Abstract
Predation plays a central role in evolutionary processes, but little is known about how predators affect the expression of heritable variation, restricting our ability to predict evolutionary effects of predation. We reared families of three-spined stickleback Gasterosteus aculeatus from two populations—one with a history of fish predation (predator sympatric) and one without (predator naive)—and experimentally manipulated experience of predators during ontogeny. For a suite of ecologically relevant behavioural (‘personality’) and morphological traits, we then estimated two key variance components, additive genetic variance (VA) and residual variance (VR), that jointly shape narrow-sense heritability (h2= VA/(VA + VR)). Both population and treatment differentially affected VA versus VR, hence h2, but only for certain traits. The predator-naive population generally had lower VA and h2 values than the predator-sympatric population for personality behaviours, but not morphological traits. Values of VR and h2 were increased for some, but decreased for other personality traits in the predator-exposed treatment. For some personality traits, VA and h2 values were affected by treatment in the predator-naive population, but not in the predator-sympatric population, implying that the latter harboured less genetic variation for behavioural plasticity.
Replication and experimental manipulation of predation regime are now needed to confirm that these population differences were related to variation in predator-induced selection. Cross-environment genetic correlations (rA) were tight for most traits, suggesting that predator-induced selection can affect the evolution of the same trait expressed in the absence of predators. The treatment effects on variance components imply that predators can affect evolution, not only by acting directly as selective agents, but also by influencing the expression of heritable variation.
Keywords: gene-environment interaction, plasticity, predation, personality, behavioural syndrome, additive genetic variance
1. Introduction
Evolution depends on both selection and narrow-sense heritability (h2), the fraction of phenotypic variance (VP) owing to additive effects of genes (VA) (Falconer & Mackay 1996). Predation regime is a major factor in the evolution of many life-history, morphological and behavioural traits (Stearns 1976; Reznick & Endler 1982; Martin 1995; Lima & Dill 2000). Yet, surprisingly little is known about how exposure to predators affects the heritability of ecologically relevant traits (Relyea 2005; Kraft et al. 2006), or how predators affect the variance components that shape h2, i.e. VA and the residual variance (VR=VP−VA).
Predators might affect variance components in two distinct ways. First, predator-induced directional selection (Dugatkin 1992; Sih et al. 2003; Biro et al. 2004; Bell & Sih 2007) might erode VA over the long term (Price & Schluter 1991), leading to differentiation in variance components (and h2) between populations with a history of predation (hereafter called predator sympatric) and those where predators have long been absent (predator naive). Alternatively, in conditions where predators induce spatio-temporal variation in selection within populations (Reimchen & Nosil 2002), predator-sympatric populations have been suggested to harbour relatively high amounts of VA (Relyea 2005).
Second, predators can induce short-term effects on prey populations by influencing the expression of VA (Relyea 2005; Kraft et al. 2006). This is the case if different genotypes existed within the same population that responded differently to predators (gene×environment interaction (G×E), i.e. genetic variation in plasticity). A growing body of evidence suggests that environmental stress (e.g. food restriction) can greatly affect the expression of variance components and h2 (Hoffmann & Merilä 1999; Charmantier & Garant 2005), but specific effects of predation on variance components have rarely been evaluated in this context (Relyea 2005; Kraft et al. 2006).
Effects of predation on the expression of VA are likely to differ between populations, depending on their evolutionary history (Nussey et al. 2007). The absence of G×E would equate to a situation where the gene sets affecting trait expression in the presence of predators are completely correlated with those affecting trait expression in the absence of predators, i.e. a cross-environment genetic correlation (rA) equal to one (Roff 1997). Evolutionary history can affect rA values by influencing the coupling of gene sets expressed under different conditions (Brodie 1993; Arnold & Phillips 1999; Whitlock et al. 2002). In the case of predation, a number of behavioural studies suggest that rA values might differ between the predator-sympatric and predator-naive populations (Brodie 1993; Bell 2005; Dingemanse et al. 2007). Selection favouring tight rA values (i.e. a lack of G×E effect) might, for instance, occur in predator-sympatric populations, if the costs of plasticity (e.g. to acquire information on predation regime; DeWitt et al. 1998) outweigh its benefits.
Predators can also have short-term and long-term effects on heritabilities if they affect VR, i.e. the sum of environmental (VE), non-additive genetic (VNA) and error (e) variances (Falconer & Mackay 1996). For example, predators often induce group living in their prey (Pulliam 1973), and such social environments might typically allow the coexistence of alternative strategies to cope with predation (Relyea 2005; Réale & Dingemanse in press). Predators might thus induce short-term effects by increasing VE (and hence VR), consequently decreasing h2. Furthermore, in cases where predator-sympatric and predator-naive populations experience different types of selection regime (e.g. fluctuating versus directional; Reimchen & Nosil 2002), we would also expect predator-induced long-term effects on VNA (Merila & Sheldon 1999).
The goals of this paper are threefold. First, we test whether evolutionary history affects VA and VR by comparing VA and VR between the two populations of three-spined stickleback Gasterosteus aculeatus that have differed in predation history for over 30 generations (Dingemanse et al. 2007). We do so by using partial North Carolina II breeding designs to estimate variance components (Lynch & Walsh 1998). Second, we test whether individual experience of predators affects the expression of VA and VR by exposing individuals from each population to either an early life experience of predators or a predator-absent control treatment. Third, we evaluate whether this experimental treatment effect and the population type interact, and assess whether rA values vary between populations.
We focus on the behavioural traits for which predator-induced plasticity and/or selection have previously been documented (Dugatkin 1992; Sih et al. 2003; Biro et al. 2004; Bell & Sih 2007), and we therefore expect effects of predators on variance components. We measured a suite of behaviours that were known to be heritable (activity, boldness towards predators, exploration and sociability; the so-called ‘personality’ traits; Réale et al. 2007), to increase the likelihood of detecting (variation in) VA. Environmental conditions have been argued to differentially affect the expression of variance components of behavioural versus morphological traits, since the latter type might exhibit less G×E (Stirling et al. 2002). For comparison, we therefore also analysed morphological traits (body size, body shape and spine length) that are shaped by predation regime in the stickleback (Hagen & Gilbertson 1972; Moodie et al. 1972; Reimchen 1980).
