Abstract
Humans are well known for their ability to keep track of social debts over extended periods of time, and for their tendency to preferentially cooperate with closely bonded partners. Non-human primates have been shown to cooperate with kin and non-kin, and reciprocate helpful acts. However, there is ongoing debate over whether they keep track of previous interactions and, if so, whether they can do it over extended periods of time, or are constrained to finalize exchanges within a single encounter. In this study, we used 3000 hours of all-day focal follows of wild chimpanzees (Pan troglodytes verus) to investigate whether both females and males reciprocate grooming within a single interaction, throughout the day, or over longer periods of time. We found that grooming was reciprocated more symmetrically when measured on a long-term, rather than on an immediate or short-term basis. Random giving, general allocation of grooming efforts, similarities among individuals and kinship do not appear to explain these highly reciprocal exchanges. Previously collected consecutive focal follows of single individuals revealed that dyads groomed an average of once every 7 days. Our findings strongly suggest that chimpanzees, similar to humans, are able to keep track of past social interactions, at least for a one-week period, and balance services over repeated encounters.
Keywords: grooming, reciprocity, chimpanzees, time frame, time lag, Pan troglodytes verus
1. Introduction
Reciprocity has been a major area of investigation due to the theoretical dilemma that helping others represents, the cognitive implications it may have in terms of memory and recognition and, therefore, its inevitable repercussions on the evolution of human trading. The theory of reciprocal altruism proposes that if giving is contingent upon receiving and if individuals give more services (i.e. helpful behaviours) or resources to those from whom they receive the most, performing helpful acts or giving resources can be an evolutionarily stable strategy (Trivers 1971; Axelrod & Hamilton 1981).
The time lag between giving and receiving of services or resources is one of the crucial aspects of reciprocity. When studying exchange behaviours, choosing an inappropriate time frame for reciprocation could lead to finding inaccurate measures of the symmetry of reciprocation (i.e. the relationship between giving and receiving) or even to concluding erroneously on the absence of reciprocity (i.e. if the time frame chosen is too short and individuals have not yet reciprocated). Much controversy surrounds this topic since researchers disagree on the length of the time frame for reciprocation in primates. Some authors have suggested that non-human primates' cognitive abilities enable them only to reciprocate acts immediately or on a short-term basis (Barrett & Henzi 2002; Stevens & Hauser 2004). Such is the case of female chacma baboons (Papio cynocephalus ursinus) and samango monkeys (Cercopithecus mitis erythrarchus), which reciprocated grooming short term (Barrett et al. 1999, 2002; Payne et al. 2003; Pazol & Cords 2005), and, as other vertebrates (Hart & Hart 1992; Connor 1995), were proposed to trade commodities immediately. Others have suggested that reciprocation takes place over a longer time frame (Manson et al. 2004; Schino et al. 2007). This was observed in female olive baboons (Papio anubis; Frank 2007), bonnet macaques (Macaca radiata; Manson et al. 2004), tufted (Schino et al. in press) and white-faced capuchins (Cebus capucinus; Manson et al. 2004), which reciprocated grooming long term and did not have balanced grooming bouts (i.e. individuals did not give and receive the same amount within a single interaction, thus the symmetry of the relationship between giving and receiving was not close to 1). It is unknown, however, whether these differences are due to exploring only limited time frames for reciprocation which could lead to erroneous conclusions on the time lag between giving and receiving, or if they are due to real species differences.
Grooming is an ideal behaviour for the investigation of the time frame of reciprocation since non-human primates tend to groom frequently (reviewed in Spruijt et al. 1992), which allows for it to be exchanged immediately or over longer time frames. As it benefits the recipient at some cost to the actor, it is proposed to function as a service that can be provided to others (de Waal 1997). Some of these costs include decreased vigilance (Mooring & Hart 1995) and resting time (Dunbar & Sharman 1984; Dunbar 1992), and exposure to microparasite transmission (Johnson et al. 2004; Nunn & Altizer 2006). However, it is a profitable commodity to exchange due to the benefits to the recipient. As grooming serves to remove parasites (Mooring et al. 1996; Hawlena et al. 2007), it provides hygienic benefits, and as it stimulates β-endorphin release (Keverne et al. 1989) and decreases heart rate (Feh & de Mazieres 1993; Aureli et al. 1999), it provides tension relief benefits. Grooming can be exchanged for other services or resources (e.g. support in agonistic conflicts, food, sex, etc., Seyfarth & Cheney 1984; Hemelrijk et al. 1992; de Waal 1997) or for itself, owing to its intrinsic hygienic and tension relief benefits (Henzi & Barrett 1999). This allows for the study of reciprocity by looking at one commodity alone. If grooming is reciprocated, individuals will give more grooming to those from whom they receive the most, and depending upon the time frame available to reciprocate, grooming will be time-matched within a bout, a day or over a longer period of time than a day (hereafter long term).
The few studies that have been conducted to date on the time frame of reciprocation have focused on monkeys (Frank 2007; Schino et al. in press), which may lack the cognitive abilities to reciprocate long term (Barrett & Henzi 2002; Stevens & Hauser 2004). Thus, chimpanzees (Pan troglodytes), which have been shown to have advanced cognitive skills (Tomasello & Call 1997; Lonsdorf et al. in press), are a suitable species in which this subject can be investigated. Furthermore, chimpanzees groom frequently (Lehmann et al. 2007); they live in large social groups with many potential exchange partners from which to choose; and they have a fission–fusion social system (Goodall 1986; Boesch & Boesch-Acherman 2000), enabling dyads to separate from the rest of the group to reciprocate previous helpful acts without being interrupted by other individuals. Grooming relationships in wild male chimpanzees have been shown to be reciprocal; however, the relationship between grooming given and received varied greatly among different populations (Boesch & Boesch-Acherman 2000; Watts 2000; Arnold & Whiten 2003; Mitani 2006). Furthermore, grooming reciprocity has not yet been studied in female dyads in the wild, even though females, despite being the dispersing sex, are an important part of the social life of the group, form strong social bonds with other individuals and interact frequently with other group members (Lehmann & Boesch 2008). Finally, the time lag between giving and receiving grooming has not been specifically addressed in this species. Studies on primates that have investigated the time frame of reciprocation have not done all-day focal follows of the same individual, precluding the assessment of reciprocity within a day. In addition, to investigate whether reciprocation occurs over time periods longer than a single interaction or a day, it is necessary to generate a large dataset to have enough interactions per pair of individuals and avoid misestimating the symmetry of reciprocation, which may not have been the case in previous studies.
