Abstract
Reproductive competition in social insects is generally mediated through specific fertility pheromones. By analysing Dufour's gland secretion in queens and workers of Bombus terrestris under varying social conditions, we demonstrate here that the volatile constituents of the secretion exhibit a context-dependent composition. The secretion of egg-laying queens is composed of a series of aliphatic hydrocarbons (alkanes and alkenes), while that of sterile workers contains in addition octyl esters, dominated by octyl hexadecanoate and octyl oleate. These esters disappear in workers with developed ovaries, whether queenright (QR) or queenless (QL), rendering their secretion queen-like. This constitutes an unusual case in which the sterile caste, rather than the fertile one, possesses extra components. Individually isolated (socially deprived) workers developed ovaries successfully, but failed to oviposit, and still possessed the octyl esters. Thus, whereas social interactions are not needed in order to develop ovaries, they appear essential for oviposition and compositional changes in Dufour's gland secretion (ester disappearance). The apparent link between high ester levels and an inability to lay eggs lends credence to the hypothesis that these esters signal functional sterility. We hypothesize that by producing a sterility-specific secretion, workers signal that ‘I am out of the competition’, and therefore are not attacked, either by the queen or by the reproductive workers. This enables proper colony function and brood care, in particular sexual brood, even under the chaotic conditions of the competition phase.
Keywords: Bombus terrestris, Dufour gland, reproduction, workers' sterility, fertility signals
1. Introduction
In social Hymenoptera, reproduction is skewed in favour of the queen or the top-ranked workers, while the majority of workers are sterile or functionally sterile. This is best explained by the inclusive fitness theory coupled with the haplodiploid sex determination, which results in relatedness asymmetry between colony members (Hamilton 1964, 1972; Trivers & Hare 1976). Consequently, while the production of gynes that are queen born is generally beneficial to both the queen and the workers, male production provides differential genetic gain. The queen gains most by producing sons over grandsons (worker sons) under all circumstances, whereas workers' greater gain from producing sons over brothers (queen sons) is conditional, and depends on the colony total reproductive output. In monogyne societies with a singly inseminated queen, workers are more related to sons (r=0.5) than nephews (r=0.375) or brothers (r=0.25) and, therefore, benefit most by producing sons; and are selected not only to unite in competition with the queen over male production, but also to compete among each other for access to reproduction (Bourke & Franks 1995; Crozier & Pamilo 1996; Bourke & Ratnieks 2001). In colonies with multiply inseminated queen, although workers are more related to sons (r=0.5) than brothers (r=0.25), raising the latter is more beneficial than raising nephews (r<0.125), and therefore policing is favourably selected. Effective policing reduces the probability of workers successfully raising sons, which consequently results in worker reproductive self-restraint (Hammond & Keller 2004; Ratnieks et al. 2006). Policing will also be favourably selected in cases in which worker reproduction seriously hampers the total reproductive output of the colony. Reproductive skew in Hymenoptera is assumed to be mediated through pheromones (reviewed in Le Conte & Hefetz 2008). To date, the honeybee Apis mellifera is the only case for which direct inhibition of worker reproduction by the queen mandibular gland primer pheromones has been empirically demonstrated (Butler 1959; Hoover et al. 2003). Fertility signals, on the other hand, have been demonstrated in several social insects to consist of caste-specific cuticular hydrocarbons (Le Conte & Hefetz 2008), the occurrence of which may indirectly affect worker reproduction. In the queenless ant Dinoponera quadriceps, for example, the top-ranking egg-laying worker (gamergate) possesses significantly larger amounts of 9-hentriacontene than the subordinate, sterile workers. This compound is also present on the gamergate's eggs, which presumably protects them from policing (Monnin & Peeters 1997; Peeters et al. 1999). In the carpenter ant Camponotus floridanus, it has further been demonstrated that queen-specific cuticular hydrocarbons are also present on the egg surface, and not only protect them from policing but also regulate worker reproduction by informing the workers of the queen's presence (Endler et al. 2004). Fertility signals are not limited to ants, but have also been demonstrated in Polistes dominulus (Bonavita-Cougourdan et al. 1991), in Bombus hypnorum (Ayasse et al. 1995), and in the honeybee (Dor et al. 2005). In general, fertility signals are considered to reliably convey the reproductive status of the individual and to provide the means to assist other members of the colony to regulate their reproduction so as to maximize their inclusive fitness when direct reproduction is impossible or unrewarding.