2. Material and methods
(a) Study populations
The two populations inhabit man-made lakes situated 5.5 km apart on the island of Anglesey (North Wales, UK). Llyn Alaw (50°20′23″N, 04°26′20″W) is a 3.09 km2 reservoir created in 1966 by the impoundment of the River Alaw, whereas Cae Mawr (50°17′06″N, 04°23′31″W) is a 214 m2 pond constructed ca 1980 with no natural input or outflow. Sticklebacks in the two populations experience very different predation regimes. Predatory fishes (perch Perca fluviatilis and rainbow trout Oncorhynchus mykiss) were introduced into Llyn Alaw immediately after impoundment and populations have since been maintained at high densities through stocking programmes (Dingemanse et al. 2007). By contrast, predatory fishes have not been introduced into Cae Mawr and have never been observed during ecological surveys (Dingemanse et al. 2007).
(b) Experimental protocol
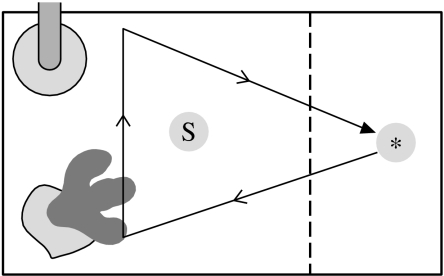
Adult sticklebacks from both populations were captured using hand nets and a small trawl during the breeding season (May 2006). Fish were returned to a temperature and day length-controlled aquarium room at Aberystwyth University and housed communally, in sex- and population-specific 50 l glass aquaria, lined with gravel and planted with artificial plants. Fish were maintained under a natural day length regime at 18±1°C. Fish were fed pre-frozen bloodworms (Chironomus sp. larvae) per individual, and females were assessed regularly for gravid status (following Barber & Arnott 2000). Two types of standard crossing schemes were applied, partial North Carolina II breeding designs and full crosses (Lynch & Walsh 1998), depending on the number of ripe females available for breeding on the same day. A total of 22 males and 15 females (Llyn Alaw) and 30 males and 28 females (Cae Mawr) were used for breeding (for further details see §2 and table S3 in the electronic supplementary material). Egg clutches of ripe females were stripped and divided over two or three moistened solid watch glasses (number dependent on crossing design; see the electronic supplementary material); each split clutch was then fertilized with the sperm of a different (randomly selected) male from the same population as the female's, following standard split-clutch in vitro fertilization techniques and egg husbandry protocols (Barber & Arnott 2000). Following fertilization (day 0), each portion of the split clutch (hereafter called a ‘full-sib family’) was incubated in isolation in a 15×10×10 cm (L×W×H) tank. At hatching (day 8), fry in each full-sib family were counted and divided equally between two 30×20×20 cm tanks (hereafter called ‘growth tanks’). One of the two growth tanks was then randomly assigned to the control treatment, and the other to the predator-exposed treatment. We, therefore generated four (population×treatment) groups (hereafter called ‘groups’); ‘Nc’ (naive population, control treatment), ‘Ne’ (naive population, exposed treatment), ‘Sc’ (predator-sympatric population, control treatment) and ‘Se’ (predator-sympatric population, exposed treatment). Fry in all groups were fed a progressive diet of Liquifry (days 6–14; twice a day), and newly hatched Artemia (from day 10 onwards, ad libitum). Each growth tank contained water (10 cm depth), a filter with aeration and a clump of artificial weed (figure 1).
Figure 1.
Diagram of the experimental set-up. Arrangement of the growth tank. The tank contained a clump of artificial weed and a filter (illustrated) and was externally lined with opaque white polythene. The dashed line inside the tank indicates the position of the opaque and transparent barriers that were inserted at the onset of the live predator and chasing tests. The asterisk denotes the position where the live perch (live predator test) or perch model (chasing test) were introduced, and the arrow denotes the pattern of model chasing.
(c) Predator exposure/non-exposure treatments
Growth tanks assigned to the predator-exposed treatment group were given a chemical, visual and behavioural stimulation regime designed to mimic that experienced by stickleback living sympatrically with predatory fishes. The treatment consisted of repeated exposure to two types of stimulus; being exposed to a live perch (‘live predator trial’), and being chased by a model perch (‘chasing trial’). The latter trial type was included to ensure that sticklebacks in the predator-exposed group perceived the perch as threatening stimuli. Each trial was executed at a randomly chosen time of day (between 08.00 and 18.00 hours). All trials were executed at about the same time on a particular day, thereby ensuring that the timing of encounters with predators was equally unpredictable.
Live predator trials were undertaken every 2 days between days 29 and 49. At the start of the trial (t0), two partitions—one opaque and one transparent—were inserted into the growth tank (figure 1). After 15 min (t15), a live perch was introduced behind the partitions and revealed, after a short settling period, by removing the opaque partition. The transparent partition prevented the perch from entering the subjects' compartment, but was loosely fitted, permitting the transfer of olfactory cues. At t45, the opaque barrier was reinserted, the perch taken out, and then both barriers were removed. In total, 70 individual perch of 12–15 cm (total length) were used as stimuli, with each growth tank experiencing a minimum of seven different live perch. Growth tanks assigned the control treatment were subjected to the same treatment, with the exception that—instead of a live perch—a similar-sized neutral object (a stone) was introduced at t15 and removed when revealing the compartment by removing the white opaque barrier. Hence, we applied the same amount of disturbance to the tank as introducing or removing a live perch.
‘Chasing’ models were constructed from three killed, eviscerated and dried perch that had been waxed (for 24 hours in waterproof microscopy grade paraffin wax, using a Gallenkamp Hotbox Oven at 65°C) and fitted with 30 cm metal rods.
