Abstract
BACKGROUND
Protocadherin-PC (PCDH-PC) expression is upregulated in apoptosis-resistant sublines of the LNCaP human prostate cancer (CaP) cell line. Here, we assess the role of PCDH-PC in CaP cells and its mRNA expression in human prostate tissues.
METHODS
LNCaP cells transfected with PCDH-PC were tested for their ability to grow in vitro and in vivo in androgen-deprived conditions. PCDH-PC mRNA expression was evaluated by semi-quantitative RT-PCR and by in situ hybridization.
RESULTS
PCDH-PC expression induced Wnt signaling in CaP cells and permitted androgen-independent growth of hormone-sensitive CaP cells. Expression of PCDH-PC-homologous transcripts was low and restricted to some epithelial cells in normal tissue and to CaP cells in tumors. However, hormone-resistant CaP cells expressed significantly higher levels of PCDH-PC-related mRNA.
CONCLUSIONS
Our findings suggest a novel mechanism for the progression of CaP involving expression of PCDH-PC. This novel protocadherin induces Wnt signaling, promotes malignant behavior and hormone-resistance of CaP cells.
Keywords: prostate cancer, apoptosis, cadherins, Wnt signaling, PCDH-PC
INTRODUCTION
Through factors as diverse as increased aging of populations and improved methods of diagnosis, prostate cancer (CaP) has become a major source of cancer-related morbidity and mortality for men in Western nations [1,2]. When detected in the advanced stages, patients are invariably treated by some form of hormonal therapy in an attempt to deplete the levels of androgenic steroids or to block the ability of these steroids to activate transcription through the androgen receptor (AR) protein [3–5]. Androgen-ablation therapy successfully shrinks primary and metastatic lesions of CaP by inducing apoptosis of androgen-dependent CaP cells [1,4,6,7]. This therapy, however, is not known to be curative. Rather, a subset of prostate tumor cells are inevitably able to survive in an androgen-deprived environment and these cells provide a repository for the eventual relapse of the tumor in a hormone-resistant form that often shows resistance to more traditional forms of therapy (radiation or chemotherapy) as well.
The molecular mechanisms through which CaP cells acquire resistance to hormonal therapies appear to be complex and diverse. Evidence supports the concept that changes in the androgen-signaling pathway play some role in this process. AR gene mutation and amplification in hormone-resistant prostate cancers (HRPC) suggest that androgen-mediated signaling may be hyperactive in these tumor cells, while cross talk between growth factor receptor and AR signaling pathways and excessive recruitment of AR transcriptional co-activators also have been postulated as mechanisms for its aberrant function [8]. Alterations in apoptosis-signaling molecules found in HRPC suggest that other molecular mechanisms related to apoptosis control might also participate in the transition to androgen independence. Overexpression of the apoptosis-suppressing protein, bcl-2 [9–11], increased Akt signaling [12,13], and inactivation of tumor suppressor genes like p53 [14,15] and ANX7 [16] have also been shown to increase resistance of CaP cells to hormonal deprivation.
In an attempt to identify other gene products that might be associated with the acquisition of apoptosis-and hormone-resistance by CaP cells, we previously created two novel apoptosis-resistant CaP cell lines, LNCaP-TR (phorbol-ester [TPA]-resistant) and LNCaP-SSR (serum starvation-resistant) by repeated transient exposure of cultured human LNCaP cells to apoptotic stimuli followed by expansion of surviving cell populations [17]. These cells were also shown to be hormone-refractory based upon their ability to form tumors in castrated male nude mice. A comparative genetic analysis of these cell lines led to the description of a gene product, protocadherin-PC (PCDH-PC) that was selectively expressed in the apoptosis-resistant and hormone-refractory cell variants [17]. PCDH-PC is encoded on the human Y chromosome (previously referred as PCDHY, at Ypll.2) and is homologous (98.1%) to a gene product (PCDHX) encoded by the human X chromosome (at Xq21.3) [18,19]. Since the area of the Y chromosome containing the PCDHY/ PCDH-PC gene lies within a region of the Y chromosome that was acquired by duplication and translocation of a portion of the X chromosome during hominid evolution, the PCDH-PC gene product is also distinctly human-specific [18,19]. Aside from occasional nucleotide differences within the coding region, PCDHY/PCDH-PC is also distinguished from PCDHX in that it lacks a small 13-bp continuous sequence that is present in the PCDHX encoded gene [17–19]. This distinction is important in that the 13-bp region lost from the PCDHY/PCDH-PC gene includes a potential AUG start site. Further analysis of the PCDH-PC transcript expressed in the resistant CaP cells revealed that it would be preferential to translate a protein that lacks a signal sequence as an apparent consequence of the missing 13-bp domain. Cellular fractionation of PCDH-PC-expressing LNCaP cells showed that the protein was cytoplasmic localized, consistent with the lack of a signal sequence [18].
While PCDH-PC expression was discovered in experimentally selected apoptosis-resistant CaP cell lines, we have also shown LNCaP cells stably transformed with PCDH-PC cDNA were able to better survive acute exposure to phorbol ester, a condition that induces apoptosis in LNCaP parental cells [17]. The PCDH-PC peptide sequence also appears to contain a β-catenin binding site localized within its COOH terminus [17] and we have demonstrated that increased expression of PCDH-PC in LNCaP cells was associated with a striking change in the intracellular localization of β-catenin protein as well as increased endogenous transcriptional activity from an LEF-1 /TCF promotor element [20,21], a function that defines activation of the canonical Wnt/β-catenin signaling pathway. Wnt signaling is involved in the differentiation of adults stem cells in epithelium of colon [23], skin [24], muscle [25], chondrocytes [26], and hematopoietic cells [27] and is also known to be causally involved in the development and progression of several human cancers [22]. The canonical Wnt signaling pathway involved in embryonic cell differentiation is driven by secreted Wnt ligands that bind to Frizzled receptor proteins on target cells [28,29]. This interaction subsequently regulates the stability of β-catenin protein by suppressing the activity of a multi-component β-catenin degradation complex containing glycogen synthase kinase 3 β (GSK-3), Axin, and adenomatous polyposis coli (APC) protein. This complex promotes phosphorylation of β-catenin and its degradation via the ubiquitin proteasome pathway. Wnt signaling antagonizes the degradation complex leading to the accumulation of β-catenin in the cytoplasm and nucleus of cells, allowing it to form a transcriptionally active complex with LEF-1/TCF transcription factors that stimulates the transcription of numerous gene products that are involved in the regulation of differentiation, cell growth, apoptosis regulation, and cell motility [30–34]. Mutations in Wnt regulatory genes (CTNNB1 [β-catenin], APC, or Axin) can also inactivate the β-catenin degradation complex and these mutations are found frequently in a variety of human malignancies including colorectal carcinomas, hepatocellular carcinomas, melanomas, uterine, and ovarian carcinomas [22].
Increasing evidence indicates that the Wnt signaling pathway may play a role in the progression of human CaP [20,35,36]. Moreover, numerous recent reports demonstrated that transient β-catenin overexpression stimulates TCF signaling in most CaP cells lines including AR-positive (CWR22-Rvl, LAPC-4, LNCaP) as well as AR-negative (DU145, PC-3) cell lines [36–38]. APC and β-catenin mutations have been reported to occur in human CaP cells [37,39,40], although the frequency of these mutations is relatively low and does not account for reports of more widespread activation of Wnt signaling pathway in human prostate tumor cells. Based on our studies, we have proposed that expression of PCDH-PC might induce apoptosis- and hormone resistance in CaP cells through the upregulation of Wnt signaling and β-catenin-mediated transcriptional activity similar to effects found when β-catenin activity is modulated during the progression of colon cancer. More recently, we showed that PCDH-PC expression, through its effects on the Wnt signaling pathway, induces a transdiff erentiation of CaP cells to a neuroendocrine cell-like phenotype that might reflect an increase in NE-like cells found in CaP progression to the hormone refractory state [41].
In this study, we further evaluated the effect of PCDH-PC on canonical Wnt signaling in various CaP cell lines and evaluated the effects of the expression of this molecule on the behavior of androgen-sensitive CaP cells under androgen-deprived conditions in vitro and in vivo. Moreover as an initial assessment of PCDH-PC’s role in human CaP progression, we ana lyzed PCDH-PC mRNA expression levels and localization in a survey of primary human tissues, including normal and cancerous specimens of human prostate. Here, we present results showing that in addition to its ability to stimulate Wnt signaling, PCDH-PC is involved in the acquisition of hormone resistance by CaP cells. The analysis of PCDH-PC mRNA expression in human tissues shows that this gene product is overexpressed in hormone refractory CaP. Together, our results suggest that PCDH-PC is a likely factor in the natural progression of CaP to androgen independence.