Colonies of Bombus terrestris provide an excellent model for testing both reproductive division of labour and the pheromonal means by which such conflicts are mediated. B. terrestris is monogynous and monandrous and, as predicted by theory, workers compete with the queen over male production (Duchateau & Velthuis 1988). Moreover, owing to this species' annual life history, the timing of reproduction is under seasonal constraint, and conflicts are predicted to arise before the end of the season. However, despite the fact that in mature colonies approximately 68 per cent of the workers have developed ovaries, and 38 per cent of the workers lay eggs, genetic analysis of the male progeny revealed that 95 per cent of them are queen descendant (Alaux et al. 2004a,b; Lopez-Vaamonde et al. 2004). Such a low percentage of worker reproductive success is explained by the considerably higher egg-laying rate of the queen, coupled with her greater ability to destroy worker eggs. Since the probability of worker eggs developing to maturity is low, one can predict either intense competition over reproduction among workers and hence high levels of aggression, or reproductive partitioning among workers with an accompanying decline in aggression consequent increase in colony productivity. In both cases, the only possibility for some of the workers is to divert to helping behaviour and by that at least to gain indirect fitness.
During their life cycle, B. terrestris colonies go through two major social phases: the ‘pre-competition phase’ (PCP) in which reproduction is exclusive to the queen and the ‘competition phase’ (CP) whereby workers compete with the queen and among each other over male parenthood, resulting in overt queen–worker as well as worker–worker aggression (Honk & Hogeweg 1981). In the PCP, the colony grows ergonomically, and workers operate in harmony, even after the queen switches to laying haploid, male-destined eggs (the ‘switch point’). It has been further demonstrated that the presence of the queen delays the reproductive development of workers as well as preventing oviposition. The precise cues that trigger the CP are still mostly unknown. Neither changes in colony size nor the switch point affect the time at which workers start to reproduce (Duchateau & Velthuis 1988; Lopez-Vaamonde et al. 2004; Alaux et al. 2005). Previous suggestions that it is a decline in the inhibitory pheromone produced by the queen that elicits worker reproduction (Honk van et al. 1980; Röseler et al. 1981) were refuted by the experiments that showed that queens heading colonies before or after the CP were equally effective in inhibiting reproduction in small groups of workers (Bloch et al. 1996). Recent studies have shown that in young colonies that are headed by an old queen, the CP starts earlier than expected, implying either qualitative changes in the queen's pheromone output, or worker reaction to the queen's senescence (Alaux et al. 2005). The strong correlation between gyne production and the onset of the CP (Duchateau & Velthuis 1988; Cnaani et al. 2000), along with experimental manipulations (Alaux et al. 2004a,b, 2005; Duchateau et al. 2004), indicate that workers postpone competition over male production until they can be certain that diploid larvae are committed to develop into gynes. Finally, the ability of egg-laying workers either in QR or QL colonies to regulate reproductive development in younger workers (Honk & Hogeweg 1981; Bloch & Hefetz 1999b) may imply a partial (if not complete) control in worker decisions to reproduce.
Many of the above studies indicate that pheromones are involved in queen–worker conflict in B. terrestris. Early reports suggesting that the queen's mandibular gland inhibits worker ovarian development (Honk van et al. 1980) have been refuted, since neither the glandular extracts nor their synthetic 3-hydroxy fatty acids' major constituents (Hefetz et al. 1996) were active, although cuticular washes had some inhibitory effects (Bloch & Hefetz 1999a). Dufour's gland may also be a source of pheromones that mediate reproductive competition. In the polygyne ant Leptothorax gredleri (Heinze et al. 1998) and the queenless ponerine species Dinoponera quadriceps (Monnin et al. 2002), queens/gamergates are occasionally engaged in dominance hierarchy contests. During such contests, the dominant queen/gamergate marks the challenging individual with Dufour's gland secretion, which elicits attacks by other workers that neutralize the pretender reproductive. In the honeybee Dufour's gland produces a caste-specific pheromone that appears to signal fertility (Katzav-Gozansky et al. 1997, 2004; Dor et al. 2005) as well as to trigger aggression during worker–worker reproductive competition (Malka et al. 2008). We therefore analysed the volatile constituents of Dufour's gland secretion in B. terrestris in an attempt to unravel its function as well as to explore how it may relate to queen–worker and worker–worker conflicts. Here, we present our results of the analysis of the chemical composition of the glandular secretion of queens and workers under different social conditions, i.e. in PCP, CP and QL colonies. Workers were also investigated at different ages in order to evaluate the influence of age on reproductive development and pheromone production.
