Abstract
This research presents the first quantitative evaluation of the olfactory acuity in extinct theropod dinosaurs. Olfactory ratios (i.e. the ratio of the greatest diameter of the olfactory bulb to the greatest diameter of the cerebral hemisphere) are analysed in order to infer the olfactory acuity and behavioural traits in theropods, as well as to identify phylogenetic trends in olfaction within Theropoda. A phylogenetically corrected regression of olfactory ratio to body mass reveals that, relative to predicted values, the olfactory bulbs of (i) tyrannosaurids and dromaeosaurids are significantly larger, (ii) ornithomimosaurs and oviraptorids are significantly smaller, and (iii) ceratosaurians, allosauroids, basal tyrannosauroids, troodontids and basal birds are within the 95% CI. Relative to other theropods, olfactory acuity was high in tyrannosaurids and dromaeosaurids and therefore olfaction would have played an important role in their ecology, possibly for activities in low-light conditions, locating food, or for navigation within large home ranges. Olfactory acuity was the lowest in ornithomimosaurs and oviraptorids, suggesting a reduced reliance on olfaction and perhaps an omnivorous diet in these theropods. Phylogenetic trends in olfaction among theropods reveal that olfactory acuity did not decrease in the ancestry of birds, as troodontids, dromaeosaurids and primitive birds possessed typical or high olfactory acuity. Thus, the sense of smell must have remained important in primitive birds and its presumed decrease associated with the increased importance of sight did not occur until later among more derived birds.
Keywords: Theropoda, olfaction, birds, olfactory bulbs, olfactory ratio
1. Introduction
The olfactory bulbs (i.e. organs associated with the sense of smell) of non-avian theropods were situated anterior to the cerebral hemispheres and olfactory tracts, and confined within a trough-shaped sphenethmoid posterior to the mesethmoid (Therrien et al. 2005; Ali et al. 2008). Fossil evidence of the olfactory bulbs is preserved as impressions on the anteroventral surface of the frontals or as bulges anterior to the olfactory tract in braincase endocasts. Based on such fossil evidence, the olfactory bulbs in non-avian theropods have been qualitatively described as small in some taxa (e.g. Dromiceiomimus, Conchoraptor; Russell 1972; Kundrát 2007) and as large in others (e.g. Allosaurus, Tyrannosaurus; Rogers 1999; Brochu 2000).
The olfactory bulbs and olfaction play an important role in the ecology (e.g. foraging, navigation, home range size, activity timing, reproduction, individual recognition) of extant archosaurian and mammalian predators (e.g. Bang & Wenzel 1985; Gittleman 1991; Verheyden & Jouventin 1994), and the same was presumably true for extinct predators, such as theropod dinosaurs. Although qualitative descriptions of olfactory bulbs in some theropods have been used to make general statements about their olfactory abilities (e.g. Russell 1969, 1972; Horner & Dobb 1997; Rogers 1999), quantitative comparisons among taxa have yet to be conducted. In this paper, we determine the relative size of the olfactory bulbs of extinct theropods in order to gain insight into their palaeobiology and to identify trends in sensory evolution among theropods.
2. Olfactory bulb size and olfaction in extant archosaurs and mammals
Olfactory bulb size has been used as an indicator of olfactory acuity (i.e. ability to discriminate between different odours contra sensitivity to odours) in extant archosaurs and mammals (e.g. Smith 1928; Cobb 1960; Bang 1971; Gittleman 1991). Recent work has revealed a positive correlation between olfactory bulb size and olfactory acuity that is related to (i) the number and size of mitral cells found in the bulb (Wenzel & Meisami 1987; Mackay-Sim & Royet 2006 and references therein), (ii) the number of odour receptors on the olfactory bulb (Mori et al. 1999) and (iii) the number of olfactory receptor genes (Steiger et al. 2008). These studies have shown that physiological mechanisms are responsible for the relationship between large olfactory bulbs and high olfactory acuity in extant birds and mammals.
Early studies on olfactory bulb size in birds revealed associations between olfactory ratio (i.e. the ratio of the greatest diameter of the olfactory bulb to the greatest diameter of the cerebral hemisphere regardless of their orientation) and foraging method, diet, nesting and breeding habits (Cobb 1960; Bang 1971; Bang & Wenzel 1985), although they lacked rigorous statistical analyses. This work showed that among carnivorous species (predators and scavengers), olfactory foragers (e.g. turkey vultures, kiwis, tube-nosed seabirds) have higher olfactory ratios (28.7–37%) than visual foragers (e.g. black vultures, peregrine falcons, owls; olfactory ratios=15.5–20%; Bang 1971; Bang & Wenzel 1985). These studies further revealed that most birds with high olfactory ratios (above 25%) are carnivorous/piscivorous, ground nesters, water-associated and colonial breeders, whereas most birds with low olfactory ratios (below 15%) are omnivorous/granivorous, tree nesters and solitary breeders (Bang 1971; Bang & Wenzel 1985). Attempts to statistically analyse the olfactory bulb size in birds in relation to diet, activity timing, nest type, development, nest dispersion and migratory behaviour have shown that a significant correlation exists only with activity timing; specifically, that taxa with longer olfactory bulbs are crepuscular or nocturnal (Healy & Guilford 1990; see electronic supplementary material). From this correlation, these authors concluded that ‘olfactory ability is generally increased to compensate for the reduced effectiveness of vision under reduced light, whatever olfaction's specific function’ (Healy & Guilford 1990, p. 343). However, a subsequent study on olfaction in procellariiformes (tube-nosed seabirds) concluded that olfaction in these and other birds ‘may have evolved as a response to environments where food is patchily distributed and provides no visual cues that could be used for detection, whatever the light conditions’ (Verheyden & Jouventin 1994, p. 291), an interpretation also supported by other empirical studies (e.g. Stager 1964; Houston 1986; Nevitt 2008). Together, these conclusions suggest that large olfactory bulbs occur in bird species for which olfaction is used to conduct activities (e.g. locating food, orientation, homing) when vision or visual cues are limited.