The chasing trial, undertaken every 4 days between days 30 and 46, started (t0) when the transparent and opaque barriers were inserted (as above) into the subjects' tank (figure 1). At t15, a model perch was introduced behind the barrier, together with 50 ml of water taken from a tank, containing 1 l of matured tap water that had held a live perch for the previous 30 min (‘perch water’). Directly following introduction, both barriers were removed, the model was moved quickly into the centre of the shoal of juvenile sticklebacks (generally located near the filter or weed), turned, moved back to the place of introduction (see arrow in figure 1) and removed from the water, all within 2 s. Growth tanks assigned the control treatment were given the same treatment, with the exception that (i) instead of a live perch, a similar-sized stone was introduced at t15 and removed when removing the barriers, (ii) instead of perch water, 50 ml of water was added from a tank, containing 1 l of matured tap water, that had not held a live perch, and (iii) instead of moving the perch model through the water, the same movement was made using a metal rod with no perch model attached.
(d) Behavioural assays
At ages 44, 46, 48, 50 and 52 days, one or two randomly selected individuals were captured from each growth tank, and then screened for personality traits. All behavioural tests were filmed from above using digital video cameras (Model GR-D340EK, JVC, Yokohama, Japan) and various behavioural measures (listed in table S4 in the electronic supplementary material) were taken from the video recordings of each trial. Sample sizes (n) were 577 (Cae Mawr), 464 (Llyn Alaw) and 1041 (grand total) individuals, but rare events (e.g. camcorder malfunctions) meant that sample sizes for some assays are slightly reduced. Personality tests were executed in a fixed order to facilitate comparison between the individuals (Dingemanse et al. 2007). Following the behavioural tests, subjects were killed with an overdose of benzocaine anaesthetic (10 mg l−1) and lateral photographs were taken.
(i) Novel environment test
From 12.30 to 13.15 hours, selected subjects were captured from their growth tanks, transferred to a confined area of a similar-sized but novel tank, and left to acclimatize for 300 s (see figure S1A in the electronic supplementary material). The barrier was then removed, allowing the subject to explore the remainder of this novel environment. The novel tank contained five large stones and was filled with water to a depth of only 5 cm to prevent the subject from swimming above the stones; information about the environment could thus only be gained by moving around the stones. Each tank was placed on top of a sheet containing 60 uniquely numbered 3×3 cm squares to facilitate recording of subject location. Each square change was recorded for a period of 300 s following the release from the confined area. Exploration behaviour was operationally defined as an activity in a novel environment (following Dingemanse et al. 2002, 2007).
(ii) Activity test
Activity in a non-novel environment was measured by recording each time a fish crossed the boundary between adjacent 3×3 cm squares (see above) during a 300 s period, both 2 hours (14.30–15.15 hours) and 4 hours (16.30–17.15 hours) following release in the novel tank.
(iii) Sociability test
Following the last activity test, four of the five stones were removed carefully from the test tank, and two transparent barriers were inserted such that the subject remained in the middle compartment of the refurbished tank (figure S1B in the electronic supplementary material). The remaining stone was then positioned in one of the empty compartments, and the subject was left to acclimatize overnight. Between 08.30 and 10.00 hours the following morning, an unrelated, age- and population-matched ‘stimulus’ conspecific was introduced into the remaining empty compartment (figure S1B in the electronic supplementary material). The test thus allowed us to assess whether the individuals preferred to stay near the shelter versus near a conspecific. The subject's behaviour was then recorded (row changes and the number of orientation bouts) for 300 s, after which the stimulus fish was removed. An orientation bout was defined as starting when the subject faced towards the stimulus fish and terminated when the subject turned away from it. Each stimulus fish was used only once to avoid pseudoreplication (Hurlbert 1984), and any variation in the behaviour of different stimulus fish was thus regarded as unbiased noise (Dingemanse et al. 2007).
(iv) Predator inspection test
Following the sociability test, the barrier furthest away from the stimulus compartment (barrier 2 in figure S1B in the electronic supplementary material) was removed and a transparent and a white opaque sheet were fitted over the remaining barrier. After 2 hours (10.30–12.00 hours), a live perch was introduced behind the barrier. After 30 s, the opaque sheet was removed and the subject's behaviour was recorded (row changes and the number of inspection bouts) for 10 min, after which the perch was removed. Inspection bouts were defined as outlined by Dingemanse et al. (2007).
(e) Morphological measurements
We took three morphological measurements from the lateral photographs (detailed in Dingemanse et al. in press): ‘standard length’ (the horizontal distance from the anterior tip of the upper lip to the caudal border of the hypural plate; landmarks 1–8 in Walker & Bell 2000; hereafter called ‘body length’); ‘body depth’ (depth of the body at the base of the first dorsal spine; landmark 3 in Walker & Bell 2000); and ‘length of the first dorsal spine’ (the horizontal distance from the base to the tip of the first dorsal spine).
(f) Statistical analyses
Principal component analyses (PCA) followed by varimax rotation (Tabachnick & Fidell 2001) was used to summarize, for each test separately, the behaviours quantified (see table S4 in the electronic supplementary material). PCA summarized the behaviours quantified into either two (sociability test) or one (all other tests) principal component(s). The results of these analyses echoed our findings from earlier behavioural studies on these populations (Dingemanse et al. 2007); for further detailed description and interpretation of emerging axes, therefore, see Dingemanse et al. (2007) and the electronic supplementary material. Notably, common PCA (using the CPCrand software; Phillips & Arnold 1999) confirmed homogeneity of phenotypic variance–covariance matrices across the four groups for each behavioural essay, justifying the use of combined analyses for all groups.
All quantitative genetic analyses were conducted using restricted maximum-likelihood (‘animal’) models, allowing analyses of unbalanced datasets and inclusion of fixed effects (Kruuk 2004), using ASReml v. 2.0 (Gilmour et al. 2006). Age was fitted as a fixed effect (continuous variable) in all models, because age at measurement varied between individuals within full-sib families. Standard length was also fitted as fixed effect in analyses of body depth and spine length, because here our interest was body shape and relative spine length, respectively. Individual was included as a random effect, with the associated variance–covariance matrix determined by the additive genetic relatedness matrix (Kruuk 2004), to estimate additive genetic (co)variance components. Environmental maternal effects were investigated by including maternal identity, as an additional random effect (Kruuk 2004); maternal effects did not explain significant variation for any of the traits and were therefore not included in the analyses presented.