MATERIALS AND METHODS
Cell Culture
LNCaP, DU145, and PC3 cells were maintained in RPMI-1640 medium. HEK293 cells were maintained in DMEM. All media are supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin. Cells were grown at 37°C/5% CO2. For androgen-free conditions, LNCaP-derivative cells were cultured in phenol red free RPMI medium containing 5% charcoal-stripped fetal bovine serum (CS-FBS).
Generation of CaP Cell Lines Expressing PCDH-PC
To establish clonal cell lines constitutively expressing PCDH-PC, stable transfections of parental LNCaP (LNCaP/PCDH-PC) and PC-3 (PC3/PCDH-PC) were performed using the 4.8-kbp PCDH-PC cDNA cloned into pcDNA3 vector previously described [17]. Corresponding empty vector was transfected to generate control cells. We used LNCaP cells to establish a variant line in which expression of PCDH-PC is controlled by ecdysone or its analog, Ponasterone A (Invitrogen, Cergy-Pontoise, France). The cDNA containing the PCDH-PC cDNA was cloned into an ecdysone inducible mammalian expression vector, pIND/neomycin (Invitrogen). LNCaP-pVgRXR (stably transfected with pVgRxR/Zeocin regulator vector and were gifts from Dr. Chang) were transfected with pIND-PCDH-PC plasmid by lipofectamine 2000 reagent according to the recommendations of the manufacturer (Invitrogen). The transfected cells were selected in medium containing G418 (400 µg/ml) and Zeocin (400 µg/ml). Stable clones, which grew after 4 weeks of selection were individually isolated by the cloning ring method and analyzed by semi-quantitative RT-PCR. Clones, which exhibited the best inducibility of PCDH-PC by ponasterone A (10 µM) were chosen for further characterization and were designated as LNCaP-pVgRXR/PCDH-PC.
Growth Curves and Soft Agar Assays
3.5 × 103 control LNCaP or LNCaP/PCDH-PC cells were plated in 12-well plates in growth medium. After 1 day, cells were washed with PBS and switched to RPMI 1640 phenol-red free medium with 5% CS-FBS represented Day 0 of the growth assay. Triplicate wells were counted using a hemocytometer at different days. For soft agar assay, 2 × l04 control or PCDH-PC-expressing cells were resuspended in 1 ml of 0.35% agar in RPMI containing 5% FBS or 5% CS-FBS. This upper layer was seeded into 6-well plates coated with 0.7% agar in RPMI containing 5% FBS or 5% CS-FBS. Every 2 days, 2 ml of corresponding fresh media was added. After 2 weeks, the culture was stained with 0.2% p-iodonitrotetrazodium violet (Sigma, Lyon, France) and analyzed in triplicate. Colonies larger than 50 µm in diameter were counted using an inverse microscope at 40× magnification.
Tumor Xenografts in Castrated Nude Mouse
Nude mice (7-week-old) (Harlan Bioproducts for Science, Inc., Indianapolis, IN) were castrated via scrotal incision and 1 week later, groups (n = 8) were subcutaneously implanted with 2 × 106 control LNCaP (empty vector) or LNCaP/PCDH-PC cells, both mixed with 100 µl of Matrigel. Tumor size was measured biweekly and tumor volume was calculated using the formula as previously reported [42]: V = π × H (H2 + 3a2) /6 where a = (L + W) /4, H = height of tumor determined by caliper measurement, L = length of tumor, and W = width of tumor.
Luciferase Reporter Assays
LNCaP/PCDH-PC, DU145, and HEK 293 were plated in 12-well dishes and incubated overnight at 37°C. For LNCaP-pVgRXR/PCDH-PC, control ethanol or ponasterone A (10 µM) was added to the medium. Transient transfection assays and measures of luciferase and β-gal activities were performed as previously described [21].
Immunocytochemistry
LNCaP-pVgRXR/PCDH-PC cells were cultured on 4-well Lab-Tek chambered cover slides (Nalge Nunc, Brumath, France). Cells were treated either with ethanol at 0.05% or with ponasterone A at 10 µM. Forty-eight hours after, cells were fixed in 4% paraformaldehyde for 15 min, permeabilized with 0.2% Triton X-100 for 10 min. Cells were incubated with anti-β-catenin antibody (BD Biosciences, St.-Quentin Yvelines, France) for 1 hr. β-catenin was visualized by using Vectastain-Elite ABC kit (Vector Laboratories, Paris, France).
Human Tissues Collection
Human tissues were obtained from the Department of Pathology at Henri Mondor Hospital, Créteil. Five group of patients were involved in this study: Group 1 were patients with normal prostate (obtained from donors, n = 15); Group 2 were patients with benign prostate hypertrophy (BPH, n = 15); Group 3 were hormone-naive (untreated) CaP patients (n = 13); Group 4 were CaP patients who received 6-month adjuvant hormonal therapy prior to radical prostatectomy (n = 9), and Group 5 were hormone refractory CaP patients (n = 11). Whole normal prostates were sampled according to McNeal’s zonal anatomy [43]. Normal human tissues (brain, kidney, liver, placenta, duodenum, lung, spleen, urothelium, and skeletal muscle) were obtained from donors. For in situ hybridization studies, prostate tissue samples were fixed for 24 hr in formalin and embedded in paraffin.
RT-PCR Quantification of PCDH-PC in Cell Cultures and in Human Tissues
RNA was extracted from frozen tissue or cells according to Chirgwin et al. [44]. The amount of PCDH-PC-homologous mRNA was determined by semi-quantitative RT-PCR by comparison with an internal control, an ubiquitous transcription factor TBP as previously reported [45]. The primers sequences for TBP were previously described [45]. The primers sequences for PCDH-PC were: 5′-AATTGGGTAACTACACCTACTA-3′ (sense) and 5′-CTCGAAGGTTGTCACTGGATA-3′ (antisense). Twenty-six cycles were used for the co-amplification of PCDH-PC and TBP. After gel electrophoresis, the PCR-amplified products were quantified with a Molecular Dynamics 300 Phosphorlmager (Sunnyvale, CA). Based on the fact that a 13-bp deletion is present in exon 2 of the PCDH-PC homolog transcripts as compared to the PCDH-X form, a set of PCR primers was designed to distinguish these two transcripts. The PCR primers allow amplifying a small (130 bp) region containing the site of the 13-bp deletion. The amplified products were analyzed by sequencing and by polyacrylamide gel electrophoresis.
Probes and Labeling
A 249-bp PCDH-PC cDNA [18] was used as a template to generate, by unidirectional PCR, a single strand cDNA probe. The sense and antisense probes were obtained by using respectively either PCDH-PC forward or reverse primer. The PCR reaction mix contained a final concentration of 100 ng cDNA, 67 mM KC1, 10 mM Tris-HCl pH 8.8, 10 mM (NH4)2SO4, 0.01% Tween 20, 1.5 mM MgCl2, 0.1 mM each of dATP, dCTP, dGTP, 0.065 mM dTTP, 0.035 mM 11-digoxigenin dUTP, and 1 µM of either forward or reverse primer. Five units of DNA polymerase (Eurobio, Courtaboeuf, France) were added to a final reaction volume of 100 µl and the amplification process was 5 min at 94°C before 35 cycles with 1 min denaturation at 94° C, 1 min annealing at 55°C, and 1 min extension at 72°C. Digoxygenin-labeled probes were purified by 0.1 M NaCl/EtOH precipitation and their specific activity was quantified by dot-blot using anti-digoxigenin as primary antibody and adjusted to a concentration of 0.5 µg/ml.