Among carnivoran mammals, olfactory bulb size has been quantified using volume rather than olfactory ratios (Gittleman 1991). Olfactory bulb size analysed in relation to various ecological and behavioural variables (diet, social interactions, group size, habitat, home range, activity timing and habitat zonation) revealed that significant correlations exist only with zonation and home range size: aquatic species possess smaller olfactory bulbs than terrestrial and arboreal species, and species with large home ranges have larger olfactory bulbs than those with small home ranges (Gittleman 1991). The relationship between home range size and olfaction may reflect the need for animals to navigate within large home ranges (e.g. Benhamou 1989), or may also reflect the need to locate food within their home range (Conover 2007).
Although crocodylians are known to have large olfactory bulbs (Starck 1979) and a keen sense of smell to locate food (Scott & Weldon 1990; Weldon et al. 1990; Weldon & Ferguson 1993), quantitative analyses of their olfactory bulb size has yet to be conducted, as has been done for birds and mammals.
3. Material and methods
Olfactory ratios of theropods and crocodylians were calculated from the greatest linear dimensions of the olfactory bulb region and the cerebral hemisphere region of the endocranial cavity (figure 1; electronic supplementary material). These linear dimensions were derived from braincases, CT scans of braincases, and digitally rendered and museum quality endocasts of 26 specimens belonging to 21 species of theropods (including allosauroids, archeopterygids, ceratosaurians, dromaeosaurids, ornithomimosaurs, oviraptorids, troodontids and tyrannosauroids) and three specimens of the extant crocodylian Alligator mississippiensis (table 1; electronic supplementary material). Although dimensions of the endocranial cavity in maniraptoriform theropods should accurately reflect the dimensions of the enclosed soft tissue structures because the brain was in contact with the braincase walls in these taxa (Russell 1969, 1972; Barsbold 1983; Currie 1995; Burnham 2004; Osmólska 2004), the same is not true of non-maniraptoriform theropods because the brain did not completely fill their endocranial cavity (Hopson 1979; Hurlburt 2005). However, olfactory ratios can be derived from all theropod braincases/endocasts by assuming that the size proportion between the soft tissue structures are equal to the size proportion of the endocranial cavity housing them (e.g. Larsson et al. 2000).
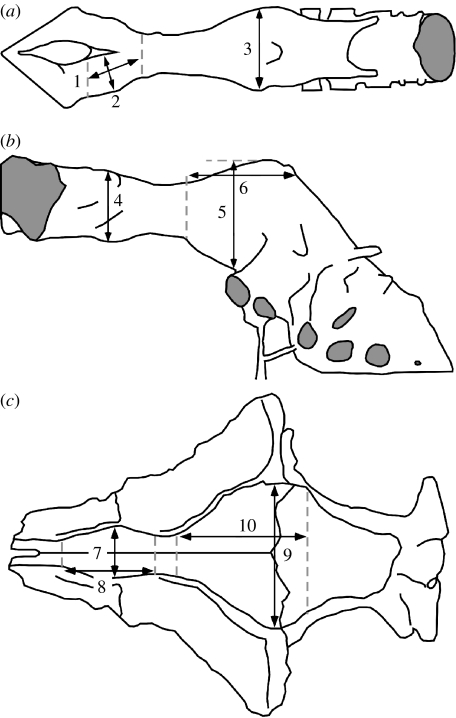
Figure 1.
Dimensions used for the calculation of olfactory ratios in studied taxa. (a) Dorsal view of an endocast: 1, length of olfactory bulb; 2, diameter of olfactory bulb; 3, mediolateral diameter of cerebral hemisphere (divided by 2). (b) Lateral view of an endocast: 4, depth of olfactory bulb; 5, depth of cerebral hemisphere (without sagittal sinus); 6, length of cerebral hemisphere. (c) Endocranial cavity on ventral surface of frontals and parietals: 7, mediolateral diameter of the olfactory bulb impression (or of olfactory fossa divided by 2); 8, length of olfactory bulb impression (or olfactory fossa); 9, mediolateral diameter of cerebral hemisphere (divided by 2); 10, length of cerebral hemispheres. Modified from Currie (1985) and Larsson (2001).
Table 1.
