Abstract
The classical version of the differential allocation hypothesis states that, when females reproduce over their lifetime with partners that differ in their genetic quality, they should invest more in reproduction with high-quality males. However, in species with lifetime monogamy, such as the zebra finch, partner quality will typically remain the same. In this case, the compensatory investment (CI) hypothesis predicts higher investment for low-quality males, because low genetic quality offspring are more dependent on maternal resources. Here, we show that female zebra finches invested more resources, both in terms of egg volume and yolk carotenoid content, when paired to a low genetic quality male, as judged from his previous ability to obtain extra-pair paternity in aviary colonies. We also found that females deposited slightly larger amounts of testosterone into eggs when paired to a low parental quality male, as judging from his previous success in rearing offspring. This is, to our knowledge, the first experimental support for the CI hypothesis in a species with lifetime monogamy. We stress that in more promiscuous species, the benefits of classical differential allocation may partly be neutralized by the supposed benefits of CI.
Keywords: carotenoids, differential allocation hypothesis, egg resources, mate attractiveness, maternal effects, reproductive investment
1. Introduction
Life-history theory predicts that females should adjust their investment in a particular breeding attempt according to the expected pay-offs of that attempt (Williams 1966). Male attractiveness can influence offspring fitness and, hence, should affect female investment in reproduction. Two opposing patterns have been found in empirical studies: either that females invest more when mated with attractive males, or, less commonly, that they invest more for unattractive males. Both these patterns can be referred to as differential allocation in the broad sense, but we suggest that it is conceptually fruitful to clearly separate the two types.
(a) Positive differential allocation
Positive differential allocation (PDA) is built on the logic that females trade off their current versus future reproduction (Trivers 1972) and that this trade-off is influenced by the attractiveness of the partner. If partner quality is expected to vary over an individual's lifetime, the female should invest more when paired to a more attractive male, since good gene effects will increase offspring value and eventually result in more grand offspring and, hence, higher fitness. This is the case in mating systems with high reproductive skew, where females should preferentially invest in the highest quality offspring. PDA has found support in a range of taxa (reviewed by Sheldon 2000). Somewhat unfortunately, the general term differential allocation has been widely accepted in this narrow sense of denoting only PDA.
(b) Compensatory investment
If low genetic quality offspring are more sensitive to environmental stress (e.g. shortage of nutrients) than high genetic quality offspring, then it may pay females to increase their investment when paired to a male of low genetic quality. This investment pattern is called compensatory investment (CI) and has been observed in a few studies (Saino et al. 2002; Michl et al. 2005; Navara et al. 2006; Gowaty et al. 2007). This is especially likely in a mating system with low reproductive skew, where even low-quality offspring will reproduce successfully. In addition to assessing the genetic quality (indirect benefits) of a partner, females could also judge the situation based on male parental quality (direct benefits). Thus, increased primary investment (for example egg nutrients) could compensate for both low genetic quality and low parental quality.
The benefits of PDA and CI have to be weighed against each other. Hence, the ecology and life history of a species will determine what process is most likely to occur. Two decisive factors are the likelihood of mating with a higher quality partner in the future and the degree of reproductive skew in the population. In addition to partner quality, a range of environmental factors could influence female investment into reproduction. We therefore use an experimental approach where we can control for a number of such factors, either experimentally (e.g. food availability, time of season, etc.) or statistically (lay order effects).
(c) Previous work on zebra finches
Burley pioneered the field of differential allocation, but in contrast to us, she concentrated on secondary investment (i.e. feeding rate, Burley 1986, 1988) rather than primary investment into eggs. Since her now classical work, the zebra finch has become a model species for studies on differential allocation (Swaddle 1996; Balzer & Williams 1998; Gil et al. 1999; Rutstein et al. 2004, 2005a,b; von Engelhardt 2004; Gorman et al. 2005; Gilbert et al. 2006; Williamson et al. 2006). However, empirical studies on female primary investment in relation to male attractiveness have had mixed results (Balzer & Williams 1998; Gil et al. 1999; Rutstein et al. 2004; von Engelhardt 2004; Gilbert et al. 2006; Williamson et al. 2006). Three of these studies used artificial ornaments (i.e. colour rings) to manipulate male attractiveness and found no significant main effects, but rather two- or three-way interactions with different investment patterns for attractive versus unattractive males, either with respect to egg laying sequence (Gilbert et al. 2006; Williamson et al. 2006) or with respect to female mass (Rutstein et al. 2004). The biological interpretation of these interactions is not straightforward. Moreover, it is unclear what aspects of male attractiveness are manipulated with coloured rings, since female zebra finches will probably choose males both for indirect (‘good genes’) and direct (for example parental abilities) benefits. The same is true for choice chamber attractiveness, that was used by Balzer & Williams (1998) and von Engelhardt (2004). To disentangle the effects of indirect and direct benefits, an experimental approach is needed which uses separate measures of male sexual attractiveness (reflecting good genes) and of male parental quality.
(d) The present study
In the present study, we look at female zebra finch primary investment into reproduction in relation to actual measures of male sexual attractiveness as ascertained by females themselves. To do this, we used birds that had participated in a breeding experiment under free-flying aviary conditions. This allowed us to obtain reliable measures of male sexual attractiveness and parental quality. A male's sexual attractiveness was measured as his success at siring extra-pair (EP) offspring, and parental quality as the success at rearing offspring. Based on these measures, we chose pairs of males with extreme values of sexual attractiveness (matched for parental quality), and pairs of males with extreme values of parental quality (matched for sexual attractiveness). We then paired females sequentially to two males, one from either extreme of one of these axes. Since investment measures differed among previous studies, and since different aspects of the investment could be influenced by either genetic or parental quality, we decided to simultaneously measure a range of egg traits. Thus, as measures of female primary investment, we use egg volume, yolk mass, an index of yolk carotenoid content, yolk testosterone content and the number of eggs laid.
