Abstract
The ability of an organism to acquire O2 from its environment is key to survival and can play an important role in dictating a species' ecological distribution. This study is the first, to our knowledge, to show a tight, phylogenetically independent correlation between hypoxia tolerance, traits involved in dictating O2 extraction capacity and the distribution of a group of closely related fish species, sculpins from the family Cottidae, along the nearshore marine environment. Sculpins with higher hypoxia tolerance, measured as low critical O2 tensions (Pcrit), inhabit the O2 variable intertidal zones, while species with lower hypoxia tolerance inhabit the more O2 stable subtidal zone or freshwater. Hypoxia tolerance is phylogenetically independently associated with an enhanced O2 extraction capacity, with three principal components accounting for 75 per cent of the variation in Pcrit: routine O2 consumption rate; mass-specific gill surface area; and whole blood haemoglobin (Hb)–O2-binding affinity (P50). Variation in whole blood Hb–O2 P50 is strongly correlated with the intrinsic O2-binding properties of the purified Hb while the differences in the concentration of the allosteric Hb modulators, ATP and GTP, provide a Hb system with substantial plasticity for survival in a highly O2 variable environment.
Keywords: phylogenetically independent contrasts, adaptation, hemoglobin, oxygen consumption, intertidal environment, Cottidae
1. Introduction
Environmental adaptation is known to play an important role in setting species distribution among heterogeneous environments because species live where they do, in part, because they can. This is especially true of organisms that are distributed along the nearshore marine environment, which is an environment characterized by a steep spatial and temporal gradient in environmental O2, temperature, salinity, pH and CO2 (Truchot & Duhamel-Jouve 1980). This steep environmental gradient has a strong effect on species distribution (Doty 1946; Stillman & Somero 1996), especially in fish from the family Cottidae (sculpins), which exhibit a pattern of vertical zonation with different species inhabiting different portions of the nearshore environment (Froese & Pauly 2007). The role of temperature in dictating vertical zonation patterns along the nearshore environment has been examined in a number of organisms (e.g. Stillman & Somero 2000), but comparatively less work has explored the role of O2 variability in setting fish species habitat selection.
The ability of an animal to acquire O2 from its environment has long been considered an important determinant of hypoxia tolerance (Hughes 1973). Organisms that possess a greater capacity for O2 extraction are able to maintain a routine metabolic rate at lower O2 tensions and exploit more O2 variable environments. Thus, modifications to the respiratory cascade that enhance O2 extraction capacity will lead to an increase in hypoxia tolerance and be a potential target of natural selection in organisms living in environments characterized by periods of hypoxia. Indeed, hypoxia-induced modifications to gill surface area (Nilsson 2007), respiration (Saint-Paul 1984), tissue O2 demands (Hopkins & Powell 2001) and haemoglobin (Hb)–O2 binding characteristics (Jensen & Weber 1982) have all been described in fish species frequently exposed to hypoxia. In addition to these physiological modifications that enhance O2 uptake, fish are also known to employ a complex suite of behavioural, biochemical and molecular responses to hypoxia exposure that are important in contributing to hypoxic survival. Among the possible selection-driven modifications underlying hypoxia tolerance, greatest attention has been afforded to Hb–O2-binding characteristics. Interspecific variation in Hb–O2-binding affinity (measured as P50) can be attributed to differences in Hb multiplicity, Hb subunit/isoform heterogeneity, as well as variation in the intracellular concentrations of allosteric modulators (Perutz 1983; Brix et al. 1999).
Although substantial evidence exists supporting the notion that modifications to the respiratory cascade that enhance O2 uptake are adaptive for hypoxia survival, most studies performed to date ignore the possible influence of phylogenetic relationships on the traits under study. In order to isolate selection-based traits from those due solely to phylogenetic history, we employed the comparative method of phylogenetically independent contrasts (PICs; Felsenstein 1985; Garland et al. 1992), using multiple species of sculpins sampled mainly from the nearshore marine environment, which experience environmental hypoxia to varying degrees. We developed a new phylogeny for sculpins based on cytochrome b (cyt b) gene sequence and used this phylogeny to understand the correlated evolution of hypoxia tolerance (assessed as critical O2 tensions; Pcrit) and the properties of several components of the respiratory cascade in normoxia acclimatized sculpins. The relationship between variation in these traits and species distribution along the nearshore environment was also examined.
2. Material and methods
(a) Experimental animals
All sculpins were caught during the summers of 2005, 2006 and 2007 near the Bamfield Marine Sciences Centre, Bamfield, British Columbia, Canada with the exception of Myoxocephalus scorpius that was brought in from the Atlantic coast (Memorial University, Newfoundland, Canada) in 2006. Sculpins were transported to the University of British Columbia (UBC) and marine sculpins were held in 12°C seawater (30 ppt), while the single freshwater species (Cottus asper) was housed in 12°C freshwater. All sculpins were allowed to recover for at least three weeks before experimentation. Throughout the study period, fish were fed daily with bloodworms and frozen fish fillets, except 24 hours before experimental trials. The UBC Animal Care Committee approved all procedures performed on animals.
Whole animal respirometry (Pcrit and routine O2 consumption rate () was performed on sculpins captured in 2005 and fish captured in 2006 and 2007 were terminally sampled for analysis of gill surface area and red blood cell (RBC) Hb characteristics. We had some initial concern that fish might differ from year to year in their physiological responses to hypoxia, but no significant differences in Pcrit were observed when the same species were examined in multiple years. The mean fish weights used in 2006 are given in table S1 in the electronic supplementary material and the weights of fish captured in 2005 and 2007 were generally not different. The magnitude of variation in fish weights seen between species has no impact on measured Pcrit (G. Lau & J. G. Richards 2006, unpublished data).
(b) Experimental protocols
Routine and Pcrit were determined according to the protocols outlined in Henriksson et al. (2008) in water of equivalent composition (seawater or freshwater) and temperature (12°C) as the acclimatization conditions. To obtain resting, normoxic tissue samples for gill and RBC analysis, individual fish were housed overnight in sampling baskets, which were submerged in well-aerated 580 l tanks containing water of appropriate salinity at 12°C. The sample baskets were 5 l plastic chambers with mesh sides and a 1 l basin at the bottom. To sample a fish, the chamber was carefully removed, confining the fish to the basin and an overdose of benzocaine (250 mg l−1, Sigma-Aldrich, USA) was introduced into the chamber. The fish lost equilibrium within approximately 1 min and was removed, patted dry, weighed, the caudal peduncle severed and a blood sample taken using a heparinized haematocrit (Hct) tube. The shortest possible anaesthetic exposure time was chosen to limit the potential impacts of benzocaine on haematological parameters (Smit et al. 1979). The right gill basket was then dissected and fixed for later determination of total gill surface area (for detail see Henriksson et al. 2008). Any fish that showed signs of distress during sampling were discarded.