2. Material and methods
(a) Bees
Colonies of B. terrestris were obtained from the Yad Mordechai apiary, Israel, usually 3–5 days after the first worker had emerged. They were maintained in the laboratory in nest-boxes (23×23×10 cm) at a temperature between 28 and 30°C and 60–70% humidity, and supplied ad libitum with a sugar solution (Bee-Happy) and fresh pollen collected from honeybee colonies. Colony development was followed daily by direct observations. The onset of competition was determined when at least one out of the following three events was observed: (i) the same egg cell was open for two consecutive days, (ii) two or more egg cells were open simultaneously, and (iii) a worker was seen to oviposit (Duchateau & Velthuis 1989; Bloch et al. 1996). Newly emerged workers were collected daily, marked individually and returned to their original colony for the entire development period of the colonies. At the end of the experiment, the colonies were killed by freezing at −20°C and kept frozen until worker dissections. Colony age was determined as days after first worker emergence. Worker age was calculated from emergence to adult.
Six queenright (QR) colonies (non-manipulated colonies with a queen) in the PCP were killed at the age of 18–19 days, before the first signs of competition were detected. Six QR colonies after the CP were killed at the age of 30–38 days, 2–6 days after the competition had started. Five queenless (QL) colonies (colonies without a queen) were established by daily introductions of two to five callow workers in order to simulate normal colony development. These colonies were killed after 20 days. Age distribution and the number of workers in each colony are shown in table 1. Age was normally distributed in 16 out of 17 colonies (Kolmogorov–Smirnov test for normality, p<0.05), except in colony 7CP (p>0.05); therefore, a non-parametric test was used to explore the differences in age distribution between the colonies. There were no significant differences in age distributions between the 17 colonies (table 1).
Table 1.
Age distribution in colonies 1–17. (Worker age was determined from adult emergence to colony freezing. Colony name reflects its social structure: PCP, pre-competition phase; CP, competition phase; QL, queenless colonies. All colonies except 7CP were normally distributed. There were no significant differences in age distributions between the 17 colonies, either considering the means (Kruskal–Wallis ANOVA test: H (16, n=783)=17.48, p=0.35) or the medians of the 17 colonies (Χ162=10.11027, p=0.86).)
| hive no. & social condition | colony's age at freezing | no. of workers | mean age ±s.e. | median | median range (25–75%) |
|---|---|---|---|---|---|
| PCP1 | 19 | 15 | 8.86±1.70 | 6 | 4–19 |
| PCP2 | 19 | 13 | 7.76±1.96 | 5 | 1–13 |
| PCP3 | 19 | 22 | 10.45±1.07 | 11 | 7–13 |
| PCP4 | 18 | 22 | 8.86±1.21 | 6 | 4–13 |
| PCP5 | 18 | 30 | 8.76±1.14 | 8 | 4–14 |
| PCP6 | 18 | 16 | 11.31±1.38 | 12 | 6–16 |
| CP7 | 38 | 93 | 12.67±1.00 | 11 | 5–19 |
| CP8 | 38 | 76 | 13.27±1.08 | 11 | 5–20 |
| CP9 | 30 | 35 | 12.74±1.54 | 12 | 5–17 |
| CP10 | 34 | 110 | 12.07±0.91 | 10 | 4–16 |
| CP11 | 34 | 73 | 11.95±1.02 | 12 | 4–16 |
| CP12 | 34 | 73 | 12.95±1.04 | 11 | 6–18 |
| QL13 | 20 | 37 | 10.59±1.00 | 10 | 6–16 |
| QL14 | 20 | 63 | 9.39±0.73 | 10 | 4–14 |
| QL15 | 20 | 55 | 11.07±0.79 | 11 | 6–16 |
| QL16 | 20 | 23 | 11.26±1.26 | 11 | 7–17 |
| QL17 | 20 | 27 | 10.51±1.15 | 11 | 5–16 |
Isolated workers (n=104) were kept in 14 cm Petri dishes for 1–20 days from the first day of emergence. Fertilized queens (n=13) were sampled from colonies in the PCP (n=6) or in the CP (n=7). Virgin queens (n=53) were obtained from laboratory-reared colonies.
(b) Bee dissections
The bees were dissected under a stereo microscope in double-distilled water, and the length of the terminal oocyte in each of the eight ovarioles was measured with a scaled ocular. Mean oocyte length for each bee was used as an index for ovarian development. Sterile workers were defined as workers with average terminal oocyte under 1 mm. Laying workers were identified either by direct in-nest observation of laying behaviour or by the presence of yellow bodies in their ovaries upon dissection. Egg laying by isolated bees was detected by scanning their Petri dishes for the presence of eggs at the end of the experiment. During the dissection, Dufour's glands were cleanly separated from the sting apparatus and extracted in 50 μl pentane containing 1 μg eicosane as internal standard. The samples were kept frozen until analysis.
