Abstract
Sperm competition theory predicts that males should invest prudently in ejaculates according to levels of female promiscuity. Males may therefore be sensitive to cues in their social environment associated with sexual competition, and tailor investment in sperm production accordingly. We tested this idea experimentally for the first time, to our knowledge, in a mammal by comparing reproductive traits of male house mice (Mus musculus domesticus) that had experienced contrasting encounter regimes with potential sexual competitors. We found that daily sperm production and numbers of sperm in the caput epididymis were significantly higher in subjects that had experienced a high encounter rate of social cues from three other males compared to those that had experienced a low encounter rate of social cues from just one other male. Epididymal sperm counts were negatively correlated with the frequency of scent-marking behaviour across all males in our study, suggesting that investment in ejaculate production may be traded off against traits that function in gaining copulations, although there was no difference in overall levels of scent marking between treatment groups. We conclude that social experience-mediated phenotypic plasticity in mammalian spermatogenesis is likely to be adaptive under sperm competition, enabling males to balance the energetic costs and paternity-enhancing benefits of ejaculate production, and is a potentially widespread explanation for intraspecific variation in ejaculate expenditure.
Keywords: Mus musculus domesticus, phenotypic plasticity, sexual selection, spermatogenesis, sperm competition
1. Introduction
Sperm competition theory predicts that males should invest prudently in ejaculates according to levels of female promiscuity (Parker 1990, 1998; Parker et al. 1996, 1997; Wedell et al. 2002; Parker & Ball 2005). Hence, ejaculate expenditure is predicted to increase in relation to the average probability or frequency of female multiple mating in a population, from low average sperm competition risk (equivalent to a low probability of female double mating) to high average intensity (many competing ejaculates) (Parker et al. 1997; Parker 1998). Increased ejaculate expenditure means that males should produce more sperm per ejaculate, which, combined with higher male mating rates (Short 1979; Stockley & Preston 2004), means that increased investment in sperm production and associated larger testes are expected wherever sperm competition is frequent or intense (Parker 1998; Parker & Ball 2005). To date, support for this prediction has come mainly from comparative studies across species, demonstrating evidence of expected variation in relative testes size (e.g. Byrne et al. 2002; Wedell et al. 2002; Pitcher et al. 2005; Ramm et al. 2005). However, the same general principle is also relevant to understanding variation in sperm production within species. As well as differences in sperm production that evolve as a selective response to consistent differences in the population risk or intensity of sperm competition (e.g. Hosken & Ward 2001), fluctuation in the level of sperm competition may also select for males to dynamically adjust their investment in sperm production to match prevailing conditions (Parker et al. 1997; Parker 1998; see also Engqvist & Reinhold 2005).
Several studies indicate that males of invertebrate taxa are sensitive to environmental factors predictive of sperm competition level, such as larval rearing density, sex ratio, group size and/or the frequency of exposure to conspecifics, and use such cues to adjust their investment in sperm production (Gage 1995; Oppliger et al. 1998; Stockley & Seal 2001; Schärer & Ladurner 2003; Bjork et al. 2007; Brauer et al. 2007). Evidence for phenotypic plasticity in ejaculate expenditure according to sperm competition level in vertebrates is currently far more limited. While there is some correlational evidence from natural populations linking variation in sperm production to environmental cues indicative of sperm competition, such as population density (Peromyscus maniculatus, Long & Montgomerie 2006), or local number of sexual competitors, (Julidochromis ornatus, Awata et al. 2006), the only study to date in which such conditions have been manipulated experimentally found no evidence of phenotypic plasticity in sperm production traits (Poecilia reticulata, Evans & Magurran 1999).
Here, we employ an experimental approach to investigate male responses to long-term manipulation of perceived sperm competition risk for the first time, to our knowledge, in a mammal. Specifically, by manipulating the encounter rates of male house mice (Mus musculus domesticus) with social cues from potential sexual competitors, we aim to test whether males respond to a perceived increase in the population risk of sperm competition by increasing their investment in sperm production (Parker et al. 1997; Parker 1998; Parker & Ball 2005). The house mouse is an ideal model to look for evidence of phenotypic plasticity in sperm production under varying sperm competition risk. Male house mice are regularly exposed to a risk of sperm competition within their mainly polygynous mating system, because females often seek extraterritorial copulations that can result in multiply sired litters (Bronson 1979; Dean et al. 2006). Moreover, sperm competition risk in this species is apparently variable and linked to population density, since the incidence of multiply sired litters is greater at higher density (Dean et al. 2006), presumably because extraterritorial copulations occur at higher frequency when rival male territories are in close proximity.
Experimentally induced changes in sperm production also make it possible to assess a key—but rarely tested—assumption of sperm competition theory; that ejaculate expenditure is traded off against investment in mating effort (Parker 1998). That is, because males are assumed to have finite resources to invest in reproduction, theory assumes that increased investment in sperm production will occur at the expense of traits that function in gaining copulations (Parker 1998; see also Gage 1995; Stockley & Seal 2001; Simmons & Emlen 2006). One such trait in house mice is scent marking, a costly behaviour that contributes to male success in defending territories and attracting females in this and many other mammal species (Ralls 1971; Gosling 1982; Hurst 1987; Rich & Hurst 1999; Gosling & Roberts 2001; Hurst & Beynon 2004; Zala et al. 2004). Hence, if ejaculate expenditure and mating effort trade-off against one another, then we might expect to find evidence of a negative correlation between male investment in sperm production and investment in scent-marking behaviour.