(e) Study species
The zebra finch is an opportunistic breeder with biparental care that form lifelong pair bonds in nature (Zann 1996). Under laboratory conditions, divorce rates of established pairs are extremely low (Forstmeier 2007), whereas re-pairing readily occurs after partner loss. Typically, several broods are reared during one rainy season (Zann 1996). Mortality between breeding seasons is rather high (Zann 1996), and since pairs remain together in the non-breeding season, both partners are likely to be affected simultaneously by harsh environmental conditions, such as droughts (Immelmann 1970). Thus, the probability of survival beyond the first partner and to reproduce with another partner is rather low. Moreover, it seems probable that there is assortative mating for quality in zebra finches (Burley 1986; Burley & Foster 2006). Thus, partner quality is expected to vary little over an individual's lifetime. Considered together with the low reproductive skew that characterizes the zebra finch mating system, we assume that there has been limited scope for PDA to evolve, while we would expect to find CI.
2. Material and methods
(a) General design
We chose the 32 females with the highest average fecundity from two breeding rounds in a previous aviary breeding experiment. They were split randomly into two groups of 16 females and each female was paired sequentially to two males. Males were chosen from the same aviary experiment based on two criteria: success at siring EP eggs and parental quality. One group of females was assigned to males based on EP attractiveness, so that the two partners of a female had maximal difference in EP attractiveness, while the parental quality was as similar as possible between the two partner males, and vice versa for the other 16 females. Within treatments, males were assigned randomly to females, but ensuring that a female had not been in the same aviary as any of her two partners.
(b) Subjects and housing
All subjects had been kept at the Max Planck Institute for Ornithology in Seewiesen, Germany, since October 2004. For details of rearing conditions for these birds, see Forstmeier (2005). During the experiments, pairs were housed in cages measuring 60×40 cm and 45 cm high in two rooms. For details of housing conditions, see Bolund et al. (2007).
(c) Aviary experiment
We measured sexual attractiveness and parental qualities under free-flying aviary conditions. Briefly, six females and six males in each of nine aviaries were allowed to breed for three months in September–November 2005. As part of a sex ratio treatment, three aviaries had an additional three females, three aviaries an additional three males, while the remaining three had no additional birds added, giving sex ratios of 0.4, 05 and 0.6, respectively. After a non-breeding period without nesting opportunities, breeding pairs were exchanged among aviaries and sex ratio treatments, such that each pair faced five new unfamiliar pairs, and allowed to breed for another three months in April–June 2006. In total, owing to the replacement of birds that died, 139 birds were used in this experiment. The paternity of eggs or offspring was determined using 10 microsatellite loci (Forstmeier et al. 2007), and 99.9 per cent of samples were assigned unambiguously to genetic parents. Calculations are based on 1727 eggs. The EP paternity rate (EPP rate) was 34 per cent of eggs or offspring. We defined EPP as eggs sired by a paired male with any female other than his partner. Each pair was allowed to rear one clutch (or two to three, if initially unsuccessful) per breeding round. Eggs were fully cross-fostered between the aviary pairs and 72 foster pairs kept in cages, and each aviary clutch was restricted to two to three eggs (2.44±0.53 mean ±s.d.). In total, 275 fertile eggs were fostered into the aviaries and they resulted in 109 fledged offspring.
(d) Measure of genetic quality
In our population, the most likely reason for females to seek EPP is for indirect benefits, i.e. good genes. This is supported by the high repeatability of male EPP success with different sets of potential EP partners in the aviary experiment (ANOVA: F59.60=3.72, p<0.001, R=0.58±0.09), suggesting that females are choosing males for EP copulations based on their perception of the male's genetic quality. However, the EPP success of a male depends both on female choice and male sex drive (i.e. the tendency to seek EP copulations). To obtain a measure reflecting female perception of male genetic quality, we used residuals of a male's average EPP success, accounting for sex ratio treatment and correcting for courtship rate measured in standardized trials, where a male and a female were allowed to interact for 5 min in a cage setting (male sex drive measured in this way is repeatable over long periods of time, R±s.e.=0.60±0.07, Forstmeier 2007). We picked the eight males with the highest and the eight with the lowest residual EPP scores that also had estimates of parental quality (see below). Out of the 16 males chosen for the current experiment based on their EPP success, the eight unattractive males sired 1.1±1.8 (range 0–4) EP offspring each, and the eight attractive sired 29.4±16.5 (range 17–57) EP offspring each (the males in the high attractiveness group sired on average 31%±13 of all available eggs in their aviary). Observations carried out in the 2006 breeding season, showed that only 1 out of 65 males never attempted an EP courtship during 22 hours of early morning observation (195 hours in total for the nine aviaries). The eight unattractive males had two unforced copulations (forced copulation attempts are practically never successful, see also Forstmeier 2004, 2007) out of 66 courtships (3% success rate, range from 1 to 25 courtships per male), while the attractive group had 24 unforced copulations out of 133 courtships (18% success rate, range from 4 to 39 courtships per male). Thus, display frequencies were still higher in the attractive group compared with the unattractive group in our experiment, but, importantly, the success rate was much higher for the attractive males. Hence, while the correction for song rate is only partly successful in disentangling female choice from male sex drive, the paternity data are still more reliable than the limited number of observed copulations. Male success at fertilizing EP eggs (EP success) was strongly dependent on the average EP female responsiveness (scored as described in Forstmeier 2007) to him in these observations (multiple regression: standardized β=0.35, p=0.01), while EP success was not dependent on EP courtship rate in the same observations (β=0.14, p=0.31). The high- and low- EP-attractiveness groups were matched as closely as possible for parental quality.