To investigate whether grooming is reciprocated in both female and male wild chimpanzees and the time frame within which giving and receiving occurs, we used 3000 hours of data collection to measure the symmetry of grooming reciprocation within bouts, within a focal day and across longer periods of time. We (i) collected all-day focal samples of the same individual, which allowed us to verify whether commodities were reciprocated within a day and not only immediately, (ii) used analyses that allowed us to statistically control for the effects of other variables on the relationship between grooming given and received, allowing us to exclude other alternative hypotheses to contingent-based reciprocity, (iii) used consecutive all-day follows of the same individual to determine the average dyadic frequency of grooming and (iv) studied both sexes, not only males. If chimpanzees reciprocate grooming within a single bout or day, then partners will alternate roles as giver and receiver within this time period, in order to balance their exchanges. The majority of grooming bouts will be bidirectional and/or partners will balance out grooming interactions later in the day. However, if they can keep track of their previous grooming interactions and can reciprocate over many encounters, they will not be constrained to always alternate roles in each bout. Thus, grooming bouts can be unidirectional and exchanges will be more symmetrical when measured over longer periods of time.
2. Material and methods
(a) Behavioural data collection
Between 2003 and 2006, C.M.G. conducted all-day focal animal sampling on adult wild chimpanzees focusing on nine adult females and five adult males from the south group of the Taï chimpanzee project in Côte d'Ivoire (Herbinger et al. 2001; Deschner et al. 2003; Boesch et al. 2006). At the start of data collection, the south group consisted of 44 individuals: 8 males (5 adults, 3 adolescents); 16 females (14 adults, 2 adolescents); and 20 juveniles and infants, all of whom were fully habituated. Throughout the study period, one adult male and three adult females disappeared or died, and there were no immigration events. Additionally, there were no obvious changes in rank, suggesting that the competitive regime of the group was stable.
Consecutive all-day focal data used to determine grooming frequency per dyad were collected between 1999 and 2001 by T. Deschner, N. O. Daurid and C. Bolé. During this period, the South group community consisted of 3 adult males and 19 adult females. Although these data were collected approximately 2 years before, we are confident that the frequency of grooming between dyads did not change substantially since the number of potential grooming partners remained rather stable (between 19 and 22 adults).
(b) Statistical analysis
We used generalized linear mixed models (GLMM, Crawley 2002; Bolker 2007; Baayen 2008) with a Gaussian error structure to determine the symmetry between giving and receiving grooming. This approach is superior to previous methods used to study reciprocity in animals (e.g. matrix permutation methods) because it allows one to include several fixed effects (i.e. predictor variables that influence the mean) and random effects (i.e. variables that influence the variance). The inclusion of many fixed effects allowed us to investigate the relationship between grooming given and received, while controlling for other potentially confounding variables that could lead to a spurious correlation between the former, thereby testing alternative explanations to contingent-based reciprocity. Including random effects allowed us to control for data points being non-independent, as is the case with repeated observations of the same individual (Crawley 2002; Bolker 2007; Baayen 2008). GLMM were carried out in R (R Development Core Team 2004) using the Lme4 package v. 0.9975-13 (Bates 2007).
To asses the overall significance of our model, we used non-parametric bootstrapping (Manly 1997). This allowed us to determine that the deviance of the null model (i.e. one that only includes the intercept and the identity of the giver and receiver) fell well above the confidence limit of the deviance of our model, indicating that the model was significant. To investigate whether the model was unstable due to multicollinearity between two or more predictor variables, the data were bootstrapped 1000 times to obtain parameter coefficients of each of the individual predictor variables. This allowed us to verify that the confidence intervals (CI) for parameter estimates of significant variables were small and did not include zeros, which is evidence for a minor effect of multicollinearity (Manly 1997). Since measures of effect size are not available for GLMM, we evaluated effect size by comparing the AIC of the full model and a reduced model that did not include the variable of interest, and performed a likelihood-ratio test. This allowed us to assess the amount of variability explained by a single variable (Baayen 2008).
(c) Model specifications
The model used for all the analyses included the following variables that were all incorporated simultaneously.
Response variable. Log-transformed values of the total amount of grooming that one individual gave another (seconds).
- Predictor variables. In addition to grooming received, we included variables that have or might have an effect on the relationship between grooming given and received. A spurious correlation between these two variables could arise if individuals with similar characteristics (the same rank, age or sex) or those who associated frequently tended to groom each other more than different individuals or those who associated less (i.e. if there was a correlation between grooming and rank, age, sex or association; de Waal & Luttrell 1988). We incorporated in the model five continuous covariates and one categorical predictor variable.
- Grooming received (seconds). Log-transformed values of the total amount of grooming that an individual received from another. To verify that the differences in observation time of each individual did not affect the relationship between grooming given and received, we ran a model with proportion of grooming given and received (i.e. the amount of grooming in relation to the time both individuals were observed) and obtained the same results as for the analysis with total amounts.