Olfactory ratios and body masses of theropod and crocodylian taxa studied. (Asterisk indicates maximum values in Albertosaurus and Gorgosaurus because they were measured on frontoparietal complexes, which preserve neither the depth of the olfactory bulbs nor the entire length of the cerebral hemispheres. For additional data, see electronic supplementary material.)
| taxon | body mass (kg) | olfactory ratio (%) | olfactory ratio residuals (log) |
|---|---|---|---|
| Ceratosauria | |||
| Ceratosaurus magnicornis | 539 | 48.1 | 0.03 |
| Majungasaurus crenatissimus | 1130 | 48.3 | −0.01 |
| Allosauroidea | |||
| Allosaurus fragilis | 1469 | 50, 51.6 | −0.01, 0.01 |
| Acrocanthosaurus atokensis | 3778 | 58.1 | 0.01 |
| Carcharodontosaurus saharicus | 7906 | 56 | −0.05 |
| Giganotosaurus carolinii | 7560 | 57.7 | −0.04 |
| Tyrannosauroidea | |||
| Dilong paradoxus | 10 | 27 | −0.01 |
| Albertosaurus sarcophagus | 2545 | 71* | 0.12 |
| Gorgosaurus libratus | 2710 | 68.5* | 0.10 |
| Tarbosaurus bataar | 2165 | 65.1 | 0.08 |
| Tyrannosaurus rex | 2237, 5855 | 66.5, 71 | 0.09, 0.07 |
| Ornithomimosauria | |||
| Garudimimus brevipes | 98 | 28.8 | −0.10 |
| Ornithomimus edmontonensis | 153 | 31.4 | −0.09 |
| Dromiceiomimus brevitertius | 207 | 29.4 | −0.14 |
| Struthiomimus altus | 278 | 32.5 | −0.11 |
| Oviraptoridae | |||
| Citipati osmolskae | 130 | 31.5 | −0.08 |
| Dromaeosauridae | |||
| Saurornitholestes langstoni | 17 | 34.8 | 0.08 |
| Bambiraptor feinbergi | 2.44 | 28.5 | 0.09 |
| Velociraptor mongoliensis | 13 | 35.7 | 0.10 |
| Troodontidae | |||
| Troodon formosus | 61 | 32.6, 33, 33.2, 33.5 | −0.02 to −0.01 |
| Aves | |||
| Archaeopteryx lithographica | 0.28 | 17.1 | −0.01 |
| Alligatoridae | |||
| Alligator mississippiensis | 162, 91, 51 | 49.8, 54.3, 55.1 | 0.11, 0.18, 0.21 |
Body mass estimates for theropods were calculated using femur length (table 1), following the method of Christiansen & Fariña (2004). If femur length for a particular specimen was unknown, then femur length of an alternative representative specimen was chosen (see electronic supplementary material). Because femur length is unknown for Majungasaurus, a published body mass estimate based on femur circumference was used (Sampson & Witmer 2007). Body mass estimates for Alligator were based on skull length using the method of Farlow et al. (2005).
Olfactory ratios were plotted as a function of body mass, both log-transformed, for theropods and Alligator. The method of independent contrasts (Felsenstein 1985) was used to correct for the phylogenetic non-independence of theropod data (electronic supplementary material) and to produce a phylogenetically corrected regression with the PDAP module v. 1.13 of Mesquite v. 2.5 (Maddison & Maddison 2008; Midford et al. 2008). Because ratios can lead to violation of the assumption for reduced major axis regression (Smith 1999), a least-squares regression was used. Only theropod taxa were included in the regression, although Albertosaurus and Gorgosaurus were plotted after calculation of the regression due to the uncertainty in their olfactory ratios (table 1; electronic supplementary material). A confidence interval around the regression slope and comparison of residuals were used to determine whether taxa were significantly different from predicted values. Residuals were statistically compared through an ANOVA and Tukey's test with GraphPad Prism v. 5.01. Estimation of the olfactory ratio for basal tyrannosauroids was done following the ancestral state reconstruction method of Garland et al. (1999) in PDAP.
4. Results
A phylogenetically corrected least-squares regression of olfactory ratio to body mass in theropods reveals a slope of 0.1237 (figure 2); this slope is not significantly altered with the exclusion of the avian theropod Archaeopteryx (p>0.05; slope=0.1260; not illustrated). The positive slope of the regression demonstrates that the olfactory ratio increases with increasing body mass and that larger taxa possess larger olfactory bulbs relative to brain size than smaller taxa. The slope of the regression also shows that the relationship between olfactory ratio and body mass is negatively allometric, indicating that olfactory bulbs become smaller relative to brain size with increasing body size. A positive slope and a negative allometric relationship are also observed in regressions of brain mass to body mass in theropods (e.g. Jerison 1973; Larsson et al. 2000).
Figure 2.
Independent contrast least-squares regression through log-transformed olfactory ratio against body mass for theropods. Dashed lines represent 95% CI. Albertosaurus, Gorgosaurus and Alligator were plotted after calculation of the regression. Tyrannosaurids and dromaeosaurids plot above predicted olfactory ratio values and ornithomimosaurs and oviraptorids plot below predicted olfactory ratio values; these clades lie outside the 95% CI. Olfactory ratios for all other theropods, including Archaeopteryx, fall near values predicted by regressions. Alligator plots above the theropod regression line. Black circles, dromaeosaurids; white circles, tyrannosauroids; grey circles, allosauroids; black square, Archaeopteryx; white squares, ornithomimosaurs; grey squares, ceratosaurians; black diamond, oviraptorids; white diamonds, troodontids; grey diamonds, Alligator.