(c) Chemical analyses
Chemical analyses of glandular extracts were performed by gas chromatography/mass spectrometry (GC/MS) (Hillbur et al. 2005). Compound separation was achieved on a DB-1 fused silica capillary column (30 m×0.25 mm ID) under temperature programme from 150 to 300°C at 5°C min−1. Compounds were identified by their mass spectra as compared with synthetic compounds and data reported in the literature (McLafferty & Stauffer 1989). Double-bond positions in unsaturated compounds were determined by an investigation of mass spectra of their adducts with dimethyl disulphide (Francis & Veland 1981; Buser et al. 1983). Structures of esters were assigned according to Francke et al. (2000). Quantitative analyses were performed using GC by peak integration compared with the internal standard (eicosane) under the same chromatographic conditions. Syntheses of esters from commercially available (Aldrich) carboxylic acids and alcohols followed laboratory standards.
(d) Statistics
Statistical analyses were performed using Statistica for windows, v. 8.0. Age distribution in each colony was tested for normality using the Kolmogorov–Smirnov test for normality. Differences in age distribution between colonies were tested using the Kruskal–Wallis test. Ovarian development and the amounts of volatiles in Dufour's gland of queens and workers were compared using one-way ANOVA followed by Tukey-type post hoc test. Nested ANOVA was used to analyse the effect of social conditions on workers' ovarian development, Dufour gland's total secretion and the proportion of esters. Simple linear regression was used to predict ovarian development, total secretion and the proportion of esters as a function of age. The data used for the nested ANOVA were the residuals of the regression lines in order to extract the influence of age. Transformation of arcsin p0.5 was made for the proportion of esters before the parametric analysis. Correlations were performed by using Pearson correlation. Statistical significance was accepted at α=0.05. Data are presented as means±s.e.
3. Results
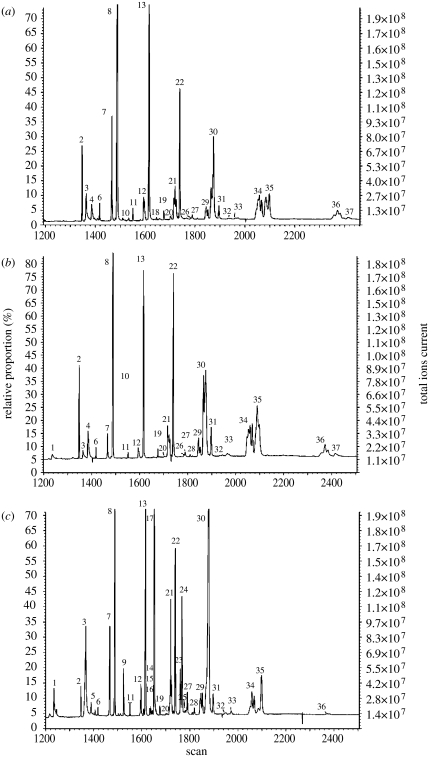
The chemical composition of the glandular secretion of queens and workers not only showed caste specificity, but also exhibited plasticity that was linked to the reproductive status of the bees (figure 1a–c, table 2). The queen secretion, either from PCP or CP colonies, was fortified by a series of saturated and unsaturated hydrocarbons. At the lower molecular weight range (i.e. from heneicosane to heptacosane), alkanes were major components. At the upper molecular weight (nonacosane to tritriacontane), however, alkenes and, to a certain extent, alkadienes were prominent, while alkanes were present either in small amounts or not at all. The secretion of sterile workers differed qualitatively from that of queens in having a series of octyl esters, the acyl part of which ranged from dodecanoic to octadecanoic acid, with octyl hexadecanoate and octyl oleate as major esters. Workers with developed ovaries (average terminal oocyte larger than 1 mm), on the other hand, contained undetectable amounts of esters and their secretion became more queen-like.
Figure 1.
(a–c) Gas chromatograms of Dufour's gland extracts of (a) egg-laying queens, (b) workers with developed ovaries (average terminal oocyte size larger 1 mm), and (c) sterile workers (average terminal oocyte smaller than 1 mm). Peak numbers correspond to the compounds listed in table 2.
Table 2.