(e) Measure of parental quality
Male parental quality is difficult to measure directly, since actual feeding rates are difficult to estimate. However, in our population, despite ad libitum food conditions, readiness to feed the chicks varies greatly among individuals, such that offspring mass at the age of 8 days varied between 1.6 and 12.2 g (Schielzeth et al. 2008). Furthermore, some birds let all or most of their offspring starve to death before starting to feed the brood. In a previous study, we measured offspring growth under cage conditions of one to two subsequent clutches for 38 females (mean ±s.d.: 1.36±0.48 clutches) and 29 males (mean ±s.d.: 1.26±0.44 clutches) with each of two different partners, i.e. each bird was a part of two different pairs. To estimate parental quality, we used a combination of the average offspring mass at day 8 (m) and fledging success (s=n/e), where n is the number of fledglings and e is the number of fertile eggs put into the nest. We combined these two measures by taking the average of (m×s) and (m). This was done to give more weight to the normally distributed m relative to the overdispersed measure s. The resulting term was z-standardized and then multiplied with (√e) to weight it by sample size. This measure was repeatable for a male with two different female partners (ANOVA: F30.31=2.27, p=0.013, R=0.39±0.15, E. Bolund, H. Schielzeth & W. Forstmeier 2005, unpublished data), whereas for females, it was not (ANOVA: F38.39=0.99, p=0.52, R=0.007±0.18). This suggests that the parental quality of males is predictable, or at least that rearing success depends on male, but not female, identity in a predictable way. Hence, for the present study, we use the same estimate of parental quality, which was measured based on male breeding success during the aviary experiment. We chose the males from the eight best and the eight worst performing pairs out of the 58 pairs with available data. This created two groups that differed substantially in parental performance (e.g. the proportion of fertile eggs resulting in fledged offspring was 0.85±0.13 versus 0.07±0.1, mean ±s.d. for the two groups, respectively). These extreme high or low levels of parental care require both the male and the female to be either ‘good’ or ‘bad’ parents. Our estimate of parental quality is less reliable for intermediate parental quality males (including the males chosen based on EP attractiveness), since an intermediate success can be achieved for example via compensation by the female following low investment of the male. The high and low parental quality groups were chosen such that they were matched for EP attractiveness, see figure 1.
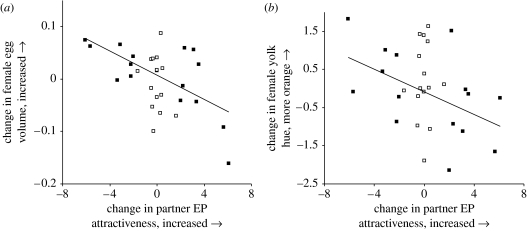
Figure 1.
(a) Change in egg volume when females were paired to two different partners (partner 2 minus partner 1) related to change in extra-pair (EP) attractiveness of the partners (partner 2 minus partner 1). Females laid significantly larger eggs with the partner with lower EP attractiveness (all females pooled: r=−0.55, n=30, p=0.002). (b) Females laid eggs with significantly more orange yolks with the less attractive partner. For ease of illustration, higher values of yolk hue correspond to more orange eggs (all females pooled: r=−0.38, n=30, p=0.038). The 15 females paired to males with maximal difference in EP attractiveness are represented by filled squares, while the 15 females paired to males with maximal difference in parental quality are represented by open squares.
Male parental quality was not correlated with his residual EP success (r=0.15, n=56, p=0.28, based on all males from the aviary experiment who had an estimate of both variables).
(f) Experimental procedure
Birds were paired in cages in January 2007, and allowed 42 days to lay two clutches (one female laid no eggs, one female laid one clutch only, while two females initiated a third clutch). After separation, they were given a non-breeding period of 52 days in same-sex groups in aviaries, with no auditory or visual contact with the previous partner. Birds were then paired to the second partner in April 2007 and allowed 40 days to lay two clutches (one female laid no eggs and four females laid only one clutch). On the day of laying, eggs were collected and replaced with plastic eggs, which were removed after a standard incubation period of 15 days after the day on which incubation was initiated. The length and width of the eggs was measured using calipers to the nearest 0.05 mm and egg volume was calculated according to the formula of an ellipsoid (1/6×π×length×width2). During the experiment, 522 eggs were laid. Three birds died between the two breeding rounds of the experiment and were replaced with birds fulfilling the above-mentioned criteria. These deaths resulted in a total of 67 birds (34 males and 33 females) used for the experiment.
At the initiation of each breeding round, we measured the mass, fat score (clavicular and abdominal fat, scored on a scale from 0 to 5) and beak colour of all birds. We used a principal component type 1 analysis of six spectrophotometric traits for beak colour analyses (described in Bolund et al. 2007). As measures of female condition, we used residuals from a regression of mass over tarsus. We used five measures of female investment, egg volume (egg volume is positively related to offspring survival to adulthood in our population, random-intercept model: t=6.0, p<0.0001, n=1661 eggs by 154 females, W. Forstmeier 2008, unpublished data), number of eggs laid per day of the experiment, yolk hue (see below), yolk androgen content and yolk mass. Correlations between female morphology and female investment can be seen in table 1.
Table 1.
Correlations between different aspects of female investment and female morphology. (Correlations are based on female mean values from the two breeding rounds. Yolk hue is used as a proxy for total carotenoid content in eggs. Female beak colour was measured before the onset of breeding with spectrophotometry. *p<0.05, **p<0.01, ***p<0.001.)
| yolk hue | egg volume | yolk mass | eggs per day | yolk testosterone | female beak colour | female mass | female condition | |
|---|---|---|---|---|---|---|---|---|
| egg volume | 0.14 | |||||||
| yolk mass | −0.12 | 0.45* | ||||||
| eggs per day | 0.40* | 0.12 | 0.28 | |||||
| yolk testosterone | −0.30 | 0.48** | 0.01 | 0.43* | ||||
| female beak colour | 0.35 | 0.03 | 0.20 | 0.14 | 0.26 | |||
| female mass | 0.04 | 0.31 | 0.20 | −0.20 | 0.065 | −0.13 | ||
| female condition | 0.05 | 0.11 | 0.16 | −0.19 | 0.020 | −0.10 | 0.86*** | |
| female fat score | −0.07 | 0.05 | 0.09 | −0.21 | −0.29 | −0.09 | 0.63*** | 0.54** |
(g) Yolk colour analyses
Carotenoids are pigments that are responsible for the hue of egg yolk and are essential to embryonic and early post-hatching development in birds (Surai 2002). Two studies using high performance liquid chromatography to measure carotenoid content in zebra finch yolks found mainly lutein, zeaxanthin and anhydrolutein, with small amounts of β-cryptoxanthin and β-carotene (McGraw et al. 2002; Royle et al. 2003). Carotenoid content decreases over the laying sequence (e.g. Royle et al. 2003; Williamson et al. 2006), but the relative percentages of component carotenoids remained unchanged in a comparison of first and last eggs in zebra finch clutches (Royle et al. 2003). The hue of zebra finch egg yolks varies dramatically among eggs and changes substantially from orange to yellow over the laying sequence within a clutch, while the saturation invariably was very close to 100 per cent (this study). This suggests that more orange yolks contain a higher total concentration of carotenoids. Studies comparing carotenoid content obtained from biochemical assays with the colour of carotenoid rich materials, such as egg yolk or feathers, have confirmed that the colour of egg yolks can be used as a reliable indicator of carotenoid content (Saks et al. 2003; Verboven et al. 2005). We thus assume that yolk hue can be used as a proxy for total carotenoid content in zebra finch egg yolks.