VA and VR were calculated using univariate animal models, and h2 was calculated with VA/(VA+VR). Significance of VA was evaluated using a likelihood ratio test (Meyer 1992). Bivariate animal models were used to estimate rA for the same trait (X) in the control (Xc) versus predator-exposed treatment (Xe) (Astles et al. 2006). Following Roff (2001), rA was estimated only when the geometric mean h2, (hXc2×hXe2)1/2, was above 0.15 to avoid unreliable estimates. Significance of rA was evaluated using a likelihood ratio test, comparing the likelihood of an unconstrained model with a constrained model where rA was fixed at either zero (to evaluate deviations from zero) or one (to evaluate deviations from one). Z-tests were used to test for population differences in rA values.
We used multivariate animal models to compare VA of the same trait among the four groups (i.e. Nc, Ne, Sc and Se, as defined above), where the trait of interest was entered as a separate y-variable for each group, the additive genetic and residual covariances between these y-variables constrained to zero and age (and standard length in the case of body shape and relative spine length) fitted as fixed effects. Support for differences in VA among groups, populations or treatments, was based on two-step analyses of the data. First, we fitted four a priori considered models that potentially described the variation in VA across the four groups (see table S2 in the electronic supplementary material). These modelled: model a equal variances across all groups (VANc=VANe=VASc=VASe), model b population differences ((VANc=VANe)≠(VASc=VASe)), model c treatment effects ((VANc=VASc)<>(VANe=VASe)), or model d group-specific variance estimates (VANc≠VANe≠VASc≠VASe). Second, we calculated the Akaikes Information Criterion (AIC) weight, a measure of relative support (Akaike 1973; Burnham & Anderson 2002), for each of the four models. AIC weight is the probability that the focal model would be the AIC best model where the data were collected again under identical circumstances. We then used the information (weights) of all models to calculate total support (Burnham & Anderson 2002) for differences between any of the population–treatment groups (Σ AIC weights of all models modelling population and/or treatment effects, i.e. models b–d), populations (Σ weights of models modelling population effects, i.e. models b and d) or treatments (Σ weights of models modelling treatment effects, i.e. models c and d; table 1). The four alternative models were constructed by constraining VA of particular groups to the same value as appropriate. We used the same procedure to investigate the variation in VR. For models on VA, VR was allowed to vary freely between the four groups, and vice versa.
Table 1.
Support for treatment and population effects in genetic parameters. (Total support for the existence of variation in additive genetic (VA) and residual variance (VR) among populations, treatments, or any of the four population–treatment groups for various personality traits (principal component axes given functional labels, alphanumeric codes in exponent refer to axes numbers in table S4 in the electronic supplementary material) and morphological traits. For each factor considered (group, population, treatment), the total support (range 0–1) was calculated by summing up the AIC weights of all models (listed in table S1 in the electronic supplementary material) that had the factor of interest in common (see §2). Effects of factors with considerable support are printed in italic. The values in brackets are the number of times any model including the factor of interest fitted better than any model that did not (support divided by one minus support).)
| comparison of VA | comparison of VR | |||||
|---|---|---|---|---|---|---|
| trait | groups | population | treatment | groups | population | treatment |
| personality traitsaxis label | ||||||
| exploration novel environmentA1 | 0.51 (1.0) | 0.32 (0.5) | 0.23 (0.3) | 0.66 (2.0) | 0.48 (0.9) | 0.28 (0.4) |
| activity 2 hours after releaseB1 | 0.75 (2.9) | 0.65 (1.9) | 0.19 (0.2) | 0.88 (7.6) | 0.31 (0.4) | 0.80 (4.1) |
| activity 4 hours after releaseC1 | 0.83 (4.9) | 0.74 (2.9) | 0.22 (0.3) | 0.48 (0.9) | 0.29 (0.4) | 0.23 (0.3) |
| sociability−D1 | 0.76 (3.2) | 0.43 (0.7) | 0.47 (0.9) | 0.92 (11.1) | 0.42 (0.7) | 0.82 (4.7) |
| exploration of novel conspecificD2 | 0.76 (3.2) | 0.58 (1.4) | 0.33 (0.5) | 0.53 (1.1) | 0.25 (0.3) | 0.36 (0.6) |
| boldness towards predators−E1 | 0.46 (0.9) | 0.25 (0.2) | 0.24 (0.3) | 0.62 (1.7) | 0.33 (0.5) | 0.38 (0.6) |
| morphological traits | ||||||
| body length | 0.52 (1.1) | 0.25 (0.3) | 0.31 (0.5) | 0.80 (4.0) | 0.72 (2.6) | 0.17 (0.2) |
| body shape | 0.50 (1.0) | 0.26 (0.4) | 0.29 (0.4) | 0.65 (1.9) | 0.26 (0.3) | 0.47 (0.9) |
| relative spine length | 0.50 (1.1) | 0.27 (0.4) | 0.27 (0.4) | 0.48 (0.9) | 0.26 (0.3) | 0.28 (0.4) |
3. Results
(a) Heritability
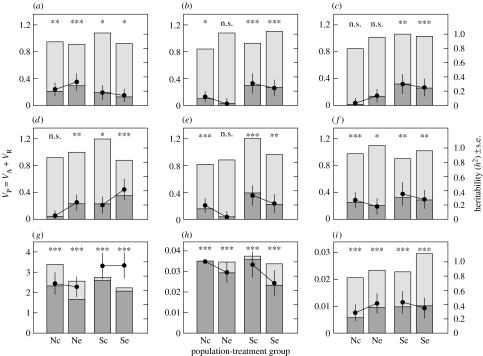
Most of the behavioural personality traits showed moderate h2 values (figure 2; see table S1 in the electronic supplementary material). For the predator-sympatric population, h2 was significant for all traits and in both predator-exposure treatments. By contrast, for the predator-naive population, only ‘exploration of novel environment’ and ‘boldness towards predators’ were significantly heritable in both treatments; ‘activity 2 hours after release’ and ‘exploration of novel conspecific’ were heritable only in the control treatment, ‘sociability’ was heritable only in the predator-exposed treatment and ‘activity 4 hours after release’ was not heritable in either treatment. When comparing across all population–treatment groups, mean h2 of personality traits was 43.4 per cent lower for the predator-naive (N) compared with the predator-sympatric (S) population, but were almost identical for the two treatment (c, control; e, predator-exposed) groups (Nc: 0.15 (mean), 0.03–0.27 (range); Ne: 0.16, 0.03–0.33; Sc: 0.28, 0.17–0.35; Se: 0.26, 0.14–0.42). With the exception of ‘exploration of novel environment’, this pattern of lower heritability in the predator-naive population held for all traits and within both treatments.