Composition of Dufour's gland secretion (mean abundance of each compound per total secretion ±s.e.) of inseminated actively nesting queens, virgin queens, sterile workers (average terminal oocyte smaller than 1 mm) and workers with developed ovaries (average terminal oocyte larger than 1 mm). Workers were taken from 17 colonies under three different social conditions as specified in table 1. ‘ND’ represents compounds that were not quantified and ‘t’ represents trace amount (see also Hefetz et al. 1996).
| queens | workers | ||||
|---|---|---|---|---|---|
| peak no. | compound | egg laying n=13 | virgin n=53 | sterile n=433 | fertile n=291 |
| 1 | hexadecanoic acid | ND | ND | ND | ND |
| 2 | heneicosane | 1.77±0.64 | 3.11±0.44 | 1.24±0.14 | 0.93±0.08 |
| 3 | oleic acid | ND | ND | ND | ND |
| 4 | geranylcitronellol | ND | ND | ND | ND |
| 5 | octyl dodecanoate | 0 | 0 | 0.05±0.01 | <0.01 |
| 6 | docosane | 0.5±0.11 | 0.31±0.11 | 1.01±0.13 | 0.57±0.1 |
| 7 | tricosene (five isomers) | 3.91±0.98 | 0.35±0.07 | 1.14±0.06 | 0.98±0.07 |
| 8 | tricosane | 29.95±4.01 | 17.05±1.1 | 8.85±0.34 | 13.13±0.51 |
| 9 | octyl tetradecanoate | 0 | 0 | 0.58±0.06 | 0.01±0.002 |
| 10 | 9-tetracosene | t | t | t | t |
| 11 | tetracosane | 1.1±0.22 | 0.21±0.02 | 0.71±0.06 | 0.83±0.11 |
| 12 | pentacosene (seven isomers) | 2.23±0.24 | 1.49±0.07 | 1.27±0.05 | 1.12±0.03 |
| 13 | pentacosane | 19.9±2.86 | 5.67±0.26 | 9.82±0.36 | 17.65±0.49 |
| 14–16 | octyl hexadecenoate (three isomers) | 0 | 0 | 0.57±0.05 | <0.01 |
| 17 | octyl hexadecanoate | 0 | 0 | 5.77±0.44 | 0.05±0.005 |
| 18 | hexacosene (several isomers) | t | t | t | t |
| 19 | hexacosane | 0.58±0.07 | 0.2±0.02 | 0.54±0.03 | 0.75±0.03 |
| 20 | heptacosadiene (two isomers) | 0.21±0.07 | 0.26±0.01 | 0.28±0.02 | 0.08±0.009 |
| 21 | heptacosene (seven isomers) | 4.59±0.84 | 23.3±0.87 | 5.77±0.14 | 4.39±0.09 |
| 22 | heptacosane | 8.85±1.17 | 5.03±0.55 | 9.3±0.28 | 14.44±0.39 |
| 23–24 | octyl linoleate + octyl oleate | 0 | 0 | 7.6±0.47 | <0.01 |
| 25 | octyl octadecanoate | 0 | 0 | 0.63±0.25 | 0.03±0.007 |
| 26 | octacosadiene (several isomers) | t | t | t | t |
| 27 | octacosene (several isomers) | t | t | 1.55±0.26 | 0.99±0.18 |
| 28 | octacosane | 0.13±0.06 | 0.08±0.01 | 0.23±0.01 | 0.25±0.01 |
| 29 | nonacosadiene (three isomers) | 2.09±0.46 | 2.7±0.15 | 3.59±0.11 | 2.78±0.09 |
| 30 | nonacosene (eight isomers) | 13.8±3.48 | 35.11±1.24 | 25.54±0.5 | 18.97±0.49 |
| 31 | nonacosane | 1.13±0.22 | 1.26±0.19 | 2.28±0.08 | 2.65±0.09 |
| 32 | triacontadienes (two isomers) | 0.15±0.03 | 0.11±0.01 | 0.66±0.06 | 0.28±0.01 |
| 33 | triacontene (seven isomers) | 0.14±0.05 | 0.33±0.02 | 0.51±0.01 | 0.63±0.01 |
| 34 | hentriacontadiene (four isomers) | 2.79±0.42 | 0.3±0.04 | 3.54±0.19 | 7.3±0.39 |
| 35 | hentriacontene (three isomers) | 5.53±1.11 | 2.99±0.32 | 6.63±0.22 | 10.84±0.3 |
| 36 | tritriacontadiene (several isomers) | ND | ND | ND | ND |
| 37 | tritriacontene | ND | ND | ND | ND |
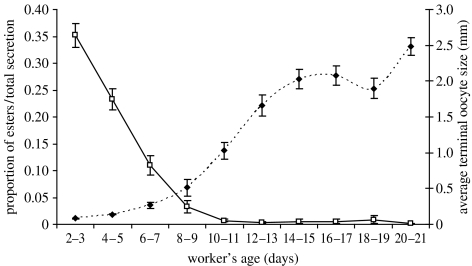
Under our experimental conditions, worker age was strongly correlated with ovarian development (Pearson correlation: n=780, r=0.65, p<0.05) and the proportion of esters per total secretion in Dufour's gland (Pearson correlation: n=727, r=−0.49, p<0.05) (figure 2). We therefore controlled for worker age in both variables as follows: two regression lines were built to predict the ovarian development and the proportion of esters in Dufour's gland using age. We then used the residuals of the regression lines as the dependent variables in the statistical analysis. By using this procedure, we controlled for the influence of age on the variables, thus ensuring that the differences were due to the effect of the social condition rather than age. We used the same procedure with Dufour gland's total secretion, although the correlation was weaker (Pearson correlation: n=727, r=0.28, p<0.05).