Out of 522 egg yolks, 514 were photographed relative to a colour standard (Q-13, Kodak Colour Separation Guide) under standardized conditions (Nikon D1x, Nikon DX80-flash), either on the day of laying or, in 12 per cent of the cases, eggs were refrigerated at 7°C on the day of laying, and photographed 1–2 days later. The albumen was removed with the use of a pipette and the yolk subsequently transferred to a slide for photographing and weighing (to the nearest 0.1 mg). We used a self-written R function to extract RGB values (red, green, blue) of the yolk and yellow standard. This function sampled a pre-defined area, covering 30–50% of the yolk. Data sampled in this automated way accurately represented the hue of the whole yolk surface in a subset of yolks: r=0.99, p<0.001, n=48. We used the function ‘grDevices’ in R to convert RGB into hue, saturation and brightness values. Each yolk was photographed twice and we used the averaged hue for further analyses. The repeatability of yolk hue between the two photographs of one yolk was extremely high (ANOVA: F516.517=235, p<0.001, R=0.99±0.0007). Brightness measured on the colour standard varied slightly among photographs (mean ±s.d.: 0.96±0.01). Hence, hue of the yolk was corrected by taking residuals from a regression over brightness of the standard. Hue is a circular variable (0–360°), but the range covered by variation in egg yolks (33–49°) is sufficiently small to use linear statistics.
To obtain one measure of yolk hue per female for each of her male partners, we used the random effect estimates for each pair from a linear mixed effect model of residual yolk hue with laying order within clutches as a fixed effect covariate, and pair identity and clutch identity as random intercepts. This model accounts for variation over the laying sequence and between successive clutches with the same male. Models were run in R 2.5.1 (R Development Core Team 2007), using the ‘lme’ function from the nlme package (Pinheiro et al. 2007).
(h) Yolk testosterone content
Testosterone (T) levels were analysed in egg 1 and 4 of each clutch where this was possible. In total, 189 eggs were analysed for T. Frozen yolks were thawed and homogenized with an equal amount of double-distilled water. Two hundred microlitres of the yolk–water mixture were used; 1500 dpm 3H-testosterone was added to measure extraction efficiency, incubated overnight, and steroids extracted by freeze decanting using a mixture of 4 ml petroleum ether/diethyl ether (30% vol./70% vol.), repeated twice. The petroleum ether/diethyl ether phase was dried at 39°C under a stream of nitrogen. Then, 90 per cent ethanol was added and samples frozen overnight to precipitate proteins. The next day, the ethanol phase was removed and washed twice with hexane to remove lipids. Then, the ethanol was dried at 50°C under a stream of nitrogen and samples resuspended in phosphate buffered saline with gelatine buffer. Extraction efficiency was 58±8 per cent (mean ±s.d.). T concentrations were measured by radioimmunoassay following Goymann et al. (2006). Because the T antibody has a cross-reactivity of 40 per cent with 5α-dihydrotestosterone our measurement refer to androgens. Samples were analysed in two assays. The detection limits were 0.6 and 0.9 pg per tube. Intra-assay variations were 5 and 3.1 per cent. The inter-assay variation was 6.1 per cent. T-values (pg per mg yolk) were ln-transformed to approach normality. To obtain one measure of T per female with each of her two partners, we used the same method as for yolk hue, i.e. we used the random effect estimates from a linear mixed effect model with laying order within clutches as a fixed effect covariate, and pair identity and clutch identity as random intercepts. T deposition was highly repeatable within females between her two different partners (ANOVA: F27.27=6.5, p<0.001, R=0.73±0.09). In a subset of 10 females, T levels were highly repeatable between the current experiment (in cages) and the previous experiment in aviaries in 2005–2006 (r=0.75, p=0.013, n=10).
(i) Interaction effects on allocation
Two previous studies looking at female investment in relation to male manipulated attractiveness found different investment patterns over the egg laying sequence for attractive versus unattractive males (Gilbert et al. 2006; Williamson et al. 2006). We therefore tested for interactions between treatment and lay order, using linear mixed effect models. Apparently, previous studies have accounted for the non-independence of data points (eggs laid by the same female) by including female identity as a random effect (allowing females to differ in their means, i.e. intercepts). However, females also differ randomly in their slopes of investment over the laying order. If random variation in slopes is not controlled for, there is a dramatically increased risk of type I errors for the interaction between laying order and treatment (Schielzeth & Forstmeier in press).
Hence, our linear mixed effect models include random intercepts as well as random slopes for female identity and clutch identity. This accounts for between-individual and between-clutch variations in overall response values as well as laying order slopes. Predictors were male EP attractiveness and lay order including both main effects and the interaction.
(j) Statistical analysis
We used SPSS (SPSS Inc. 2004) and R 2.5.1 (R Development Core Team 2005) for statistical analyses. All statistical tests are two-tailed and α was set to 0.05. Data were transformed if necessary to approach normality. Repeatabilities and their standard errors were calculated following Lessells & Boag (1987) and Becker (1984). G*Power 3.0.8 (Franz Faul, Universität Kiel, Germany, 2006) was used for power analysis.
3. Results
The repeatability of female investment between the two different partners was generally very high (table 2).
Table 2.