In Situ Hybridization
Five µm paraffinized sections were deparaffinized, rehydrated, and incubated for 20 min in 0.2 N HC1 at room temperature. After washing with 5 mM MgCl2/PBS, sections were incubated for 15 min with 0.3% TritonX-100/PBS. Tissues were then digested with 10 µg/ml of proteinase K for 30 min at 37°C in 20 mM Tris pH 7.4 containing 5 mM EDTA. Inactivation of enzyme was performed with 0.2% glycine/PBS for 10 min. After washing with PBS, tissues were refixed with 4% formaldehyde/PBS for 5 min at room temperature. After two washes with PBS, sections were incubated for 15 min at 45°C with 10 mM DTT/PBS and acetylated for 10 min in 0.25% acetic anhydride diluted in 0.1 M triethanolamine. Slides were rinsed in 2× SSC and prehybridized for 3 hr at 60°C with hybridization buffer containing 4 × SSC, l × Denhart, 10% Dextran sulfate, 100 µg/ml of salmon sperm DNA, 100 µg/ml tRNA, and 50% formamide. Hybridization was carried out by incubation at 60°C overnight in hybridization buffer supplemented with 5 µg/ml of sense or antisense digoxigenin probe. Slides were washed 30 min at 2 × SSC with 50% formamide and 45 min at 42°C in 20 mM β-mercaptoethanol diluted in 0.1 × SSC, respectively. After saturation of non-specific binding sites with saturation buffer containing 1% blocking buffer, 2% normal sheep serum diluted in 0.15 M NaCl, 0.1 M maleic acid, pH 7.5, the alkaline phosphataselabeled antidigoxigenin conjugated antibody (Roche, France) was added, diluted in saturation buffer. After four washes, antibody complex was revealed by alkaline phosphatase substrate (nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate in 0.1 M Tris-HC1, 0.1 M NaCl, 0.05 M MgCl2; pH 9.5) containing 1 mM levamisol.
Statistical Analysis
The data obtained by RT-PCR was analyzed for statistical significance by using Mann-Whitney U-test. A P-value below 0.05 was considered to denote statistical significance.
RESULTS
Expression of PCDH-PC mRNA in LNCaP Cell Variants and in Primary Human Prostate Tissues
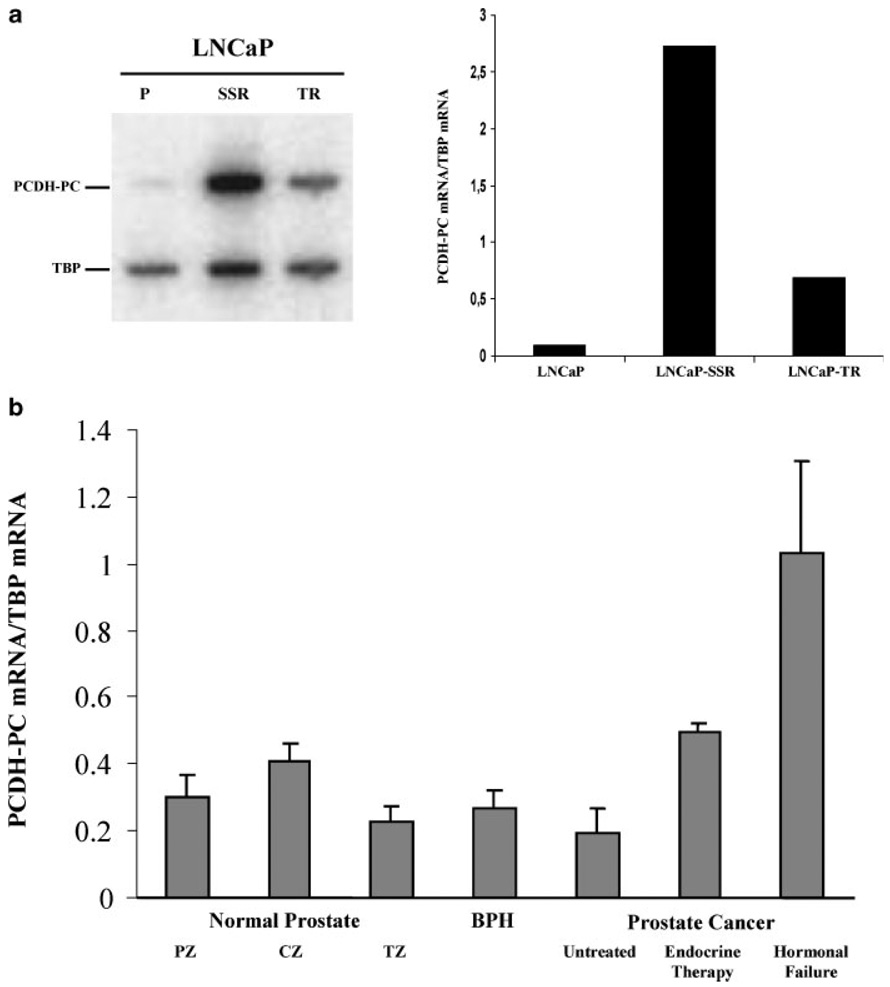
PCDH-PC was first described in apoptosis-resistant variants of the human CaP cell line, LNCaP-TR, and LNCaP-SSR [17]. In order to measure its relative mRNA expression level in these cell lines, we have used a semi quantitative RT-PCR technique with an internal expression control (TBP mRNA). As is shown in Figure 1a, the results of our assay indicated that PCDH-PC mRNA levels were much lower in parental (apoptosis-sensitive) LNCaP cells than in the apoptosis-resistant-TR and -SSR derivatives, confirming results previously obtained by Northern blot analysis of RNAs from these cell types. We have also measured relative PCDH-PC mRNA expressions in cultured human prostate cells (C4-2b, CWR22rv-l, DU145, PC3, primary epithelial and stromal cell cultures). We found that only C4-2b and CWR22rv-l cell lines endogenously expressed PCDH-PC mRNA (data not shown).
Fig. 1.
PCDH-PC mRNA expression in human prostatic tissue, hormone sensitive, and apoptosis resistant cell lines, a: Expression of PCDH- PC mRNA was investigated by semi-quantitative RT-PCR and by comparison with an internal control, the TBP mRNA. PCDH-PC mRNA expression was higher in apoptosis resistant lines LNCaP-TR and LNCaP-SSR compared to LNCaP parental cell line, b: Relative expression of PCDH-PC mRNA in prostatic tissues was also determined by semi-quantitative RT-PCR. Values corresponded to the mean of PCDH-PC expression levels in RNAs extract from different groups: the peripheral (n = 7), central (n = 9), and transitional (n = 6) zones of the normal prostate, the benign hyperplastic prostate (n = 15), untreated (n = 10), and treated (n = 8) prostate tumors and hormonal refractory patients (n = 9). Of note, PCDH-PC expression is significantly upregulated in hormone failure group as compared to either non-CaP, treated, or untreated CaP groups.
We utilized the semi-quantitative RT-PCR assay to examine expression levels of PCDH-PC in RNAs extracted from 63 different specimens consisting of normal or diseased (benign and malignant) human prostates. The results of this survey (Fig. 1b) showed a low-level expression of PCDH-PC-related mRNA in all normal prostate tissues, regardless as to whether they were derived from the peripheral, central, or transitional zones of the prostate (mean relative expression of 0.302 ± 0.169, 0.411 ± 0.119, and 0.231 ± 0.134, respectively). This low level of expression was maintained in several specimens of diseased prostate tissues consisting of BPH or untreated (localized) CaPs (0.287 ± 0.131, BPH; 0.196 ± 0.204, untreated cancers). A small number (8) of primary localized prostate tumors obtained from patients who had received 6 months of hormonal therapy prior to their surgery also demonstrated this low mean level expression of PCDH-PC mRNA (0.495 ± 0.656). In contrast, tumors obtained from patients that were experiencing hormonal failure had a mean expression of PCDH-PC mRNA that was significantly greater than any of the other types of tissue or tumor (mean relative expression levels in hormonal failure patients = 1.031 ± 0.896 vs. 0.307 ± 0.507 for all other tumor specimens; P=0.017).