- Rank of the giver and rank of the receiver. We established dominance relationships among individuals based on greeting vocalizations (Wittig & Boesch 2003). The hierarchy was linear (improved linearity test: h′=0.94, p=0.0001, de Vries 1995), with all males dominant over all females, and it remained stable throughout the whole data collection period. The model was analysed twice, once with rank of the giver and once with rank of the receiver, since the information obtained from these two variables and rank difference was redundant (see below).
- Rank difference. Difference between the ranks of the giver and receiver.
- Age difference. Absolute difference between the age of the giver and the receiver.
- Dyadic association index (DAI). DAI was used as a measure of how frequently two individuals associated with each other (i.e. were present in the same party). It was calculated in the following way:
where Ti is the total amount of time subject i was seen; Tj is the total amount of time subject j was seen; and Tij is the time i and j were seen together in the same party (Nishida 1968). - Sex combination of the dyad. Sex of both individuals involved in a grooming interaction was included in the model as a predictor categorical variable with four levels (♂♂, ♀♀, ♂♀, ♀♂, i.e. three dummy-coded predictor variables).
- Besides the predictor variables mentioned above, which were considered as fixed effects in these models, we included the identity of the giver and the receiver as random effects. This relieves the problem of dependency of data by controlling for variation between individuals in their tendency to perform certain acts (e.g. grooming others, Bolker 2007).
(d) Time frame of grooming reciprocation
To investigate the time lag between giving and receiving grooming, we measured grooming symmetry within bouts, within a focal day and across longer periods of time.
(i) Details of the within-bouts analysis
A bout was defined as a period of time during which two individuals were involved in a grooming interaction, without any change of behaviour, and ended when both individuals stopped grooming for more than 85 s. We chose this time as the limit for considering two grooming events as part of the same bout, based on the frequency distribution of the pauses between grooming events, which showed a minimum at 85 s.
In this analysis, we included a temporal autocorrelation parameter as an additional covariate into the model, to control for the possibility of dependency of data between grooming interactions happening close to one another. This autocorrelation covariate was defined as the weighted average of residuals (from a full model excluding the autocorrelation parameter), with the weight equalling the inverse time lag between two grooming bouts. To calculate the autocorrelation covariate for a given data point, we used all data from the same dyad and excluded the residuals of this particular data point. However, this parameter was not significant (t=0.27, p=0.34), indicating that there was no apparent temporal autocorrelation between grooming bouts, and was therefore not retained in the model reported.
(ii) Details of the within-days analysis
We summed all the grooming that each focal animal gave and received, to and from all other individuals each day, and used each combination of grooming per day/per dyad as a data point. In this analysis, we included a daily temporal autocorrelation parameter as an additional covariate into the model, to control for the possibility of dependency of data between grooming interactions happening on days close to one another. However, this parameter was not significant (t=1.12, p=0.27), indicating that there was no apparent temporal autocorrelation between days, and was therefore not retained in the model reported.
(iii) Details of the dyadic long-term analysis
First, we investigated whether dyadic grooming interactions were stable over time. To do this, we divided the complete dataset into six different periods of three to four months each. We then correlated, separately for grooming given and received, the amount of grooming per dyad for each pair of periods using matrix correlation analyses (Sokal & Rohlf 1995). An average ρ=0.4 and an average p=0.005 clearly suggest that grooming among dyads remained relatively stable throughout the observation period. We studied the symmetry in grooming reciprocity over 7, 11, 15, 19 and 22 months, hence increasing the amount of data that contributed to the dataset. In this analysis, unlike in the within-bouts and within-days analyses, there was an increased coverage of grooming interactions as more data were included in the analysis (i.e. the amount of data that contributed to each data point increased with observation time). This allowed us to determine the minimum dataset required to obtain an accurate and precise estimate of the symmetry of grooming reciprocity (i.e. how many months of data collection were necessary to obtain sufficient interactions per dyad for this estimate to remain rather stable).
(iv) Details of the comparison between the analyses of different time frames
The combination of each pair of giver–receiver and bout or day was a data point in the within-bouts and within-days analyses, whereas each giver–receiver pair was a data point in the dyadic long-term analysis. Since we were interested in the time lag between giving and receiving grooming, and non-reciprocated grooming could reflect defection or interchange for other commodities (Henzi & Barrett 1999), as in other studies (Barrett et al. 1999; Manson et al. 2004), we only included data points in which both individuals groomed. Thus, the within-bouts differed from the within-days and dyadic long-term analyses in that unidirectional bouts were never included in the former, but were usually included in the latter. They were included in the latter when days or dyads were reciprocal because members of a dyad changed roles as ‘groomer’ and ‘groomee’ at any time during the day, or time period of the dyadic long-term analysis. However, when including all data points in the analyses, the patterns remained the same with regard to their statistical significance (see §3). This shows that although our analyses were appropriate given the implications of including unidirectional bouts in the within-bouts analysis, our approach was conservative because including unidirectional bouts would lead to a decrease in the estimate of within-bouts reciprocity compared with the other two. To determine whether the differences in the symmetry of grooming reciprocity between each time frame used were statistically significant, we calculated the CI for each estimate of symmetry. We then verified whether the CI between each pair of estimates did not overlap, and whether the CI of the difference between each pair did not include zero (0), which would indicate that the differences between the pair of estimates were significant (Brandstätter 1999).
We examined whether symmetry could increase solely due to more data contributing to each data point, by permuting the amount of grooming given in the dyadic long-term dataset and progressively increasing the amount of data included in the analysis. We used matrix permutation as in a Mantel test (Sokal & Rohlf 1995) and determined the estimate of reciprocity for each permutation using the same analysis as for the original data.