Repeatability of female investment with two different partners. Yolk hue is used as a proxy for carotenoid content.
| d.f. | F | p-value | repeatability±s.e. | |
|---|---|---|---|---|
| yolk hue | 29, 30 | 9.90 | <0.001 | 0.82±0.06 |
| egg volume | 29, 30 | 16.4 | <0.001 | 0.89±0.039 |
| yolk mass | 29, 30 | 9.13 | <0.001 | 0.80±0.064 |
| eggs per day | 30, 31 | 6.83 | <0.001 | 0.74±0.080 |
| yolk testosterone | 27, 27 | 7.28 | <0.001 | 0.76±0.083 |
(a) Effects of extra-pair attractiveness
In the group of females that were assigned males based on EP attractiveness, the change in female investment was correlated with the change in the male trait. When paired to a male with lower EP attractiveness, females laid eggs that were significantly larger (egg volume: r=−0.69, n=15, p=0.005, mean ±s.d. with attractive male: 1.20±0.11, mean ±s.d. with unattractive male: 1.23±0.12, this result remained significant after the most conservative Bonferroni correction, i.e. for conducting 10 tests) and produced more orange yolks (yolk hue: r=0.52, n=15, p=0.046 mean ±s.d. with attractive male: 43.3±1.84, mean ±s.d. with unattractive male: 42.9±2.35, lower hue values correspond to more orange yolks). Both relationships remain significant when including the second group of females that were assigned to males based on parental quality (figure 1). Results remained qualitatively the same when using an alternative analysis with female absolute values of investment as the response and controlling for male parental quality in linear mixed effect models (data not shown). Change in T levels in the yolk, yolk mass and the number of eggs laid were not influenced by change in male EP attractiveness (all r<0.24, all p>0.38). There were no significant interaction effects of male attractiveness on female investment over the laying order of a clutch (egg volume: F1,392=0.75, p=0.39, yolk hue: F1,390=0.19, p=0.67).
(b) Effects of parental quality
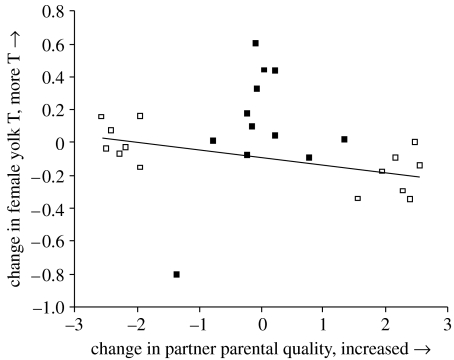
The change in T levels in the yolk was correlated with the change in male parental quality (figure 2). However, the changes in T levels in response to this treatment were small compared with the changes observed in the other group of females that were assigned males based on EP attractiveness. Hence, the correlation across all females was clearly non-significant (figure 2). The change in all other investment traits, i.e. egg volume, yolk hue, yolk mass and the number of eggs laid, were not correlated with the change in male parental quality (all r<0.31, all p>0.24). There were no significant interaction effects of male parental quality on female investment over the laying order of a clutch (all F<1.75, all p>0.18).
Figure 2.
Change in female yolk testosterone (T) deposition when paired to two different males (partner 2 minus partner 1) related to change in parental quality of the partners (partner 2 minus partner 1). Females assigned males based on parental quality deposited more T when paired to a low parental quality male (r=−0.70, n=14, p=0.0052, see regression line). When all females are pooled, this is not significant (r=−0.17, n=26, p=0.42). Females paired to males with maximal difference in EP attractiveness are represented by filled squares, while females paired to males with maximal difference in parental quality are represented by open squares.
4. Discussion
In concordance with the CI hypothesis, females invested more resources into their eggs (larger eggs and more orange yolks) when paired to a male of lower sexual attractiveness. Females paired to a male of low attractiveness could be making the most of a bad situation. This is similar to the finding of Navara et al. (2006), who concluded that female house finches may alter the condition of lower quality offspring to salvage an otherwise unsuccessful breeding attempt. We found no effect of male attractiveness or parental quality on the number of eggs laid or yolk mass. Similarly, in earlier studies of female primary investment in relation to male attractiveness in zebra finches, no or only very weak effects on clutch size have been found (Balzer & Williams 1998; Rutstein et al. 2004; von Engelhardt 2004; Gilbert et al. 2006).
Female T deposition was not related to male attractiveness. Thus, the hypothesis that low genetic quality offspring would not be able to tolerate higher levels of T (see Groothuis et al. 2005) is not supported by our data. Interestingly, females deposited more T in the yolk when paired to a male of low parental quality. While this was highly significant, the effect was only minor in relation to the overall variation, see figure 2. Thus, it is questionable whether this difference in T levels confers any biological relevance. Still it should be mentioned that the large within-female changes in T levels in the EP attractiveness treatment group does not invalidate the significant effect obtained in the parental quality treatment group, because our estimates of male parental quality may be valid only for extreme cases, i.e. the latter group (see §2). Nevertheless, the pattern is consistent with the previous finding of Gil et al. (1999), showing that females increased androgen deposition when paired to a red-ringed male compared with when the same females were paired to a green-ringed male. Red-ringed males have been found to provide less parental care (Burley 1986, 1988; Burley et al. 1994, 1996; von Engelhardt 2004). Thus, both studies imply that females might adjust T deposition in response to expected male parental quality rather than in response to male perceived genetic quality. This is in accordance with the idea that females might use T to manipulate partner parental contribution (see Groothuis et al. 2005; Hinde & Kilner 2007), since higher T levels in the yolk causes chicks to beg more intensely (Schwabl 1996; Groothuis et al. 2005). The other primary investment traits measured were not influenced by male parental quality. It might be that egg nutrients are an inefficient compensation for the decreased male chick provisioning. Male parental quality would then probably influence female secondary, rather than primary, investment (see Burley 1988).
(a) Possible ultimate and proximate explanations
If one assumes that CI is an adaptive strategy for the female, this would require that an increase in egg nutrients confers larger fitness benefits for low genetic quality offspring than for high genetic quality offspring. It is possible that this is the case in a mating system with low reproductive skew where most individuals will be able to find a partner. If provided enough resources during development, low genetic quality offspring might be able to reproduce nearly as successfully as high genetic quality offspring, whereas with more restricted resources, they might not reproduce at all. Thus, CI might generally confer a fitness advantage in species with low reproductive skew. By contrast, if reproductive skew is high, disproportionate investment in the highest genetic quality offspring would seem to pay more in terms of the production of grandchildren, since only the highest quality males will be able to reproduce.
A possible proximate explanation to the CI pattern is that male behaviour influences female investment. Males of low EP attractiveness are expected to focus their reproductive investment on their social partner instead of seeking EP copulations. This increase in within-pair investment could stimulate the reproductive physiology of the partner, so that she invests more in egg quality. One pathway of reproductive stimulation would be via stimulatory singing (Kroodsma & Byers 1991). We have some evidence that this is the case in our zebra finch population (Bolund et al. in preparation).