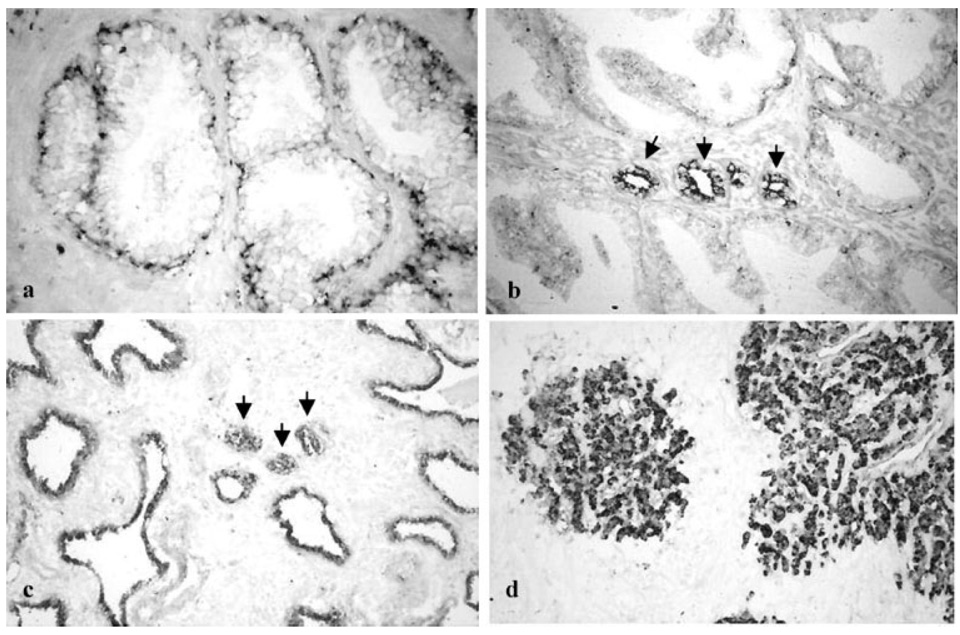
This difference in PCDH-PC mRNA expression was also found when we examined tissue sections from similar groups of patients that were subjected to in situ hybridization procedures to evaluate PCDH-PC expression (Fig. 2). In all prostate tissues analyzed, hybridization of the PCDH-PC antisense probe was mainly localized to epithelial cells, although occasional endothelial cells or smooth muscle cells appeared to be weakly stained. In the normal prostate tissues, PCDH-PC expression was predominantly found in the basal epithelium with less than 5% of ductal or acinar epithelial cells showing weak hybridization (Fig. 2a). In regions derived from the central zone of normal prostates, we observed more extensive hybridization with the non-basal epithelium and in some regions up to 48% of the epithelial cells were weakly labeled. For specimens containing BPH, the hybridization pattern was very similar to that found in normal transitional zone epithelium with labeling of basal cells and rare and weak labeling of acinar epithelial cells. In specimens containing prostate tumors from untreated patients, all tumor cells were found to be positive for hybridization to the PCDH-PC antisense probe and these cells generally had a higher intensity of staining when compared with cells in the benign regions of these specimens (Fig. 2b). No difference in staining level was observed when we compared the staining of epithelial cells in benign regions directly adjacent to tumors with normal peripheral or transition zone tissues. However, significantly more intense hybridization was observed in the cells of all (localized) tumors obtained from patients with 6 months or more of hormonal therapy prior to surgery as well as in the epithelial cells present in the benign but atrophic glands present in these specimens (Fig. 2c,d). These data enforce the results of our semi-quantitative RT-PCR assay and suggest that hormonal deprivation induces PCDH-PC-related mRNA expression in both normal (but atrophic) and cancerous prostate epithelial cells, similar to our findings in cultured and xenograft CaP cells [17,21].
Fig. 2.
In situ localization of PCDH-PC m RNA in prostatic tissues. In situ hybridization technique was performed on formalin fixed paraffin-embedded tissue using digoxigenin-labeled PCDH-PC antisense probes, a: Tissues from normal prostate. Note the staining corresponding to PCDH-PC mRNA was mainly localized in the basal epithelial cells. Differentiated glandular cells were faint or negative-stained. Representative results of ISH performed on primary (untreated) cancers were presented in (b). Tumor cells (indicated by arrows) were strongly positive for PCDH-PC staining compared to adjacent normal epithelial cells. In tissues obtained from patients treated by hormonal therapy (c) and from hormone-refractory human CaPs (d), strong staining corresponding to PCDH-PC mRNA was localized in all tumor cells (indicated by arrows) and in normal (atrophic) epithelial cells. Magnification: a–d 200×.
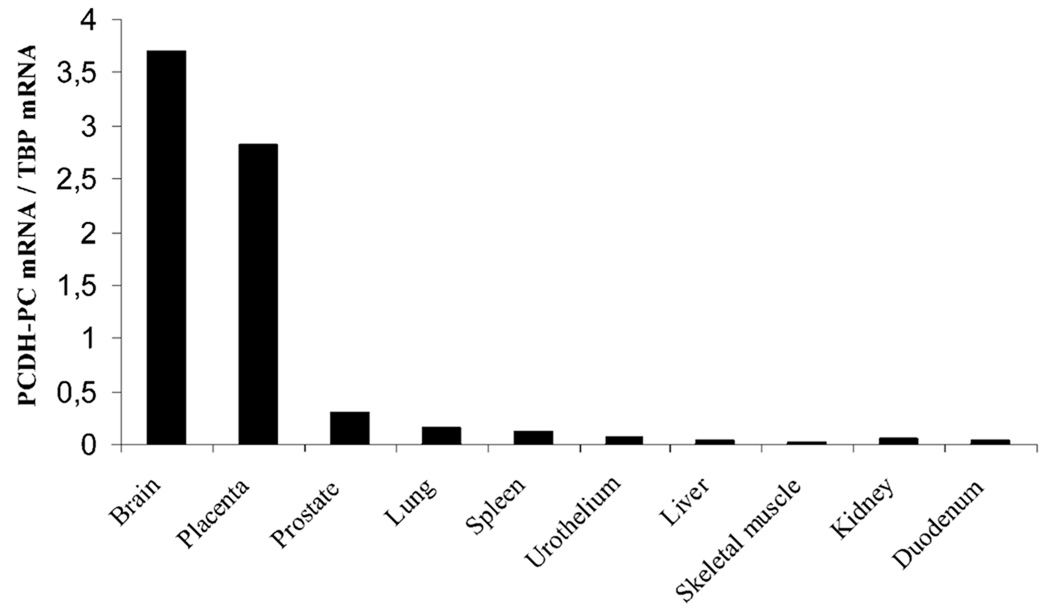
Expression of PCDH-PC-Homologous mRNA in Other Normal Human Tissues
RNAs from a variety of other normal human tissues (brain, liver, lung, spleen, skeletal muscle, duodenum, prostate, urothelium, kidney, and placenta) were also evaluated for PCDH-PC expression using the semi-quantitative RT-PCR assay and this was compared to the levels found to be expressed in normal human prostates (Fig. 3). The results of our surveys show that some form of PCDH-PC mRNA is expressed in normal prostate (at a low level), in human placenta and brain (at much higher levels). All other tissues examined lacked expression of PCDH-PC-related transcripts. Based upon our previous finding that the sequence of PCDH-PC cDNA, cloned from apoptosis-resistant CaP cells lacked a 13-bp region that is found in the X-related homolog (PCDHX), we performed an RT-PCR procedure on mRNAs isolated from the various human tissues that expressed the related transcript in order to specifically identify whether the expression was from the X- (PCDHX) or Y-linked (PCDH-PC) gene. Using a set of PCR primers that allow amplification of a small (130 bp) region from within exon 2 of the PCDH-PC transcript (containing the site of the 13-bp deletion as defined from the genomic sequence of X-chromosome gene), we performed RT-PCR amplification of mRNA extracted from two normal human prostate, two untreated human prostate tumors, two hormone-resistant human prostate tumors, and normal human brain and placenta. The PCR amplification product obtained from each of these procedures was directly sequenced and the sequence demonstrated that the brain and placenta expressed a form of PCDHX mRNA that contained the 13-bp sequence (data not shown). In striking contrast, the sequence of the PCR product amplified from the hormone-resistant CaP lacked the 13 base pair sequence corresponding to the PCDH-PC-encoded homolog. However, in normal prostate and in untreated prostate tumor, no definite sequence was obtained because of the presence of several PCR amplified products (data not shown). These results were in line with our recent observation on apoptosis-resistant cell lines [17] suggesting that the expression of the PCDH-PC homolog (as opposed to the PCDHX homolog) was preferentially expressed in hormone-resistant CaP.
Fig. 3.
PCDH-PC mRNA is expressed in some human normal tissues. Relative expression of PCDH-PC mRNA in different human normal tissues (brain, duodenum, kidney, liver, lung, placenta, prostate, skeletal muscle, spleen, and urothelium) was determined by semi-quantitative RT-PCR.