Figure 2.
Sources of variation in heritabilities. For personality and morphological traits, and for each of four population–treatment groups (‘Nc’, naive population, control treatment; ‘Ne’, naive population, exposed treatment; ‘Sc’, predator-sympatric population, control treatment; ‘Se’, predator-sympatric population, exposed treatment). Stacked bars (y-axis, left) indicate the total phenotypic variance (VP) decomposed into its additive genetic (VA; dark grey bars) and residual (VR; light grey bars) variance components, and dots (y-axis, right) indicate the estimated narrow-sense heritabilities (h2)±s.e. ((a) Exploration novel environment, (b) activity 2 hours after release, (c) activity 4 hours after release, (d) sociability, (e) exploration novel conspecific, (f) boldness towards predator, (g) body length, (h) body shape and (i) relative length of first dorsal spine.) We give the significance of each h2 value (n.s., p>0.05; *p<0.05; **p<0.01; ***p<0.001; for statistical analyses see table S1 in the electronic supplementary material.).
All morphological traits had significant h2 values for both populations and treatments (figure 2; see table S1 in the electronic supplementary material). When comparing across all population–treatment groups, mean h2 of the morphological traits was slightly (9.8%) lower for the predator-naive compared with the predator-sympatric population, but slightly higher (10.2%) for the control compared with the predator-exposed treatment: (Nc: 0.66 (mean), 0.29–1.00 (range); Ne: 0.64, 0.41–0.85; Sc: 0.78, 0.43–0.96; Se: 0.66, 0.35–0.94).
(b) Variance components
For ‘activity 2’ and ‘4 hours after release’, ‘sociability’, ‘exploration of novel conspecific’ and ‘body length’, the data supported the notion that treatment, and/or population, differentially affected the variance components VA versus VR and therefore h2 (table 1; figure 2). The morphological traits showed a higher ratio of VA relative to VR (i.e. higher h2) compared with the behavioural traits, except for ‘relative spine length’, which showed moderate heritability (figure 2; see table S1 in the electronic supplementary material).
For ‘activity 2’ and ‘4 hours after release’, ‘sociability’ and ‘exploration of a novel conspecific’, statistical models incorporating differences in VA between population–treatment groups were 2.9–4.9 times better supported than those with no differences between groups (table 1), because VA was either higher in the predator-sympatric compared with the predator-naive population (‘activity 2’ and ‘4 hours after release’) or because treatment effects were restricted to the predator-naive population only (exploration of a novel conspecific: VA control>exposed; sociability VA control<exposed; figure 2). For activity 2 hours after release and sociability, also VR varied between groups (i.e. presence of variation was 7.6 and 11.1 times better supported than its absence), because VR was either increased (activity 2 hours after release) or decreased (sociability) in the predator-exposed compared with the control treatment.
None of the morphological traits showed a pattern of increased VA in the predator-sympatric population compared with the predator-naive population (table 1 and figure 2), implying that the two populations did not generally differ in VA. Body length was the only trait where we detected population variation in VR (naive>sympatric).
(c) Cross-environment genetic correlations
All cross-environment rA values were positive and relatively tight (greater than 0.67), regardless of the type of trait or population under consideration (table 2). Thus, rank-order differences between individuals raised in the control treatment that were owing to additive effects of genes would largely have been maintained had these individuals instead been raised in the predator-exposed treatment. Body length in the predator-sympatric population was the only exception, where rA was low (0.38) and non-significant (but note that the upper estimate was relatively high (95% CI=0.80) owing to large s.e.). None of the values of rA differed between the populations (all Z<0.49, all p>0.624).
Table 2.
Cross-environment genetic correlations. Genetic correlations (rA±s.e.) across the control versus predator-exposed environments are given with their geometric mean heritability (Avg. h2; not given when Avg. h2 <0.15) for various personality traits and morphological traits and for two populations.