The occurrence of EPP has the potential to shift the expected investment pattern from CI to PDA (see Sheldon 2000). If the availability and genetic quality of EP males varies over the lifetime of a female, she is expected to invest more when having EPP with a high genetic quality male. However, it is questionable whether a female can adaptively adjust her investment in response to EPP, since the clutch will probably contain a mixture of within-pair and EP offspring (Birkhead et al. 2000). Also, in a colonial breeding species, such as the zebra finch, there should always be the opportunity to have EP copulations with a highly attractive male, leading to little variation in the attractiveness of genetic sires between clutches.
(b) Previous studies
Two previous studies have found interaction effects of male attractiveness on female investment patterns over the egg laying sequence of a clutch (Gilbert et al. 2006; Williamson et al. 2006). Caution is required when assessing the statistical significance of such interaction effects (see §2 and Schielzeth & Forstmeier in press).We found no interaction between male attractiveness and female investment over the lay order. While an increased investment in the last eggs of a clutch could help in alleviating the disadvantages to last hatched chicks, under the framework of CI, all offspring of an unattractive male would be of lower genetic quality and hence would all benefit from increased investment.
Previous studies on differential allocation in zebra finches have found no significant main effects regarding egg size or yolk carotenoid level. One study looked at carotenoid levels in egg yolks, and found a trend for an increased level of carotenoids in eggs laid for green-ringed (i.e. unattractive) males (Williamson et al. 2006). Four studies have looked at effects of natural or manipulated male attractiveness on egg size and found no consistent trends (Balzer & Williams 1998; Rutstein et al. 2004; von Engelhardt 2004; Gilbert et al. 2006).
Our significant results of male attractiveness on female investment beg the question of why such an effect has not been found in previous studies. We can think of three possible explanations that will be dealt with in detail below: (i) differences in experimental design, (ii) the strength of the experimental treatment and (iii) differences in information content of the traits used as indicators of male attractiveness.
(c) Experimental design and effect sizes
Earlier studies on DA in zebra finches have often used a between-female design (Rutstein et al. 2004; von Engelhardt 2004; Gilbert et al. 2006; Williamson et al. 2006). While this design has the advantage of avoiding possible carry-over effects on female investment, i.e. that females would be ‘primed’ by the attractiveness level of their first partner (Rutstein et al. 2005a), unfortunately, it also confers a considerable loss of statistical power. This is because between-individual differences in investment traits are much larger than within-individual flexibility, as signified by the high individual repeatability of investment (table 2). In our experiment, the observed within-individual change in egg volume (figure 1a), for the 16 females assigned males with maximum difference in EP attractiveness, was only 0.27 s.d. of the between-individual variation in our population (mean within-individual change in egg volume: 0.032±0.058 cm3, between-individual variation in s.d. =0.11 cm3). This is not surprising, considering egg size is highly heritable (h2=0.63±0.09 in our population), with a limited flexibility (repeatability=0.89±0.04 in this study). To detect an effect of d=0.27 using a between-female design, 330 females would be necessary for a power of 80 per cent.
Balzer & Williams (1998) used the more powerful within-female approach, and paired a female sequentially to her most and least preferred male from a six-way choice chamber (i.e. each female was paired to the extremes of six males). In our experiment, each female was instead paired to one of the eight most, and one of the eight least, attractive males out of 58 males. Using a simulated dataset of a male trait drawn from a standard normal distribution, one can compare the expected difference in trait value between a female's two partners if males are chosen in the two different ways described above. With our approach, the mean difference is 3.13 s.d., which can be compared with 2.15 s.d. for the approach by Balzer & Williams. If one assumes that EP attractiveness and choice chamber attractiveness are traits with similar information content to females, the expected effect size in the study by Balzer & Williams would be 0.21. With a power of 80 per cent, 27 females would be required to detect this effect. The actual sample size was close to this, with 22 females, yet no effect was found. This might imply that the assumption above is not correct, i.e. that the information content of choice chamber attractiveness and EP attractiveness might be different from female zebra finches. This is supported by the low correlation between choice chamber and EP attractiveness in our population (r=0.24, p=0.098, n=50, E. Bolund & H. Schielzeth 2008, unpublished data).
(d) The use of colour rings to manipulate attractiveness
Using paternity data from a free-flying aviary situation, we were able to determine male actual success at obtaining EPP, thus overcoming the difficulty of obtaining reliable measures of male sexual attractiveness to females. The EP success of a male was highly repeatable under two different social situations, suggesting that it represents female perception of male genetic quality, if we assume that females choose EP males based on additive genetic benefits. By contrast, most previous studies have manipulated male attractiveness with coloured leg rings (Rutstein et al. 2004; Gilbert et al. 2006; Williamson et al. 2006) and it can thus be useful to consider what red and green rings might signal to female zebra finches. While a preference for red over green rings has been found in several populations in choice chamber experiments (Burley et al. 1982; Hunt et al. 1997, but see Ratcliffe & Boag 1987), to our knowledge, Burley and her co-workers are the only ones who have studied the effects of red and green rings on male EP success under aviary conditions. Burley et al. (1994) found that red-ringed males were more successful at obtaining EP copulations in aviaries. The success rate of red males in EP courtships was 2.4 times that of green males (based on 906 EP-courtships, with 40 unforced copulations). This can be compared to our data. In the 16 males that were chosen on the basis of their EP success in aviaries, the attractive group had a success rate that was 5.8 times as high as that of the unattractive group (based on 198 EP courtships, with 26 unforced copulations).
This weaker treatment effect of rings when compared with success in siring EP offspring would reduce the effect size from 0.27 s.d. to an expected effect size of 0.11 s.d. Combined with a between-female design, this effect would require approximately 2000 females to be detected with a probability of 80 per cent.
In ringed birds, red-ringed males are more attractive for EP copulations and provide less parental care, while green-ringed males are less sexually attractive, but provide more parental care (Burley 1986, 1988; Burley et al. 1994, 1996). This apparent trade-off between EP attractiveness and parental quality in ringed birds, might not be observable under unmanipulated conditions, since males that are naturally more successful at obtaining EP copulations, will also be of higher genetic quality and hence also able to provide good parental care.