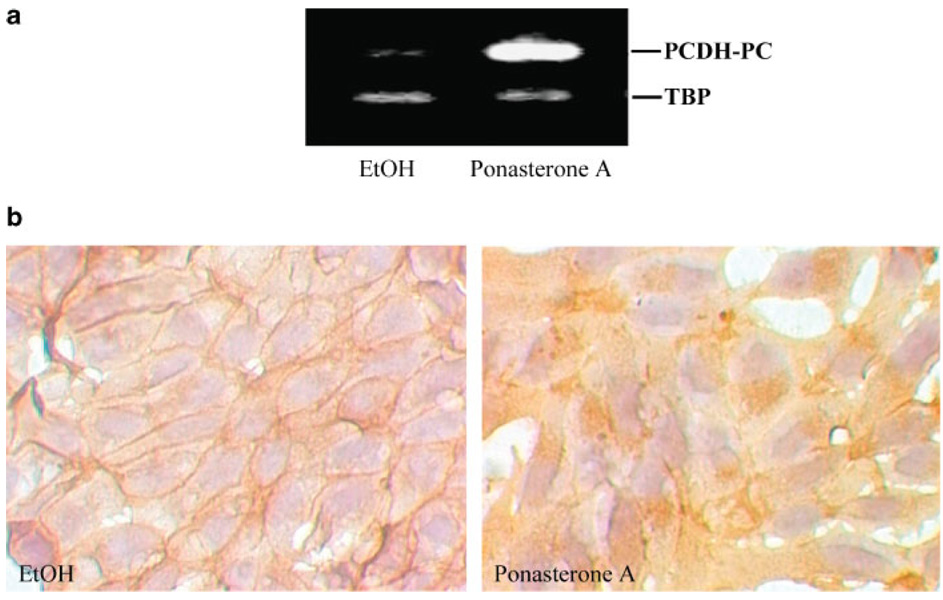
PCDH-PC Enhances Endogenous Tcf Transcriptional Activity
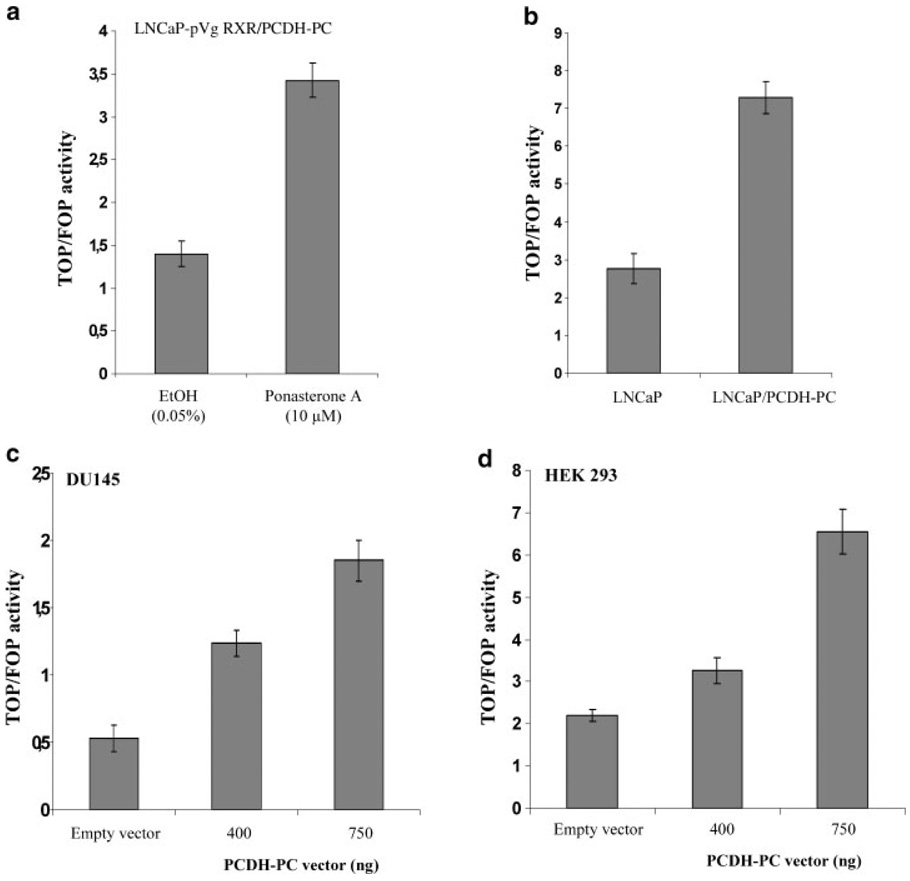
It is well established that the formation of nuclear β-catenin/TCF complexes plays a pivotal role in the activation of wnt target genes [46]. We have recently demonstrated that expression of PCDH-PC was associated with a redistribution of β-catenin protein from the membrane to the cytoplasm and nucleus of LNCaP cells and increases Wnt signaling [17,21]. In further test to prove that PCDH-PC is critical to the wnt signaling pathway, we utilized the ecdysone inducible expression system to generate PCDH-PC-inducible expression in LNCaP cells. The line, which exhibited the best inducibility of PCDH-PC was designated as LNCaP-pVgRXR/PCDH-PC. As illustrated in Figure 4a, cells treated with ponasterone A at 10 µM for 48 hr strongly upregulated PCDH-PC mRNA compared to control cells treated with ethanol at 0.05%. Using this model, we examined by immunocytochemistry the effect of PCDH-PC on β-catenin localization. β-catenin immunostaining is mainly restricted to the plasma membrane of ethanol-treated cells, whereas induction of PCDH-PC expression results in increased immunostaining within the cytosolic compartment of ponasterone A-treated cells (Fig. 4b). In order to confirm that TCF transactivation is modulated by the expression of PCDH-PC, we co-transfected cells with either the wild type TCF reporter vector (pTOP-flash) or mutated reporter plasmid (pFOP-flash) and a β-gal reporter plasmid. The comparison of normalized pTOP-flash reporter activity to normalized pFOP-flash reporter activity indicated that the TOP/FOP ratio was significantly higher in PCDH-PC-expressed cell lines (LNCaP-pVgRXR/PCDH-PC cells treated with ponasterone A and LNCaP that stably expressing PCDH-PC (LNCaP/PCDH-PC)) compared to control-cells (Fig. 5a,b). Furthermore, by using transient PCDH-PC-co-transfected cells (DU145 and HEK 293), we demonstrated that PCDH-PC increases TCF activity in a dose-dependant manner (Fig. 5c,d). These results confirm that PCDH-PC can activate the canonical Wnt pathway in CaP cell lines as well as in other cell lines like colon (HCT116) [21] and embryonic kidney (HEK 293) cells.
Fig. 4.
Inducible expression of PCDH-PC causes dramatic changes in β-catenin localization, a: LNCaP-pVgRXR/PCDH-PC cell line generated from Ecdysone system was treated with either ponA (IO µM) or ethanol 0.05% for 48 hr. Relative expression of PCDH-PC transcripts in both conditions was evaluated with a standard RT-PCR procedure. Note expression of PCDH-PC is very low in absence of ponasterone and extends in presence of PonA demonstrating inducibility of the system, b: LNCaP-pVgRXR/PCDH-PC cells were treated with vehicule (ethanol) or with ponA (10 µM) for 48 hr, and stained for β-catenin. Interestingly, immunostaining shows that hormone exposure is concomitant with a change in β-catenin expression pool from the cell-cell border to the cytoplasm and occasionally in the nucleus. Magnification 400×.
Fig. 5.
PCDH-PC augments β-catenin /TCF-mediated transcription in various cell lines. β-catenin/TCF-mediated transcription activation was measured by luciferase assay subsequent to 48 hr transfection with a TOPFLASH or FOPFLASH reporter construct (1 µg) together with a β-gal reporter plasmid (100 ng). Various cells lines were used: (a) LNCaP-pVgRXR-PCDH-PC, stable treated with ethanol at 0.05% or with ponA (10 µM) for 48 hr; (b) LNCaP that stably expresses PCDH-PC (LNCaP-PCDH-PC) and Mock-transfected control cells; (c) DUI45 and (d) HEK 293. HEK 293 and DUI45 were co-transfected with various doses of either pcDNA3 or pcDNA3-PCDH-PC expression plasmids (0,400, or 750 ng) as indicated. TCF-dependent transcription was defined as the ratio TOPFLASH/FOPFLASH luciferase activities each corrected internally for β-gal activities.