(c) Analytical procedures
From the sampled blood, [Hb] was determined using Drabkin's reagent (Sigma). Whole blood Hb–O2 P50 and Hill's coefficient (n) were determined using the procedures originally outlined by Reeves (1980) and described in Henriksson et al. (2008). Briefly, approximately 1 μl of freshly sampled whole blood was sandwiched between two gas permeable membranes and loaded into a prototype PWee50 regulated at 12°C. CO2 was kept constant at 0.5 per cent, but O2 levels were varied throughout the experiment, ranging from 0 to 100% O2, in order to determine the per cent saturation of Hb at different O2 levels. Measurements were recorded at seven to nine different O2 tensions to construct a linear section of the Hill plot for the determination of Hb P50 with n determined as the slope around half saturation in a plot of log(Y/(1−Y)) versus log PO2. The remaining blood was centrifuged, Hct measured, and RBCs were separated from plasma and both frozen in liquid N2 and stored at −80°C. On a separate group of fish (2007), blood samples were taken as described above and used to determine RBC intracellular pH (pHi) using the freeze-thaw method (Zeidler & Kim 1977).
Frozen RBC pellets from 2006 were thawed on ice and 20 mM Tris buffer (pH 7.4) was added at 12 times the estimated RBC volume. Samples were vortexed and left on ice for 5 min before centrifugation at 15 000g for 10 min at 4°C. From the resulting supernatant, aliquots were used for analysis of [ATP], [GTP] and [Hb]. The remaining cell haemolysate was immediately stripped of Hb modulators (ATP and GTP) using Micro Bio-spin P-30 Tris chromatography columns (Bio-Rad Laboratories) and aliquots of the stripped Hb were set aside for the determination of stripped blood Hb–O2 P50 (as described above), met-Hb analysis, Hb isoform profiles and reconstituted Hb–O2 P50. All four aliquots were frozen and stored at −80°C. Met-Hb was determined using the protocols of Benesch et al. (1973) and samples always contained below 15 per cent met-Hb.
Red blood cell [ATP], [GTP] and Hb isoform profiles were determined via HPLC using published protocols (Feuerlein & Weber 1994) with minor modifications. Briefly, separate HPLC runs were performed for triphosphates and Hb isoform determinations. For the analysis of ATP and GTP, aliquots of RBC haemolysates were first deproteinized with 3 per cent HClO4 and then neutralized with 3 M Tris base. Samples were clarified by centrifugation and immediately injected onto an anion exchange Mono-Q 5/50 GL column (GE Health Care, USA) and separated according to Feuerlein & Weber (1994). ATP and GTP were detected at 254 nm and the peaks were identified by comparison with retention times of known standards and quantified by comparisons to a standard curve prepared daily. [ATP] and [GTP] were standardized to [Hb] determined on the same RBC haemolysate. Hb isoform profiles were determined on an aliquot of stripped haemolysates that was diluted 7.2 times in high performance liquid chromatography buffer A (20 mM Tris, pH 8.0) and analysed according to Feuerlein & Weber (1994). To confirm that there was consistency between this study and Feuerlein & Weber (1994), blood from rainbow trout (Oncorhynchus mykiss) was treated identically to that described above, resulting in similar isoform elution patterns.
In order to determine if ATP and GTP were the primary RBC Hb modulators in sculpins, we reconstituted the purified, stripped Hb samples with measured RBC [ATP]/[Hb] and [GTP]/[Hb] and determined Hb–O2 P50. Briefly, samples of stripped haemolysates were thawed on ice and [Hb] quantified. Following Hb determination, samples of stripped Hb were reconstituted to the same [ATP]/[Hb] and [GTP]/[Hb] ratio measure as whole cell lysates and immediately analysed for Hb–O2 P50 as described above. To verify that we achieved the appropriate [ATP] and [GTP] in the reconstituted samples, concentrations of total nucleotide triphosphates (NTP) were determined spectrophotometrically using the enzyme-coupled assays as described by Bergmeyer (1983). There was no significant difference between the nominal sum of [ATP] and [GTP] and measured total [NTP] (data not shown).
(d) Phylogenetic analyses
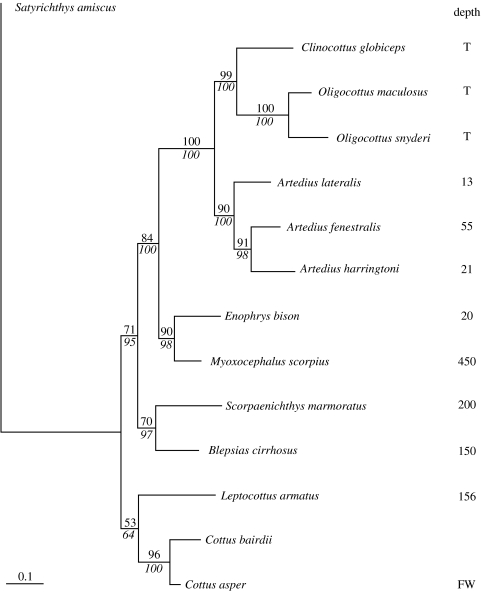
Genomic DNA was extracted from liver of three individuals of each species using a DNeasy Tissue Kit (Qiagen, Canada). Cyt b was amplified from each DNA sample by PCR using primers L14724 and H15915 from Schmidt & Gold (1993). PCR products were gel purified, extracted using a commercial kit (GenElute, Sigma), and sequenced directly using BigDye Terminator v. 3.1 chemistry and high-throughput sequence analysis (Applied Biosystems 3730S 48-capillary sequencer). For each sample, the resulting PCR product was sequenced in both directions and from all three individuals a consensus sequence for each species was established. Sequences have been submitted to GenBank with accession numbers of EU836693 to EU836704. Sequences were aligned using ClustalW and formatted as a nexus file in Mega v. 3.1 (Kumar et al. 2004). Sequences were imported into PAUP* (v. 4, Sinauer Associates Inc. Publishers, USA) to construct both maximum-likelihood and maximum-parsimony gene trees. Although both analyses gave similar results, the maximum-likelihood gene tree was chosen and Modeltest (Posada & Crandall 1998) was used to determine the likelihood ratio test that best fit the sequence data. A heuristics search was used to create the tree with bootstrap analysis of 100 pseudoreplicates. A consensus tree that was created from 100 000 Bayesian trees generated using MrBayes v. 3.0 (Ronquist & Huelsenbeck 2003) was similar to the maximum-likelihood tree (figure 1).
Figure 1.