The distribution of theropod taxa along the least-squares regression reveals that tyrannosaurids and dromaeosaurids have high olfactory ratios that plot above the 95% CI, whereas ornithomimosaurs and oviraptorids have low olfactory ratios that plot below it. All other theropod taxa fall within the 95% CI and are considered typical. Comparison of the residuals reveals that the differences between these categories (high, low and typical) are significant (p<0.0001; figure 3; table 1).
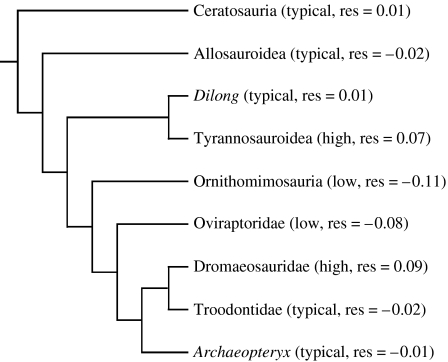
Figure 3.
Phylogenetic distribution of olfactory ratios in theropods (low: lower than predicted values; high: higher than predicted values; typical: near predicted values). Mean of residuals is reported for each clade. Statistical tests for each category (low, high and typical) were conducted on individual residuals reported in table 1. Phylogenetic hypothesis of Holtz & Osmólska (2004).
Olfactory ratios of Alligator are significantly higher than those predicted for theropods of similar body mass (figure 2), which suggests that the allometric relationship between olfactory bulb and cerebral hemisphere size in crocodylians may be different from that of theropods.
5. Palaeobiological implications of olfactory ratios in theropods
The relationship between olfactory ratio and body mass in theropods reveals that olfactory ratios are influenced by body size (figure 2). Consequently, olfactory ratios should not be used to directly compare olfactory acuity among theropods without consideration of body size. The fact that the olfactory ratios of tyrannosaurids, dromaeosaurids, ornithomimosaurs and oviraptorids differ significantly from predicted values indicates that their ratio values are not just an allometric consequence of body size, but probably reflect palaeobiological differences in these taxa. Because large olfactory bulbs occur in extant predators for which olfaction is used to conduct activities when vision or visual cues are limited (see §2), we interpret our results of olfactory bulb size in theropods accordingly.
Tyrannosaurids and dromaeosaurids have higher olfactory ratios than predicted for theropods of similar body size (figures 2 and 3), which suggests a keener sense of smell and a greater reliance on olfaction than in other theropods. These taxa also possess a higher degree of stereoscopic vision (similar to that of modern birds of prey) than other non-avian theropods, suggestive of predatory lifestyles (Stevens 2006). Among tyrannosaurids, however, Tyrannosaurus rex has been deemed a scavenger, partly based on the large size of its olfactory bulbs (Horner & Dobb 1997). Given the biological implications of olfactory bulb size in extant predators, the large olfactory bulbs of tyrannosaurids and dromaeosaurids may indicate that they were active in low-light conditions or may reflect the importance of olfaction for certain activities (e.g. location of food sources, navigation in large home ranges), rather than indicating a particular feeding strategy (i.e. predator versus scavenger).
Ornithomimosaurs and oviraptorids possess olfactory ratios that are much lower than predicted for theropods of their body size (figures 2 and 3), which indicates a low olfactory acuity. The enlarged orbits and optic lobes of these taxa have been interpreted as indicative of high visual acuity (Russell 1972; Makovicky et al. 2004; Osmólska et al. 2004; Kundrát 2007). Together, these features indicate that ornithomimosaurs and oviraptorids may have relied more on sight than on olfaction. If the trend observed between bird diets and olfactory ratios (Bang 1971; Bang & Wenzel 1985) can be applied to non-avian theropods, then the low olfactory ratios of ornithomimosaurs and oviraptorids would be consistent with earlier claims, based on skeletal features, that these animals may have been omnivorous (see Osmólska et al. (2004) and Barrett (2005) for recent reviews).
Allosauroids, ceratosaurians and basal tyrannosauroids all have olfactory ratios near those predicted for theropods of their respective body size (figures 2 and 3), which suggests that their olfactory acuities represent the typical (and inferred primitive) condition for theropods. If the olfactory ratios between theropods and Alligator can be directly compared, then theropods of body masses similar to Alligator would have had a lower olfactory acuity than Alligator (figure 2). The primitive theropod condition of laterally facing orbits in allosauroids has also been shown to result in a lower degree of stereoscopic vision than that of extant crocodylians (Stevens 2006), which probably also applies to ceratosaurians and basal tyrannosauroids based on the position of their orbits (see illustrations in Gilmore 1920; Madsen & Welles 2000; Xu et al. 2004; Sampson & Witmer 2007). It thus appears that the primitive condition for olfactory acuity and stereoscopic vision in theropods may have been lower than that of Alligator.
