Abstract
Camouflage is an important strategy in animals to prevent predation. This includes disruptive coloration, where high-contrast markings placed at an animal's edge break up the true body shape. Successful disruption may also involve non-marginal markings found away from the body outline that create ‘false edges’ more salient than the true body form (‘surface disruption’). However, previous work has focused on breaking up the true body outline, not on surface disruption. Furthermore, while high contrast may enhance disruption, it is untested where on the body different contrasts should be placed for maximum effect. We used artificial prey presented to wild avian predators in the field, to determine the effectiveness of surface disruption, and of different luminance contrast placed in different prey locations. Disruptive coloration was no more effective when comprising high luminance contrast per se, but its effectiveness was dramatically increased with high-contrast markings placed away from the body outline, creating effective surface disruption. A model of avian visual edge processing showed that surface disruption does not make object detection more difficult simply by creating false edges away from the true body outline, but its effect may also be based on a different visual mechanism. Our study has implications for whether animals can combine disruptive coloration with other ‘conspicuous’ signalling strategies.
Keywords: camouflage, disruptive coloration, crypsis, predation, signalling, animal coloration
1. Introduction
Avoiding predation is a crucial aspect of many animals' fitness, and various strategies have evolved to achieve this, including colours and patterns to prevent detection or recognition; camouflage (Ruxton et al. 2004; Stevens 2007). This includes background matching, where an animal resembles the colour, luminance and pattern of the background(s) on which it is found (Ruxton et al. 2004). Although often incorporating background matching features, disruptive coloration specifically uses markings to break up the appearance of the body outline. Disruption is widely thought to be an important aspect of successful camouflage because just matching the background often leaves an object's outline unbroken and detectable (Thayer 1909; Cott 1940; Stevens & Merilaita in press). Cott (1940) described various aspects related to disruption, including ‘differential blending’, where some of the marginal markings blend into the background, and ‘maximum disruptive contrast’, where adjacent markings have high contrast. However, disruptive coloration has only recently received significant empirical investigation, including analysing the markings of real animals (Merilaita 1998), and demonstrating a survival advantage above background matching in artificial prey presented to wild avian predators (Cuthill et al. 2005), followed by further experimental work demonstrating its importance in concealment (e.g. Merilaita & Lind 2005; Schaefer & Stobbe 2006; Stevens et al. 2006b; Fraser et al. 2007). Mechanistically, disruptive coloration works by breaking up visual edge information corresponding to the true body outline, and creating false internal (‘inside’) edges, preventing detection or recognition of the object (Stevens & Cuthill 2006). Disruptive coloration has been proposed to occur in a range of animals across many taxonomic groups (reviewed by Stevens et al. 2006a), and given its effectiveness in producing concealment, one expects disruptive markings to be widespread in nature.
However, various key questions remain regarding how disruptive coloration works. In particular, Stevens & Merilaita (in press) have reorganized the ‘sub-principles’ that Thayer (1909) and Cott (1940) suggested with respect to disruption. These sub-principles include not only differential blending and maximum disruptive contrast, but also the concepts of ‘marginal pattern elements’, where markings are specifically placed at the body edge to break up the outline, and ‘surface disruption’, where markings found away from the body outline create false edges not corresponding to any identifiable feature of the animal. A significant gap in our understanding of how disruptive coloration functions, concerns the relative importance of the marginal pattern elements and disruption of surface, and how these principles are influenced by the contrast of the pattern elements. Previous work has focused on the importance of marginal markings, with no study explicitly investigating the independent role of the non-marginal (inside) makings found away from the body edge. As Stevens & Merilaita (in press) state, it is unknown whether the inside markings in a disruptive pattern are required simply to maintain a high level of background matching, or whether they play an important role in disruption, independent of the marginal markings; i.e. no one has ever tested Cott's (1940) idea of surface disruption and how important it is in concealment.