3. Results
(a) General grooming patterns
The 91 dyads sampled were observed grooming for a total of 87 hours. The majority of the dyads (70%) were seen, at some point, grooming each other at the same time (i.e. mutual grooming). Out of the 87 grooming hours, 22 (25%) were of mutual grooming, while only 1 individual of the pair groomed, at a given time, during the remaining 65 hours (75%). This suggests that if grooming was reciprocated, individuals frequently alternated roles as giver and receiver. However, out of the 1236 grooming bouts observed, only 400 (32%) were bidirectional (i.e. individuals alternated roles as giver and receiver within the same bout), while 836 (68%) were unidirectional (i.e. only one individual of the pair groomed within a bout) indicating that in most cases individuals did not alternate roles within bouts.
(b) Reciprocity and the time frame of grooming reciprocation
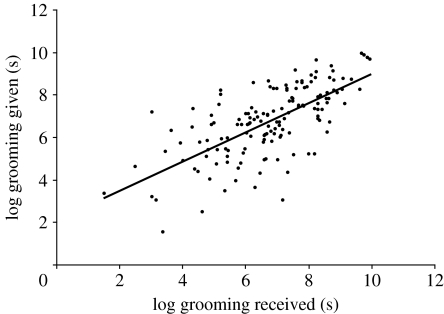
We found that grooming received significantly predicted the amount of grooming given in the dyadic long-term analysis (b±CI=0.83±0.08, t151=16.16, p<0.0001, figure 1). A model that did not include grooming received proved to have a significantly inferior fit (model with grooming received: AIC=435, d.f.=11; model without grooming received: AIC=546, d.f.=10; likelihood-ratio test: Χ2=113, p<0.0001), indicating that grooming received explained an important amount of the variability of grooming given. Furthermore, the symmetry in grooming reciprocity (i.e. the estimated slope of the relationship between grooming given and received) was close to 1 (b±CI=0.83±0.08). This indicates that, in the long term, a subject giving small amounts of grooming to a certain partner also received small amounts of grooming from that partner, while one giving large amounts of grooming to a particular individual also received large amounts of grooming from that partner (figure 1). Exclusion of mutual grooming (25%) from the analysis did not change these patterns, showing that the relationship between giving and receiving was not due to individuals grooming each other at the same time.
Figure 1.
Dyadic long-term relationship between grooming given and received. The estimated slope of the relationship between grooming given and received (b=0.83±0.08) reflects the symmetry in grooming reciprocation, which is perfectly symmetrical when equal to 1.
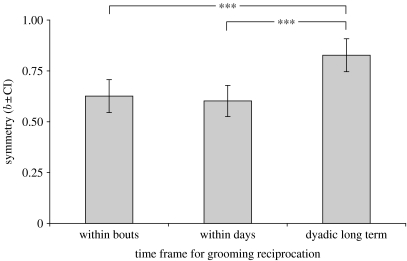
Grooming was also reciprocated within bouts and within days (within bouts: b±CI=0.63±0.08, t400=18.26, p<0.0001; within days: b±CI=0.61±0.08, t343=15.46, p<0.0001), although less symmetrically than long term (figure 2). The lack of overlap between the CI of the dyadic long-term analysis and the within-bouts and within-days analyses, and the exclusion of zero (0) from the CI of the difference between each pair of estimates (CIbouts-dyadiclong-term=0.09–0.31, CIdays-dyadiclong-term=0.11–0.34), indicates that these differences were significant. The large overlap of CI between the within-bouts and within-days symmetry measures, and the inclusion of zero in the CI of the difference between these two estimates (CIbouts–days=−0.9 to 0.13), indicates that the symmetry in grooming reciprocity did not differ significantly between these two time frames.
Figure 2.
Effect of the time frame for reciprocating grooming on the symmetry in grooming reciprocation. The relationship between grooming given and received was significant in the three analyses (within bouts: b±CI=0.63±0.08, t400=18.26, p<0.0001; within days: b±CI=0.61±0.08, t343=15.46, p<0.0001, dyadic long term: b±CI=0.83±0.08, t151=16.16, p<0.0001). The overlap of confidence intervals (CI) between the within-bouts and within-days analyses indicates that the differences in the symmetry in grooming reciprocation were not significant. The lack of overlap of CI between the dyadic long-term and other two analyses indicates that these differences were significant. (*** indicates p<0.0001.)
Although progressively including more data in the dyadic long-term analysis (from 7 to 22 months) did not lead to significant changes in the estimate of symmetry in grooming reciprocity, the measure became more precise and accurate. This was reflected by the decrease in the CI and the increase in the estimate of symmetry as more data were included in the analysis, until the estimate reached a plateau at approximately 15 months of data collection (b±CI: 7 months, 0.53±0.16; 11 months, 0.65±0.15; 15 months, 0.78±0.09; 19 months, 0.83±0.078; 22 months, 0.79±0.078). The stabilization of the estimate of symmetry and the decrease in the CI indicates that increasing the sample size led to a decrease in variance, and that a minimum of 15 months of data collection was necessary to obtain a reliable estimate of reciprocity on a long-term basis. However, the 15-month time lag probably does not have a biological meaning and will vary among studies depending on the species' grooming frequency and the data collection method used. In addition, we found that if grooming was given to a certain partner irrespective of its identification and the amount received from it, there would not be a relationship between grooming given and received across dyads, independent of the amount of data included in the analysis (matrix permutation, average b∼0, p∼0.5). This indicates that the increase in symmetry in the dyadic long-term analysis compared with the within-bouts and within-days analyses (figure 2) was not an analytical artefact (i.e. the result of using larger sample sizes).