(e) Conclusions
Our study highlights the usefulness of real measures of male quality, as opposed to manipulating unknown aspects of male attractiveness. When we use separate measures reflecting direct and indirect benefits, a new picture of female investment in relation to male attractiveness emerges in the zebra finch. Yet, importantly, our findings are not contradicted by earlier studies in any point (see §4 above) and some of our findings are even in line with earlier results (Gil et al. 1999; Williamson et al. 2006). Our study further suggests that it is important to consider the mating pattern and life history of a species when investigating patterns of female resource allocation into reproduction. In less monogamous systems than the zebra finch, the benefits of PDA and of CI might have to be balanced against each other. If the benefits cancel each other out, neither PDA nor CI should evolve.
Acknowledgments
The study was approved by the animal care and ethics representative of the Max Planck Institute for Ornithology.We thank Bart Kempenaers for providing facilities and other support. James Dale, Alain Jacot and three anonymous referees provided helpful suggestions that improved the manuscript. We thank Wolfgang Goymann and Ingrid Schwabl for invaluable support with hormone analyses, and Melanie Schneider for performing molecular and hormone work. Our gratitude also goes to our animal caretakers: Edith Bodendörfer, Annemarie Grötsch, Johann Hacker, Markus Lehr, Jenny Minshull, Frances Preininger and Agnes Türk. Funding was provided by the German Science Foundation (DFG), Emmy Noether fellowship (FO 340/2).
References
- Balzer A.L., Williams T.D. Do female zebra finches vary primary reproductive effort in relation to mate attractiveness? Behaviour. 1998;135:297–309. [Google Scholar]
- Becker W.A. Academic Enterprises, Pullman; Washington, DC: 1984. A manual of quantitative genetics. [Google Scholar]
- Birkhead T., Schwabl H., Burke T. Testosterone and maternal efforts: integrating mechanisms and function. Trends Ecol. Evol. 2000;15:86–87. doi: 10.1016/s0169-5347(99)01803-0. doi:10.1016/S0169-5347(99)01803-0 [DOI] [PubMed] [Google Scholar]
- Bolund E., Schielzeth H., Forstmeier W. Intrasexual competition in zebra finches, the role of beak colour and body size. Anim. Behav. 2007;74:715–724. doi:10.1016/j.anbehav.2006.10.032 [Google Scholar]
- Bolund, E., Schielzeth, H. & Forstmeier, W. In preparation. Honest signalling turned upside down: high signalling effort may reflect low male quality.
- Burley N. Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 1986;127:415–445. doi:10.1086/284493 [Google Scholar]
- Burley N. The differential-allocation hypothesis: an experimental test. Am. Nat. 1988;132:611–628. doi:10.1086/284877 [Google Scholar]
- Burley N.T., Foster V.S. Variation in female choice of mates: condition influences selectivity. Anim. Behav. 2006;72:713–719. doi:10.1016/j.anbehav.2006.01.017 [Google Scholar]
- Burley N., Krantzberg G., Radman P. Influence of colour-banding on the conspecific preferences of zebra finches. Anim. Behav. 1982;30:444–455. doi:10.1016/S0003-3472(82)80055-9 [Google Scholar]
- Burley N.T., Enström D.A., Chitwood L. Extra-pair relations in zebra finches: differential male success results from female tactics. Anim. Behav. 1994;48:1031–1041. doi:10.1006/anbe.1994.1336 [Google Scholar]
- Burley N.T., Parker P.G., Lundy K. Sexual selection and extrapair fertilization in a socially monogamous passerine, the zebra finch (Taeniopygia guttata) Behav. Ecol. 1996;7:218–226. doi:10.1093/beheco/7.2.218 [Google Scholar]
- Forstmeier W. Female resistance to male seduction in zebra finches. Anim. Behav. 2004;68:1005–1015. doi:10.1016/j.anbehav.2004.02.003 [Google Scholar]
- Forstmeier W. Quantitative genetics and behavioural correlates of digit ratio in the zebra finch. Proc. R. Soc. B. 2005;272:2641–2649. doi: 10.1098/rspb.2005.3264. doi:10.1098/rspb.2005.3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmeier W. Do individual females differ intrinsically in their propensity to engage in extra-pair copulations? PLoS ONE. 2007;2:e952. doi: 10.1371/journal.pone.0000952. doi:10.1371/journal.pone.0000952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmeier W., Schielzeth H., Schneider M., Kempenaers B. Development of polymorphic microsatellite markers for the zebra finch (Taeniopygia guttata) Mol. Ecol. Notes. 2007;7:1026–1028. doi:10.1111/j.1471-8286.2007.01762.x [Google Scholar]
- Gil D., Graves J., Hazon N., Wells A. Male attractiveness and differential testosterone investment in zebra finch eggs. Science. 1999;286:126–128. doi: 10.1126/science.286.5437.126. doi:10.1126/science.286.5437.126 [DOI] [PubMed] [Google Scholar]
- Gilbert L., Williamson K., Hazon N., Graves J. Maternal effects due to male attractiveness affect offspring development in the zebra finch. Proc. R. Soc. B. 2006;273:1765–1771. doi: 10.1098/rspb.2006.3520. doi:10.1098/rspb.2006.3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman H.E., Arnold K.E., Nager R.G. Incubation effort in relation to male attractiveness in zebra finches Taeniopygia guttata. J. Avian Biol. 2005;36:413–420. doi:10.1111/j.2005.0908-8857.03464.x [Google Scholar]
- Gowaty P.A., Anderson W.W., Bluhm C.K., Drickamer L.C., Kim Y.K., Moore A.J. The hypothesis of reproductive compensation and its assumptions about mate preferences and offspring viability. Proc. Natl Acad. Sci. USA. 2007;104:15 023–15 027. doi: 10.1073/pnas.0706622104. doi:10.1073/pnas.0706622104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W., Trappschuh M., Jensen W., Schwabl I. Low ambient temperature increases food intake and dropping production, leading to incorrect estimates of hormone metabolite concentrations in European stonechats. Horm. Behav. 2006;49:644–653. doi: 10.1016/j.yhbeh.2005.12.006. doi:10.1016/j.yhbeh.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G., Muller W., von Engelhardt N., Carere C., Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. doi:10.1016/j.neubiorev.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Hinde C.A., Kilner R.M. Negotiations within the family over the supply of parental care. Proc. R. Soc. B. 2007;274:53–60. doi: 10.1098/rspb.2006.3692. doi:10.1098/rspb.2006.3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S., Cuthill I.C., Swaddle J.P., Bennett A.T.D. Ultraviolet vision and band-colour preferences in female zebra finches, Taeniopygia guttata. Anim. Behav. 1997;54:1383–1392. doi: 10.1006/anbe.1997.0540. doi:10.1006/anbe.1997.0540 [DOI] [PubMed] [Google Scholar]
- Immelmann K. Die Neue Brehm Buecherei, Ziemsen Verlag; Wittenberg, Lutherstadt, Germany: 1970. Der Zebrafink. [Google Scholar]
- Kroodsma D.E., Byers B.E. The function(s) of bird song. Am. Zool. 1991;31:318–328. [Google Scholar]
- Lessells C.M., Boag P.T. Unrepeatable repeatabilites: a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- McGraw K.J., Adkins-Regan E., Parker R.S. Anhydrolutein in the zebra finch: a new, metabolically-derived carotenoid in birds. Comp. Biochem. Physiol. B. 2002;132:811–818. doi: 10.1016/s1096-4959(02)00100-8. doi:10.1016/S1096-4959(02)00100-8 [DOI] [PubMed] [Google Scholar]
- Michl G., Török J., Péczely P., Garamszegi L.Z., Schwabl H. Female collared flycatchers adjust yolk testosterone to male age, but not to attractiveness. Behav. Ecol. 2005;16:383–388. doi:10.1093/beheco/ari002 [Google Scholar]
- Navara K.J., Hill G.E., Mendonça M.T. Yolk androgen deposition as a compensatory strategy. Behav. Ecol. Sociobiol. 2006;60:392–398. doi:10.1007/s00265-006-0177-1 [Google Scholar]
- Pinheiro, J., Bates, D., DebRoy, S. & Sarkar, D. 2007 nlme: linear and nonlinear mixed effects models R package v. 3.1-84.