It is also unclear what level of contrast is most effective in disruptive markings. Cuthill et al. (2005) found that disruptive markings were particularly effective with high-contrast colours and luminances found in the background. By contrast, Stevens et al. (2006b), Fraser et al. (2007) and Stobbe & Schaefer (2008) all found that disruptive coloration had reduced effectiveness with markings of higher contrast. However, these three studies involved markings where the high-contrast versions mismatched the background. Therefore, the work of Cuthill et al. (2005) remains the only study testing the importance of contrast with background matching colours and luminances. Finally, the markings involved in surface disruption to create false edges may require a different level of contrast to the marginal markings involved in breaking up the real outline.
Here, we test the relative importance of marginal markings in breaking up the true body outline, compared with inside markings producing surface disruption, and the role of pattern contrast in this. We test these questions by undertaking a predation experiment, presenting artificial prey to wild avian predators (as Cuthill et al. 2005), and by analysing the effect different treatment types have on a model of avian visual processing (Stevens & Cuthill 2006).
2. Material and methods
(a) Predation experiment
(i) Artificial prey creation
The method of stimuli creation was similar to Stevens et al. (2006b). Triangular artificial prey 54 mm wide and 28 mm high were created from waterproof paper (HP LaserJet Tough Paper; Palo Alto, USA), and printed with specific patterns on a Hewlett Packard LaserJet 2605dn printer at 300 dpi. As with previous work, these were not intended to mimic any real species, but designed to match the pattern of mature ash tree Fraxinus excelsior bark to which the targets were pinned (‘field psychophysics’; Cuthill et al. 2005). Patterns were made from samples of digital photos (uncompressed TIFF files) of ash tree trunks at 1 : 1 reproduction, taken with a calibrated Fuji Finepix S7000 camera (Stevens et al. 2007). Images were independently thresholded at 50 per cent to binary (black/white) images using Photoshop Elements v. 5.0 (Adobe Systems Inc., San Jose, CA, USA) to retain the variation in bark pattern, so that there was approximately the same proportion of light and dark markings on each target. Different samples, from different trees, were used for each set of replicate targets (figure 1). As with Stevens et al. (2006b), targets comprised different shades of grey and were calibrated to avian luminance vision, in terms of double cone responses, as these seem involved in bird luminance perception (see Osorio & Vorobyev 2005). We modelled blue tit Cyanistes caeruleus double cone photon catches (Hart et al. 2000) using reflectance spectra taken with an Ocean Optics (Dunedin, FL, USA) USB4000 spectrometer, with illumination by a PX-2 pulsed Xenon lamp and irradiance spectra taken in the study site with the cosine corrected spectrometer (e.g. Endler & Meilke 2005). We took five measurements each from a sample of 30 pieces of ash tree bark at 45° to normal. Calibrations for luminance alone allowed precise manipulations of contrast, and targets lacking colour information are still effective in providing camouflage (Stevens et al. 2006b), probably because for textural discrimination and detection of small objects, luminance rather than colour contrast is important (e.g. Jones & Osorio 2004; Osorio & Vorobyev 2005). We calibrated the stimuli such that the printed grey values corresponded to the required luminance by printing calibration charts of grey squares with values increasing in steps of five from 0–255, and by modelling the photon catches of a blue tit's double cones. See Stevens et al. (2006b) for further details.
Figure 1.
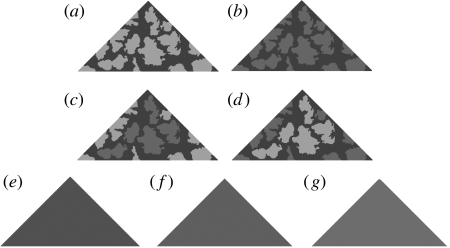
Stimuli used in the experiment: (a) disruptive with all markings of high contrast (AH); (b) disruptive with all markings of low contrast (AL); (c) disruptive with high-contrast edge and low-contrast internal markings (EH); (d) disruptive with low-contrast edge and high-contrast inside markings (IH); (e) average of the markings on treatment AL (L); (f) average of the markings on treatments EH/IH (M); and (g) average of the markings on treatment AH (H). Within a single replicate of marked treatments (IH, EH, AH and AL) the pattern was the same, with only the contrast level and contrast placement differing. Across replicates, targets were based on different samples of bark, and so each replicate of targets had a unique pattern.