Figure 2.
Ovarian development and the proportion of esters in Dufour's gland secretion in workers of different age groups ±s.e. The data presented here include workers from colonies 1–17. The number of workers analysed in each age group is: 2–3 days, 91/100 (esters/ovaries, respectively) workers; 4–5 days, 95/101; 6–7 days, 60/64; 8–9 days, 47/48; 10–11 days, 79/84; 12–13 days, 54/64; 14–15 days, 63/67; 16–17 days, 46/48; 18–19 days, 59/60; 20–21 days, 28/31 (squares, proportion of esters/total secretion; diamonds, average terminal oocyte size).
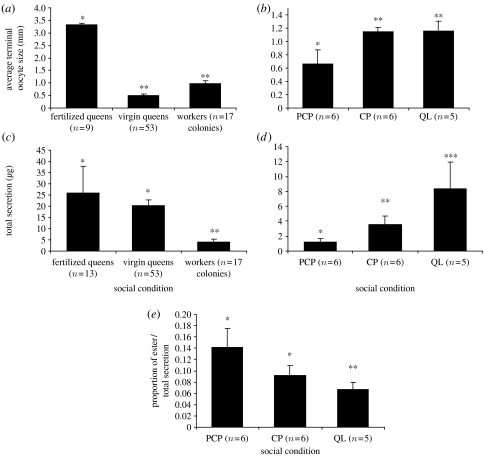
The mean oocyte size of actively laying queens was significantly higher than that of either virgin queens or workers taken from different social conditions (one-way ANOVA, f2,76=167.22, p<0.001 followed by Tukey-type post hoc multiple comparisons p<0.001) (figure 3a). Social conditions had a significant effect on the degree of ovarian development in workers. Among the various worker types, only PCP workers had significantly lower ovarian development (nested ANOVA, hive (social condition): f14,763=2.8, p<0.001; social condition f2,763=7.96, p<0.001 followed by Tukey-type post hoc multiple comparisons p<0.01) (figure 3b). There were also differences in the percentage of workers with developed ovaries (mean terminal oocyte greater than 1 mm) between treatments. While among the CP and QL workers, 41.8 and 41.9 per cent had developed ovaries, respectively; only 29.6 per cent of the PCP workers had developed ovaries. Oviposition was also affected by the social structure of the colonies. The youngest worker to oviposit among the QL workers was 9 days old, compared with 25 days old among the CP workers. None of the PCP workers was observed ovipositing, and none had yellow bodies in their ovaries (indicative of egg laying).
Figure 3.
Ovarian development, total secretion of Dufour's gland and the proportion of esters in queens and workers under different social conditions. (a) Average terminal oocyte size in queens (virgin or inseminated) and workers. (b) Average terminal oocyte size in workers sampled from colonies under different social conditions: those in the PCP, those in the CP and those without a queen. (c) Total secretion of Dufour's gland in queens and workers. (d) Total secretion of Dufour's gland in workers under the above social conditions. (e) The proportion of esters (per total secretion) in Dufour's gland in workers under the above social conditions. The data are presented as mean ±s.e. Different asterisks above columns denote statistical differences at p<0.05.
The total amount of Dufour's gland secretion was also affected by caste and social condition. Glands of queens, whether fertilized or virgin, contained significantly more copious secretion than glands of workers, irrespective of their social condition or reproductive status (one-way ANOVA, f2,80=4.19, p=0.018 followed by Tukey-type post hoc multiple comparisons p<0.05) (figure 3c). Although there were no quantitative differences between the total secretion of virgin and fertilized queens, there were some qualitative differences: virgin queens contained relatively large amounts of heptacosenes and nonacosenes whereas the volatiles of actively nesting queens were dominated by tricosane and pentacosane. This matched the differences in ovarian development between these two queen groups (virgin queens had undeveloped ovaries). Among workers, significant differences were found between the three different social conditions. The total secretion in Dufour's gland in the QL workers was the highest, whereas the PCP workers had the lowest amount of compounds in their Dufour's gland, in accordance with their low ovarian development (nested ANOVA, hive (social condition): f14,712=13.94, p<0.001, social condition: f2,712=37.87, p<0.001 followed by Tukey-type post hoc multiple comparisons p<0.05) (figure 3d).