2. Material and methods
(a) Subject males
Subject males (n=30) were from a colony of wild house mice that had been outbred for six or fewer generations in captivity and originally derived from local populations in Cheshire. Each male was individually housed in a 48 cm×11.5 cm×12 cm cage for the duration of the experiment (M3, North Kent Plastic Cages Ltd., UK), with Corn Cob Absorb 10/14 substrate and paper-wool bedding material, and ad libitum access to food (LabDiet 5002) and water. All subjects were maintained under controlled environmental conditions in the same room (with no other animals present) throughout the duration of the experiment: temperature 20–21°C, relative humidity 45–65% and a reversed 12 : 12 hour light cycle (lights off at 08.00).
(b) Manipulation of social experience
To test for an influence of perceived population-level sperm competition risk on ejaculate expenditure of subject males, we manipulated the social experience of recently weaned males over a period of 22 weeks. This period exceeds the duration of spermatogenesis in this species of 34.5 days (Oakberg 1956). The rationale for our experimental design was to simulate a natural situation in which individual male territory owners, each with access to an equivalent number of females, would be subject to varying encounter rates and territorial intrusion by neighbouring males (see below). Shortly after being weaned at approximately 28 days (mean age at start of experiment: 36 days; range 27–42 days), subject males were assigned to two long-term treatment groups: a ‘high-competition’ group, in which subjects received regular exposure to three other males, and a ‘low-competition’ group, in which subjects received regular exposure to one other male. Treatment groups were matched for subject age (mean±s.e.: high: 35.50±1.34 days; low: 35.93±1.34 days; t=−0.225, d.f.=28, p=0.82) and body mass (mean±s.e.: high: 15.37±0.48 g; low: 15.50±0.40 g; t=−0.201, d.f.=28, p=0.84) at the start of the experiment. Throughout the experiment, subjects in both the high- and low-competition treatment groups received the same level of exposure to females and their odours, in order to stimulate the development of normal social and sexual behaviour (Vandenbergh 1971), while balancing the absolute number of females to which males in each treatment group were exposed. To reduce the possibility that subject males might be exposed to additional social cues outside of the treatments provided, their cages were spread out across 11 separate high-sided enclosures (each 1.2 m×1.2 m), in groups of four and two cages per enclosure for the high- and low-competition treatments, respectively. Thus, of the 30 experimental males, 16 (4×4) and 14 (2×7) subjects were allocated to the high-and low-competition treatments, respectively. This arrangement allowed us to maintain the animals in closest proximity to those individuals with which the experimental design permitted regular exposure, while shielding them from incidental exposure to social cues from other subjects.
(i) Social experience: rival male exposure
Subject males were exposed to both odours and direct encounters with rival males, spread over the total experimental period of 22 weeks. Conspecific odours are an appropriate stimulus in this context since olfaction is the primary sensory modality in rodents: conspecifics are recognized by individually distinct scent signals (Hurst & Beynon 2004), and olfactory cues influence sperm allocation in some cases (delBarco-Trillo & Ferkin 2006, 2007). Exposure to odour was performed in blocks, with each block lasting for two weeks and commencing in weeks 2, 4, 6, 8, 12, 19 and 21. In each block, subjects' cages were cleaned on day 1 and odours were introduced on days 4, 8 and 11 (±1 day). The odour exposure regime for the high- and low-competition groups was designed to simulate contrasting levels of territorial intrusion by neighbouring males, both with respect to the frequency of intrusion, and the number of different rivals intruding into the subject male's territory. Hence, on each day of odour exposure, males in the high-competition group were each exposed to a territorial ‘intrusion’ by one of their three potential rivals, with the odour of each used in rotation to provide a balanced exposure to three different intruding males per subject. By contrast, males in the low-competition group were exposed to either the odour of their potential rival (one-third of exposures), or their own odour (two-thirds of exposures), to simulate a lower frequency of territorial intrusion by a single rival male, while also controlling for potential cage-handling effects. In each case, odour was introduced by taking approximately 25 g of soiled bedding using a plastic weighing boat from the front of the donor's cage and introducing it to the rear of the recipient's cage, or in the case of control treatments, removing soiled bedding in the same way from the front of the male's cage and then re-introducing it at the rear. In total, each male received 21 such odour introductions.
To supplement this odour exposure, in weeks 2, 6 and 10, males were also given an opportunity to encounter their sexual rivals directly. This was achieved by releasing each male in turn from his home cage into the larger enclosure in which the group of (four or two) males were housed, for a period of 30 min each; in this way, each male in the high-competition treatment received 2 hours of possible interaction with rivals (when either he or a rival was able to roam freely in the enclosure) per session, and each male in the low-competition treatment received 1 hour of possible interaction per session. Note that at any one time only one male was free to roam within each enclosure, such that mice were always separated by cage bars; this allowed physical contact, but prevented the escalation of any aggressive interactions.