Troodontids also had olfactory ratios close to predicted values for theropods of their body size (figures 2 and 3), which suggests a typical olfactory acuity among theropods. In contrast to other theropods with typical olfactory ratios, however, Troodon had large, anteriorly facing orbits that probably resulted in well-developed stereoscopic vision (Russell 1969; Russell & Séguin 1982; Currie 1985; Stevens 2006). Thus, although troodontids retained the primitive condition among theropods for olfactory acuity, these animals evolved better depth perception of the surrounding environment than other theropods, perhaps indicating an increased reliance on vision.
The primitive bird Archaeopteryx had an olfactory ratio near values predicted for theropods of similar size (figures 2 and 3). This closeness of fit of Archaeopteryx to the regression reveals that the size of the olfactory bulbs, and hence the sense of smell, of the earliest birds would have been comparable with that of small non-avian theropods. Enlarged orbits and optic lobes in Archaeopteryx have been interpreted as indicative of well-developed sense of sight (e.g. Domínguez Alonso et al. 2004). Thus, this basal bird appears to have retained the primitive theropod condition for olfactory acuity but evolved a better sense of sight, generally regarded as an adaptation for flight (e.g. Domínguez Alonso et al. 2004).
6. Phylogenetic implications
Olfactory ratios of primitive members of various theropod clades studied in this analysis give insight into phylogenetic trends in the evolution of olfaction among theropods. Within tyrannosauroids, increased olfactory acuity characterizes only derived members of the clade (figure 3). The condition in the basal tyrannosauroid Dilong, with an olfactory bulb size typical of small theropods, differs from the enlarged olfactory bulbs of tyrannosaurids (figures 2 and 3). Ancestral state reconstruction (see electronic supplementary material) for the common ancestor of Dilong and tyrannosaurids reveals that it would have possessed an olfactory ratio close to that predicted for a theropod of its size (ancestor olfactory ratio=44.55, 95% CI on olfactory ratio: 34.9–56.9; ancestor body mass=470 kg, 95% CI on body mass: 62.8–3515.6 kg). From this result, it can be determined that, among tyrannosauroids, larger olfactory bulbs and greater olfactory acuity evolved closer to the clade Tyrannosauridae.
Among ornithomimosaurs, the low olfactory ratios of ornithomimids and of their sister taxon Garudimimus (Makovicky et al. 2004; Kobayashi & Barsbold 2005; figure 2) indicate that reduced reliance on olfaction was present in their common ancestor (figure 3). Such similarity between these taxa may reflect similar behaviour, possibly related to diet, as Garudimimus is the sole edentulous non-ornithomimid and presumably possessed ramphothecae (Kobayashi & Barsbold 2005). Given that feeding adaptations and skeletal proportions of basal ornithomimosaurs differ markedly from those of more derived taxa studied here (Makovicky et al. 2004; Kobayashi & Barsbold 2005), it is possible that the olfactory acuity of basal forms may have been closer to the typical condition for theropods. Study of basal ornithomimosaurs may reveal if reduced olfactory acuity is an autapomorphy of Ornithomimosauria or if it evolved within the clade.
The phylogenetic distribution of olfactory ratios within Theropoda also sheds light on avian sensory evolution. Recent studies have suggested that a general decrease in the importance of olfaction, associated with an increased reliance in sight, occurred through theropod evolution leading to birds (Rogers 1999; Franzosa 2004). Our study, however, demonstrates that reliance on olfaction does not decrease during theropod evolution, with the closest avian relatives (dromaeosaurids and troodontids) and primitive birds (Archaeopteryx) characterized by typical or high olfactory ratios (figure 3). Our results support Kurochkin et al.'s (2007) statement that the brain of early birds had not undergone major remodelling (i.e. enlarged cerebellum and reduced olfactory bulbs) relative to the brain of non-avian theropods to meet flight requirements. Indeed, many studies have suggested that the brain architecture of extant birds may not have evolved until the advent of more derived, and presumably more agile, ornithurine birds (Larsson et al. 2000; Kurochkin et al. 2007).
7. Conclusions
Our study reveals important palaeobiological information and evolutionary trends from the relationship between olfactory bulb size (relative to cerebral hemisphere size) and body mass in theropods. The larger-than-predicted olfactory bulbs of tyrannosaurids and dromaeosaurids suggest greater olfactory acuity and reliance on olfaction than that in other theropods, possibly to compensate for the diminished effectiveness of vision under low-light conditions and/or to locate prey and navigate larger home ranges. The smaller-than-predicted olfactory bulbs of ornithomimosaurs and oviraptorids suggest a reduced reliance on smell and possibly an omnivorous diet. Allosauroids, ceratosaurs, basal tyrannosauroids, troodontids and basal birds have olfactory bulb sizes predicted for theropods of their respective body masses, which suggests that these taxa had a typical sense of smell for theropods. Phylogenetic trends in olfaction in Theropoda reveal that the sense of smell does not decrease in the ancestry of birds, as troodontids, dromaeosaurids and even primitive birds possessed typical or high olfactory acuity. Thus, olfaction must have remained important in primitive birds and its presumed decrease associated with the increased importance of sight probably occurred among more derived ornithurine birds.