| predator-naive population | predator-sympatric population | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| test of rA =0 | test of rA =1 | test of rA=0 | test of rA=1 | |||||||||
| trait | Avg. h2 | rA±s.e. | Χ12 | p-value | Χ12 | p-value | Avg. h2 | rA±s.e. | Χ12 | p-value | Χ12 | p-value |
| personality traitsaxis label | ||||||||||||
| exploration novel environmentA1 | 0.27 | 0.65±0.27 | 4.238 | 0.04 | 2.626 | 0.105 | 0.15 | 0.84±0.45 | 2.74 | 0.098 | 0.154 | 0.695 |
| activity 2 hours after releaseB1 | 0.06 | — | — | — | — | — | 0.27 | 0.78±0.28 | 4.312 | 0.038 | 0.78 | 0.377 |
| activity 4 hours after releaseC1 | 0.06 | — | — | — | — | — | 0.27 | 1.17±0.09 | 18.722 | <0.001 | 2.146 | 0.143 |
| sociability−D1 | 0.11 | — | — | — | — | — | 0.29 | 0.74±0.33 | 3.28 | 0.07 | 0.77 | 0.38 |
| exploration of novel conspecificD2 | 0.08 | — | — | — | — | — | 0.27 | 0.83±0.31 | 4.166 | 0.041 | 0.348 | 0.555 |
| boldness towards predators−E1 | 0.22 | 0.87±0.34 | 5.466 | 0.019 | 0.202 | 0.653 | 0.32 | 1.13±0.11 | 16.092 | <0.001 | 1.21 | 0.271 |
| morphological traits | ||||||||||||
| body length | 0.67 | 0.67±0.14 | 12.982 | <0.001 | 2.968 | 0.085 | 0.94 | 0.38±0.21 | 2.674 | 0.102 | 38.656 | <0.001 |
| body shape | 0.92 | 0.64±0.13 | 13.61 | <0.001 | 27.278 | <0.001 | 0.82 | 0.83±0.11 | 15.13 | <0.001 | 7.564 | 0.006 |
| relative spine length | 0.34 | 0.81±0.21 | 8.068 | 0.005 | 0.992 | 0.319 | 0.39 | 0.81±0.20 | 7.392 | 0.007 | 1.454 | 0.228 |
Irrespective of the population, rA also deviated from 1 (or tended to do so) for both body length and body shape, implying that the gene sets causing heritable variation in the control treatment did not overlap completely with those causing heritable variation in the predator-exposed treatment. For personality traits, deviations from 1 were not detected; however, estimates of rA for all personality traits had high s.e.'s, suggesting that we may have had insufficient power to test whether rA deviated from 1.
4. Discussion
Predators can affect evolution of prey not only by acting as selective agents, but also by influencing prey variance components, as shown in this study. Heritabilities of various behavioural personality—but not morphological—traits were affected by experience with predators during ontogeny. The mechanism by which predator-exposure treatment affected heritabilities (via VA or VR), as well as the direction of its effect (increasing versus decreasing), differed between personality traits. To our knowledge, only two other studies, both focusing on the morphological traits in frogs (Rana sp.), have investigated how exposure to predators affects variance components (Relyea 2005; Kraft et al. 2006). Both showed that exposure increased VA values for certain traits, and it has been hypothesized that this is a general pattern in nature (Relyea 2005; Kraft et al. 2006). Our results do not support this idea. Instead, it appears that predation can have diverse effects on variance components, depending on the evolutionary history of the population. This latter notion is illustrated by our finding that for certain personality traits, values of VA and h2 were affected by treatment only in the predator-naive and not in the predator-sympatric population. Whether or not the reported effects of evolutionary history were related causally to predation regime requires further clarification, and cannot be judged based on the present study owing to lack of replication.
Although predator-exposure treatment did not directly affect VA or h2 values for morphological traits, estimates of cross-environment rA values were significantly below one in three out of six cases for both populations, revealing that experimental predator exposure influenced the expression of genetic variation for this type of trait. Altogether it appears that genetic variation in predator-induced morphological plasticity existed in both populations, whereas genetic variation in behavioural plasticity was less apparent, in the predator-sympatric one. Assuming for now that variation in predation regime has resulted in these population differences, such patterns would suggest that the predator-sympatric population inhabits an environment where selection has removed genetic variation in behavioural plasticity (Nussey et al. 2007). This idea is consistent with the observation that predator-sympatric populations exhibit behavioural syndromes (tight positive phenotypic correlations between the personality traits activity, aggressiveness, boldness and exploration) whereas predator-naive populations do not (Dingemanse et al. 2007). In other words, predator-sympatric populations are also characterized by limited plasticity across behavioural contexts and situations (Sih et al. 2004).
For two personality traits (activity 2 hours following release and sociability), we found evidence that the predator-exposure treatment affected h2 via VR. We do not know of other studies that have investigated the effects of predators on VR, although other factors, including territory quality, are well known to influence this component (Charmantier & Garant 2005). Observed changes in VR might imply that predators affected the amount of environmental heterogeneity, hence VE, for instance by increasing the number of social interactions (e.g. by inducing shoaling), in turn promoting individual differentiation in behaviour within tanks (Hemelrijk & Wiantia 2005). Alternatively, individual differentiation might have resulted from the differential expression of non-additive effects of genes in the two treatments. Although we cannot currently differentiate between these two scenarios, our findings exemplify the importance of considering variance components other than VA when studying environmentally induced variation in h2 (Charmantier & Garant 2005).
The observed population variation in h2 of personality traits implies that evolutionary history affects h2 of personality traits, either via stochastic processes (drift and founder effects) or spatial variation in selection. Our predator-naive populations inhabit ponds of relatively small size, whereas predator-sympatric ones occur in reservoirs of sufficient size to maintain populations of predatory fishes (Dingemanse et al. 2007). Founder effects or drift might therefore readily explain the lowered VA (and h2) values in the predator-naive population. However, stochasticity would not readily explain why reduced levels of VA were found for personality but not for morphological traits. Hence, the exciting alternative explanation for these patterns is that a history of directional selection has reduced VA in these specific traits in the predator-naive population, whereas fluctuating selection (Reimchen & Nosil 2002) might maintain high levels of VA in the predator-sympatric population (Frank & Slatkin 1990). Experimental manipulation of predation regime, combined with the measurement of fitness landscapes in manipulated populations, could offer a fruitful next step in studying the role of predators in shaping variance components.
Acknowledgments
The experiment was designed within the guidelines of the Association for the Study of Animal Behaviour, and undertaken under a UK Home Office project license (PPL 40.2969, held by I.B.).We are grateful to K. Boeke, R. Geoghegan, K. Meijer, C. Oosterhof and T. Sparrow for assistance, Welsh Water for permission to capture sticklebacks on their property, D. Garant and A. Gilmour for statistical advice and Anahita Kazem for discussion. N.J.D. was supported by The Netherlands Organisation for Scientific Research (grant no. 863.05.002), and N.J.D. and J.W. by the Research Council of Norway (STORFORSK project ‘Population genetics in an ecological context’).