(ii) Social and sexual experience: female exposure
All subject males were given equivalent exposure to female odours in weeks 2, 6 and 10, respectively, receiving on each occasion approximately 25 g of soiled substrate removed from a stock cage containing two wild female mice. In addition, all males received opportunities to interact with a laboratory BALB/c female in oestrus (as determined by vaginal cytology) in weeks 11, 16 or 18, and 20. Interactions were conducted in a clean enclosure (60 cm×60 cm) and lasted for 40 min or until the first ejaculation. To prevent risk of injury, males were removed if they displayed persistent aggression towards the female. In weeks 11 and 20, any males that did not mate at the first opportunity were given a second interaction 2–6 days later. To ensure that all males received a similar number of interactions with females and copulations, we also restricted mating opportunities in week 20 to only those males that had not mated previously; all other males received instead a ‘control’ female exposure in which a female was placed inside the enclosure containing the male but was contained within an M3 cage. Overall, there were no differences between treatments in either the number of female interactions per male (high-competition mean±s.e.: 4.69±0.15; low-competition mean±s.e: 4.71±0.16; t=−0.121, d.f.=28, p=0.91) or number of matings per male (high-competition mean±s.e: 0.63±0.13; low-competition mean±s.e: 0.64±0.17; t=−0.086, d.f.=28, p=0.93). Moreover, at the end of the experiment, males who mated in the two different treatment groups did not differ in their mean time since last mating (high-competition mean±s.e: 37.40±8.72 days; low-competition mean±s.e: 37.75±10.86 days; t=0.025; d.f.=16, p=0.98).
(c) Scent-marking assays
For each male, scent-marking behaviour was measured at the end of the experiment (week 20). This was achieved by placing each subject for 2 hours in a clean M3 cage lined with Benchkote paper. The paper was streaked with a 10 μl urine stimulus from a male C57BL/6 laboratory mouse in a standardized position to stimulate scent marking. Marking patterns were recovered using a Bio-Rad Fluor-S MultiImager (Bio-Rad Laboratories Ltd., Hemel Hempstead, UK) and QuantityOne software (BioRad) with parameters adjusted for mouse urine (12 second exposure duration, 530DF60 filter, UV light source Epi illumination, high-resolution mode). Scent mark numbers were counted automatically based on images obtained from QuantityOne using the ‘Analyze Particles’ tool in ImageJ version 1.38x (http://rsb.info.nih.gov/ij/). The threshold range was set to 120–255, although in three cases the lower threshold was adjusted upwards after manual inspection to avoid miscounting.
(d) Male morphology and sperm production
(i) Morphology
Subject male body masses were recorded at the middle (week 8) and end (week 22) of the experiment. At the end of the experiment (day 153±1), males were killed using an overdose of halothane, and the wet mass of both testes, seminal vesicles, preputial glands and the right epididymis were recorded for each subject (with measurements recorded ‘blind’ to treatment group).
(ii) Epididymal sperm counts
The concentration of sperm in the caput of the left epididymis was estimated immediately after dissection as follows. The dissected tissue was placed in a Petri dish with 0.1 ml of 1 per cent citrate solution and macerated for 1 min with a scalpel blade. A further 0.9 ml 1 per cent citrate was then added and the mixture was left to stand for a further minute. After mixing using a pipette, a small amount of the preparation was added to each chamber of an improved Neubauer haemocytometer, which was left to stand for 15 min in a sealed container on moist cotton wool before sperm were counted manually under a microscope. Sperm counts for each chamber were duplicated, and conducted blind to the origin of the sample.
(iii) Estimates of daily sperm production
We estimated daily sperm production based on spermatid head counts from testicular homogenates (Amann 1970). Because the timing of spermatogenesis in mice is known precisely (Oakberg 1956), a static measure of sperm cells at the homogenization-resistant stage of spermatogenesis can be converted into a dynamic estimate of daily sperm production (sperm per testis per day). We followed the protocol described by Seung et al. (2003). Briefly, frozen right testes were thawed for 1 min, the tunica albuginea removed, and remaining tissue was weighed. The material was then homogenized in 2×1 min stages in 10 ml dimethyl sulphoxide/saline solution using an Ystral X10/20 homogenizer with 10T shaft. Spermatids were then stained with Trypan blue, and spermatid heads were counted using an improved Neubauer haemocytometer under 40× magnification.
Since one of the subject males in the low-competition treatment had extremely small testes on dissection (less than 25% of the next smallest recorded mass) and no sperm were recovered from the epididymis, this animal was excluded from all subsequent analyses. In addition, we were unable to measure spermatid head counts for two males (both in the high competition group) due to problems with sample processing. All data were log transformed prior to analysis to improve normality.
3. Results
The male house mice in our high- and low-competition treatment groups had comparable body masses throughout the experiment (mean±s.e.: week 8, high: 18.11±0.42 g; low: 18.18±0.80 g; t=−0.080; d.f.=27, p=0.94; week 22, high: 21.45±0.55 g; low: 20.91±0.77 g; t=−0.684; d.f.=27, p=0.50), and a repeated-measures general linear model incorporating body masses at the start, middle and end of the experiment revealed no effect of treatment group (F1,27=0.074, p=0.79). Thus, neither group grew faster or attained a greater mature weight in response to differing encounter regimes with sexual rivals.