PCDH-PC Expression Influences Pro-Malignant Behavior and Hormonal Sensitivity of CaP cells In Vitro and In Vivo
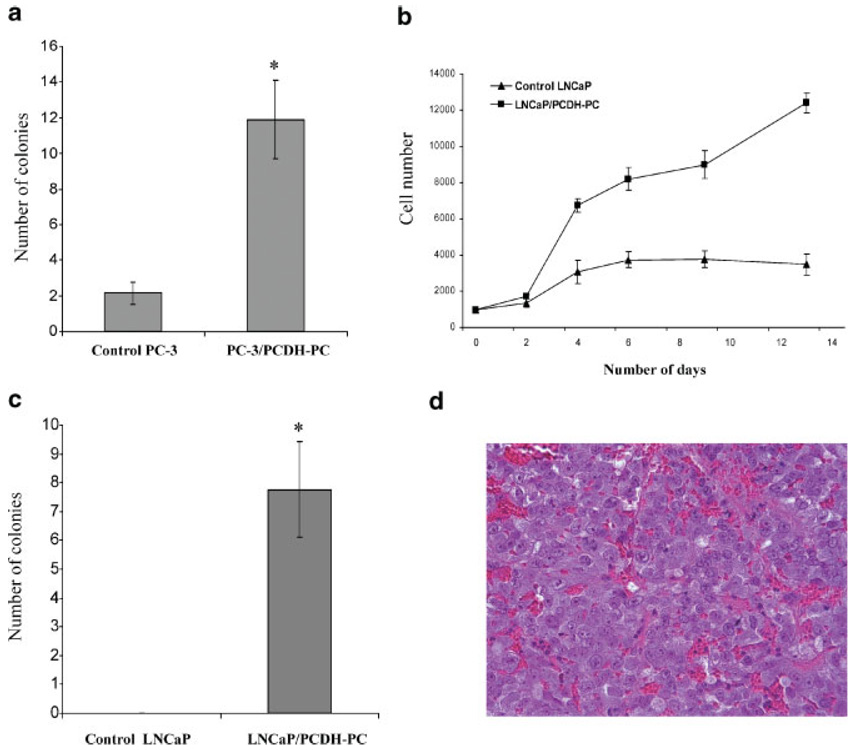
Anchorage-independent growth, assessed by colony formation in soft agar, is a marker of pro-malignant behavior of cancer cells. In order to study the potential role of PCDH-PC in tumorigenesis of CaP, we examined the anchorage-independent growth of PC-3 stably expressing PCDH-PC in a semisolid agar medium. As shown in Figure 6A, the PC-3/PCDH-PC cells, cultivated in complete medium (RMPI with 5% FCS), formed a significant greater number of colonies compared with the control-PC-3 cells transfected with an empty pcDNA3 vector. Similar results were obtained with LNCaP stably expressing PCDH-PC (data not shown). To determine whether increased PCDH-PC expression might induce a hormone-sensitive to hormone-refractory transition, we designed assays to mimic the clinical circumstances of hormone refractory disease. We first measured the ability of LNCaP cells to grow in steroid-depleted media in vitro. As expected, control LNCaP cells switched to steroid-depleted medium stopped growing after a very short time. In contrast, PCDH-PC expressing LNCaP cells continue to grow in steroid-depleted medium, displaying a high rate of proliferation (Fig. 6b) and formed colonies in soft agar (Fig. 6c) in this medium. To address whether PCDH-PC expression promotes androgen-independent growth in vivo, control transfected LNCaP or LNCaP/PCDH-PC cells were implanted subcutaneously into nude mouse that had been castrated 1 week prior and tumor formation was monitored. After 7 weeks, mice injected with control cells had no visible or palpable tumor (0/8) whereas all mice receiving PCDH-PC transformed cells had tumor (8/8) and their average size was 114.6 ± 21.8 (mean ± SEM) mm3. These tumors were extremely vascularized and a photomicrograph of a thin section from one of these tumors is shown in Figure 6d. Together, these data demonstrate that PCDH-PC expression might directly convert parental LNCaP cells to a hormone-resistant state.
Fig. 6.
PCDH-PC promotes anchorage and androgen-independent CaP cell growth, a: Soft agar assays of PC-3 stably expressing PCDH-PC (PC-3/PCDH-PC) or mock transfected PC-3 (control PC-3) cells. Cells (20,000) were seeded in triplicate in 6-wells plates. The colonies were counted after 2 weeks of culture in completed medium (RMPI/5% FCS). The number of soft agar colonies presented is the mean of colony counts in 10 40× microscopic fields from three wells, b: Growth curve assays. LNCaP/PCDH-PC cells or empty-vector-transfected LNCaP cells (3,500) were seeded in triplicate in 12-well plates. The cells were cultivated in hormone-deprived medium (RMPI/5% CS-FCS). At 2–3 day intervals, the cell number was analyzed. In contrast to control cells, LNCaP/PCDH-PC continued to proliferate in androgen-depleted medium, c: Anchorage-independent growth in hormone-deprived medium. LNCaP/PCDH-PC cells or empty-vector-transformed LNCaP cells (20,000) were seeded in triplicate in 6-well plates. The colonies were counted after 2 weeks of culture in RMPI/5% CS-FCS. Error bars indicate standard errors. *P < 0.001 compared with control-cells. d: Tumorigenicity of PCDH-PC overexpressed LNCaP cells in castrated nude mice. 2 × 106 of either control LNCaP cells or LNCaP/PCDH-PC cells were injected into eight nude mice castrated 1 week before injection. After 7 weeks, mice injected with control cells had no visible or palpable tumor (0/8) whereas 100% of castrated mice xenografted with LNCaP/PCDH-PC cells formed tumors (8/8). Hematoxylin and eosin staining showed these tumors were highly vascularized. Magnification: 200×.
DISCUSSION
Hormone treatment for advanced CaP, albeit initially effective, is invariably complicated by the development of hormone resistance. There is experimental evidence to support the concept that some hormone-resistant CaP cells might be present in prostate tumors even before therapy is applied and that hormonal therapies might simply select these hormone-resistant cells, allowing their eventual expansion [47,48]. Other evidence suggests that the application of hormonal therapy may enable some CaP cells to acquire hormone resistance through specific genetic changes that occur during adaptation to the low androgen environment of the hormonally treated patient [49–52]. Regardless of whether either one or both of these paradigms are correct, it is likely that the androgen-resistant CaP cell is genetically different from the androgen-sensitive CaP cell and our ability to identify the genetic differences that confer hormone resistance to CaP cells is a prelude to the development of better and more effective therapies for the disease.
The work described here significantly extends our previous study in which we described a unique genetic change in CaP cell lines that had acquired resistance to apoptosis following repeated exposure to apoptotic agents [17]. The loss of apoptosis-sensitivity was attributed to the induced expression of one particular gene product in the apoptosis-resistant cell lines, PCDH-PC, that was not found to be expressed in the apoptosis-sensitive parental cell line (LNCaP) from which they were selected. Structural and experimental analysis showed that PCDH-PC is a cytoplamic protein and contains a β-catenin binding domain [17]. The interaction between these two proteins was demonstrated by immunoprecipitation assay [17]. In this study and in our previous reports, we show that the expression of PCDH-PC was associated with a redistribution of endogenous β-catenin from the membrane to the cytosol of LNCaP cells. Furthermore, the upregulation of PCDH-PC significantly increases TCF signaling in CaP cell lines as well in other cells like colon (HCT116, HT29 (data not shown)) and embryonic kidney (HEK 293) cells. Our results are in line with numerous reports showing that cytoplasmic or nuclear stabilization of β-catenin is associated with increase transcriptional activity of Tcf/Lef factors at the end-point of the canonical Wnt signaling pathway. By using specific Wnt signaling pathway microarray, we have recently demonstrated that elevated PCDH-PC expression is also associated with increase expression of Wnt target genes (c-Myc, Cyclin D, c-Ret, and Cox-2) and Wnt signaling molecules (Wnt-3, -7B, 10A, 11, and FZD2,4,10) [21]. Together, we have proposed that the coincidence of cytoplasmic PCDH-PC expression in conjunction with dysregulation of β-catenin activity associated with increase Wnt signaling could mechanistically explain the basis for acquired apoptosis- and hormone-resistance in CaP cells.
