. While p402 was unable to induce the expression of IL-16 in primary microglia isolated from IL-12Rβ1 (−/−) mice, IL-12Rβ2 knockdown had no effect on p402-induced expression of IL-16 in microglia. This is an interesting observation because only IL-12Rβ2 subunit has been reported to function as the signal transducing component of the high-affinity receptor complex (Gately et al., 1998). This has been suggested due to the absence of tyrosine residues in the cytoplasmic domain of IL-12Rβ1 and the presence of conserved tyrosine residues in the cytoplasmic domain of IL-12Rβ2 (Gately et al., 1998). Therefore, the question remains: How does the non signal transducing receptor IL-12Rβ1 engage itself for the transcription of IL-16 by p402? Probably, one could foresee the engagement of another putative signal transducing receptor with the binding receptor IL-12Rβ1. Works are underway in this laboratory to investigate this possibility.
One previous report indicates that the activation of T lymphocytes by antigens, PHA, and other mitogens strongly increases the transcription of IL-16 and the activation of IL-16 promoter (Bannert et al., 1999). Bannert et al. (1999) have also demonstrated that IL-16 promoter lacking any TATA box harbors two CAAT box-like motifs and three binding sites for GA-binding protein (GABP) transcription factors which are required for full IL-16 promoter activity. Therefore, it is possible that p402 is able to induce the activation of GABP transcription factor in microglia via IL-12Rβ1. However, at present, we do not know why p70 capable of binding both IL-12Rβ1 and IL-12Rβ2 does not induce IL-16? We Wondered if IL-12Rβ2 was transducing a negative regulatory signal for the expression of IL-16. However, p70 remained ineffective in inducing the expression of IL-16 in IL-12Rβ2 (−/−) microglia shooting down our hypothesis. Therefore, it is likely that engagement of both IL-12Rβ1 and IL-12 Rβ2 by p70 induces putative negative regulatory signal(s) for the expression of IL-16 in microglia.
Microglia are considered as CNS-resident professional macrophages and sensor cells that function as the principal immune effectors cells of the CNS responding to any pathological event. Activation of microglia has been implicated in the pathogenesis of a variety of neurodegenerative diseases, including multiple sclerosis (MS) (Gonzalez-Scarano and Baltuch, 1999; Minagar et al., 2002). It has been found that activated microglia accumulate at sites of demyelination in MS patients. Although the in vitro situation of mouse and human microglia in culture and its treatment with p402 may not truly resemble the in vivo situation of microglia in the brain of patients with MS, our results identify p402 as a possible candidate for controlling the leukocyte chemoattractant factor IL-16.
Acknowledgements
This study was supported by grants from NIH (NS39940 and NS48923) and National Multiple Sclerosis Society (RG3422A1/1).
References
- Bannert N, Avots A, Baier M, Serfling E, Kurth R. GA-binding protein factors, in concert with the coactivator CREB binding protein/p300, control the induction of the interleukin 16 promoter in T lymphocytes. Proc. Natl. Acad. Sci. USA. 1999;96:1541–1546. doi: 10.1073/pnas.96.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright JJ, Musuro BF, Du C, Sriram S. Expression of IL-12 in CNS and lymphoid organs of mice with experimental allergic encephalitis. J. Neuroimmunol. 1998;82:22–30. doi: 10.1016/S0165-5728(97)00184-7. [DOI] [PubMed] [Google Scholar]
- Constantinescu CS, Goodman DB, Hilliard B, Wysocka M, Cohen JA. Murine macrophages stimulated with central and peripheral nervous system myelin or purified myelin proteins release inflammatory products. Neurosci. Lett. 2000;287:171–174. doi: 10.1016/s0304-3940(00)01184-8. [DOI] [PubMed] [Google Scholar]
- Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–38. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Cruikshank WW, Kornfeld H, Center DM. Signaling and functional properties of interleukin-16. Int. Rev. Immunol. 1998;16:523–540. doi: 10.3109/08830189809043007. [DOI] [PubMed] [Google Scholar]