4. Discussion
Chimpanzees in the Taï forest reciprocated grooming, even though, as in other chimpanzee communities (e.g. Gombe community, Goodall 1986; Mahale community, Takahata 1990; Sonso community, Arnold & Whiten 2003), they spent a smaller fraction of their time involved in mutual grooming. Removing mutual grooming events from the analyses revealed that the relationship between giving and receiving was not due to individuals grooming each other at the same time, and that partners frequently alternated roles as giver and receiver. The proportion of unidirectional grooming bouts (i.e. non-reciprocated grooming bouts) varies considerably within and across species (bonnet macaques: 93–95%, Manson et al. 2004; tufted capuchins (Cebus apella): 80%, Schino et al. in press; white-faced capuchins: 73–88%, Manson et al. 2004; olive baboons: 66%, Frank 2007; chacma baboons: 49–69%, Barrett et al. 1999). However, since most of the grooming bouts were unidirectional in this group of chimpanzees, the dyadic long-term analysis would only lead to more symmetrical grooming exchanges if imbalances in grooming interactions (due to unidirectional or skewed bidirectional bouts) were reciprocated by the other partner of a dyad at a later time. The lack of a significant difference in the symmetry of grooming reciprocation between the within-bouts and within-days time frames suggests that when dyads groomed more than once during the day they did not tend to balance out previous grooming interactions that had occurred that day. Our finding that grooming interactions measured long term were significantly more symmetrical than when measured within bouts or days suggests that chimpanzees are able to keep track of their grooming interactions over a longer period of time than a bout or a day, and to balance their grooming exchanges mainly over repeated interactions.
Reciprocal relationships could also arise due to mechanisms that do not require keeping track of past interactions. Rules that may govern the direction of social exchanges without any direct concern for contingent reciprocity include random giving, giving equally to everyone in the group (Gurven 2006), and preferentially giving to similar individuals (e.g. to individuals of the same sex or similar rank, or to kin; de Waal & Luttrell 1988). If individuals directed grooming equally to everyone in the group, the slope of the relationship between giving and receiving would be close to zero, because there would not be any variation between the amount of grooming given and received among dyads. However, the amount of time dyads were observed grooming varied considerably (figure 1), showing clearly that individuals were not directing grooming equally to all other individuals in the group. We excluded random giving (i.e. giving more to those with whom the target spent more time with) and giving to similar individuals by statistically controlling for association among individuals in the former case, and sex, age and rank in the latter. Reciprocal relationships could also occur if individuals were preferentially grooming kin. Using current genetic techniques, it is not possible to reliably estimate the dyadic degree of relatedness between adults in natural populations in the absence of at least some information on the parentage of those adults (Csillery et al. 2006; Van Horn 2008). Because we did not have such information for this group of chimpanzees, we were unable to control for kinship. Nonetheless, the high levels of symmetry in grooming exchanges found in this study group would only be explained through kin selection if the majority of the interacting dyads were related. Studies on this group of chimpanzees (Vigilant et al. 2001) and on a range of vertebrate species (Csillery et al. 2006) suggest that at most, 5 per cent of the dyads in a population are closely related. Furthermore, the majority of cooperative dyads in another group of chimpanzees were unrelated or distantly related (Langergraber et al. 2007), suggesting that most of the cooperative acts in chimpanzees cannot be explained through kin selection. In addition, studies on humans and other primates found that kin reciprocated less symmetrically than non-kin (Gurven 2006; Frank 2007). These findings conjointly indicate that the high levels of reciprocity found in this study are unlikely to be explained solely through kin selection.
Neither random giving, uniform allocation of grooming efforts, similarities among individuals nor kinship can adequately explain the highly reciprocal patterns observed in this group of chimpanzees. We suggest that chimpanzees are able to keep track of previous grooming interactions with other individuals and reciprocate over repeated encounters, and not only within a single bout as has been suggested for non-human primates (Barrett & Henzi 2002). Previously collected consecutive focal follows of single individuals of 6–19 days (see §2) revealed that within this period of time chimpanzees only groomed 12 (range: 6–19 individuals) out of the 21 available adult grooming partners and on average dyads groomed every 7 days (range: 2–18 days). This indicates that many of the dyads groomed at most once or twice per month. Therefore, in order to maintain reciprocal grooming relationships, individuals had to keep track of their past interactions for at least 7 days and up to 18 or more days.
The high levels of grooming reciprocity observed in this group were similar to those found in other groups of chimpanzees (matrix correlations, average τKr; Ngogo: τKr∼0.64, Watts 2000; Sonso: τKr∼0.61, Arnold & Whiten 2003; Mahale: τKr∼0.78, Nishida & Hosaka 1996), suggesting that our findings are not specific to the Taï chimpanzees. However, similar data and analyses will be necessary to explore the time frame used by individuals to reciprocate helpful acts in other groups of chimpanzees and other non-human primates.
(a) Proximate mechanisms of long-term reciprocity
Although the relationship between grooming given and received may superficially seem to be justified by mechanisms such as affiliation towards particular individuals (i.e. ‘friendship’), this alone is not a plausible explanation. In the absence of mechanisms that would allow individuals to keep track of services given and received, they would be susceptible to cheating and reciprocity would probably break down. Models of reciprocity based on the iterated two-person prisoners' dilemma game (Axelrod & Hamilton 1981) show how cooperation is enforced through repeated interactions between dyads. Keeping track on a long-term basis of past interactions and possessing the ability to identify ‘good’ and ‘bad’ reciprocators would be selected for if individuals were following exchange strategies such as ‘tit for tat’ (Axelrod & Hamilton 1981; Trivers 1985). Based on this strategy, individuals should tend to exchange with good reciprocators, and if partner choice is possible (Noë & Hammerstein 1994; Nesse 2001), form long-lasting stable social bonds with them. Therefore, a tendency to exchange with particular individuals or ‘friends’ probably complements these reciprocal relationships. Our findings, as well as recent evidence from chimpanzees and baboons showing that individuals have strong and enduring social bonds (Boesch & Boesch-Acherman 2000; Silk et al. 2006a; Lehmann & Boesch 2008), groom more equitably with these preferred partners (Silk et al. 2006b) and choose, as exchange partners, individuals who have been good reciprocators in the past (Melis et al. 2006), support this interpretation.