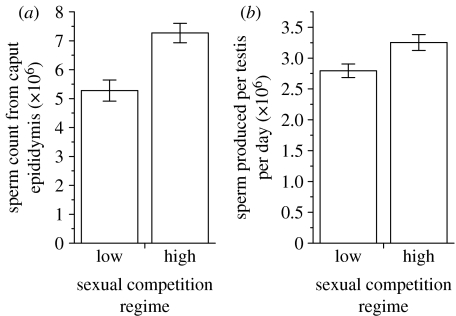
By contrast, the sperm production rates of these mice differed significantly according to experimental treatment group. Males in the high-competition treatment group had significantly higher epididymal sperm counts compared to those in the low-competition group (mean±s.e.: high: 7.27±0.33×106; low: 5.28±0.36×106; t=−4.277, d.f.=27, p<0.001; figure 1a), and produced significantly more sperm per testis per day on the basis of counts of homogenization-resistant spermatids (mean±s.e.: high: 3.25±0.13×106; low: 2.79±0.11×106; t=−2.670, d.f.=25, p=0.013; figure 1b). Analyses to test for differences between treatment groups based on enclosure means (n=4 high-competition enclosures and n=7 low-competition enclosures) produced qualitatively identical results (mean±s.e.: epididymal sperm counts, high: 7.34±0.14×106; low: 5.20±0.36×106; t=−3.667, d.f.=9, p=0.005; spermatogenic activity, high: 3.29±0.16×106; low: 2.77±0.07; t=−3.350, d.f.=9, p=0.009). Thus, our data support the prediction that males adjust sperm production according to environmental (in this case social) cues of sperm competition risk.
Figure 1.
Differences in sperm production by male house mice under a high (three competitors) compared to a low (one competitor) sexual competition regime. Males in the ‘high-competition’ group have (a) significantly more sperm in their caput epididymis (t=−4.277, d.f.=27, p<0.001) and (b) significantly higher daily sperm production rates (t=−2.670, d.f.=25, p=0.013) than males in the ‘low-competition’ treatment group. Bars represent mean±s.e.
We found no differences between the high- and low-competition treatment groups in the mean absolute mass of the testes, seminal vesicles, right epididymis or preputial glands (mean±s.e.: testes, high: 0.209±0.006 g; low: 0.199±0.006 g; t=−1.209, d.f.=27, p=0.24; seminal vesicles, high: 0.146±0.006 g; low: 0.153±0.017 g; t=0.008, d.f.=27, p=0.99; right epididymis, high: 0.033±0.001 g; low: 0.033±0.001 g; t=−0.402, d.f.=27, p=0.69; preputial glands, high: 0.044±0.003 g; low: 0.061±0.012 g; t=1.255, d.f.=27, p=0.22). Similar results were obtained when comparing relative organ masses, using general linear models with the organ mass as the dependent variable, treatment group as a fixed factor and body mass as a covariate (all treatment group effects non-significant, not shown), except that males in the low-competition group had significantly larger preputial glands for their body size than did males in the high-competition group (treatment group effect: F1,26=4.90, p=0.04). All organ masses were highly correlated with body mass among the low-competition males (all p≤0.016), but among the high-competition males, seminal vesicle mass was significantly correlated with body mass (p=0.01), while testis mass, preputial gland mass and epididymis mass were not (all p≥0.16).
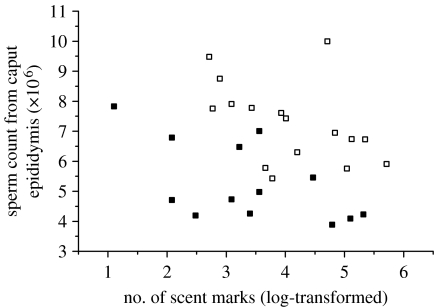
Our data also provide partial support for an investment trade-off between male traits under pre- and postcopulatory sexual selection, because males investing most in sperm production also tended to invest least in scent-marking behaviour (figure 2). Thus, there was a significant negative correlation between epididymal sperm counts and the number of scent marks in the low-competition treatment group (n=13, r=−0.581, p=0.04) and a similar, though marginally non-significant, trend in the high-competition treatment group (n=16, r=−0.464, p=0.07). Overall, we observed a significant negative association between scent marking and sperm counts, as indicated by the significant negative effect of scent marking in a general linear model containing epididymal sperm count as the dependent variable and treatment group as a fixed factor (scent-marking effect: F1,26=10.23, p=0.004; treatment group effect: F1,26=32.31, p<0.001). Nevertheless, there was no significant difference in average levels of scent marking between the high- and low-competition treatments (high: 89.50±20.84 marks; low: 57.92±18.19 marks; t=1.629; d.f.=27, p=0.12), suggesting that any upregulation of sperm production in the high-competition males does not occur at the expense of scent marking.
Figure 2.
Negative association between the number of sperm recovered from the caput epididymis and the number of scent marks deposited in a standardized scent-marking assay among both ‘high-competition’ (open squares; n=16, r=−0.464, p=0.07) and ‘low-competition’ (filled squares; n=13, r=−0.581, p=0.04) males. Overall, there is a significant negative association between scent marking and sperm counts, as indicated by the significant effect of scent marking in a general linear model containing epididymal sperm count as the dependent variable and treatment group as a fixed factor (scent-marking effect: F1,26=10.23, p=0.004; treatment group effect: F1,26=32.31, p<0.001).