To further explore this hypothesis, we evaluated involvement of PCDH-PC on prostate tumor growth both in vivo and in vitro. Our results demonstrated that PCDH-PC expression promotes a growth advantage of CaP cells in soft agar and in androgen-deprived media. As further evidence to the potential link between PCDH-PC and hormone-resistant CaP, we tested whether LNCaP cells, long known to have an androgen-sensitive phenotype with regards to their inability to form tumor xenografts in castrated male nude mice, might gain a hormone-resistant phenotype following transfection with PCDH-PC. Indeed, PCDH-PC transformed LNCaP cells readily formed tumors in castrated male nude mice in contrast to LNCaP cells transfected with an empty expression vector, thus directly demonstrating that PCDH-PC transfection not only confers an apoptosis-resistant phenotype but also a hormone-resistant phenotype. In addition, we have completed an initial survey of normal and cancerous human prostate tissues to determine whether PCDH-PC expression is associated with hormone-resistance of human CaP. Semi-quantitative comparative analysis of our prostate tissues suggest that PCDH-PC expression was low in normal prostate and in non-treated and early-treatment cancers whereas it was significantly higher in hormone-resistant CaPs. In situ hybridization showed that expression of the PCDH-PC-homologous transcripts was restricted to basal cells and occassional acinar epithelial cells in normal prostate tissue and to CaP cells in tumor. Significantly more intense hybridization was observed in tumor cells derived from hormone-treated patients and in tumors from patients failing hormone therapy. Our findings suggested that PCDH-PC mRNA expression is acquired by tumor cells after hormonal deprivation and this is consistent with our observations that LNCaP cells cultured in androgen-free medium upregulate PCDH-PC expression [17,21]. We note here that, because of the extensive homology between the PCDH-PC and PCDHX gene products, most of our assays for PCDH-PC expression applied to the tissues and tumors would likely detect expression of either homolog. However, our sequence analysis of PCR-amplified transcripts from hormone-resistant prostate tumors definitively showed that it was the PCDH-PC-specific transcript that was amplified from these tissues whereas our amplification of homologous transcripts from normal brain selectively detected the PCDHX homolog.
In a previous report, the hormone-resistant prostate tumors that were used in this study were also immunohistochemically surveyed for β-catenin. These tumors showed evidence for abnormal distribution of β-catenin within the cytoplasm and/or nucleus of the tumor cells [20]. This abnormal distribution was rare in the small number of untreated CaPs examined.
Immunohistochemical analysis of these specimens showed that β-catenin was almost always restricted to the membranes of the untreated cancer cells. The fact that we failed to find any mutations in the β-catenin molecule expressed in the hormone-resistant cancer cells, suggests that β-catenin dysregulation found in the hormone-resistant CaP cells is likely the consequence of increasing expression of PCDH-PC as we had proposed in our in vitro studies.
In our laboratory analysis of CaP cells, both naturally selected and genetically manipulated CaP cells that express PCDH-PC acquire a hormone-refractory phenotype. There are reasons to believe that this effect might be related to the ability of PCDH-PC expression (through its actions on the Wnt signaling pathway) to induce neuroendocrine (NE) transdifferentiation of CaP cells. Studies from other laboratories support the idea that NE transdifferentiation associated with CaP progression after hormonal treatment increases the production of growth stimulating neuropeptides (like bombesin or neurotensin) that can lead to androgen independence through autocrine or paracrine pathways [53,54]. However, there is increasing evidence that Wnt signaling cross-talks to the androgen signaling pathway through direct interaction of β-catenin protein with the AR. Additionally, we have recently obtained evidence that the human AR gene is a direct target of the canonical Wnt signaling (via LEF-1/TCF-mediated transcription) and that AR mRNA is drastically increased in CaP cells that express PCDH-PC [55].
CONCLUSIONS
Our collective results strongly support the idea that PCDH-PC expression may be a novel mechanism that promotes progression of human CaP to hormone resistance and aggressive states. This work suggests that the effects of PCDH-PC are mediated by its ability to influence signaling through the Wnt signaling pathway in the CaP cell, a signaling pathway that has proven to be very involved in oncogenesis in other organ systems. As well, increasing evidence suggests that the Wnt signaling pathway specifically crosstalks to the androgen signaling pathway identifying a secondary mechanism through which PCDH-PC expression has the potential to drastically influence malignant behavior of CaP cells that express it. These data continue to identify PCDH-PC as a potential target for therapeutics designed to suppress aggressiveness of CaP.
ACKNOWLEDGMENTS
We are very grateful to Dr. Glenn T.G. Chang (Rotterdam, The Netherlands) for providing us the LNCaP-pVgRxR cell line. We thank Pascale Maillé for excellent technical assistance. This article is dedicated to the memory of the professor Dominique K. Chopin. This work was supported by INSERM; Université Paris XII; grants from the Association de Recherche contre le Cancer (ARC) and from the Association pour la Recherche sur les Tumeurs de la Prostate (ARTP) (to FV), the U.S. Department of Defense (DAMD-PC050402) (to RB), NIH, USA (CA111618) (to RB) the T.J. Martell Foundation (to RB) and Certus. S.Terry is a recipient of a fellowship from the Ministère de la Recherche et de la Technologie.
REFERENCES
- 1.Gittes RF. Carcinoma of the prostate. N Engl J Med. 1991;324(4):236–245. doi: 10.1056/NEJM199101243240406. [DOI] [PubMed] [Google Scholar]
- 2.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49(1):8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Schulze H, Isaacs JT, Coffey DS. A critical review of the concept of total androgen ablation in the treatment of prostate cancer. Prog Clin Biol Res. 1987;243A:1–19. [PubMed] [Google Scholar]
- 4.Grayhack JT, Keeler TC, Kozlowski JM. Carcinoma of the prostate. Hormonal therapy. Cancer. 1987;60(3 Suppl):589–601. doi: 10.1002/1097-0142(19870801)60:3+<589::aid-cncr2820601526>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Carter HB, Isaacs JT. Overview of hormonal therapy for prostate cancer. Prog Clin Biol Res. 1990;350:129–140. [PubMed] [Google Scholar]
- 6.Kyprianou N, English HF, Isaacs JT. Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation. Cancer Res. 1990;50(12):3748–3753. [PubMed] [Google Scholar]
- 7.Westin P, Stattin P, Damber JE, Bergh A. Castration therapy rapidly induces apoptosis in a minority and decreases cell proliferation in a majority of human prostatic tumors. Am J Pathol. 1995;146(6):1368–1375. [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;l(l):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 9.Colombel M, Olsson CA, Ng PY, Buttyan R. Hormone-regulated apoptosis results from reentry of differentiated prostate cells onto a defective cell cycle. Cancer Res. 1992;52(16):4313–4319. [PubMed] [Google Scholar]
- 10.Miyake H, Tolcher A, Gleave ME. Antisense Bcl-2 oligodeoxynucleotides inhibit progression to androgen-independence after castration in the Shionogi tumor model. Cancer Res. 1999;59(16):4030–4034. [PubMed] [Google Scholar]
- 11.Raffo AJ, Perlman H, Chen MW, Day ML, Streitman JS, Buttyan R. Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res. 1995;55(19):4438–4445. [PubMed] [Google Scholar]
- 12.Paweletz CP, Charboneau L, Bichsel VE, Simone NL, Chen T, Gillespie JW, Emmert-Buck MR, Roth MJ, Petricoin IE, Liotta LA. Reverse phase protein microarrays which capture disease progression show activation of pro-survival pathways at the cancer invasion front. Oncogene. 2001;20(16):1981–1989. doi: 10.1038/sj.onc.1204265. [DOI] [PubMed] [Google Scholar]
- 13.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, Kreisberg JI. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8(4):1168–1171. [PubMed] [Google Scholar]