The proximate mechanisms that enable an individual to keep track of past interactions could be attitudinal reciprocity (de Waal 2000; Aureli & Schino 2004; Schino et al. 2007) or calculated reciprocity (de Waal & Luttrell 1988). Attitudinal reciprocity or ‘emotionally mediated’ scorekeeping depends on an animal having a positive emotional attitude towards a partner as a consequence of prior friendly interactions (e.g. grooming), which could be mediated hormonally (e.g. receiving more grooming from a specific partner leads to increased β-endorphin secretion, which leads to a stronger positive emotional feeling towards that partner, which leads an individual to give more grooming to that partner, Schino et al. 2007). Calculated reciprocity requires a detailed cognitive accounting of the amount of services given and received (de Waal & Luttrell 1988). Non-human primates are thought to lack the cognitive abilities, such as good memory and planning, that may be required to do long-term calculated reciprocity (Stevens & Hauser 2004). As a result of this, non-human primates have been thought to perceive relationships as a series of discrete events (Barrett & Henzi 2002). However, an emotionally mediated mechanism would not require such cognitive capacities, and this might explain how some non-human primates, even in the absence of such capacities, can reciprocate long term (Schino et al. 2007). Nevertheless, further research specifically addressing this question is needed to provide more insight into the proximate mechanisms explaining our results.
Acknowledgements
This research complied with the ethics guidelines of the Max Planck Society and supported by the Ivorian authorities (Ivory Coast Park Authorities and Ministry of Science and Research, Ivory Coast).
We thank the ‘Ministère de l'Environnement et des Eaux et Forets’, the ‘Ministère de la Recherche Scientifique’, the O.I.P.R., the director of the Taï National Park and the ‘Centre Suisse de Recherche Scientifique’ in Abidjan. Special thanks are due to T. Deschner for contributing data to this paper and to N. O. Daurid, C. Bolé and N. B. Gouyan for support in collecting behavioural data. We would like to thank T. Deschner, H. Baayen, C. Sanz, R. Walker, K. Zuberbühler, B. Preston, C. Rowney, M. Gurven and three anonymous reviewers for their helpful input and comments on earlier versions of this manuscript. This research was funded by the Wenner-Gren Foundation and the Max Planck Society.
References
- Arnold K., Whiten A. Grooming interactions among the chimpanzees of the Budongo Forest, Uganda: tests of five explanatory models. Behaviour. 2003;140:519–552. doi:10.1163/156853903322127968 [Google Scholar]
- Aureli F., Schino G. The role of emotions in relationships. In: Thierry B., Singh M., Kaumanns W., editors. Macaque societies: a model for the study of social organization. Cambridge University Press; Cambridge, MA: 2004. pp. 38–60. [Google Scholar]
- Aureli F., Preston S.D., de Waal F.B.M. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J. Comp. Psychol. 1999;113:59–65. doi: 10.1037/0735-7036.113.1.59. doi:10.1037/0735-7036.113.1.59 [DOI] [PubMed] [Google Scholar]
- Axelrod R., Hamilton W.D. The evolution of cooperation. Science. 1981;211:1390–1396. doi: 10.1126/science.7466396. doi:10.1126/science.7466396 [DOI] [PubMed] [Google Scholar]
- Baayen R.H. Cambridge University Press; Cambridge, MA: 2008. Analyzing linguistic data. A practical introduction to statistics. [Google Scholar]
- Barrett L., Henzi S.P. Constraints on relationship formation among female primates. Behaviour. 2002;139:263–289. doi:10.1163/156853902760102672 [Google Scholar]
- Barrett L., Henzi S.P., Weingrill T., Lycett J.E., Hill R.A. Market forces predict grooming reciprocity in female baboons. Proc. R. Soc. B. 1999;266:665–670. doi:10.1098/rspb.1999.0687 [Google Scholar]
- Barrett L., Gaynori D., Henzi S.P. A dynamic interaction between aggression and grooming reciprocity in female chacma baboons. Anim. Behav. 2002;63:1047–1053. doi:10.1006/anbe.2002.3008 [Google Scholar]
- Bates, D. 2007 The lme4 package: linear mixed-effects models using S4 classes. See http://www.R-project.org
- Boesch C., Boesch-Achermann H. Oxford University Press; Oxford, UK: 2000. The chimpanzees of the Taï Forest. [Google Scholar]
- Boesch C., Boesch H., Vigilant L. Cooperative hunting in chimpanzees: kinship or mutualism. In: Kappeler P.M., van Schaik C.P., editors. Cooperation in primates and humans: mechanisms and evolution. Springer; Berlin, Germany: 2006. pp. 139–150. [Google Scholar]
- Bolker B. Princeton University Press; Princeton, NJ: 2007. Ecological models and data in R. [Google Scholar]
- Brandstätter E. Confidence intervals as an alternative to significance testing. Methods Psychol. Res. Online. 1999;4:33–46. [Google Scholar]