Phylogenetic relationship of 13 species of sculpins based on a maximum-likelihood tree using cyt b sequences. Satyrichthys amiscus is included as an out-group. Numbers above lines show per cent of 100 maximum-likelihood bootstrap replicates with values shown for groups with more than 50% support. Italicized numbers below lines show posterior probability from Bayesian Markov Chain Monte Carlo analysis. Character data are presented for maximum depth of a species (metres), with T representing tide pool and FW representing freshwater.
(e) Phylogenetic independent contrasts
The maximum-likelihood tree and branch lengths were imported into Mesquite (Maddison & Maddison 2007) and the PDAP module (Midford et al. 2003) was used to analyse PIC. Additionally, we randomly sampled 10 000 trees from 100 000 trees created through Bayesian analysis into Mesquite and performed the standardized contrasts on the simulated trees in order to determine the robustness of the PIC analysis in terms of the uncertainty in topology and branch lengths (Martins & Housworth 2002). The PIC analysis using either the maximum-likelihood tree or Bayesian trees yielded similar results (Bayesian analysis shown in figures S1 and S2 in the electronic supplementary material). The results of PIC analysis also did not change appreciably when branch lengths of the maximum-likelihood tree were set to one (data not shown).
Since analysis can only be performed on complete datasets, we pruned the phylogenetic tree in Mesquite to include only the species for which there were available character data. Therefore, we excluded the out-group, Satyrichthys amiscus whose cyt b sequence (accession no. AP004441) was used to root the tree. The cyt b sequence from Cottus bairdii (accession no. AY833333) was included in order to resolve a polytomy between Leptocottus armatus, C. asper and the remainder of the sculpin family (cf. sculpin phylogeny in Kinziger & Wood 2003). Cottus bairdii was subsequently pruned from the tree for PIC analysis. An additional tree was constructed (results not shown) containing only the species of sculpins with available character data and there was no significant effect on the results generated by PIC analysis (results not shown).
(f) Statistical analyses
Data are presented as means±s.e. PIC correlations were analysed for significance in Mesquite, while conventional (non-PIC) correlations were analysed for significance using SigmaStat v. 3.0. Multiple linear regression models were developed on phylogenetically standardized contrasts and analysed for significance using SPSS 11. Statistical significance was assumed at p<0.05.
3. Results and discussion
(a) Phylogeny and species distribution
The phylogenetic comparative method aims to identify the repeated evolution of a trait correlated with one or more putatively selective variables while factoring out the possible confounding effects of shared ancestry among species (Felsenstein 1985). The application of PIC (Garland et al. 2005) assists in identifying selection-driven traits but requires an understanding of the phylogenetic relationships among the species under study. In the present study, we collected 11 species of sculpin from the nearshore marine environment (figure 1; maximum depth from Froese & Pauly 2007, but all species were collected within 5 m of the shore during the lowest tides), plus a freshwater species (C. asper) and constructed a well-resolved maximum-likelihood phylogeny based upon approximately 1090 bp of cyt b sequence from each species (figure 1). The resulting phylogeny is statistically robust with high bootstrap values and the overall topology is in general agreement with published molecular and morphological phylogenies (e.g. Ramon & Knope 2008). In further agreement with Ramon & Knope (2008), the mapping of maximum habitat depth of each species on to our phylogeny (figure 1) clearly demonstrates that the more ancestral groups of sculpins are primarily subtidal while the more derived species are found in the intertidal or tide pool environment.
(b) Species distribution and hypoxia tolerance
The nearshore environment displays a steep environmental gradient over a narrow geographical range with environments that show severe diurnal fluctuations in O2, temperature, pH, salinity and CO2 (tide pools) in close proximity to environments that show little fluctuation in these environmental parameters (subtidal; Truchot & Duhamel-Jouve 1980). Of relevance to the present study, fluctuations in environmental O2 along the nearshore environment have been shown to exert strong effects on species distribution (Brix et al. 1999; Altieri 2006), with more hypoxia tolerant species located in the more O2 variable intertidal zone. The distribution of sculpins along the nearshore environment was tightly correlated with hypoxia tolerance as determined by critical oxygen tension (Pcrit). Pcrit is the environmental O2 tension at which an organism's O2 consumption rate () transitions from being independent of environmental O2 to being dependent on environmental O2 and as such, Pcrit is considered an indicator of an animal's hypoxia tolerance (Chapman et al. 2002). Specifically there was a significant, positive correlation between species' maximum depth (a proxy for habitat breadth in the nearshore environment) and Pcrit, and the correlation was significant with both conventional correlation analysis (r2=0.52, p<0.01, correlation not shown) and the application of PIC (r2=0.57, p=0.01, correlation not shown). Sculpins located in the O2 variable tide pools have the lowest Pcrit values and are therefore more hypoxia tolerant than sculpins that inhabit the more O2 stable subtidal zone.
(c) Correlated evolution of hypoxia tolerance
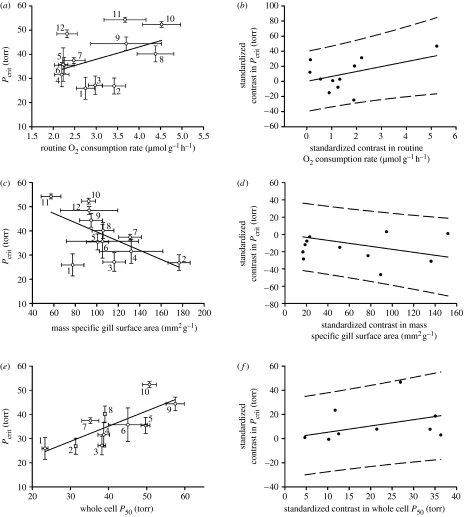
There is a vast literature supporting the notion that adjustments to the respiratory cascade that enhance O2 uptake are adaptive for hypoxia survival (e.g. Saint-Paul 1984; Hopkins & Powell 2001); however, this is the first study, to our knowledge, to apply rigorously PIC to understand the correlated evolution of hypoxia tolerant traits. Phylogenetically corrected multiple linear regression analysis revealed that routine , mass-specific gill surface area and whole blood Hb–O2 P50 assessed in normoxia acclimatized sculpins are the primary determinants of Pcrit in sculpins and the combined variation in these traits explain approximately 75 per cent of the variation in Pcrit (adjusted r2 of 0.747; F3,6=9.869, p<0.01). Species with a low Pcrit have a low routine (figure 2a), a large gill surface area (figure 2c), and high whole blood Hb–O2 binding affinity (figure 2e).
Figure 2.