Acknowledgments
We thank Dr P. J. Currie (University of Alberta), Dr D. J. Varricchio (Montana State University) and Dr G. M. Erickson (Florida State University) for sharing unpublished data on theropod braincases and femur length. The authors are also grateful to Dr H. C. E. Larsson (McGill University) for allowing them to measure Carcharodontosaurus and Ornithomimus endocasts in his possession, Dr X. Xing (IVPP) for allowing us to CT scan Dilong and M. O'Neill (JHMI) for discussion about independent contrasts. The authors are indebted to Dr A. Kantzas and Y. Li (University of Calgary) for CT scanning numerous theropod braincases. Translation of Barsbold (1983) was done by C. Siskron, S. P. Welles and M. Carrano and obtained courtesy of the Polyglot Paleontologist website (http://ravenel.si.edu//paleoglot). The authors are grateful to Dr L. Witmer and Dr H. Larsson for their constructive comments on the manuscript.
Supplementary Material
Review of the studies of olfactory ratios in birds and description of the methods and analyses
References
- Ali F., Zelenitsky D.K., Therrien F., Weishampel D.B. Homology of the ‘ethmoid complex’ of tyrannosaurids and its implications for the reconstruction of the olfactory apparatus of non-avian theropods. J. Vert. Paleontol. 2008;28:123–133. doi:10.1671/0272-4634(2008)28[123:HOTECO]2.0.CO;2 [Google Scholar]
- Bang B.G. Functional anatomy of the olfactory system in 23 orders of birds. Acta Anat. 1971;79:1–76. doi: 10.1159/isbn.978-3-318-01866-0. doi:10.1159/000143668 [DOI] [PubMed] [Google Scholar]
- Bang B.G., Wenzel B.M. Nasal cavity and olfactory system. In: King A.S., McLelland J., editors. Form and function in birds. Academic Press; New York, NY: 1985. pp. 195–225. [Google Scholar]
- Barrett P.M. The diet of ostrich dinosaurs (Theropoda: Ornithomimosauria) Palaeontology. 2005;48:347–358. doi:10.1111/j.1475-4983.2005.00448.x [Google Scholar]
- Barsbold R. Carnivorous dinosaurs from the Cretaceous of Mongolia. Trans. Joint Sov.-Mongol. Paleontol. Exped. 1983;19:5–119. [Google Scholar]
- Benhamou S. An olfactory orientation model for mammals' movements in their home ranges. J. Theor. Biol. 1989;139:379–388. doi:10.1016/S0022-5193(89)80216-4 [Google Scholar]
- Brochu C.A. A digitally-rendered endocast for Tyrannosaurus rex. J. Vert. Paleontol. 2000;20:1–6. doi:10.1671/0272-4634(2000)020[0001:ADREFT]2.0.CO;2 [Google Scholar]
- Burnham D.A. New information on Bambiraptor feinbergi (Theropoda: Dromaeosauridae) from the Late Cretaceous of Montana. In: Currie P.J., Koppelhus E.B., Shugar M.A., Wright J.L., editors. Feathered dragons: studies on the transition from dinosaurs to birds. Indiana University Press; Bloomington, IN: 2004. pp. 67–111. [Google Scholar]
- Christiansen P., Fariña R.A. Mass prediction in theropod dinosaurs. Hist. Biol. 2004;16:85–92. [Google Scholar]
- Cobb S. A note on the size of the avian olfactory bulbs. Epilepsia. 1960;1:394–402. doi: 10.1111/j.1528-1157.1959.tb04276.x. doi:10.1111/j.1528-1157.1959.tb04276.x [DOI] [PubMed] [Google Scholar]
- Conover M.R. CRC Press; Boca Raton, FL: 2007. Predator–prey dynamics: the role of olfaction. [Google Scholar]
- Currie P.J. Cranial anatomy of Stenonychosaurus inequalis (Saurischia, Theropoda) and its bearing on the origin of birds. Can. J. Earth Sci. 1985;22:1643–1658. [Google Scholar]
- Currie P.J. New information on the anatomy and relationships of Dromaeosaurus albertensis (Dinosauria, Theropoda) J. Vert. Paleontol. 1995;15:576–591. [Google Scholar]
- Domínguez Alonso P., Milner A.C., Ketcham R.A., Cookson M.J., Rowe T.B. The avian nature of the brain and inner ear of Archaeopteryx. Nature. 2004;430:666–669. doi: 10.1038/nature02706. doi:10.1038/nature02706 [DOI] [PubMed] [Google Scholar]
- Farlow J.O., Hurlburt G.R., Elsey R.M., Britton A.R.C., Langston W., Jr Femoral dimensions and body size of Alligator mississippiensis: estimating the size of extinct mesoeucrocodylians. J. Vert. Paleontol. 2005;25:354–369. doi:10.1671/0272-4634(2005)025[0354:FDABSO]2.0.CO;2 [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. doi:10.1086/284325 [DOI] [PubMed] [Google Scholar]
- Franzosa, J. W. 2004 Evolution of the brain in Theropoda (Dinosauria). PhD dissertation, University of Texas at Austin, Austin, Texas.