We selected the luminance values of the prey based on a histogram of ash bark double cone values. Unlike Stevens et al. (2006b), all shades selected were found in the bark to which the targets were pinned. For the dark background shade (‘base’ shade) of all patterned targets, we selected a grey value that, when printed, corresponded to a double cone photon catch of approximately 0.14. This was the darkest luminance value regularly found in the ash bark (approximately 10% of all shades measured), although darker values were measured. For the low- and high-contrast shades (against the base shade), we selected grey values corresponding to double cone catches of 0.25 and 0.45, respectively (both approximately 15% of all shades measured). These two values were both equally common in ash bark and so all target shades should, on average, contrast equally with the tree backgrounds to which the targets were pinned. Although the level of matching will be influenced by the presence of three-dimensional aspects of the bark (such as shadows), our calibrations were towards the average bark values measured. Because there is variation between the trees in luminance, this inevitably means that no one target will perfectly match any tree, as is presumably true with many real animals. However, the luminance values of the markings were all approximately, equally common in the bark, such that the main difference between treatments was in terms of contrast level and location. We created seven treatments (four patterned and three controls; figure 1). Two treatments comprised disruptive patterns with two shades of grey; the base shade plus one other. These had either markings with a high contrast against the base shade (‘two-shade high contrast’; AH) or a low contrast against the base shade (‘two-shade low contrast’; AL). Two treatments had three shades of grey; the dark base shade, plus both high and low-contrast markings. For treatment ‘three-shade edge high’ (EH), the markings touching the target edge (‘marginal’ markings) had high contrast with the base shade, and the markings not touching the body edge (‘inside’ markings) had a low contrast with the base shade. By contrast, treatment ‘three-shade inside high’ (IH) had marginal markings of low contrast, but inside markings of high contrast with the background target grey. The relative area of the marginal and inside markings was approximately the same for all replicates, with no difference in overall luminance between the three-shade treatments. Finally, three controls matched the average of the AH (treatment ‘average high’; H), the AL (treatment ‘average low’; L) and the three-shade (‘average all shades’; M) prey. Each set of disruptive targets had an entirely unique pattern (figure 1).
(ii) Field experimental procedure
The experimental procedure followed Cuthill et al. (2005). Targets were pinned to ash trees at a height of 1–2 m in the mixed deciduous University of Cambridge Madingley Woods Cambridgeshire, UK (0°3.2′ E, 52°12.9′ N), in a randomized block design. Each block comprised eight replicates of each treatment (56 targets in total per block), randomly allocated and pinned to suitable trees along nonlinear transects of approximately 1–2 km long and 20 m wide, using no more than 5 per cent of the available trees, with each block taking place in a different woodland region. Ten blocks were conducted in July and August 2008 (total sample=560, 80 targets per treatment). The low target density and use of different woodland areas minimized the chance that the same bird would encounter multiple targets. Attached underneath each target, partially projecting out (as Schaefer & Stobbe 2006) was a dead mealworm (Tenebrio molitor larvae) providing an edible component for avian predators. Targets were checked at regular time intervals of 4, 24, 48 and 72 hours. The woodland has a range of avian predators, primarily small songbirds (see Stevens et al. 2008a), with avian predation revealed by the disappearance of all or most of the mealworm from the target. Other forms of predation were identified by slime trails (slugs) or hollow mealworm exoskeletons (spiders).
(iii) Statistical analysis
Survival analysis was by Cox proportional hazards regression (Cox 1972; Klein & Moeschberger 2003; Cuthill et al. 2005), with significance tested with the Wald statistic (abbreviated W). Non-avian predation, complete target disappearance or ‘survival’ to the end of the experiment were incorporated as censored values in the analysis (Cuthill et al. 2005). We used planned pairwise contrasts (Ruxton & Beauchamp 2008) to compare specific treatments, using no more tests than remaining degrees of freedom (six tests in total), meaning p-value correction of post hoc tests was not needed (Rosenthal et al. 2000). These comparisons best tested our experimental aims, and we tested for an effect of pattern (AH versus average high), overall luminance (average high versus average low), disruptive pattern contrast (AH versus AL), and contrast placement (AH versus EH; AH versus IH; and, EH versus IH). These comparisons determine if disruptive targets survive better than the controls, whether there is a difference between the control targets with different overall luminance, and if changing the level and location of contrast changes the effectiveness of disruptive coloration. Effect sizes are odds ratios (OR), where a value of 1.00 indicates two treatments have identical survival probabilities.