Relationship between Pcrit and routine (a: y=5.15x+22.44, r2=0.20, p=0.15; b: y=6.52x, r2=0.35, p=0.04), Pcrit and mass-specific gill surface area (c: y=−1.81x+176.03, r2=0.35, p=0.04; d: y=−0.17x, r2=0.38, p=0.03) and Pcrit and whole blood Hb–O2 P50 (e: y=0.63x+9.94, r2=0.57, p=0.01; f: y=0.52x, r2=0.45, p=0.03). Conventional correlations are shown in (a,c,e), and standardized independent contrasts are shown in (b,d,f). Data are represented as mean±s.e. in (a,c,e), and 95% prediction intervals are shown in (b,d,f). 1 torr=0.133 kPa. Cottus asper has been omitted from panels (e) and (f) for reasons outlined in Henriksson et al. (2008). Numbers 1–12 represent different species: (1) Oligocottus maculosus, (2) Chaetopsylla globiceps, (3) Oligocottus snyderi, (4) Enophrys bison, (5) Artedius fenestralis, (6) Artedius lateralis, (7) Leptocottus armatus, (8) Scorpaenichthys marmoratus, (9) Blepsias cirrhosus, (10) M. scorpius, (11) C. asper, and (12) Artedius harringtoni.
Routine is an important factor shaping the respiratory cascade because it is an index of the overall O2 demand and an organism's resting energy requirements. Using conventional correlation analysis there was no significant relationship between Pcrit and routine (figure 2a); however, after correcting for the phylogenetic relatedness among sculpins using PIC, a significant positive relationship between Pcrit and routine was observed (figure 2b). In order to account for the possible effects of tree topology and branch length uncertainty on our conclusions, we performed PIC analysis using 10 000 randomly sampled trees generated via Bayesian analysis and 98 per cent of the resulting correlations had p<0.05 (figure S1a in the electronic supplementary material). Clearly, possible variability in tree topology does not appreciably affect the correlation between routine and Pcrit. Hypoxia tolerant sculpins have a low routine and therefore lower metabolic demands compared with hypoxia intolerant species.
Modifications to gill surface area in response to hypoxia exposure have been noted in several fish species and it has been proposed that these modifications enhance O2 uptake (Nilsson 2007). In normoxia acclimatized sculpins, there was an inverse relationship between Pcrit and mass-specific gill surface area (figure 2c), such that species with lower mass-specific gill surface area have a higher Pcrit. The relationship was significant when tested by conventional correlation analysis (figure 2c) and improved when corrected for phylogeny (figure 2d). This PIC correlation was robust and not affected by possible variation in tree topology as all analyses performed on trees generated via Bayesian analysis were significant (figure S1b in the electronic supplementary material). The large differences in total gill surface area observed between species were primarily due to large differences in filament number, filament length, lamellar area, and body weight (table S1 in the electronic supplementary material). No significant relationship existed between species body weight and mass-specific gill surface area (r2<0.01, p=0.94; correlation not shown), indicating that the variation in fish weight seen among species had only a minor impact on mass-specific gill surface area. Large gill surface area has previously been shown in fish species that inhabit periodically hypoxic environments such as the Amazonian Colossoma macropomum (Saint-Paul 1984), salt marsh-dwelling Poecilia latipinna (Timmerman & Chapman 2004b) and populations of Pseudocrenilabrus multicolour, Gnathonemus victoriae and Petrocephalus catostoma inhabiting dense swamp regions (Chapman et al. 2002).
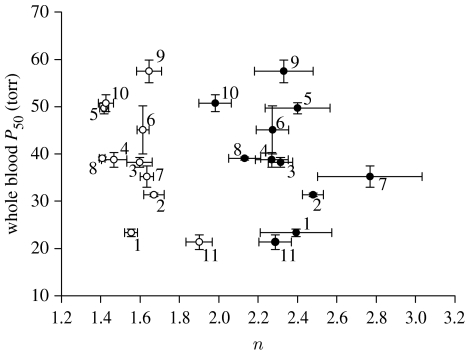
Conflicting views exist on whether or not evolutionary adaptation acts upon Hb–O2-binding affinity. Jensen (1991) proposed that since a low whole blood Hb–O2 P50 is often associated with hypoxia tolerant animals there must be a strong positive selection on Hb–O2-binding affinity. On the other hand, a recent synthesis by Milo et al. (2007) showed that whole blood Hb–O2 P50 did not vary substantially among groups of mammals, but cooperativity between Hb subunits during O2 binding (measured as Hill's coefficient; n) varied drastically. Under changing physiological conditions the opposite trend was noted and n remained constant, while the greatest variation was observed in whole blood Hb–O2 P50. From these results, Milo et al. (2007) concluded that evolutionary adaptation acts on n, while ‘physiological adaptations’ or rather physiological plasticity through acclimatization acts on Hb–O2 P50. Inferring adaptive value of traits, especially through the application of correlative analysis, however, requires the careful selection of species that inhabit a range of habitats in which the environmental condition under study varies and to which species appear to be ecologically isolated in these environments. The study of Milo et al. (2007) focused on mammals that do not experience large variations in environmental O2, therefore, their ability to comment on adaptations to hypoxia is limited.
Among sculpins living in environments that vary in magnitude and duration of hypoxia exposure, we noted very little variation in n (figure 3), while there was clear variation in whole blood Hb–O2 P50 that was phylogenetically independently correlated with Pcrit (figure 2f) and not influenced by possible variation in tree topology (figure S1c in the electronic supplementary material). These data strongly suggest that evolutionary adaptation to hypoxia within sculpins predominantly acts upon whole blood Hb–O2 P50, and sculpins with high Pcrit values have a high whole blood Hb–O2 P50 and are found in environments that infrequently experience hypoxic episodes (subtidal and freshwater). By contrast, sculpins with inherently low whole blood Hb–O2 P50 are able to extract more O2 at lower environmental O2 levels (ie. low Pcrit) and therefore can tolerate more hypoxic conditions such as those routinely encountered in isolated tide pools at night.
Figure 3.
Relationship between Hb–O2 P50 and Hill's coefficient (n) measured in whole blood (open circles) and stripped haemolysates (filled circles). Error bars around the symbols represent s.e. for x- and y-values. See figure 2 caption for more details.
Previous studies have suggested that high [Hb] and Hct may be beneficial to organisms inhabiting hypoxic environments (Chapman et al. 2002; Timmerman & Chapman 2004a). In sculpins, however, there was no relationship between blood [Hb] or Hct and Pcrit (tables 1 and 2). These findings are in contrast to Chapman et al. (2002), who found that fish species dwelling in hypoxic swamps showed greater O2 carrying capacity through higher [Hb] and Hct than normoxic lake-dwelling fish species. It must be emphasized, however, that our analysis was carried out on normoxia acclimatized sculpins in order to evaluate the inherent properties of the O2 cascade that might predispose fish to survive or succumb to hypoxia. Analysis of the effects of hypoxia exposure on these species is necessary in order to understand whether the degree of trait plasticity (e.g. hypoxia induced increases in [Hb]) is under selection and perhaps a better predictor of longer-term hypoxia tolerance.