- Garland T., Jr, Midford P.E., Ives A.R. An introduction to phylogenetically based statistical methods, with a new method for confidence intervals on ancestral values. Am. Zool. 1999;39:374–388. [Google Scholar]
- Gilmore C.W. Osteology of the carnivorous Dinosauria in the United States National Museum, with special reference to the genera Antrodemus (Allosaurus) and Ceratosaurus. US Nat. Mus. Bull. 1920;10:1–159. [Google Scholar]
- Gittleman J.L. Carnivore olfactory bulbs: allometry, phylogeny and ecology. J. Zool. 1991;225:253–272. [Google Scholar]
- Healy S., Guilford T. Olfactory-bulb size and nocturnality in birds. Evolution. 1990;44:339–346. doi: 10.1111/j.1558-5646.1990.tb05203.x. doi:10.2307/2409412 [DOI] [PubMed] [Google Scholar]
- Holtz Jr, T. J. & Osmólska, H. 2004 Saurischia. In The Dinosauria (eds D. B. Weishampel, P. Dodson & H. Osmólska), pp. 21–24, 2nd edn. Berkeley, CA: University of California Press.
- Hopson J.A. Paleoneurology. In: Gans C., editor. Biology of the Reptilia. Neurology A. vol. 9. Academic Press; New York, NY: 1979. pp. 39–146. [Google Scholar]
- Horner J.R., Dobb E. Harcourt Brace & Company; San Diego, CA: 1997. Dinosaur lives: unearthing an evolutionary saga. [Google Scholar]
- Houston D.C. Scavenging efficiency of turkey vultures in tropical forest. Condor. 1986;88:318–323. doi:10.2307/1368878 [Google Scholar]
- Hurlburt G.R. Alligator cerebrum occupies less than half of corresponding endocast region: implications for relative forebrain size in dinosaurs including Tyrannosaurus rex. J. Vert. Paleontol. 2005;25(Suppl. 65th Ann. Meet. Abstracts):73A. [Google Scholar]
- Jerison H.J. Academic Press; New York, NY: 1973. Evolution of the brain and intelligence. [Google Scholar]
- Kobayashi Y., Barsbold R. Reexamination of a primitive ornithomimosaur, Garudimimus brevipes Barsbold, 1981 (Dinosauria: Theropoda) from the Late Cretaceous of Mongolia. Can. J. Earth Sci. 2005;42:1501–1521. doi:10.1139/e05-044 [Google Scholar]
- Kundrát M. Avian-like attributes of a virtual brain model of the oviraptorid theropod Conchoraptor gracilis. Naturwissenschaften. 2007;94:499–504. doi: 10.1007/s00114-007-0219-1. doi:10.1007/s00114-007-0219-1 [DOI] [PubMed] [Google Scholar]
- Kurochkin E.N., Dyke G.J., Saveliev S.V., Pervushov E.M., Popov E.V. A fossil brain from the Cretaceous of European Russia and avian sensory evolution. Biol. Lett. 2007;3:309–313. doi: 10.1098/rsbl.2006.0617. doi:10.1098/rsbl.2006.0617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H.C.E. Endocranial anatomy of Carcharodontosaurus saharicus (Theropoda: Allosauroidea) and its implications for theropod brain evolution. In: Tanke D.H., Carpenter K., editors. Mesozoic vertebrate life. Indiana University Press; Bloomington, IN: 2001. pp. 19–33. [Google Scholar]
- Larsson H.C.E., Sereno P.C., Wilson J.A. Forebrain enlargement among nonavian theropod dinosaurs. J. Vert. Paleontol. 2000;20:615–618. doi:10.1671/0272-4634(2000)020[0615:FEANTD]2.0.CO;2 [Google Scholar]
- Mackay-Sim A., Royet J.-P. Structure and function of the olfactory system. In: Brewer W., Castle D., Pantelis C., editors. Olfaction and the brain. Cambridge University Press; Cambridge, UK: 2006. pp. 3–27. [Google Scholar]
- Maddison, W. P. & Maddison, D. R. 2008 Mesquite: a modular system for evolutionary analysis, v. 2.5. See http://mesquiteproject.org
- Madsen J.H., Jr, Welles S.P. Ceratosaurus (Dinosauria, Theropoda), a revised osteology. UT Geol. Surv. Misc. Publ. 2000;00-2:1–80. [Google Scholar]
- Makovicky, P. J., Kobayashi, Y. & Currie, P. J. 2004 Ornithomimosauria. In The Dinosauria (eds D. B. Weishampel P. Dodson & H. Osmólska), pp. 137–150, 2nd edn. Berkeley, CA: University of California Press.