4. Discussion
Our results show that male house mice vary their investment in sperm production according to social experience: daily sperm production and numbers of sperm in the caput epididymis were significantly higher in subjects that had experienced a high encounter rate of social cues from three other males, compared to those that had experienced a low encounter rate of social cues from just one other male. Since an increased encounter rate with rival males is likely to reflect an increased risk of extraterritorial copulations by females in this species (multiple paternity is significantly more common in high-density populations compared to low-density populations; Dean et al. 2006), the observed higher sperm production in our high-competition males may be interpreted as an adaptive long-term response to a greater risk of sperm competition (Parker 1998). Such a response is predicted in mammals and other taxa for which the outcome of sperm competition is influenced by a ‘raffle principle’ (Parker 1990, 1998), such that a male's chances of winning fertilizations increase with the number of sperm transferred in a given contest (Stockley 1997; Preston et al. 2003; Schulte-Hostedde & Millar 2004).
Our experimental results in a mammal extend the range of taxa for which plasticity in sperm production based on sperm competition level has been demonstrated, since previous work has focused almost exclusively on invertebrates (Gage 1995; Oppliger et al. 1998; Stockley & Seal 2001; Schärer & Ladurner 2003; Bjork et al. 2007; Brauer et al. 2007). As noted by Bjork et al. (2007), a potentially confounding factor in many of these previous studies has been that the effects of perceived sperm competition level could not be separated from the effects due to differences in mating rates. Because we controlled male mating rate, our results provide evidence that plasticity in sperm production rate can be based solely on social cues in the male's environment, without the need for feedback mechanisms based on differences in mating rate. Further work is now required to investigate the potential mechanisms involved in regulating plasticity in mammalian sperm production rate, including olfactory-mediated processes (Hurst & Beynon 2004; Koyama 2004), as well as other relevant social or environmental cues. Future studies might also focus on whether sperm production rate varies due to developmental plasticity (e.g. Schärer & Ladurner 2003), phenotypic flexibility (e.g. Brauer et al. 2007) or both (see Piersma & Drent 2003), and on the importance of sperm production rate to realized sperm competition success. Irrespective of the mechanism involved, our results emphasize the importance of considering variation in both population and immediate levels of sperm competition when interpreting variation in male reproductive phenotypes and testing predictions from sperm competition theory (Engqvist & Reinhold 2005). In this case, for example, understanding how male mice allocate the variable numbers of sperm they produce according to the population risk of sperm competition may help to explain their complex response to the immediate risk of sperm competition (Preston & Stockley 2006; Ramm & Stockley 2007).
Despite the clear social experience-induced differences reported here in measures of sperm production, we did not observe a significant concomitant response in testis size, or other reproductive organs such as the seminal vesicles responsible for producing the seminal fluid fraction of the ejaculate. This is perhaps surprising, given that Long & Montgomerie (2006) found a positive correlation between average testis size and population density across different years in field studies of deer mice (P. maniculatus), which may reasonably be interpreted as indirect evidence of adaptive plasticity in sperm production. However, other authors have explained intraspecific variation in testis size without invoking phenotypic plasticity (Ribble & Millar 1992; Pitcher & Stutchbury 1998; Brown & Brown 2003). For example, facultative adjustment by males was deemed an unlikely explanation for the observed correlation between the testis size of cliff swallows (Petrochelidon pyrrhonota) and the size of their breeding colonies, because testis development in this species occurs before males have settled in a group (Brown & Brown 2003). Male mice in our study presumably did have the opportunity to alter investment in testis size in response to local conditions, since their exposure to differing social regimes began shortly after weaning and prior to sexual maturity. However, while testis size is often closely linked to sperm production (Amann 1970), there is also evidence of variation in the relationship between these traits (e.g. Sorex araneus, Stockley et al. 1996; Rattus norvegicus, Pound & Gage 2004). Moreover, in a study of laboratory mice, Koyama & Kamimura (2000) found no significant differences in the testis size of males differing in social status, despite significant differences in sperm density in the cauda epididymis. Our findings are also consistent with recent results for the hermaphroditic flatworm Macrostomum lignano, for which increases in sperm production in response to increased group size cannot be wholly explained by differences in testis size (Schärer & Ladurner 2003; Schärer et al. 2004; Schärer & Vizoso 2007). Similarly, Oppliger et al. (1998) found that males of the freshwater snail Viviparus ater produce more oligopyrene (but not eupyrene) sperm in response to a male-biased sex ratio, despite there being no significant response in testis size. An obvious focus for future studies, then, will be to determine precisely how physiological and evolutionary changes in the testis bring about adjustments in sperm production (Schärer et al. 2008; Lüpold et al. in press).
Finally, our results also suggest an overall negative correlation between scent marking (an important component of mating effort) and sperm production. We stress, however, that we found no significant evidence that the experimentally induced modulation of sperm production in our experiment occurs at the expense of scent marking, and that previous data in house mice suggest that dominant males (which scent mark at a high rate) tend to have higher densities of sperm in the cauda epididymis (Koyama & Kamimura 2000). We did though observe a significant difference in the relative size of the preputial glands between treatments, which may be relevant since the products of this gland are deposited in scent marks and are implicated in intermale aggression and the establishment of social dominance (Bronson & Marsden 1973; Thompson et al. 2007). It should also be noted that laboratory conditions may not be ideal for investigating energetic trade-offs, which should they exist, would presumably most readily be detected under less benign conditions (e.g. dietary restriction). Nevertheless, a trade-off between traits under pre- and postcopulatory sexual selection is not inevitable, and the relationship between such traits can be positive in some cases (e.g. Malo et al. 2005; Locatello et al. 2006). The relative importance of traits under pre- and postcopulatory sexual selection to reproductive success is also likely to vary substantially within species (e.g. Preston et al. 2003). Further investigation of the potential investment trade-offs between traits under sexual selection (e.g. Simmons & Emlen 2006) remains a high priority for a more integrated understanding of how sexual selection shapes reproductive phenotypes.