(b) Avian visual model
In addition to the field predation experiment, we analysed the effect that each treatment had on a model of avian visual processing, to determine the mechanism underlying how the different patterns and contrasts may work (Stevens 2007). We used a computational model of avian vision and edge processing, based on analysis of calibrated digital images (Stevens & Cuthill 2006). The model has been used to test the idea that disruptive coloration works by exploiting edge detection algorithms operating in vertebrate visual systems (sensu Marr & Hildreth 1980), previously showing that targets with disruptive markings are harder to detect than those with non-disruptive background matching patterns (Stevens & Cuthill 2006). The model has several main stages, outlined in detail in Stevens & Cuthill (2006). Digital images of targets of each treatment pinned to trees in situ were calibrated to correspond to the photon catches of a birds photoreceptors (Stevens et al. 2007), followed by processing with an edge detection filter (a Laplacian of Gaussian algorithm; Marr & Hildreth 1980), which characterizes edges as sharp changes in intensity in an image at different spatial scales based on the presence of ‘zero crossings’. Once the edge images are calculated, a line detection algorithm (a Hough transform; based on Gonzalez et al. 2004) is used to search for the most salient line information present in an image (such as the three edges of a triangular target). Our targets are all grey scale, and so, unlike Stevens & Cuthill (2006), we model only the luminance channel, based on blue tit double cones. We photographed a sample of targets on ash trees used in the predation experiment (15 per treatment; 105 in total), ran these through the model, and used the Hough transform line detector to search for the three most salient lines in each of the edge images (between zero and three correct edges). For targets where the true body edge has not been successfully disrupted, the programme should detect more lines corresponding to the true outline. By contrast, treatments with more effective disruptive coloration should have fewer target edges detected. We used a Kruskal–Wallis test to analyse the difference between treatments and six Wilcoxon–Mann–Whitney tests for pairwise comparisons (same contrasts as the predation experiment). Finally, we also repeated the modelling with a Sobel edge detector (as Stevens & Cuthill 2006) to determine if the results are consistent between these two methods. The results were directly analogous, and so we do not discuss these further.
3. Results
(a) Predation experiment
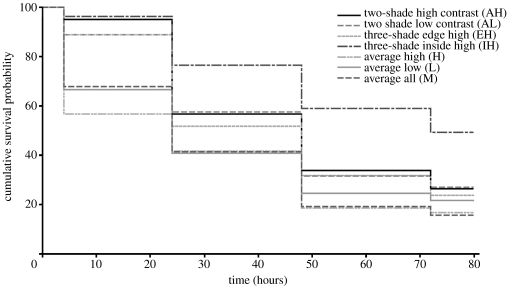
There was a significant effect of treatment (W6=36.275, p<0.001; figure 2) and block (W9=28.826, p=0.001). Disruptively marked targets survived significantly better than the uniform controls (AH versus average high; W1=8.229, p=0.004, OR=1.694), and there was no difference in survival between the best and worst surviving controls of different overall luminance (average high versus average low; W1=0.598, p=0.439, OR=1.151). There was no difference in survival between the two-shade disruptive prey with either high- or low- contrast markings (AH versus AL; W1=0.025, p=0.874, OR=1.029), and no difference between the treatment with just high-contrast markings (AH) and treatment EH (W1=0.267, p=0.605, OR=1.101). However, having high-contrast inside and low-contrast marginal markings conferred a survival advantage as treatment IH survived significantly better than the targets with just high-contrast markings (AH; W1=6.726, p=0.010, OR=1.730), and high-contrast edge and low-contrast inside markings (EH; W1=9.353, p=0.002, OR=1.906). As such, the disruptive targets survived better than the controls, and treatment IH survived better than all other treatments.
Figure 2.
Non-parametric survival plot of the treatments with curves the probability of surviving bird predation over time. Survival top to bottom: IH>AH=AL=EH>L=M=H.