- R Development Core Team 2005 R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing.
- Ratcliffe L.M., Boag P.T. Effects of colour bands on male competition and sexual attractiveness in zebra finches (Poephila guttata) Can. J. Zool. 1987;65:333–338. [Google Scholar]
- Royle N.J., Surai P.F., Hartley I.R. The effect of variation in dietary intake on maternal deposition of antioxidants in zebra finch eggs. Funct. Ecol. 2003;17:472–481. doi:10.1046/j.1365-2435.2003.00752.x [Google Scholar]
- Rutstein A.N., Gilbert L., Slater P.J.B., Graves J.A. Mate attractiveness and primary resource allocation in the zebra finch. Anim. Behav. 2004;68:1087–1094. doi:10.1016/j.anbehav.2004.02.011 [Google Scholar]
- Rutstein A.N., Gilbert L., Tomkins J.L. Experience counts: lessons from studies of differential allocation. Behav. Ecol. 2005a;16:957–960. doi:10.1093/beheco/ari061 [Google Scholar]
- Rutstein A.N., Gorman H.E., Arnold K.E., Gilbert L., Orr K.J., Adam A., Nager R., Graves J.A. Sex allocation in response to paternal attractiveness in the zebra finch. Behav. Ecol. 2005b;16:763–769. doi:10.1093/beheco/ari052 [Google Scholar]
- Saino N., Bertacche V., Ferrari R.P., Martinelli R., Møller A.P., Stradi R. Carotenoid concentration in barn swallow eggs is influenced by laying order, maternal infection and paternal ornamentation. Proc. R. Soc. B. 2002;269:1729–1733. doi: 10.1098/rspb.2002.2088. doi:10.1098/rspb.2002.2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks L., McGraw K., Hõrak P. How feather colour reflects its carotenoid content. Funct. Ecol. 2003;17:555–561. doi:10.1046/j.1365-2435.2003.00765.x [Google Scholar]
- Schielzeth, H. & Forstmeier, W. In press. Conclusions beyond support: over-confident estimates in mixed models. Behav. Ecol [DOI] [PMC free article] [PubMed]
- Schielzeth H., Burger C., Bolund E., Forstmeier W. Sexual imprinting on continuous variation: do female zebra finches prefer or avoid unfamiliar sons of their foster parents? J. Evol. Biol. 2008;21:1274–1280. doi: 10.1111/j.1420-9101.2008.01568.x. doi:10.1111/j.1420-9101.2008.01568.x [DOI] [PubMed] [Google Scholar]
- Schwabl H. Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem. Physiol. Part A: Physiol. 1996;114:271–276. doi: 10.1016/0300-9629(96)00009-6. doi:10.1016/0300-9629(96)00009-6 [DOI] [PubMed] [Google Scholar]
- Sheldon B.C. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 2000;15:397–402. doi: 10.1016/s0169-5347(00)01953-4. doi:10.1016/S0169-5347(00)01953-4 [DOI] [PubMed] [Google Scholar]
- SPSS Inc. 2004 SPSS for Windows, rel. 15.0.0. Chicago, IL: SPSS Inc.
- Surai P. Nottingham University Press; Nottingham, UK: 2002. Natural antioxidants in avian nutrition and reproduction. [Google Scholar]
- Swaddle J.P. Reproductive success and symmetry in zebra finches. Anim. Behav. 1996;51:203–210. doi:10.1006/anbe.1996.0017 [Google Scholar]
- Trivers R.L. Parental investment and sexual selection 1871–1971. In: Campbell B.G., editor. Sexual selection and the descent of man. Aldine; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- von Engelhardt, N. 2004 Proximate control of avian sex allocation: a study on zebra finches. PhD thesis, The Netherlands, University of Groningen.
- Verboven N., Evans N.P., D'Alba L., Nager R.G., Blount J.D., Surai P.F., Monaghan P. Intra-specific interactions influence egg composition in the lesser black-backed gull (Larus fuscus) Behav. Ecol. Sociobiol. 2005;57:357–365. doi:10.1007/s00265-004-0862-x [Google Scholar]
- Williams G.C. Natural selection, the costs of reproduction and a refinement of Lack's principle. Am. Nat. 1966;100:687–690. doi:10.1086/282461 [Google Scholar]
- Williamson K.A., Surai P.F., Graves J.A. Yolk antioxidants and mate attractiveness in the zebra finch. Funct. Ecol. 2006;20:354–359. doi:10.1111/j.1365-2435.2006.01087.x [Google Scholar]
- Zann R. Oxford University Press; New York, NY: 1996. The zebra finch: a synthesis of field and laboratory studies. [Google Scholar]