In summary, we provide clear experimental evidence, and the first such evidence, to our knowledge, in mammals, that social factors alone can influence the resources males allocate to spermatogenesis. This social experience-mediated phenotypic plasticity in spermatogenesis is likely to be adaptive under sperm competition, enabling males to balance the energetic costs and paternity-enhancing benefits of ejaculate production, and is a potentially widespread explanation for intraspecific variation in ejaculate expenditure.
Acknowledgments
This research adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research, the legal requirements of the country in which the work was carried out and all institutional guidelines.
We thank J. Hurst and members of the Mammalian Behaviour and Evolution group for their support and constructive feedback, L. Burgess, F. Fair, R. Humphries, S. Jopson, J. F. Lemaître and J. Waters for help in conducting the experiment, R. Beynon for the loan of a homogenizer to carry out the spermatid counts, and The Leverhulme Trust for funding. We also thank two anonymous reviewers for their helpful comments on an earlier draft of the manuscript.
References
- Amann R.P. Sperm production rates. In: Johnson A.D., Gomes W.R., Vandemark N.L., editors. The testis. Academic Press; New York, NY: 1970. pp. 433–482. [Google Scholar]
- Awata S., Heg D., Munehara H., Kohda M. Testis size depends on social status and the presence of male helpers in the cooperatively breeding cichlid Julidochromis ornatus. Behav. Ecol. 2006;17:372–379. doi:10.1093/beheco/arj043 [Google Scholar]
- Bjork A., Dallai R., Pitnick S. Adaptive modulation of sperm production rate in Drosophila bifurca, a species with giant sperm. Biol. Lett. 2007;3:517–519. doi: 10.1098/rsbl.2007.0219. doi:10.1098/rsbl.2007.0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer V.S., Schärer L., Michiels N.K. Phenotypically flexible sex allocation in a simultaneous hermaphrodite. Evolution. 2007;61:216–222. doi: 10.1111/j.1558-5646.2007.00018.x. doi:10.1111/j.1558-5646.2007.00018.x [DOI] [PubMed] [Google Scholar]
- Bronson F.H. The reproductive ecology of the house mouse. Q. Rev. Biol. 1979;54:265–299. doi: 10.1086/411295. doi:10.1086/411295 [DOI] [PubMed] [Google Scholar]
- Bronson F.H., Marsden H.M. The preputial gland as an indicator of social dominance in male mice. Behav. Biol. 1973;9:625–628. doi: 10.1016/s0091-6773(73)80056-2. doi:10.1016/S0091-6773(73)80056-2 [DOI] [PubMed] [Google Scholar]
- Brown C.R., Brown M.B. Testis size increases with colony size in cliff swallows. Behav. Ecol. 2003;14:569–575. doi:10.1093/beheco/arg030 [Google Scholar]
- Byrne P.G., Roberts J.D., Simmons L.W. Sperm competition selects for increased testes mass in Australian frogs. J. Evol. Biol. 2002;15:347–355. doi:10.1046/j.1420-9101.2002.00409.x [Google Scholar]
- Dean M.D., Ardlie K.G., Nachman M.W. The frequency of multiple paternity suggests that sperm competition is common in house mice (Mus domesticus) Mol. Ecol. 2006;15:4141–4151. doi: 10.1111/j.1365-294X.2006.03068.x. doi:10.1111/j.1365-294X.2006.03068.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- delBarco-Trillo J., Ferkin M.H. Male meadow voles respond differently to risk and intensity of sperm competition. Behav. Ecol. 2006;17:581–585. doi:10.1093/beheco/ark001 [Google Scholar]
- delBarco-Trillo J., Ferkin M.H. Increased sperm numbers in the vas deferens of meadow voles, Microtus pennsylvanicus, in response to odors of conspecific males. Behav. Ecol. Sociobiol. 2007;61:1759–1764. doi:10.1007/s00265-007-0408-0 [Google Scholar]
- Engqvist L., Reinhold K. Pitfalls in experiments testing predictions from sperm competition theory. J. Evol. Biol. 2005;18:116–123. doi: 10.1111/j.1420-9101.2004.00792.x. doi:10.1111/j.1420-9101.2004.00792.x [DOI] [PubMed] [Google Scholar]
- Evans J.P., Magurran A.E. Male mating behaviour and sperm production characteristics under varying sperm competition risk in guppies. Anim. Behav. 1999;58:1001–1006. doi: 10.1006/anbe.1999.1212. doi:10.1006/anbe.1999.1212 [DOI] [PubMed] [Google Scholar]
- Gage M.J.G. Continuous variation in reproductive strategy as an adaptive response to population density in the moth Plodia interpunctella. Proc. R. Soc. B. 1995;261:25–30. doi:10.1098/rspb.1995.0112 [Google Scholar]
- Gosling L.M. A reassessment of the function of scent marking in territories. Z. Tierpsychol. 1982;60:89–118. [Google Scholar]
- Gosling L.M., Roberts S.C. Scent-marking by male mammals: cheat-proof signals to competitors and mates. Adv. Study Behav. 2001;30:169–217. doi:10.1016/S0065-3454(01)80007-3 [Google Scholar]