Table 1.
Blood haematocrit (Hct), haemoglobin (Hb), mean cellular haemoglobin content (MCHC), haemoglobin modulators, RBC intracellular pH and Hb isoforms from sculpins. (Data are means±s.e. Hct is presented in %, Hb in mM, MCHC in [Hb]/Hct, [ATP] and [GTP] are presented relative to [Hb]. pHi represents RBC intracellular pH, Hb isoforms represent total number of isoforms, anodic represents the number of anodic Hb isoforms and cathodic represents the number of cathodic Hb isoforms. Numbers in brackets indicate sample size. A representative from each species was used to determine the total number of Hb isoforms, as well as the number of cathodic and anodic isoforms.)
| O. maculosus | C. globiceps | O. snyderi | E. bison | A. fenestralis | A. lateralis | L. armatus | S. marmoratus | B. cirrhosus | M. scorpius | C. asper | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hct | 35±2 (8) | 35±1 (8) | 37±3 (8) | 22±2 (8) | 27±2 (8) | 24±2 (4) | 11±1 (8) | 23±1 (8) | 29±1 (8) | 25±2 (8) | 27±2 (8) |
| Hb | 1.3±0.1 (5) | 1.3+0.1 (6) | 1.3+0.1 (3) | 0.6±0.1 (7) | 0.9±0.1 (8) | 0.9±0.1 (4) | 0.1±0.1 (8) | 0.8±0.1 (16) | 0.7±0.1 (6) | 0.7±0.1 (6) | 0.8±0.1 (15) |
| MCHC | 3.7±0.2 (3) | 3.4±0.3 (2) | 3.2±0.7 (2) | 2.5±0.2 (7) | 3.5±0.2 (8) | 3.5±0.4 (4) | 1.3±0.2 (7) | 2.8±0.1 (15) | 2.7±0.1 (14) | 2.7±0.2 (6) | 3.0±0.1 (15) |
| ATP/Hb | 1.82±0.18 (8) | 2.43±0.09 (8) | 2.18±0.06 (3) | 0.04±0.01 (6) | 0.91±0.06 (8) | 1.44±0.09 (3) | 1.47±0.13 (8) | 0.01±0.01 (7) | 1.38±0.04 (3) | 0.69±0.13 (6) | 1.04±0.17 (8) |
| GTP/Hb | 0.25±0.06 (8) | 0.51±0.03 (8) | 0.10±0.01 (3) | 0.28±0.08 (6) | 2.33±0.16 (8) | 0.40±0.07 (3) | 0.33±0.05 (8) | 0.02±0.01 (7) | 0.18±0.02 (3) | 0.22±0.03 (6) | 0.19±0.04 (8) |
| pHi | 7.35±0.03 (12) | 7.26±0.05 (2) | 7.26±0.02 (7) | 7.27 | 7.32±0.04 (6) | 7.30±0.06 (4) | 7.29±0.02 (8) | 7.18 | 7.42±0.02 (12) | N/A | 7.28±0.01 (8) |
| total isoforms | 4 | 9 | 4 | 9 | 9 | 10 | 5 | 4 | 8 | 3 | 10 |
| anodic | 4 | 6 | 3 | 8 | 6 | 7 | 5 | 4 | 8 | 3 | 9 |
| cathodic | 0 | 3 | 1 | 1 | 3 | 3 | 0 | 0 | 0 | 0 | 1 |
Table 2.
Relationship between Pcrit and haematological parameters, Hb modulators and Hb isoforms of sculpins using conventional and PIC correlations.
| conventional | PIC | |||||
|---|---|---|---|---|---|---|
| parameter | slope | r2 | p-value | slope | r2 | p-value |
| Hct | −0.53 | 0.16 | 0.23 | 0.31 | 0.04 | 0.57 |
| Hb | −13.81 | 0.24 | 0.12 | 3.70 | 0.01 | 0.77 |
| MCHC | −4.42 | 0.09 | 0.37 | 4.41 | 0.07 | 0.44 |
| ATP/Hb | −5.79 | 0.21 | 0.15 | −0.47 | <0.01 | 0.92 |
| GTP/Hb | −1.97 | 0.02 | 0.70 | −0.50 | <0.01 | 0.91 |
| total Hb isoforms | 0.17 | <0.01 | 0.89 | −0.45 | 0.02 | 0.66 |
| anodic Hb isoforms | 1.24 | 0.07 | 0.43 | −0.47 | 0.02 | 0.72 |
| cathodic Hb isoforms | −2.46 | 0.11 | 0.33 | −1.32 | 0.02 | 0.64 |
(d) Accounting for variation in whole blood Hb–O2 P50
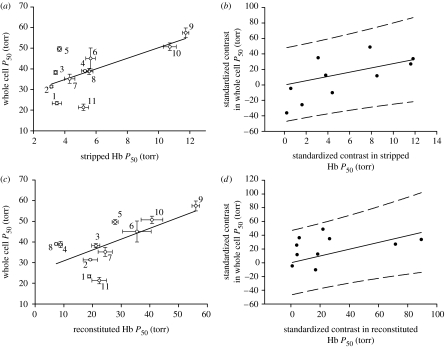
Whole blood Hb–O2 P50 can be set by both the intrinsic properties of the Hb protein and allosteric interactions between Hb and its modulators (Weber & Lykkeboe 1978). Variation in whole blood Hb–O2 P50 among sculpins is primarily dictated by the intrinsic properties of the Hb protein. Purified ‘stripped’ Hb isolated from different species of sculpins showed a similar pattern of Hb–O2 P50 as whole blood Hb–O2 P50, but at much reduced O2 tensions. Both conventional (figure 4a) and PIC (figure 4b) correlations showed significant positive relationships between whole blood Hb–O2 P50 and stripped Hb–O2 P50 and the PIC correlation was robust with the degree of significance not affected by variation in tree topology (figure S2a in the electronic supplementary material). Differences in intrinsic Hb–O2 P50 could be the result of variation in Hb protein sequence or the functional heterogeneity of Hb isoforms (Brix et al. 1999; Rutjes et al. 2007). Brix et al. (1999) showed that triplefin fishes located in O2 variable tide pools had a greater number of the higher affinity cathodic Hb isoforms, while those fish inhabiting mid-depth and deeper waters expressed fewer total Hb isoforms and of those present there was a greater proportion of lower affinity anodic isoforms. Furthermore, isoform switching to higher affinity Hb isoforms was observed during hypoxia acclimatization in the Lake Victoria cichlid, Haplochromis ishmaeli, pointing to Hb multiplicity as an important response to hypoxia exposure (Rutjes et al. 2007). Hb isoform characterization using anion-exchange chromatography showed no relationship between the number of total, anodic or cathodic Hb isoforms expressed in normoxia acclimatized sculpins and their Pcrit or whole blood Hb–O2 P50 (tables 1–3). These results should, however, be viewed tentatively as more careful isoform analysis (e.g. two-dimensional gel electrophoresis) and isoform specific functional characterization are required before we can discount the importance of Hb multiplicity in contributing to sculpin hypoxia tolerance.