(b) Avian visual model
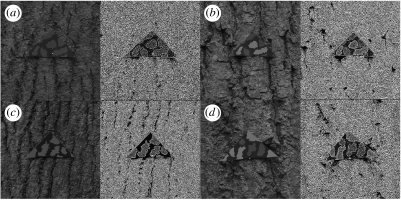
There was a significant difference between the treatments in terms of the number of correct target edges that were detected (H6=37.830, p<0.001; figure 3). Disruptive prey were harder to detect than the uniform controls; treatment AH had significantly fewer body edges detected than treatment average high (W1=160.000, p=0.001), and there was no difference in body edge detection between the controls (average high versus average low; W1=230.500, p=0.947). There was no difference between the two-shade prey with either high- or low-contrast markings (AH versus AL; W1=235.000, p=0.928), or between treatments AH and EH (W1=215.500, p=0.458), AH and IH (W1=228.500, p=0.875) or IH and EH (W1=244.500, p=0.608). As such, the disruptive targets had fewer true body edges detected than the controls, but there was no difference between the disruptive treatments.
Figure 3.
Example targets of the patterned treatments, (a) AL, (b) IH, (c) AH and (d) EH, pinned to trees, with the edge processed images from the visual model to the right of the images of each target.
4. Discussion
Disruptive camouflage offered a survival advantage over uniform camouflaged controls. High-contrast markings per se did not provide a survival advantage compared with targets with low-contrast markings, but the three-shade targets with high-contrast inside markings (‘IH’) survived significantly better than all other treatments. The results from the avian visual model showed that the true body outlines of the disruptive targets were harder to detect than those of the uniform controls, but that there was no difference between the different disruptive target types.
Our result that targets with only high-contrast disruptive markings did not survive better than those with only low-contrast markings does not support the findings of Cuthill et al. (2005) who found that disruptive targets survived better when they had higher contrast. We also found no difference between the number of correct body edges detected by the model for the high- and low-contrast prey. As such, the issue of optimal contrast in disruptive markings is not simple, and Cott's (1940) idea that the contrast in disruptive patterns should be maximized (‘maximum disruptive contrast’) seems oversimplified. Cuthill et al.'s (2005) targets comprised a range of different shades of colour, and it is possible that maximum disruptive contrast is more relevant to colour than luminance contrast. This is supported by previous work, where disruption may be effective when not matching the background colour (Schaefer & Stobbe 2006), but is compromised when mismatching the background for luminance (Stevens et al. 2006b; Fraser et al. 2007).
The most intriguing finding of our experiment regards the dramatic survival advantage conferred by having high-contrast inside and low-contrast marginal markings compared with other contrast placements (treatment IH survived almost twice as well as the other disruptive treatments). The higher survival of IH is unlikely to be due to better blending with the background because both the high- and low-contrast shades were equally common. Finally, it is possible that the increased survival of the targets with high-contrast inside markings could have been if there were aggregations of high-, but not low, contrast markings in the background. However, after inspecting bandpass filtered images of the stimuli and bark, capturing information at different spatial scales, we could find no evidence of aggregations of markings corresponding to either the low- or high-contrast patterns in the background.
By contrast to the survival study, the avian edge model found no difference between the disruptive treatments. This implies that the benefit from the high-contrast inside markings did not simply stem from the creation of salient false edges per se. Rather, an additional visual mechanism may underlie the benefit that these markings confer. The inside markings may have worked by a distractive effect, drawing predator ‘attention’ away from the true target outline, preventing detection or recognition (Thayer 1909; Stevens 2007). However, the potential survival value of distractive markings is controversial, and recent evidence has indicated that high-contrast distractive markings increase predation compared with unmarked controls (Stevens et al. 2008b). However, in Stevens et al. (2008b), the distractive markings were costly when mismatching the background luminance, whereas here, the high-contrast markings were still common in the bark. Subjectively, we found targets of treatment IH difficult to detect, and the outline of the body hard to determine because the inside markings had a higher contrast than the peripheral markings, meaning that the most ‘salient’ shape information was highly irregular and did not correspond to the true body outline. Thus, we do not discount the possibility of a distractive effect. An alternative, physiological rather than attentional explanation, is that the markings worked by producing a ‘crowding’ or ‘contour interaction’ effect, involving interference of adjacent retinal receptors due to lateral inhibition, stemming from the high-contrast markings being close to, but not touching, the true body outline (see Stevens 2007). This would be a useful avenue for future computational vision modelling. Overall, our results provide the first support for the idea that inside non-marginal markings to create false edges have a key role in disruptive camouflage independent of the marginal markings breaking up the real body outline, and our study provides the first support for Cott's (1940) idea of surface disruption. Our results also imply that in animals with disruptive coloration, the non-marginal markings should have higher contrast than those found at the body edges, even if the different contrasts are equally common in the background. This finding may help researchers to identify disruptive coloration in real animals.