- Hosken D.J., Ward P.I. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 2001;4:10–13. doi:10.1046/j.1461-0248.2001.00198.x [Google Scholar]
- Hurst J.L. The functions of urine marking in a free-living population of house mice (Mus domesticus Rutty) Anim. Behav. 1987;35:1433–1442. doi:10.1016/S0003-3472(87)80016-7 [Google Scholar]
- Hurst J.L., Beynon R.J. Scent wars: the chemobiology of competitive signalling in mice. Bioessays. 2004;26:1288–1298. doi: 10.1002/bies.20147. doi:10.1002/bies.20147 [DOI] [PubMed] [Google Scholar]
- Koyama S. Primer effects by conspecific odors in house mice: a new perspective in the study of primer effects on reproductive activities. Horm. Behav. 2004;46:303–310. doi: 10.1016/j.yhbeh.2004.03.002. doi:10.1016/j.yhbeh.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Koyama S., Kamimura S. Influence of social dominance and female odor on the sperm activity of male mice. Physiol. Behav. 2000;71:415–422. doi: 10.1016/s0031-9384(00)00361-9. doi:10.1016/S0031-9384(00)00361-9 [DOI] [PubMed] [Google Scholar]
- Locatello L., Rasotto M.B., Evans J.P., Pilastro A. Colourful male guppies produce faster and more viable sperm. J. Evol. Biol. 2006;19:1595–1602. doi: 10.1111/j.1420-9101.2006.01117.x. doi:10.1111/j.1420-9101.2006.01117.x [DOI] [PubMed] [Google Scholar]
- Long T.A.F., Montgomerie R. Ejaculate investment in a promiscuous rodent, Peromyscus maniculatus: effects of population density and social role. Evol. Ecol. Res. 2006;8:345–356. [Google Scholar]
- Lüpold, S., Linz, G. M., Rivers, J. W., Westneat, D. F. & Birkhead, T. R. In press. Sperm competition selects beyond relative testes size in birds. Evolution [DOI] [PubMed]
- Malo A.F., Roldan E.R.S., Garde J., Soler A.J., Gomendio M. Antlers honestly advertise sperm production and quality. Proc. R. Soc. B. 2005;272:149–157. doi: 10.1098/rspb.2004.2933. doi:10.1098/rspb.2004.2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakberg E.F. Duration of spermatogenesis in the mouse and timing of the stages of the seminiferous epithelium. Am. J. Anat. 1956;99:507–516. doi: 10.1002/aja.1000990307. doi:10.1002/aja.1000990307 [DOI] [PubMed] [Google Scholar]
- Oppliger A., Hosken D.J., Ribi G. Snail sperm production characteristics vary with sperm competition risk. Proc. R. Soc. B. 1998;265:1527–1534. doi:10.1098/rspb.1998.0468 [Google Scholar]
- Parker G.A. Sperm competition games: raffles and roles. Proc. R. Soc. B. 1990;242:120–126. doi:10.1098/rspb.1990.0114 [Google Scholar]
- Parker G.A. Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead T.R., Møller A.P., editors. Sperm competition and sexual selection. Academic Press; London, UK: 1998. pp. 3–54. [Google Scholar]
- Parker G.A., Ball M.A. Sperm competition, mating rate and the evolution of testis and ejaculate sizes: a population model. Biol. Lett. 2005;1:235–238. doi: 10.1098/rsbl.2004.0273. doi:10.1098/rsbl.2004.0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A., Ball M.A., Stockley P., Gage M.J.G. Sperm competition games: assessment of sperm competition intensity by group spawners. Proc. R. Soc. B. 1996;263:1291–1297. doi:10.1098/rspb.1996.0189 [Google Scholar]
- Parker G.A., Ball M.A., Stockley P., Gage M.J.G. Sperm competition games: a prospective analysis of risk assessment. Proc. R. Soc. B. 1997;264:1793–1802. doi: 10.1098/rspb.1997.0249. doi:10.1098/rspb.1997.0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma T., Drent J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 2003;18:228–233. doi:10.1016/S0169-5347(03)00036-3 [Google Scholar]
- Pitcher T.E., Stutchbury B.J.M. Latitudinal variation in testis size in six species of North American songbirds. Can. J. Zool. 1998;76:618–622. doi:10.1139/cjz-76-4-618 [Google Scholar]
- Pitcher T.E., Dunn P.O., Whittingham L.A. Sperm competition and the evolution of testes size in birds. J. Evol. Biol. 2005;18:557–567. doi: 10.1111/j.1420-9101.2004.00874.x. doi:10.1111/j.1420-9101.2004.00874.x [DOI] [PubMed] [Google Scholar]
- Pound N., Gage M.J.G. Prudent sperm allocation in Norway rats, Rattus norvegicus: a mammalian model of adaptive ejaculate adjustment. Anim. Behav. 2004;68:819–823. doi:10.1016/j.anbehav.2004.02.004 [Google Scholar]
- Preston B.T., Stockley P. Risk of sexual competition stimulates premature and repeated ejaculation in a mammal. Curr. Biol. 2006;16:239–241. doi: 10.1016/j.cub.2006.03.018. doi:10.1016/j.cub.2006.03.018 [DOI] [PubMed] [Google Scholar]