Figure 4.
Relationship between whole Hb–O2 P50 and stripped Hb–O2 P50 (a: y=2.58x+24.56, r2=0.45, p=0.03; b: y=2.76x, r2=0.44, p=0.03) and whole blood Hb–O2 P50 and reconstituted Hb–O2 P50 (c: y=0.83x−6.86, r2=0.44, p=0.03; d: y=0.49x, r2=0.46, p=0.02). Conventional correlations are shown in (a,c), and standardized independent contrasts are shown in (b,d). Data are represented as mean ±s.e. in (a,c), and 95% prediction intervals are shown in (b,d). See figure 2 caption for more details.
Table 3.
Relationship between RBC Hb–O2 P50 and Hb modulators, Hb isoforms, RBC pHi and Hill coefficients of sculpins using conventional and PIC correlations.
| conventional | PIC | |||||
|---|---|---|---|---|---|---|
| parameter | m | r2 | p-value | m | r2 | p-value |
| ATP/Hb | −3.58 | 0.06 | 0.46 | 1.43 | <0.01 | 0.83 |
| GTP/Hb | 0.02 | 0.08 | 0.41 | 3.80 | 0.04 | 0.54 |
| total Hb isoforms | −0.01 | <0.01 | 0.99 | −0.83 | 0.04 | 0.54 |
| anodic Hb isoforms | −0.18 | <0.01 | 0.92 | −1.35 | 0.07 | 0.43 |
| cathodic Hb isoforms | 0.43 | <0.01 | 0.88 | −0.02 | <0.01 | 0.99 |
| RBC pHi | 62.14 | 0.12 | 0.32 | 48.05 | 0.09 | 0.40 |
| n (whole) | −38.79 | 0.25 | 0.11 | −0.01 | 0.17 | 0.21 |
| n (stripped) | −16.89 | 0.09 | 0.37 | −0.01 | 0.03 | 0.61 |
| n (reconstituted) | −7.24 | 0.01 | 0.76 | −0.01 | 0.02 | 0.67 |
Whole blood cell Hb–O2 P50 can also be influenced by the concentration of allosteric modulators such as ATP and GTP, whose binding to the Hb causes an increase in Hb–O2 P50. During hypoxia exposure, Hb–O2 P50 decreases through reductions in the concentration of allosteric modulators and this brings about a short-term improvement in O2 uptake from the environment (Weber & Lykkeboe 1978). ATP and GTP are the two major Hb modulators in most sculpins examined as indicated by our ability to nearly fully reconstitute whole blood Hb–O2 P50 by adding measured [ATP] and [GTP] back to purified, stripped Hb samples (figure 4d, S2b in the electronic supplementary material and table 1). These correlations were significant despite two notable exceptions, Enophrys bison and Scorpaenichthys marmoratus, which have extremely low ATP/Hb and GTP/Hb values (table 1), suggesting that these two species may possess different Hb modulators (e.g. 2,3-diphosphoglycerate). Despite ATP and GTP being the main Hb allosteric modulators in most species of sculpins, there was no significant relationship, either conventional or PIC (table 3), between blood [ATP] and [GTP] and whole blood Hb–O2 P50. Furthermore, there was no relationship between RBC pHi, another known modulator of Hb–O2 P50, and whole blood Hb–O2 P50 (table 3). Overall, the variation in whole blood Hb–O2 P50 seen among normoxia acclimatized sculpins appears to be primarily dictated by the intrinsic properties of the Hb protein and not the interactions with known Hb modulators.
Rutjes et al. (2007) demonstrated that hypoxia tolerant Lake Victoria cichlids have higher [ATP] and [GTP] under normoxic conditions compared with the relatively hypoxia-intolerant salmonids. In sculpins a similar, although non-significant, trend was observed with hypoxia tolerant sculpins (i.e. those with low Pcrit values) having higher [ATP]/[Hb] than the more hypoxia intolerant subtidal and freshwater species (tables 1 and 2). This relationship between Pcrit and RBC [ATP]/[Hb] was significant, however, when E. bison and S. marmoratus, the two species that potentially use alternate Hb modulators, were removed and the analysis repeated (r2=0.48 between ATP and Pcrit, p=0.04). Higher [ATP] coupled with a low Hb–O2 P50 in hypoxia tolerant sculpins possibly instils a significant capacity to endure not only severe hypoxia, but also rapid changes in environmental O2. RBC [ATP] and [GTP] are extremely responsive to O2 tension and decrease during hypoxia exposure in many fish species (e.g. Jensen & Weber 1982) causing a decrease in whole blood Hb–O2 P50. Presumably a similar regulation of Hb modulators occurs in sculpins, especially the most hypoxia tolerant sculpins that have very low intrinsic Hb–O2 P50 and high [ATP] and [GTP], overall providing greater capacity to respond to the fluctuating environmental O2 tensions typical in the tide pool. Analysis of the response of RBC [ATP] and [GTP] during hypoxia exposure is necessary to elucidate the importance of modulating these allosteric modulators in the hypoxic response.
The removal of the organic phosphates, ATP and GTP from the blood caused an increase in n that is consistent in all species examined (figure 3) and reversible with the addition of ATP and GTP to stripped Hb lysates (data not shown). There was no significant correlation between n determined in whole, stripped or reconstituted blood and whole blood Hb–O2 P50 (table 3), suggesting that cooperativity between Hb subunits during O2 binding is not significantly related to normoxic Hb–O2 binding affinity (table 3). Under hypoxic conditions, however, when RBC [ATP] and [GTP] decrease, increased cooperativity of Hb–O2 binding, along with decreased Hb–O2 P50 could work synergistically to enhance O2 extraction from the O2 poor environment in the most hypoxia tolerant sculpins.