The occurrence of esters in worker Dufour's gland secretions appears to be affected by multiple factors, including age, ovarian development and social status. The proportion of esters was found to be age dependent (figure 2), with their amount gradually decreasing in young workers as they aged from 1 to 7 days. However, esters could also be present in Dufour's gland of older workers (with undeveloped ovaries) or absent in young workers that had developed ovaries. With respect to ovarian development, esters were present in bees with average terminal oocyte smaller than 1 mm and disappeared in bees possessing average terminal oocyte size above 1 mm. Among the bees with undeveloped ovaries, the QL bees had a significantly lower proportion of esters compared with either the PCP or the CP workers, with the latter two not being different. (Nested ANOVA, hive (social condition): f14,711=2.89, p<0.01; social condition f2,711=4.13, p=0.016 followed by Tukey-type post hoc multiple comparisons p<0.05) (figure 3e).
Isolated bees constituted an exceptional case. Their ovaries developed with time in a similar way to the QL workers (1.08±0.08 mm, n=104), but they had a significantly higher ester proportion than any of the other groups (on average 0.41±0.02 esters/total secretion, n=104). The higher proportion of esters may have been partially due to the age distribution in the isolated bees, which was skewed towards young workers compared with the colonies in this study (57% of the isolated workers were under the age of 7 days compared with 41.2±2.57%, on average, in the other 17 colonies; mean age of the isolated bees was 9.09±0.47, median: 7 days. See table 1 for means and medians of workers from colonies). However, worker age in itself cannot explain the large differences in ester occurrence. For example, isolated bees older than 14 days had developed ovaries (2.03±0.12 mm in average, n=22), but they nonetheless still possessed esters amounting to 16.2±0.02% of the total secretion. Interestingly, none of these workers (or the other isolated workers) had laid eggs even by the age of 20 days, whereas a typical QL worker lays eggs within 7 days after becoming QL. Moreover, isolated workers showed higher amounts of secretion (on average 7.87±0.59 μg) compared with either QL or PCP, or CP workers.
4. Discussion
The bumble-bee B. terrestris provides a good model for studying both the ultimate and the proximate mechanisms underlying the transition from sterility to fertility in workers. However, despite the wealth of behavioural studies pertaining to the behaviours involved, data pertaining to the role of pheromones in this process remain scanty. Unravelling the pheromones involved may also shed light on the controversy over whether pheromones play a coercive role in inhibiting worker reproduction, or act as fertility signals that advertise the quality of the reproducing individuals and, thus, allow workers to respond with the appropriate reproductive decision that will impart maximum inclusive fitness (Keller & Nonacs 1993). The aim of the present study was to explore whether Dufour's gland secretion might include such a regulatory pheromone. We thus deciphered the composition of the secretion and searched for differences among queens and workers in correlation with their reproductive status. Measurement of ovarian development of queens and workers under different social conditions largely confirmed previous reports, in particular with respect to the fact that PCP workers had significantly lower ovarian development than QL or CP workers (Duchateau & Velthuis 1989). Interestingly, socially isolated workers also developed ovaries but did not lay eggs, even after three weeks. This is in contrast to the honeybee, in which isolated workers fail to develop ovaries (Dor et al. 2005).
Chemical analyses of Dufour's gland secretions of the above queens and workers showed both caste specificity and reproductive-dependent plasticity: while glands of queens and workers with developed ovaries had very similar compositions, glands of sterile workers, either from QL or QR colonies, possessed a series of octyl esters that were correlated with both their age and reproductive condition. Sterile workers under all the social conditions contained varying amounts of the octyl esters, which disappeared when workers started to develop ovaries. Socially isolated workers were exceptional in that they possessed considerable amounts of esters even at an older age, and although these amounts declined when the workers started to develop ovaries, they did not disappear entirely, unlike the case of socially interacting workers. This indicates that lack of social interactions does not affect ovarian development but does affect the chemical composition of Dufour's gland secretions and the ability to lay eggs.