- Connor R.C. Impala allogrooming and the parceling model of reciprocity. Anim. Behav. 1995;49:528–530. doi:10.1006/anbe.1995.0070 [Google Scholar]
- Crawley M.J. Wiley; New York, NY: 2002. Statistical computing: an introduction to data analysis using S-Plus. [Google Scholar]
- Csillery K., Johnson T., Beraldi D., Clutton-Brock T., Coltman D., Hansson B., Spong G., Pemberton J.M. Performance of marker-based relatedness estimators in natural populations of outbred vertebrates. Genetics. 2006;173:2091–2101. doi: 10.1534/genetics.106.057331. doi:10.1534/genetics.106.057331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries H. An improved test of linearity in dominance hierarchies containing unknown or tied relationships. Anim. Behav. 1995;50:1375–1389. doi:10.1016/0003-3472(95)80053-0 [Google Scholar]
- de Waal F.B.M. The chimpanzee's service economy: food for grooming. Evol. Hum. Behav. 1997;18:375–386. doi:10.1016/S1090-5138(97)00085-8 [Google Scholar]
- de Waal F.B.M. Attitudinal reciprocity in food sharing among brown capuchin monkeys. Anim. Behav. 2000;60:253–261. doi: 10.1006/anbe.2000.1471. doi:10.1006/anbe.2000.1471 [DOI] [PubMed] [Google Scholar]
- de Waal F.B.M., Luttrell L.M. Mechanisms of social reciprocity in three primate species: symmetrical relationship characteristics or cognition? Ethol. Sociobiol. 1988;9:101–118. doi:10.1016/0162-3095(88)90016-7 [Google Scholar]
- Deschner T., Heistermann M., Hodges K., Boesch C. Timing and probability of ovulation in relation to sex skin swelling in wild West African chimpanzees, Pan troglodytes verus. Anim. Behav. 2003;66:551–560. doi:10.1006/anbe.2003.2210 [Google Scholar]
- Dunbar R.I. Time: a hidden constraint on the behavioural ecology of baboons. Behav. Ecol. Sociobiol. 1992;31:35–49. doi:10.1007/BF00167814 [Google Scholar]
- Dunbar R.I., Sharman M. Is social grooming altruistic? Z Tierpsychol. 1984;64:163–173. [Google Scholar]
- Feh C., de Mazieres J. Grooming at a preferred site reduces heart-rate in horses. Anim. Behav. 1993;46:1191–1194. doi:10.1006/anbe.1993.1309 [Google Scholar]
- Frank, R. 2007 The role of contingent reciprocity and market exchange in the lives of female olive baboons. PhD dissertation, Anthropology Department, UCLA.
- Goodall J. Belknap Press of Harvard University; Cambridge, MA: 1986. The chimpanzees of Gombe: patterns of behavior. [Google Scholar]
- Gurven M. The evolution of contingent reciprocity. Curr. Anthropol. 2006;47:185–192. doi:10.1086/499552 [Google Scholar]
- Hart B., Hart L. Reciprocal allogrooming in impala, Aepyceros melampus. Anim. Behav. 1992;44:1073–1083. doi:10.1016/S0003-3472(05)80319-7 [Google Scholar]
- Hawlena H., Bashary D., Abramsky Z., Krasnov B.R. Benefits, costs and constraints of anti-parasitic grooming in adult and juvenile rodents. Ethology. 2007;113:394–402. doi:10.1111/j.1439-0310.2007.01332.x [Google Scholar]
- Hemelrijk C.K., van Laere G.J., van Hooff J.A.R.A.M. Sexual exchange relationships in captive chimpanzees. Behav. Ecol. Sociobiol. 1992;30:269–275. doi:10.1007/BF00166712 [Google Scholar]
- Henzi S.P., Barrett L. The value of grooming to female primates. Primates. 1999;40:47–59. doi: 10.1007/BF02557701. doi:10.1007/BF02557701 [DOI] [PubMed] [Google Scholar]
- Herbinger I., Boesch C., Rothe H. Territory characteristics among three neighboring chimpanzee communities in the Taï National Park, Côte d'Ivoire. Int. J. Primatol. 2001;22:143–167. doi:10.1023/A:1005663212997 [Google Scholar]
- Johnson D.D.P., Stopka P., MacDonald D.W. Ideal flea constraints on group living: unwanted public goods and the emergence of cooperation. Behav. Ecol. 2004;15:181–186. doi:10.1093/beheco/arg093 [Google Scholar]
- Keverne E.B., Martensz N., Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology. 1989;14:155–161. doi: 10.1016/0306-4530(89)90065-6. doi:10.1016/0306-4530(89)90065-6 [DOI] [PubMed] [Google Scholar]
- Langergraber K.E., Mitani J.C., Vigilant L. The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl Acad. Sci. 2007;104:7786–7790. doi: 10.1073/pnas.0611449104. doi:10.1073/pnas.0611449104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J., Boesch C. Sexual differences in chimpanzee sociality. Int. J. Primatol. 2008;29:65–81. doi: 10.1007/s10764-007-9230-9. doi:10.1007/s10764-007-9230-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J., Korstjens H., Dunbar R.I.M. Group size, grooming and the social cohesion in primates. Anim. Behav. 2007;74:1617–1629. doi:10.1016/j.anbehav.2006.10.025 [Google Scholar]
- Lonsdorf, E. V., Ross, S. R. & Matsuzawa, T. (eds) In press. The mind of the chimpanzee: ecological and experimental perspectives Chicago, IL: University of Chicago Press.