Supplementary Material
Electronic supplementary material text
Electronic supplementary material figure S1
References
- Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petran B.N., Csáki F., editors. International symposium on information theory. Akadémiai Kiadi; Budapest, Hungary: 1973. pp. 267–281. [Google Scholar]
- Arnold S.J., Phillips P.C. Hierarchical comparison of genetic variance–covariance matrices. II. Coastal–inland divergence in the garter snake, Thamnophis elegans. Evolution. 1999;53:1516–1527. doi: 10.1111/j.1558-5646.1999.tb05415.x. doi:10.2307/2640897 [DOI] [PubMed] [Google Scholar]
- Astles P.A., Moore A.J., Preziosi R.F. A comparison of methods to estimate cross-environment genetic correlations. J. Evol. Biol. 2006;19:114–122. doi: 10.1111/j.1420-9101.2005.00997.x. doi:10.1111/j.1420-9101.2005.00997.x [DOI] [PubMed] [Google Scholar]
- Barber I., Arnott S.A. Split-clutch IVF: a technique to examine indirect fitness consequences of mate preferences in sticklebacks. Behaviour. 2000;137:1129–1140. doi:10.1163/156853900502484 [Google Scholar]
- Bell A.M. Behavioral differences between individuals and two populations of stickleback (Gasterosteus aculeatus) J. Evol. Biol. 2005;18:464–473. doi: 10.1111/j.1420-9101.2004.00817.x. doi:10.1111/j.1420-9101.2004.00817.x [DOI] [PubMed] [Google Scholar]
- Bell A.M., Sih A. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus) Ecol. Lett. 2007;10:828–834. doi: 10.1111/j.1461-0248.2007.01081.x. doi:10.1111/j.1461-0248.2007.01081.x [DOI] [PubMed] [Google Scholar]
- Biro P.A., Abrahams M.V., Post J.R., Parkinson E.A. Predators select against high growth rates and risk-taking behaviour in domestic trout populations. Proc. R. Soc. B. 2004;271:2233–2237. doi: 10.1098/rspb.2004.2861. doi:10.1098/rspb.2004.2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie E.D. Homogeneity of the genetic variance–covariance matrix for antipredator traits in 2 natural populations of the garter snake Thamnophis ordinoides. Evolution. 1993;47:844–854. doi: 10.1111/j.1558-5646.1993.tb01238.x. doi:10.2307/2410188 [DOI] [PubMed] [Google Scholar]
- Burnham K.P., Anderson D.R. 2nd edn. Springer-Verlag; New York, NY: 2002. Model selection and multimodel inference: a practical information—theoretic approach. [Google Scholar]
- Charmantier A., Garant D. Environmental quality and evolutionary potential: lessons from wild populations. Proc. R. Soc. B. 2005;272:1415–1425. doi: 10.1098/rspb.2005.3117. doi:10.1098/rspb.2005.3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt T.J., Sih A., Wilson D.S. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 1998;13:77–81. doi: 10.1016/s0169-5347(97)01274-3. doi:10.1016/S0169-5347(97)01274-3 [DOI] [PubMed] [Google Scholar]
- Dingemanse N.J., Both C., Drent P.J., Van Oers K., Van Noordwijk A.J. Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim. Behav. 2002;64:929–937. doi:10.1006/anbe.2002.2006 [Google Scholar]
- Dingemanse N.J., Wright J., Kazem A.J.N., Thomas D.K., Hickling R., Dawnay N. Behavioural syndromes differ predictably between 12 populations of stickleback. J. Anim. Ecol. 2007;76:1128–1138. doi: 10.1111/j.1365-2656.2007.01284.x. doi:10.1111/j.1365-2656.2007.01284.x [DOI] [PubMed] [Google Scholar]
- Dingemanse, N. J., Oosterhof, C., van der Plas, F. & Barber, I. In press. Variation in stickleback head morphology associated with parasite infection. Biol. J. Linn. Soc
- Dugatkin L.A. Tendency to inspect predators predicts mortality risk in the guppy. Behav. Ecol. 1992;3:124–127. doi:10.1093/beheco/3.2.124 [Google Scholar]
- Falconer D.S., Mackay T.F.C. Longman; New York, NY: 1996. Introduction to quantitative genetics. [Google Scholar]
- Frank S.A., Slatkin M. Evolution in a variable environment. Am. Nat. 1990;136:244–260. doi:10.1086/285094 [Google Scholar]
- Gilmour A.R., Gogel B.J., Cullis B.R., Welham S.J., Thompson R. VSN International; Hemel Hempstead, UK: 2006. ASreml user guide. Release 1.0. [Google Scholar]
- Hagen D.W., Gilbertson L.G. Geographic variation and environmental selection in Gasterosteus aculeatus L. in the Pacific Northwest. Am. Evol. 1972;26:32–51. doi: 10.1111/j.1558-5646.1972.tb00172.x. doi:10.2307/2406981 [DOI] [PubMed] [Google Scholar]
- Hemelrijk C.K., Wiantia J. Individual variation by self-organisation. Neurosci. Biobehav. Rev. 2005;29:125–136. doi: 10.1016/j.neubiorev.2004.07.003. doi:10.1016/j.neubiorev.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Hoffmann A.A., Merilä J. Heritable variation and evolution under favourable and unfavourable conditions. Trends Ecol. Evol. 1999;14:96–101. doi: 10.1016/s0169-5347(99)01595-5. doi:10.1016/S0169-5347(99)01595-5 [DOI] [PubMed] [Google Scholar]
- Hurlbert S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984;54:187–211. doi:10.2307/1942661 [Google Scholar]
- Kraft P.G., Wilson R.S., Franklin C.E., Blows M.W. Substantial changes in the genetic basis of tadpole morphology of Rana lessonae in the presence of predators. J. Evol. Biol. 2006;19:1813–1818. doi: 10.1111/j.1420-9101.2006.01185.x. doi:10.1111/j.1420-9101.2006.01185.x [DOI] [PubMed] [Google Scholar]