Finally, it has often been suggested that because disruptive coloration may involve high-contrast markings, the coloration of some animals may serve a duel function by combining, for example, warning or sexual signals with disruptive camouflage (e.g. Gamberale-Stille 2001). However, given the lack of unambiguous support for the idea that disruption works better with high-contrast potentially ‘conspicuous’ markings, it may be difficult for animals to combine warning signals with a disruptive camouflage function. Conversely, our present work indicates that the placement of high contrast dramatically influences disruptive camouflage, and so it may be possible to combine warning coloration with disruption effectively if the ‘conspicuous’ high-contrast signal components are distributed away from the body edge. Overall, the effectiveness of duel coloration may be strongly influenced by the spatial distribution of the markings, as well as the contrast itself. Clearly, much work is needed to investigate the value and optimization of disruptive coloration, and how different strategies may be combined in animals.
Acknowledgments
We thank, two anonymous referees for various helpful suggestions, Catherine Young for assistance. M.S. was supported by a Research Fellowship from Girton College, Cambridge, I.S.W. by a Department of Zoology J. Arthur Ramsay Trust Fund, J.G. by an Association for the Study of Animal Behaviour Undergraduate Project Scholarship and A.C. by a Nuffield Undergraduate Science Bursary.
References
- Cott H.B. Methuen & Co. Ltd; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Cox D.R. Regression models and life-tables. J. R. Stat. Soc. B. 1972;34:187–220. [Google Scholar]
- Cuthill I.C., Stevens M., Sheppard J., Maddocks T., Párraga C.A., Troscianko T.S. Disruptive coloration and background pattern matching. Nature. 2005;434:72–74. doi: 10.1038/nature03312. doi:10.1038/nature03312 [DOI] [PubMed] [Google Scholar]
- Endler J.A., Meilke P.W.J. Comparing color patterns as birds see them. Biol. J. Linn. Soc. 2005;86:405–431. doi:10.1111/j.1095-8312.2005.00540.x [Google Scholar]
- Fraser S., Callahan A., Klassen D., Sherratt T.N. Emperical tests of the role of disruptive coloration in reducing detectability. Proc. R. Soc. B. 2007;274:1325–1331. doi: 10.1098/rspb.2007.0153. doi:10.1098/rspb.2007.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamberale-Stille G. Benefit by contrast: an experiment with live aposematic prey. Behav. Ecol. 2001;12:768–772. doi:10.1093/beheco/12.6.768 [Google Scholar]
- Gonzalez R.C., Woods R.E., Eddins S.L. Pearson Education Ltd; London, UK: 2004. Digital image processing using Matlab. [Google Scholar]
- Hart N.S., Partridge J.C., Cuthill I.C., Bennett A.T.D. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.) J. Comp. Physiol. A. 2000;186:375–387. doi: 10.1007/s003590050437. doi:10.1007/s003590050437 [DOI] [PubMed] [Google Scholar]
- Jones C.D., Osorio D. Discrimination of orientated visual textures by poultry chicks. Vis. Res. 2004;44:83–89. doi: 10.1016/j.visres.2003.08.014. doi:10.1016/j.visres.2003.08.014 [DOI] [PubMed] [Google Scholar]
- Klein J.P., Moeschberger M.L. Springer; New York, NY: 2003. Survival analysis: techniques for censored and truncated data. [Google Scholar]
- Marr D., Hildreth E. Theory of edge detection. Proc. R. Soc. B. 1980;207:187–217. doi: 10.1098/rspb.1980.0020. doi:10.1098/rspb.1980.0020 [DOI] [PubMed] [Google Scholar]
- Merilaita S. Crypsis through disruptive coloration in an isopod. Proc. R. Soc. B. 1998;265:1059–1064. doi:10.1098/rspb.1998.0399 [Google Scholar]