(e) Adaptations to interacting stressors
O2 is only one of many environmental factors that are known to vary along the nearshore environment. Significant temporal and spatial variation in temperature, salinity, pH and CO2 (Truchot & Duhamel-Jouve 1980) have been noted and tolerance to these environmental perturbations, along with other ecological factors, contribute to dictating species distribution along the nearshore environment. In contrast to the remarkably stable subtidal environment (measured at 1 m depth; O2 between 70 and 120% air saturation, 11–13°C, pH approx. 9), we have measured dramatic variation in tide pools in O2 (less than 5 to approx. 400% air saturation), temperature (11–24°C in pools isolated at midday) and pH (7–9; D. G. Harley & J. G. Richards 2007, unpublished data). Therefore, sculpins inhabiting tide pools (T in figure 1) are exposed to a variety of environmental stressors, albeit at different times of the day and to different degrees depending on the tidal cycle and local weather conditions, compared with those species living in the subtidal. Although, the present study focused on the mechanisms underlying hypoxia tolerance, many of the attributes described in our most hypoxia tolerant sculpins should be viewed as beneficial for surviving the large changes in temperature, pH and CO2 typical in the tide pool environment. For example, an intrinsically low Hb–O2 P50 in sculpins inhabiting tide pools is not only beneficial for aiding in hypoxia survival at night, but also potentially beneficial in counteracting the effects of elevated temperature during the day, which is known to increase Hb–O2 P50 and impair O2 uptake. Clearly, the selective pressures driving trait evolution in the intertidal environment are complex, and a thorough assessment of hypoxia, temperature, pH, CO2 and salinity tolerance may help elucidate which environmental variables are responsible for the patterns of species zonation along the heterogeneous nearshore environment.
(f) Concluding remarks
In normoxia acclimatized sculpins, hypoxia tolerance is primarily associated with the maintenance of a low routine , high gill surface area and low whole blood Hb–O2 P50 all of which function to maintain an O2 uptake sufficient to meet metabolic demands. The adaptive value of maximizing O2 uptake is that it prolongs the period of time an animal can remain in a hypoxic environment prior to eliciting a hypoxia response, such as a downregulation of Hb allosteric modulators or a decrease in metabolic rate. For organisms living in the nearshore marine environment this is ideal, as it allows for the ability to cope with diurnal fluctuations in O2 levels without impacting cellular function and overall physiological performance. The responses to hypoxia exposure (e.g. decreases in RBC [ATP]), however, are also probably under strong selection and remain to be examined.
Acknowledgments
The UBC Animal Care Committee approved all procedures performed on animals.
This work was funded by a Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grant to J.G.R., a postgraduate scholarship to M.M. and a postdoctoral fellowship to A.E.T. We thank Dr Peter Frappell, LaTrobe Unversity for loaning us essential equipment and Dr Wayne Maddison, UBC, for their valuable insight into applying phylogenetically independent contrasts.
Supplementary Material
Distribution of p-values resulting from analyses performed with 10 000 randomly sampled trees from 100 000 trees generated by Bayesian analysis for correlation between (a) Pcrit and routine, (b) Pcrit and mass specific gill surface area and (c) Pcrit and RBC Hb-O2 P50
Distribution of p-values resulting from analyses performed with 10 000 randomly sampled trees from 100 000 trees generated by Bayesian analysis for correlation between (a) RBC Hb-O2 P50 and stripped Hb-O2 P50 and (b) whole RBC Hb-O2 P50 and reconstituted Hb-O2 P50
Fish weight and gill morphometrics from 12 species of sculpins
References
- Altieri A.H. Inducible variation in hypoxia tolerance across the intertidal-subtidal distribution of the blue mussel Mytilus edulis. Mar. Ecol. Prog. Ser. 2006;325:295–300. doi:10.3354/meps325295 [Google Scholar]
- Benesch R.E., Benesch R., Yung S. Equations for the spectrophotometric analysis of hemoglobin mixtures. Anal. Biochem. 1973;55:245–248. doi: 10.1016/0003-2697(73)90309-6. doi:10.1016/0003-2697(73)90309-6 [DOI] [PubMed] [Google Scholar]
- Bergmeyer H.U. Academic Press; New York, NY: 1983. Methods of enzymatic analysis. [Google Scholar]
- Brix O., Clements K.D., Wells R.M.G. Haemoglobin components and oxygen transport in relation to habitat selection in triplefin fishes (Tripterygiidae) J. Comp. Physiol. B. 1999;169:329–334. doi:10.1007/s003600050228 [Google Scholar]
- Chapman L.J., Chapman C.A., Nordlie F.G., Rosenberger A.E. Physiological refugia: swamps, hypoxia tolerance and maintenance of fish diversity in the Lake Victoria region. Comp. Biochem. Physiol. A. 2002;133:421–437. doi: 10.1016/s1095-6433(02)00195-2. doi:10.1016/S1095-6433(02)00195-2 [DOI] [PubMed] [Google Scholar]
- Doty M.S. Critical tide factors that are correlated with the vertical distribution of marine algae and other organisms along the Pacific Coast. Ecology. 1946;27:315–328. doi:10.2307/1933542 [Google Scholar]
- Felsenstein J. Phylogenies and the comparative methods. Am. Nat. 1985;125:1–15. doi:10.1086/284325 [Google Scholar]
- Feuerlein R.J., Weber R.E. Rapid and simultaneous measurement of anodic and cathodic haemoglobins and ATP and GTP concentrations in minute quantities of fish blood. J. Exp. Biol. 1994;189:273–277. doi: 10.1242/jeb.189.1.273. [DOI] [PubMed] [Google Scholar]
- Froese, R. & Pauly, D. 2007 FishBase. See www.fishbase.org
- Garland T.J., Harvey P.H., Ives A.R. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 1992;41:18–32. doi:10.2307/2992503 [Google Scholar]
- Garland T.J., Bennett A.F., Rezende E.L. Phylogenetic approaches in comparative physiology. J. Exp. Biol. 2005;208:3015–3035. doi: 10.1242/jeb.01745. doi:10.1242/jeb.01745 [DOI] [PubMed] [Google Scholar]
- Henriksson P., Mandic M., Richards J. The osmo-respiratory compromise in sculpins: impaired gas exchange is associated with freshwater tolerance. Physiol. Biochem. Zool. 2008;81:310–319. doi: 10.1086/587092. doi:10.1086/587092 [DOI] [PubMed] [Google Scholar]