- Cruikshank WW, Kornfeld H, Center DM. Interleukin-16. J. Leukoc. Biol. 2000;67:757–766. doi: 10.1002/jlb.67.6.757. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Bandopadhyay M, Pahan K. Generation of functional blocking monoclonal antibodies against mouse interleukin-12 p40 homodimer and monomer. Hybridoma (Larchmt) 2008;27:141–151. doi: 10.1089/hyb.2007.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S, Schluesener H, Mittelbronn M, Seid K, Adjodah D, Wehner HD, Meyermann R. Dynamics of microglial activation after human traumatic brain injury are revealed by delayed expression of macrophage-related proteins MRP8 and MRP14. Acta Neuropathol. 2000;100:313–322. doi: 10.1007/s004019900172. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Ragoschke A, Rossol S, Schwartz A, Mielke O, Paulig A, Hennerici M. Increased release of interleukin-12p40 in MS: association with intracerebral inflammation. Neurology. 1998;51:753–758. doi: 10.1212/wnl.51.3.753. [DOI] [PubMed] [Google Scholar]
- Franz JK, Kolb SA, Hummel KM, Lahrtz F, Neidhart M, Aicher WK, Pap T, Gay RE, Fontana A, Gay S. Interleukin-16, produced by synovial fibroblasts, mediates chemoattraction for CD4+ T lymphocytes in rheumatoid arthritis. Eur. J. Immunol. 1998;28:2661–2671. doi: 10.1002/(SICI)1521-4141(199809)28:09<2661::AID-IMMU2661>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J. Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Guo LH, Mittelbronn M, Brabeck C, Mueller CA, Schluesener HJ. Expression of interleukin-16 by microglial cells in inflammatory, autoimmune, and degenerative lesions of the rat brain. J. Neuroimmunol. 2004;146:39–45. doi: 10.1016/j.jneuroim.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Jana M, Pahan K. Redox regulation of cytokine-mediated inhibition of myelin gene expression in human primary oligodendrocytes. Free Radic Biol. Med. 2005;39:823–831. doi: 10.1016/j.freeradbiomed.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: implications for multiple sclerosis. J. Neuroimmune Pharmacol. 2007;2:184–193. doi: 10.1007/s11481-007-9066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J. Biol. Chem. 2001;276:44527–44533. doi: 10.1074/jbc.M106771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Dasgupta S, Saha RN, Liu X, Pahan K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J. Neurochem. 2003;86:519–528. doi: 10.1046/j.1471-4159.2003.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Anderson JA, Saha RN, Liu X, Pahan K. Regulation of inducible nitric oxide synthase in proinflammatory cytokine-stimulated human primary astrocytes. Free Radic. Biol. Med. 2005;38:655–664. doi: 10.1016/j.freeradbiomed.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Jana M, Jana A, Liu X, Ghosh S, Pahan K. Involvement of phosphatidylinositol 3-kinase-mediated up-regulation of I kappa B alpha in anti-inflammatory effect of gemfibrozil in microglia. J. Immunol. 2007a;179:4142–4152. doi: 10.4049/jimmunol.179.6.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M, Jana A, Pal U, Pahan K. A simplified method for isolating highly purified neurons, oligodendrocytes, astrocytes, and microglia from the same human fetal brain tissue. Neurochem. Res. 2007b;32:2015–2022. doi: 10.1007/s11064-007-9340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keates AC, Castagliuolo I, Cruickshank WW, Qiu B, Arseneau KO, Brazer W, Kelly CP. Interleukin 16 is up-regulated in Crohn’s disease and participates in TNBS colitis in mice. Gastroenterology. 2000;119:972–982. doi: 10.1053/gast.2000.18164. [DOI] [PubMed] [Google Scholar]
- Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, Ghilardi N, Desauvage FJ, Lund FE, Cooper AM. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J. Exp. Med. 2006;203:1805–1815. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge S, Ernst P, Ghaffar O, Cruikshank WW, Kornfeld H, Center DM, Hamid Q. Increased expression of interleukin-16 in bronchial mucosa of subjects with atopic asthma. Am. J. Respir. Cell. Mol. Biol. 1997;17:193–202. doi: 10.1165/ajrcmb.17.2.2750. [DOI] [PubMed] [Google Scholar]
- Mannie MD, Abbott DJ. A fusion protein consisting of IL-16 and the encephalitogenic peptide of myelin basic protein constitutes an antigen-specific tolerogenic vaccine that inhibits experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:1458–1465. doi: 10.4049/jimmunol.179.3.1458. [DOI] [PubMed] [Google Scholar]
- Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J. Neurol. Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- Mueller CA, Schluesener HJ, Conrad S, Pietsch T, Schwab JM. Spinal cord injury-induced expression of the immune-regulatory chemokine interleukin-16 caused by activated microglia/macrophages and CD8+ cells. J. Neurosurg. Spine. 2006;4:233–240. doi: 10.3171/spi.2006.4.3.233. [DOI] [PubMed] [Google Scholar]