- Merilaita S., Lind J. Background-matching and disruptive coloration, and the evolution of cryptic coloration. Proc. R. Soc. B. 2005;272:665–670. doi: 10.1098/rspb.2004.3000. doi:10.1098/rspb.2004.3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio D., Vorobyev M. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. R. Soc. B. 2005;272:1745–1752. doi: 10.1098/rspb.2005.3156. doi:10.1098/rspb.2005.3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R., Rosnow R.L., Rubin D.B. Cambridge University Press; Cambridge, UK: 2000. Contrasts and effect sizes in behavioral research. [Google Scholar]
- Ruxton G.D., Beauchamp G. Time for some a priori thinking about post hoc testing. Behav. Ecol. 2008;19:690–693. doi:10.1093/beheco/arn020 [Google Scholar]
- Ruxton G.D., Sherratt T.N., Speed M.P. Oxford, UK; Oxford University Press: 2004. Avoiding attack.doi:10.1093/acprof:oso/9780198528609.003.0012 [Google Scholar]
- Schaefer M.H., Stobbe N. Disruptive coloration provides camouflage independent of background matching. Proc. R. Soc. B. 2006;273:2427–2432. doi: 10.1098/rspb.2006.3615. doi:10.1098/rspb.2006.3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M. Predator perception and the interrelation between protective coloration. Proc. R. Soc. B. 2007;274:1457–1464. doi: 10.1098/rspb.2007.0220. doi:10.1098/rspb.2007.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Cuthill I.C. Disruptive coloration, crypsis and edge detection in early visual processing. Proc. R. Soc. B. 2006;273:2141–2147. doi: 10.1098/rspb.2006.3556. doi:10.1098/rspb.2006.3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, M. & Merilaita, S. In press. Defining disruptive coloration and distinguishing its functions. Phil. Trans. R. Soc. B364 (doi:10.1098/rstb.2008.0216). [DOI] [PMC free article] [PubMed]
- Stevens M., Cuthill I.C., Párraga C.A., Troscianko T. The effectiveness of disruptive coloration as a concealment strategy. In: Alonso J.-M., Macknik S., Martinez L., Tse P., Martinez-Conde S., editors. Progress in brain research. vol. 155. Elsevier; Amsterdam, The Netherlands: 2006a. pp. 49–65. [DOI] [PubMed] [Google Scholar]
- Stevens M., Cuthill I.C., Windsor A.M.M., Walker H.J. Disruptive contrast in animal camouflage. Proc. R. Soc. B. 2006b;273:2433–2438. doi: 10.1098/rspb.2006.3614. doi:10.1098/rspb.2006.3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Párraga C.A., Cuthill I.C., Partridge J.C., Troscianko T.S. Using digital photography to study animal coloration. Biol. J. Linn. Soc. 2007;90:211–237. doi:10.1111/j.1095-8312.2007.00725.x [Google Scholar]
- Stevens M., Hardman C.J., Stubbins C.L. Conspicuousness, not eye mimicry, makes ‘eyespots’ effective anti-predator signals. Behav. Ecol. 2008a;19:525–531. doi:10.1093/beheco/arm162 [Google Scholar]
- Stevens, M., Graham, J., Winney, I. S. & Cantor, A. 2008b Testing Thayer's hypothesis: can camouflage work by distraction? Biol. Lett (doi:10.10.98/rsbl.2008.0486). [DOI] [PMC free article] [PubMed]
- Stobbe N., Schaefer M.H. Enhancement of chromatic contrast increases predation risk for striped butterflies. Proc. R. Soc. B. 2008;275:1535–1541. doi: 10.1098/rspb.2008.0209. doi:10.1098/rspb.2008.0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer G.H. Macmillan; New York, NY: 1909. Concealing-coloration in the animal kingdom: an exposition of the laws of disguise through color and pattern: being a summary of Abbott H. Thayer's discoveries. [Google Scholar]