- 14.Navone NM, Troncoso P, Pisters LL, Goodrow TL, Palmer JL, Nichols WW, von Eschenbach AC, Conti CJ. p53 protein accumulation and gene mutation in the progression of human prostate carcinoma. J Natl Cancer Inst. 1993;85(20):1657–1669. doi: 10.1093/jnci/85.20.1657. [DOI] [PubMed] [Google Scholar]
- 15.Heidenberg HB, Sesterhenn IA, Gaddipati JP, Weghorst CM, Buzard GS, Moul JW, Srivastava S. Alteration of the tumor suppressor gene p53 in a high fraction of hormone refractory prostate cancer. J Urol. 1995;154(2 Pt 1):414–421. doi: 10.1097/00005392-199508000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava M, Bubendorf L, Srikantan V, Fossom L, Nolan L, Glasman M, Leighton X, Fehrle W, Pittaluga S, Raffeld M, Koivisto P, Willi N, Gasser TC, Kononen J, Sauter G, Kallioniemi OP, Srivastava S, Pollard HB. ANX7, a candidate tumor suppressor gene for prostate cancer. Proc Natl Acad Sci USA. 2001;98(8):4575–4580. doi: 10.1073/pnas.071055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MW, Vacherot F, De La Taille A, Gil-Diez-De-Medina S, Shen R, Friedman RA, Burchardt M, Chopin DK, Buttyan R. The emergence of protocadherin-PC expression during the acquisition of apoptosis-resistance by prostate cancer cells. Oncogene. 2002;21(51):7861–7871. doi: 10.1038/sj.onc.1205991. [DOI] [PubMed] [Google Scholar]
- 18.Blanco P, Sargent CA, Boucher CA, Mitchell M, Affara NA. Conservation of PCDHX in mammals; expression of human X/Y genes predominantly in brain. Mamm Genome. 2000;11(10):906–914. doi: 10.1007/s003350010177. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida K, Sugano S. Identification of a novel protocadherin gene (PCDH11) on the human XY homology region in Xq21.3. Genomics. 1999;62(3):540–543. doi: 10.1006/geno.1999.6042. [DOI] [PubMed] [Google Scholar]
- 20.de la Taille A, Rubin MA, Chen MW, Vacherot F, de Medina SG, Burchardt M, Buttyan R, Chopin D. Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin Cancer Res. 2003;9(5):1801–1807. [PubMed] [Google Scholar]
- 21.Yang X, Chen MW, Terry S, Vacherot F, Chopin DK, Bemis DL, Kitajewski J, Benson MC, Guo Y, Buttyan R. A human- and male-specific protocadherin that acts through the wnt signaling pathway to induce neuroendocrine transdifferentiation of prostate cancer cells. Cancer Res. 2005;65(12):5263–5271. doi: 10.1158/0008-5472.CAN-05-0162. [DOI] [PubMed] [Google Scholar]
- 22.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–1851. [PubMed] [Google Scholar]
- 23.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;lll(2):241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 24.Alonso L, Fuchs E. Stem cells of the skin epithelium. Proc Natl Acad Sci USA. 2003;100 Suppl 1:11830–11835. doi: 10.1073/pnas.1734203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113(7):841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 26.Church V, Nohno T, Linker C, Marcelle C, Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115(Pt 24):4809–4818. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
- 27.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 28.Eastman Q, Grosschedl R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr Opin Cell Biol. 1999;ll(2):233–240. doi: 10.1016/s0955-0674(99)80031-3. [DOI] [PubMed] [Google Scholar]
- 29.Miller JR. The Wnts. Genome Biol. 2002;3(1) doi: 10.1186/gb-2001-3-1-reviews3001. REVIEWS3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 31.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 32.Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci USA. 1999;96(4):1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He TC, Chan TA, Vogelstein B, Kinzler KW. PPAR delta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99(3):335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich SM, Fields JZ, Boman BM. Evidence that APC regulates survivin expression: A possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;61(24):8664–8667. [PubMed] [Google Scholar]
- 35.Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, Rabbani SA. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: Potential pathogenetic and prognostic implications. Cancer. 2004;101(6):1345–1356. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- 36.Chesire DR, Ewing CM, Gage WR, Isaacs WB. In vitro evidence for complex modes of nuclear beta-catenin signaling during prostate growth and tumorigenesis. Oncogene. 2002;21(17):2679–2694. doi: 10.1038/sj.onc.1205352. [DOI] [PubMed] [Google Scholar]
- 37.Chesire DR, Ewing CM, Sauvageot J, Bova GS, Isaacs WB. Detection and analysis of beta-catenin mutations in prostate cancer. Prostate. 2000;45(4):323–334. doi: 10.1002/1097-0045(20001201)45:4<323::aid-pros7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 38.Cronauer MV, Schulz WA, Ackermann R, Burchardt M. Effects of WNT/beta-catenin pathway activation on signaling through T-cell factor and androgen receptor in prostate cancer cell lines. Int J Oncol. 2005;26(4):1033–1040. doi: 10.3892/ijo.26.4.1033. [DOI] [PubMed] [Google Scholar]
- 39.Gerstein AV, Almeida TA, Zhao G, Chess E, Shih Ie M, Buhler K, Pienta K, Rubin MA, Vessella R, Papadopoulos N. APC/CTNNB1 (beta-catenin) pathway alterations in human prostate cancers. Genes Chromosomes Cancer. 2002;34(1):9–16. doi: 10.1002/gcc.10037. [DOI] [PubMed] [Google Scholar]
- 40.Voeller HJ, Truica CI, Gelmann EP. Beta-catenin mutations in human prostate cancer. Cancer Res. 1998;58(12):2520–2523. [PubMed] [Google Scholar]
- 41.Huss WJ, Gregory CW, Smith GJ. Neuroendocrine cell differentiation in the CWR22 human prostate cancer xenograft: Association with tumor cell proliferation prior to recurrence. Prostate. 2004;60(2):91–97. doi: 10.1002/pros.20032. [DOI] [PubMed] [Google Scholar]
- 42.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405(6784):354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 43.McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2(l):35–49. doi: 10.1002/pros.2990020105. [DOI] [PubMed] [Google Scholar]
- 44.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 45.Gil-Diez de Medina S, Salomon L, Colombel M, Abbou CC, Bellot J, Thiery JP, Radvanyi F, Van der Kwast TH, Chopin DK. Modulation of cytokeratin subtype, EGF receptor, and androgen receptor expression during progression of prostate cancer. Hum Pathol. 1998;29(9):1005–1012. doi: 10.1016/s0046-8177(98)90208-8. [DOI] [PubMed] [Google Scholar]
- 46.Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol. 2003;129(4):199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PubMed] [Google Scholar]
- 47.Isaacs JT, Schulze H, Coffey DS. Development of androgen resistance in prostatic cancer. Prog Clin Biol Res. 1987;243A:21–31. [PubMed] [Google Scholar]
- 48.Craft N, Chhor C, Tran C, Belldegrun A, DeKernion J, Witte ON, Said J, Reiter RE, Sawyers CL. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 1999;59(19):5030–5036. [PubMed] [Google Scholar]
- 49.Isaacs WB, Bova GS, Morton RA, Bussemakers MJ, Brooks JD, Ewing CM. Genetic alterations in prostate cancer. Cold Spring Harb Symp Quant Biol. 1994;59:653–659. doi: 10.1101/sqb.1994.059.01.075. [DOI] [PubMed] [Google Scholar]
- 50.Nupponen NN, Kakkola L, Koivisto P, Visakorpi T. Genetic alterations in hormone-refractory recurrent prostate carcinomas. Am J Pathol. 1998;153(1):141–148. doi: 10.1016/S0002-9440(10)65554-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stubbs AP, Abel PD, Golding M, Bhangal G, Wang Q, Waxman J, Stamp GW, Lalani EN. Differentially expressed genes in hormone refractory prostate cancer: Association with chromosomal regions involved with genetic aberrations. Am J Pathol. 1999;154(5):1335–1343. doi: 10.1016/S0002-9440(10)65387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(l):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 53.Jin RJ, Wang Y, Masumori N, Ishii K, Tsukamoto T, Shappell SB, Hayward SW, Kasper S, Matusik RJ. NE-10 neuroendocrine cancer promotes the LNCaP xenograft growth in castrated mice. Cancer Res. 2004;64(15):5489–5495. doi: 10.1158/0008-5472.CAN-03-3117. [DOI] [PubMed] [Google Scholar]
- 54.Lee LF, Guan J, Qiu Y, Kung HJ. Neuropeptide-induced androgen independence in prostate cancer cells: Roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol Cell Biol. 2001;21(24):8385–8397. doi: 10.1128/MCB.21.24.8385-8397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X, Chen M, Terry S, Vacherot F, Bemis D, Capodice J, Kitajewski J, de la Taille A, Benson M, Guo Y, Buttyan R. Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells. Oncogene. 2006 Feb 13; doi: 10.1038/sj.onc.1209366. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]