- Pahan K, Sheikh FG, Liu X, Hilger S, McKinney M, Petro TM. Induction of nitric-oxide synthase and activation of NF-kappaB by interleukin-12 p40 in microglial cells. J. Biol. Chem. 2001;276:7899–7905. doi: 10.1074/jbc.M008262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahan K, Jana M, Liu X, Taylor BS, Wood C, Fischer SM. Gemfibrozil, a lipid-lowering drug, inhibits the induction of nitric-oxide synthase in human astrocytes. J. Biol. Chem. 2002;277:45984–45991. doi: 10.1074/jbc.M200250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell TD, Yan Q, Fan G, Khalifah AP, Bishop DK, Brody SL, Walter MJ. IL-12 p40 homodimer-dependent macrophage chemotaxis and respiratory viral inflammation are mediated through IL-12 receptor beta 1. J. Immunol. 2003;171:6866–6874. doi: 10.4049/jimmunol.171.12.6866. [DOI] [PubMed] [Google Scholar]
- Saha RN, Jana M, Pahan K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J. Immunol. 2007;179:7101–7109. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluesener HJ, Seid K, Kretzschmar J, Meyermann R. Leukocyte chemotactic factor, a natural ligand to CD4, is expressed by lymphocytes and microglial cells of the MS plaque. J. Neurosci. Res. 1996;44:606–611. doi: 10.1002/(SICI)1097-4547(19960615)44:6<606::AID-JNR11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Schoenhaut DS, Chua AO, Wolitzky AG, Quinn PM, Dwyer CM, McComas W, Familletti PC, Gately MK, Gubler U. Cloning and expression of murine IL-12. J. Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- Schwab JM, Nguyen TD, Meyermann R, Schluesener HJ. Human focal cerebral infarctions induce differential lesional interleukin-16 (IL-16) expression confined to infiltrating granulocytes. CD8+ T-lymphocytes and activated microglia/macrophages. J. Neuroimmunol. 2001a;114:232–241. doi: 10.1016/s0165-5728(00)00433-1. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Schluesener HJ, Seid K, Meyermann R. IL-16 is differentially expressed in the developing human fetal brain by microglial cells in zones of neuropoesis. Int. J. Dev. Neurosci. 2001b;19:93–100. doi: 10.1016/s0736-5748(00)00063-0. [DOI] [PubMed] [Google Scholar]
- Seegert D, Rosenstiel P, Pfahler H, Pfefferkorn P, Nikolaus S, Schreiber S. Increased expression of IL-16 in inflammatory bowel disease. Gut. 2001;48:326–332. doi: 10.1136/gut.48.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skundric DS, Cai J, Cruikshank WW, Gveric D. Production of IL-16 correlates with CD4+ Th1 inflammation and phosphorylation of axonal cytoskeleton in multiple sclerosis lesions. J. Neuroinflammation. 2006;3:13. doi: 10.1186/1742-2094-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skundric DS, Dai R, Zakarian VL, Bessert D, Skoff RP, Cruikshank WW, Kurjakovic Z. Anti-IL-16 therapy reduces CD4+ T-cell infiltration and improves paralysis and histopathology of relapsing EAE. J. Neurosci. Res. 2005a;79:680–693. doi: 10.1002/jnr.20377. [DOI] [PubMed] [Google Scholar]
- Skundric DS, Zhou W, Cruikshank WW, Dai R. Increased levels of bioactive IL-16 correlate with disease activity during relapsing experimental autoimmune encephalomyelitis (EAE) J. Autoimmun. 2005b;25:206–214. doi: 10.1016/j.jaut.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- Wolf SF, Temple PA, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick RM, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J. Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- Yoshida N, Arima M, Cheng G, Eda F, Hirata H, Honda K, Fukushima F, Fukuda T. Interleukin (IL)-4/IL-9 and exogenous IL-16 induce IL-16 production by BEAS-2B cells, a bronchial epithelial cell line. Cell Immunol. 2001;207:75–80. doi: 10.1006/cimm.2000.1745. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Fauser U, Schluesener HJ. Early attenuation of lesional interleukin-16 up-regulation by dexamethasone and FTY720 in experimental traumatic brain injury. Neuropathol. Appl. Neurobiol. 2007 doi: 10.1111/j.1365-2990.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- Zhao ML, Si Q, Lee SC. IL-16 expression in lymphocytes and microglia in HIV-1 encephalitis. Neuropathol. Appl. Neurobiol. 2004;30:233–242. doi: 10.1046/j.0305-1846.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- Zipris D, Greiner DL, Malkani S, Whalen B, Mordes JP, Rossini AA. Cytokine gene expression in islets and thyroids of BB rats, IFN-gamma and IL-12p40 mRNA increase with age in both diabetic and insulin-treated nondiabetic BB rats. J. Immunol. 1996;156:1315–1321. [PubMed] [Google Scholar]