- Kruuk L.E.B. Estimating genetic parameters in natural populations using the ‘animal model’. Proc. R. Soc. B. 2004;359:873–890. doi: 10.1098/rstb.2003.1437. doi:10.1098/rstb.2003.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima S.L., Dill L.M. Behavioural decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 2000;68:619–640. doi:10.1139/z90-092 [Google Scholar]
- Lynch M., Walsh B. Sinauer Associates; Sunderland, MA: 1998. Genetics and analysis of quantitative traits. [Google Scholar]
- Martin T.E. Avian life-history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 1995;65:101–127. doi:10.2307/2937160 [Google Scholar]
- Merila J., Sheldon B.C. Genetic architecture of fitness and nonfitness traits: empirical patterns and development of ideas. Heredity. 1999;83:103–109. doi: 10.1046/j.1365-2540.1999.00585.x. doi:10.1038/sj.hdy.6885850 [DOI] [PubMed] [Google Scholar]
- Meyer K. Variance components due to direct and maternal effects for growth traits of Australian beef cattle. Livest. Prod. Sci. 1992;31:179–204. doi:10.1016/0301-6226(92)90017-X [Google Scholar]
- Moodie G.E.E., McPhail J.D., Hagen D.W. Experimental demonstration of selective predation on Gasteroteus aculeatus. Behaviour. 1972;47:95–105. doi:10.1163/156853973X00292 [Google Scholar]
- Nussey D.H., Wilson A.J., Brommer J.E. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 2007;20:831–844. doi: 10.1111/j.1420-9101.2007.01300.x. doi:10.1111/j.1420-9101.2007.01300.x [DOI] [PubMed] [Google Scholar]
- Phillips P.C., Arnold S.J. Hierarchical comparison of genetic variance–covariance matrices. I. Using the Flury hierarchy. Evolution. 1999;53:1506–1515. doi: 10.1111/j.1558-5646.1999.tb05414.x. doi:10.2307/2640896 [DOI] [PubMed] [Google Scholar]
- Price T., Schluter D. On the low heritability of life-history traits. Evolution. 1991;45:853–861. doi: 10.1111/j.1558-5646.1991.tb04354.x. doi:10.2307/2409693 [DOI] [PubMed] [Google Scholar]
- Pulliam H.R. Advantages of flocking. J. Theor. Biol. 1973;38:419–422. doi: 10.1016/0022-5193(73)90184-7. doi:10.1016/0022-5193(73)90184-7 [DOI] [PubMed] [Google Scholar]
- Reimchen T.E. Spine deficiency and polymorphism in a population of Gasterosteus aculeatus: an adaptation to predators? Can. J. Zool. 1980;58:1232–1244. [Google Scholar]
- Reimchen T.E., Nosil P. Temporal variation in divergent selection on spine number in threespine stickleback. Evolution. 2002;56:2472–2483. doi: 10.1111/j.0014-3820.2002.tb00172.x. doi:10.1554/0014-3820(2002)056[2472:TVIDSO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Relyea R.A. The heritability of inducible defenses in tadpoles. J. Evol. Biol. 2005;18:856–866. doi: 10.1111/j.1420-9101.2005.00882.x. doi:10.1111/j.1420-9101.2005.00882.x [DOI] [PubMed] [Google Scholar]
- Reznick D.N., Endler J.A. The impact of predation on life-history evolution in Trinidadian guppies (Poecilia reticulata) Evolution. 1982;36:160–177. doi: 10.1111/j.1558-5646.1982.tb05021.x. doi:10.2307/2407978 [DOI] [PubMed] [Google Scholar]
- Roff D.A. Chapman & Hall; New York, NY: 1997. Evolutionary quantitative genetics. [Google Scholar]
- Roff D.A. The threshold model as a general purpose normalizing transformation. Heredity. 2001;86:404–411. doi: 10.1046/j.1365-2540.2001.00844.x. doi:10.1046/j.1365-2540.2001.00844.x [DOI] [PubMed] [Google Scholar]
- Réale, D. & Dingemanse, N. J. In press. Personality and individual social specialisation. In Social behaviour: genes, ecology and evolution (eds T. Szekely, A. Moore & J. Komdeur), Cambridge, UK: Cambridge University Press.
- Réale D., Reader S.M., Sol D., McDougall P., Dingemanse N.J. Integrating temperament in ecology and evolutionary biology. Biol. Rev. 2007;82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x. doi:10.1111/j.1469-185X.2007.00010.x [DOI] [PubMed] [Google Scholar]
- Sih A., Kats L.B., Maurer E.F. Behavioural correlations across situations and the evolution of ineffective antipredator behaviour in a sunfish–salamander system. Anim. Behav. 2003;65:29–44. doi:10.1006/anbe.2002.2025 [Google Scholar]
- Sih A., Bell A., Johnson J.C. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. doi:10.1016/j.tree.2004.04.009 [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Life-history tactics: review of ideas. Q. Rev. Biol. 1976;51:3–47. doi: 10.1086/409052. doi:10.1086/409052 [DOI] [PubMed] [Google Scholar]
- Stirling D.G., Réale D., Roff D.A. Selection, structure and the heritability of behaviour. J. Evol. Biol. 2002;15:277–289. doi:10.1046/j.1420-9101.2002.00389.x [Google Scholar]
- Tabachnick B.G., Fidell L.S. Allyn & Bacon; Boston, MA: 2001. Using multivariate statistics. [Google Scholar]
- Walker J.A., Bell M.A. Net evolutionary trajectories of body shape evolution within a microgeographic radiation of threespine sticklebacks (Gasterosteus aculeatus) J. Zool. 2000;252:293–302. doi:10.1111/j.1469-7998.2000.tb00624.x [Google Scholar]
- Whitlock M.C., Phillips P.C., Fowler K. Persistence of changes in the genetic covariance matrix after a bottleneck. Evolution. 2002;56:1968–1975. doi: 10.1111/j.0014-3820.2002.tb00122.x. doi:10.1554/0014-3820(2002)056[1968:POCITG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material text
Electronic supplementary material figure S1