- Hopkins S.R., Powell F.L. Common themes of adaptation to hypoxia: insights from comparative physiology. Adv. Exp. Med. Biol. 2001;502:153–167. doi: 10.1007/978-1-4757-3401-0_11. [DOI] [PubMed] [Google Scholar]
- Hughes G.M. Respiratory responses to hypoxia in fish. Am. Zool. 1973;13:475–489. [Google Scholar]
- Jensen F.B. Multiple strategies in oxygen and carbon dioxide transport by hemoglobin. In: Woakes A.J., Grieshaber M.K., Bridges C.R., editors. Physiological strategies for gas exchange and metabolism. Cambridge University Press; Cambridge, UK: 1991. pp. 55–78. [Google Scholar]
- Jensen F.B., Weber R.E. Respiratory properties of tench blood and hemoglobin: adaptation to hypoxic-hypercapnic water. Mol. Physiol. 1982;2:235–250. [Google Scholar]
- Kinziger A.P., Wood R.M. Molecular systematics of the polytypic species Cottus hypselurus (Teleostei: Cottidae) Copeia. 2003;3:624–627. doi:10.1643/CI-02-240R [Google Scholar]
- Kumar S., Tamura K., Nei M. Mega3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. doi:10.1093/bib/5.2.150 [DOI] [PubMed] [Google Scholar]
- Maddison, W. P. & Maddison, D. R. 2007 Mesquite: a modular system for evolutionary analysis. See http://mesquiteproject.org
- Martins E.P., Housworth E.A. Phylogeny shape and the phylogenetic comparative method. Syst. Biol. 2002;51:873–880. doi: 10.1080/10635150290102573. doi:10.1080/10635150290102573 [DOI] [PubMed] [Google Scholar]
- Midford, P. E., Garland, T. J. & Maddison, W. P. 2003 PDAP : PDTREE: a translation of the PDTREE application of Garland et al's phenotypic diversity analysis programs. See http://mesquiteproject.org/pdap_mesquite
- Milo R., Hou J.H., Springer M., Brenner M.P., Kirschner M.W. The relationship between evolutionary and physiological variation in hemoglobin. Proc. Natl Acad. Sci. USA. 2007;104:16 998–17 003. doi: 10.1073/pnas.0707673104. doi:10.1073/pnas.0707673104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G.E. Gill remodeling in fish: a new fashion or an ancient secret? J. Exp. Biol. 2007;210:2403–2409. doi: 10.1242/jeb.000281. doi:10.1242/jeb.000281 [DOI] [PubMed] [Google Scholar]
- Perutz M.F. Species adaptation in a protein molecule. Mol. Biol. Evol. 1983;1:1–28. doi: 10.1093/oxfordjournals.molbev.a040299. [DOI] [PubMed] [Google Scholar]
- Posada D., Crandall K.A. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. doi:10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- Ramon M.L., Knope M.L. Molecular support for maine sculpins (Cottidae; Oligocottinae) diversification during the transition from the subtidal to intertidal habitat in the Northeastern Pacific Ocean. Mol. Phylogenet. Evol. 2008;46:475–483. doi: 10.1016/j.ympev.2007.11.005. doi:10.1016/j.ympev.2007.11.005 [DOI] [PubMed] [Google Scholar]
- Reeves R.B. A rapid micro method for obtaining oxygen equilibrium curves on whole blood. Respir. Physiol. 1980;42:299–315. doi: 10.1016/0034-5687(80)90121-8. doi:10.1016/0034-5687(80)90122-X [DOI] [PubMed] [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. doi:10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Rutjes H.A., Nieveen M.C., Weber R.E., Witte F., Van den Thillart G.E.E.J.M. Multiple strategies of Lake Victoria cichlids to cope with life long hypoxia include hemoglobin switching. Am. J. Physiol. 2007;293:R1376–R1383. doi: 10.1152/ajpregu.00536.2006. [DOI] [PubMed] [Google Scholar]
- Saint-Paul U. Physiological adaptation to hypoxia of a neotropical characoid fish Colossoma macropomum, Serrasalmidae. Environ. Biol. Fish. 1984;11:53–62. doi:10.1007/BF00001845 [Google Scholar]
- Schmidt T.R., Gold J.R. Complete sequence of the mitochondrial cytochrome b gene in the Cherryfin Shiner, Lythrurus roseipinnis (Teleostei: Cyprinidae) Copeia. 1993;3:880–883. doi:10.2307/1447258 [Google Scholar]
- Smit G.L., Hattingh J., Burger A.P. Haematological assessment of the effects of the anaesthetic MS222 in natural and neutralized form in three freshwater fish species: interspecies differences. J. Fish. Biol. 1979;15:633–643. doi:10.1111/j.1095-8649.1979.tb03672.x [Google Scholar]
- Stillman J.H., Somero G.N. Adaptation to temperature stress and aerial exposure in congeneric species of intertidal porcelain crabs (genus Petrolisthes): correlation of physiology, biochemistry and morphology with vertical distribution. J. Exp. Biol. 1996;199:1845–1855. doi: 10.1242/jeb.199.8.1845. [DOI] [PubMed] [Google Scholar]
- Stillman J.H., Somero G.N. A comparative analysis of the upper thermal tolerance limits of eastern Pacific porcelain crabs, genus Petrolisthes: influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol. Biochem. Zool. 2000;73:200–208. doi: 10.1086/316738. doi:10.1086/316738 [DOI] [PubMed] [Google Scholar]
- Timmerman C.M., Chapman C.A. Behavioural and physiological compensation for chronic hypoxia in the Sailfin Molly (Peocilia latipinna) Physiol. Biochem. Zool. 2004a;77:601–610. doi: 10.1086/421754. doi:10.1086/421754 [DOI] [PubMed] [Google Scholar]
- Timmerman C.M., Chapman L.J. Hypoxia and interdemic variation in Poecilia latipinna. J. Fish. Biol. 2004b;65:635–650. doi:10.1111/j.0022-1112.2004.00474.x [Google Scholar]
- Truchot J.P., Duhamel-Jouve A. Oxygen and carbon dioxide in the marine intertidal environment: diurnal and tidal changes in rockpools. Respir. Physiol. 1980;39:241–254. doi: 10.1016/0034-5687(80)90056-0. doi:10.1016/0034-5687(80)90056-0 [DOI] [PubMed] [Google Scholar]
- Weber R.E., Lykkeboe G. Respiratory adaptations in carp blood: influences of hypoxia, red cell organic phosphates, divalent cations and CO2 on hemoglobin–oxygen affinity. Biochem. Exp. Biol. 1978;128:127–137. [Google Scholar]
- Zeidler R., Kim H.D. Preferential hemolysis of postnatal calf red cells induced by internal alkalinization. J. Gen. Physiol. 1977;70:385–401. doi: 10.1085/jgp.70.3.385. doi:10.1085/jgp.70.3.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of p-values resulting from analyses performed with 10 000 randomly sampled trees from 100 000 trees generated by Bayesian analysis for correlation between (a) Pcrit and routine, (b) Pcrit and mass specific gill surface area and (c) Pcrit and RBC Hb-O2 P50
Distribution of p-values resulting from analyses performed with 10 000 randomly sampled trees from 100 000 trees generated by Bayesian analysis for correlation between (a) RBC Hb-O2 P50 and stripped Hb-O2 P50 and (b) whole RBC Hb-O2 P50 and reconstituted Hb-O2 P50
Fish weight and gill morphometrics from 12 species of sculpins