- Manly B. Chapman & Hall; New York, NY: 1997. Randomization, bootstrap and Monte Carlo methods in biology. [Google Scholar]
- Manson J.H., Navarrete C.D., Silk J.B., Perry S. Time-matched grooming in female primates? New analyses from two species. Anim. Behav. 2004;67:493–500. doi:10.1016/j.anbehav.2003.05.009 [Google Scholar]
- Melis A., Hare B., Tomasello M. Chimpanzees recruit the best collaborators. Science. 2006;311:1297–1300. doi: 10.1126/science.1123007. doi:10.1126/science.1123007 [DOI] [PubMed] [Google Scholar]
- Mitani J.C. Reciprocal exchange in chimpanzees and other primates. In: Kappeler P., van Schaik C.P., editors. Cooperation in primates and humans: mechanisms and evolution. Springer; New York, NY: 2006. pp. 107–119. [Google Scholar]
- Mooring M.S., Hart B.L. Costs of allogrooming in impala—distraction from vigilance. Anim. Behav. 1995;49:1414–1416. doi:10.1006/anbe.1995.0175 [Google Scholar]
- Mooring M.S., McKenzie A.A., Hart B.L. Grooming in impala: role of oral grooming in removal of ticks and effects of ticks in increasing grooming rate. Physiol. Behav. 1996;59:965–971. doi: 10.1016/0031-9384(95)02186-8. doi:10.1016/0031-9384(95)02186-8 [DOI] [PubMed] [Google Scholar]
- Nesse R. Russel Sage Press; New York, NY: 2001. Evolution and the capacity for commitment. [Google Scholar]
- Nishida T. The social group of wild chimpanzees in the Mahale Mountains. Primates. 1968;9:167–224. doi:10.1007/BF01730971 [Google Scholar]
- Nishida T., Hosaka K. Coalition strategies among adult male chimpanzees of the Mahale Mountains, Tanzania. In: Mc Grew W.C., Marchant L.A., Nishida T., editors. Great Ape Societies. Cambridge University Press; Cambridge, MA: 1996. pp. 114–134. [Google Scholar]
- Noë R., Hammerstein P. Biological markets—supply-and-demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 1994;35:1–11. doi:10.1007/BF00167053 [Google Scholar]
- Nunn C.L., Altizer S.M. Oxford University Press; Oxford, UK: 2006. Infectious diseases in primates: behavior, ecology and evolution. [Google Scholar]
- Payne H., Lawes M.J., Henzi P.S. Competition and the exchange of grooming among female samango monkeys (Cercopithecus mitis erythrarchus) Behaviour. 2003;160:453–471. doi:10.1163/156853903322127931 [Google Scholar]
- Pazol K., Cords M. Seasonal variation in feeding behavior, competition and female social relationships in a forest dwelling guenon, the blue monkey (Cercopithecus mitis stuhlmanni), in the Kakamega Forest, Kenya. Behav. Ecol. Sociobiol. 2005;58:566–577. doi:10.1007/s00265-005-0953-3 [Google Scholar]
- R Development Core Team. 2004 R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna. See http://www.R-project.org
- Schino G., Polizzi di Sorrentino E., Tiddi B. Grooming and coalitions in Japanese macaques (Macaca fuscata): partner choice and the time frame of reciprocation. J. Comp. Psych. 2007;121:181–188. doi: 10.1037/0735-7036.121.2.181. doi:10.1037/0735-7036.121.2.181 [DOI] [PubMed] [Google Scholar]
- Schino G., Di Giuseppe F. & Visalberghi E. In press. The time frame of partner choice in the grooming reciprocation of Cebus apella Ethology
- Seyfarth R.M., Cheney D.L. Grooming, alliances and reciprocal altruism in vervet monkeys. Nature. 1984;308:541–542. doi: 10.1038/308541a0. doi:10.1038/308541a0 [DOI] [PubMed] [Google Scholar]
- Silk J.B., Altmann J., Alberts S.C. Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behav. Ecol. Sociobiol. 2006;61:183–195. doi:10.1007/s00265-006-0249-2 [Google Scholar]
- Silk J.B., Alberts S.C., Altmann J. Social relationships among adult female baboons (Papio cynocephalus) II. Variation in the quality and stability of social bonds. Behav. Ecol. Sociobiol. 2006;61:197–204. doi:10.1007/s00265-006-0250-9 [Google Scholar]
- Sokal R., Rohlf F. 3rd edn. W.H. Freeman; New York, NY: 1995. Biometry—the principles and practice of statistics in biological research. [Google Scholar]
- Spruijt B.M., van Hooff J.A.R.A.M., Gispen W.H. Ethology and neurobiology of grooming behavior. Physiol. Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Stevens J.R., Hauser M.D. Why be nice? Psychological constraints on the evolution of cooperation. Trends Cogn. Sci. 2004;8:60–65. doi: 10.1016/j.tics.2003.12.003. doi:10.1016/j.tics.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Takahata Y. University of Tokyo Press; Tokyo, Japan: 1990. The chimpanzees of the Mahale Mountains. [Google Scholar]
- Tomasello M., Call J. Oxford University Press; Oxford, UK: 1997. Primate cognition. [Google Scholar]
- Trivers R.L. Evolution of reciprocal altruism. Q. Rev. Biol. 1971;46:35–57. doi:10.1086/406755 [Google Scholar]
- Trivers R. The Benjamin/Cummings Publishing Company; Menlo Park, CA: 1985. Social evolution. [Google Scholar]
- Van Horn R. Can't get from here to there: inferring kinship from pairwise genetic relatedness. Anim. Behav. 2008;75:1173–1180. doi:10.1016/j.anbehav.2007.08.027 [Google Scholar]
- Vigilant L., Hofreiter M., Siedel H., Boesch C. Paternity and relatedness in wild chimpanzee communities. Proc. Natl Acad. Sci. 2001;98:12 890–12 895. doi: 10.1073/pnas.231320498. doi:10.1073/pnas.231320498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D.P. Grooming between male chimpanzees at Ngogo, Kibale National Park. I. Partner number and diversity and grooming reciprocity. Int. J. Prim. 2000;21:189–210. doi:10.1023/A:1005469302911 [Google Scholar]
- Wittig R.M., Boesch C. Food competition and linear dominance hierarchy among female chimpanzees of the Tai National Park. Int. J. Primatol. 2003;24:847–867. doi:10.1023/A:1024632923180 [Google Scholar]