- Midford, P. E., Garland Jr, T. & Maddison, W. P. 2008 PDAP package of Mesquite, v. 1.13. See http://mesquiteproject.org/pdap_mesquite/index.html
- Mori K., Nagao H., Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. doi:10.1126/science.286.5440.711 [DOI] [PubMed] [Google Scholar]
- Nevitt G.A. Sensory ecology on the high seas: the odor world of the procellariiform seabirds. J. Exp. Biol. 2008;211:1706–1713. doi: 10.1242/jeb.015412. doi:10.1242/jeb.015412 [DOI] [PubMed] [Google Scholar]
- Osmólska H. Evidence on relation of brain to endocranial cavity in oviraptorid dinosaurs. Acta Palaeontol. Pol. 2004;49:321–324. [Google Scholar]
- Osmólska, H., Currie, P. J. & Barsbold, R. 2004 Oviraptorosauria. In The Dinosauria (eds D. B. Weishampel P. Dodson & H. Osmólska), pp. 165–183, 2nd edn. Berkeley, CA: University of California Press.
- Rogers S.W. Allosaurus, crocodiles, and birds: evolutionary clues from spiral computed tomography of an endocast. Anat. Rec. 1999;257:162–173. doi: 10.1002/(SICI)1097-0185(19991015)257:5<162::AID-AR5>3.0.CO;2-W. doi:10.1002/(SICI)1097-0185(19991015)257:5<162::AID-AR5>3.0.CO;2-W [DOI] [PubMed] [Google Scholar]
- Russell D.A. A new specimen of Stenonychosaurus from the Oldman Formation (Cretaceous) of Alberta. Can. J. Earth Sci. 1969;6:595–612. [Google Scholar]
- Russell D.A. Ostrich dinosaurs from the Late Cretaceous of Western Canada. Can. J. Earth Sci. 1972;9:375–402. [Google Scholar]
- Russell D.A., Séguin R. Reconstructions of the small Cretaceous theropod Stenonychosaurus inequalis and a hypothetical dinosauroid. Syllogeus. 1982;37:1–43. [Google Scholar]
- Sampson S.D., Witmer L.M. Craniofacial anatomy of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. J. Vert. Paleontol. 2007;27(Suppl., Memoir 8):32–102. doi:10.1671/0272-4634(2007)27[32:CAOMCT]2.0.CO;2 [Google Scholar]
- Scott T.P., Weldon P.J. Chemoreception in the feeding behaviour of adult American alligators, Alligator mississippiensis. Anim. Behav. 1990;39:398–405. doi:10.1016/S0003-3472(05)80887-5 [Google Scholar]
- Smith L. A comparison of the number of nerve cells in the olfactory bulbs of domesticated albino and wild Norway rats. J. Comp. Neurol. 1928;45:483–501. doi:10.1002/cne.900450204 [Google Scholar]
- Smith R.J. Statistics of sexual size dimorphism. J. Hum. Evol. 1999;36:423–459. doi: 10.1006/jhev.1998.0281. doi:10.1006/jhev.1998.0281 [DOI] [PubMed] [Google Scholar]
- Stager K.E. The role of olfaction in food location by the turkey vulture (Cathartes aura) Los Angel. Count. Mus. Contr. Sci. 1964;81:1–63. [Google Scholar]
- Starck D. Cranio-cerebral relations in recent reptiles. In: Gans C., editor. Biology of the Reptilia. Neurology A. vol. 9. Academic Press; New York, NY: 1979. pp. 1–38. [Google Scholar]
- Steiger S.S., Fidler A.E., Valcu M., Kempenaers B. Avian olfactory receptor gene repertoires: evidence for a well-developed sense of smell in birds? Proc. R. Soc. B. 2008;275:2309–2317. doi: 10.1098/rspb.2008.0607. doi:10.1098/rspb.2008.0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens K.A. Binocular vision in theropod dinosaurs. J. Vert. Paleontol. 2006;26:321–330. doi:10.1671/0272-4634(2006)26[321:BVITD]2.0.CO;2 [Google Scholar]
- Therrien F., Ali F., Weishampel D.B. Olfactory bulb size as an indicator of olfactory acuity in non-avian theropods. J. Vert. Paleontol. 2005;25(Suppl. 65th Ann. Meet. Abstracts):121A. [Google Scholar]
- Verheyden C., Jouventin P. Olfactory behavior of foraging procellariforms. Auk. 1994;111:285–291. [Google Scholar]
- Weldon P.J., Ferguson M.W.J. Chemoreception in crocodilians: anatomy, natural history, and empirical results. Brain Behav. Evol. 1993;41:239–245. doi: 10.1159/000113845. doi:10.1159/000113845 [DOI] [PubMed] [Google Scholar]
- Weldon P.J., Swenson D.J., Olson J.K., Brinkmeier W.G. The American alligator detects food chemicals in aquatic and terrestrial environments. Ethology. 1990;85:191–198. [Google Scholar]
- Wenzel B.M., Meisami E. Number, size, and density of mitral cells in the olfactory bulbs of the Northern Fulmar and Rock Dove. In: Roper S., Atema J., editors. Olfaction and taste 9. Annals of the New York Academy of Sciences; New York, NY: 1987. pp. 700–702. [Google Scholar]
- Xu X., Norell M.A., Kuang X., Wang X., Zhao Q., Jia C. Basal tyrannosauroids from China and evidence for protofeathers in tyrannosauroids. Nature. 2004;431:680–684. doi: 10.1038/nature02855. doi:10.1038/nature02855 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Review of the studies of olfactory ratios in birds and description of the methods and analyses