- Preston B.T., Stevenson I.R., Pemberton J.M., Coltman D.W., Wilson K. Overt and covert competition in a promiscuous mammal: the importance of weaponry and testes size to male reproductive success. Proc. R. Soc. B. 2003;270:633–640. doi: 10.1098/rspb.2002.2268. doi:10.1098/rspb.2002.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralls K. Mammalian scent marking. Science. 1971;171:443–449. doi: 10.1126/science.171.3970.443. doi:10.1126/science.171.3970.443 [DOI] [PubMed] [Google Scholar]
- Ramm S.A., Stockley P. Ejaculate allocation under varying sperm competition risk in the house mouse, Mus musculus domesticus. Behav. Ecol. 2007;18:491–495. doi:10.1093/beheco/arm003 [Google Scholar]
- Ramm S.A., Parker G.A., Stockley P. Sperm competition and the evolution of male reproductive anatomy in rodents. Proc. R. Soc. B. 2005;272:949–955. doi: 10.1098/rspb.2004.3048. doi:10.1098/rspb.2004.3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribble D.O., Millar J.S. Intraspecific variation in testes size among northern populations of Peromyscus. Funct. Ecol. 1992;6:455–459. doi:10.2307/2389283 [Google Scholar]
- Rich T.J., Hurst J.L. The competing countermarks hypothesis: reliable assessment of competitive ability by potential mates. Anim. Behav. 1999;58:1027–1037. doi: 10.1006/anbe.1999.1217. doi:10.1006/anbe.1999.1217 [DOI] [PubMed] [Google Scholar]
- Schärer L., Ladurner P. Phenotypically plastic adjustment of sex allocation in a simultaneous hermaphrodite. Proc. R. Soc. B. 2003;270:935–941. doi: 10.1098/rspb.2002.2323. doi:10.1098/rspb.2002.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schärer L., Vizoso D.B. Phenotypic plasticity in sperm production rate: there's more to it than testis size. Evol. Ecol. 2007;21:295–306. doi:10.1007/s10682-006-9101-4 [Google Scholar]
- Schärer L., Ladurner P., Rieger R.M. Bigger testes do work more: experimental evidence that testis size reflects testicular cell proliferation activity in the marine invertebrate, the free-living flatworm Macrostomum sp. Behav. Ecol. Sociobiol. 2004;56:420–425. doi:10.1007/s00265-004-0802-9 [Google Scholar]
- Schärer L., Da Lage J.L., Joly D. Evolution of testicular architecture in the Drosophilidae: a role for sperm length. BMC Evol. Biol. 2008;8:143. doi: 10.1186/1471-2148-8-143. doi:10.1186/1471-2148-8-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Hostedde A.I., Millar J.S. Intraspecific variation of testes size and sperm length in the yellow-pine chipmunk (Tamias amoenus): implications for sperm competition and reproductive success. Behav. Ecol. Sociobiol. 2004;55:272–277. doi:10.1007/s00265-003-0707-z [Google Scholar]
- Seung H., Wolfe G., Rocca M. Performing a testicular spermatid head count. Curr. Protocols Toxicol. Suppl. 2003;16:1671–1676. doi: 10.1002/0471140856.tx1607s16. [DOI] [PubMed] [Google Scholar]
- Simmons L.W., Emlen D.J. Evolutionary trade-off between weapons and testes. Proc. Natl Acad. Sci. USA. 2006;103:16 346–16 351. doi: 10.1073/pnas.0603474103. doi:10.1073/pnas.0603474103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P. No evidence of sperm selection by female common shrews. Proc. R. Soc. B. 1997;264:1497–1500. doi: 10.1098/rspb.1997.0207. doi:10.1098/rspb.1997.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P., Preston B.T. Sperm competition and diversity in rodent copulatory behaviour. J. Evol. Biol. 2004;17:1048–1057. doi: 10.1111/j.1420-9101.2004.00742.x. doi:10.1111/j.1420-9101.2004.00742.x [DOI] [PubMed] [Google Scholar]
- Stockley P., Seal N. Plasticity in reproductive effort of male dung flies (Scatophaga stercoraria) as a response to larval density. Funct. Ecol. 2001;15:96–102. doi:10.1046/j.1365-2435.2001.00496.x [Google Scholar]
- Stockley P., Searle J.B., Macdonald D.W., Jones C.S. Correlates of reproductive success within alternative mating tactics of the common shrew. Behav. Ecol. 1996;7:334–340. doi:10.1093/beheco/7.3.334 [Google Scholar]
- Thompson R.N., Napier A., Wekesa K.S. Chemosensory cues from the lacrimal and preputial glands stimulate production of IP3 in the vomeronasal organ and aggression in male mice. Physiol. Behav. 2007;90:797–802. doi: 10.1016/j.physbeh.2007.01.008. doi:10.1016/j.physbeh.2007.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh J.G. The influence of the social environment on sexual maturation in male mice. J. Reprod. Fertil. 1971;24:383–390. doi: 10.1530/jrf.0.0240383. [DOI] [PubMed] [Google Scholar]
- Wedell N., Gage M.J.G., Parker G.A. Sperm competition, male prudence and sperm limited females. Trends Ecol. Evol. 2002;17:313–320. doi:10.1016/S0169-5347(02)02533-8 [Google Scholar]
- Zala S.M., Potts W.K., Penn D.J. Scent-marking displays provide honest signals of health and infection. Behav. Ecol. 2004;15:338–344. doi:10.1093/beheco/arh022 [Google Scholar]