The exact role of Dufour's gland secretion in B. terrestris queen–worker and worker–worker conflicts over reproduction is still unknown, but the correlation of its composition with reproductive status is highly supportive of such a role. In this respect, the B. terrestris case seems to be unique among social insects. In all cases studied so far, fertility signals have been characterized in containing supplemental compounds, which are found in reproductive individuals but are absent in sterile workers (Hefetz 2007; Le Conte & Hefetz 2008): for example, the cuticular hydrocarbons 3, 11-dimethylheptacosane in Pachycondyla inverse (Heinze et al. 2002; D'Ettorre et al. 2004) and 9-pentacosene and 3-methylpentacosane in Myrmecia gulosa (Dietemann et al. 2003) in ants, and the wax-type esters in the honeybee Dufour's gland. (Katzav-Gozansky et al. 1997). In contrast to all these results, in B. terrestris sterile workers are the ones that exhibit extra components, the octyl esters, which are characteristic of these workers but are absent in fertile individuals, queens or workers. Notwithstanding, it should be noted that both queens and workers with developed ovaries possess small amounts of a terpene that, according to its mass spectrum (Bergström & Svensson 1973), has been tentatively identified as geranylcitronellol. However, because of its low quantities its identification in the bees was erratic and could not be properly quantified, and its enantiomeric composition remained unknown. Interestingly, this diterpene is a main component in the cephalic secretion that males of B. lapponicus use during odour marking (Bergström & Svensson 1973).
In general, although sterile workers can behaviourally manipulate reproductive competition (by policing for example), they are assumed to play a passive role in the field of chemical signals, which are typically used by the fertile or dominant individuals. Here, we show for the first time that sterile workers too actively advertise their reproductive status by producing exclusive compounds. We further postulate that by advertising their sterility, such workers are also players in the race for reproduction. The gain from advertising sterility, in contrast to fertility advertisement, is not immediately obvious, and, therefore, necessitates further explanation of how it confers advantage to the signalling bees. We postulate that in the cases in which workers may have an equivalent reproductive potential, as in the case of B. terrestris, correct assessment of every individual's reproductive status is crucial. While fertility signals may regulate (indirectly) ovarian development in nest-mates, as well as attract helpers, they may also be costly, because the signalling bee becomes the focus of aggression both by the queen and by competing workers. This seems to be the case in the honeybee, where workers with developed ovaries are recognized as such through their specific Dufour's gland composition, and, therefore, are more exposed to aggression (Visscher & Dukas 1995; Malka et al. 2008). Advertising sterility, by contrast, may protect the non-reproductive workers from attack and harassment, and thus enhance social harmony by maintaining some division of tasks in the seemingly chaotic CP. Such task partitioning facilitates brood care (mostly gynes and males at this stage of colony development). Such a positive effect on a colony's reproductive output provides inclusive fitness for the sterile workers and, therefore, can be selected at the colony level. The finding that QL and CP workers had lower amounts of esters compared with PCP workers of the same age supports this hypothesis and reinforces the connection between the signal and reproductive status. Under PCP conditions, an unrestricted outbreak of aggression during the CP may hamper colony gyne production and, therefore, maintaining the reproductive division of labour among workers is important. Consequently, non-reproductive workers are predicted to invest most effort in survival and brood care rather than in reproduction, and hence advertising sterility confers an advantage to both the reproductive and the non-reproductive workers. Under QL or CP conditions, on the other hand, a higher fraction of workers enters the reproductive competition and, as a result, advertising fertility and dominance confers an advantage to the signaller. Moreover, the proportion of esters under the CP condition, although not significantly differing from the proportion of esters under the QL condition, did show a decrease, which may indicate the gradual change in CP workers from harmonious PCP workers to competitive QL workers.
The results obtained with isolated workers shed additional light on the correlation between the ovarian development and the octyl esters biosynthesis. Although these workers developed ovaries, none laid eggs, and all had relatively high amounts of octyl esters (one-to-one correlation, n=104). These findings suggest that social interactions play an important role in both ester disappearance and oviposition ability, and may indicate that these two traits are linked. Moreover, the greater amounts of total secretion in these workers indicate that in the absence of social interactions, the glandular exudate is not used and thus accumulates to maximum in the gland. The nature of such a link may lie in a common regulatory system; and if oviposition is correlated to the presence of the octyl esters, the latter may provide an uncheatable measure (‘index’ sensu Maynard Smith & Harper 2003) of functional sterility. To test this hypothesis, it is necessary to manipulate experimentally either ovarian development/oviposition or glandular expression and to examine whether the two are mutually and irreversibly affected. Such a study was performed recently in A. mellifera (Malka et al. 2008), but awaits further experimentation in B. terrestris.
The lack of esters in virgin queens seems at first to negate the sterility-signalling hypothesis, because virgin queens are not attacked by their sister workers despite not having the octyl esters. One possible explanation is that queens are recognized as such by additional means (size, additional queen-specific pheromones) and since they are of high value to workers they are not attacked. Lending credence to this hypothesis is the quantitative differences found in glandular composition between virgin gynes and actively laying queens, which enable workers to discriminate between gynes and the actively laying mother queen, with which they compete for male production. We further postulate that queens had already lost their capacity to produce the specific octyl esters at the larval stage during caste determination. This is in accordance with the hypothesis that in the B. terrestris queen caste, this is the normal course of development (similar to solitary bees) whereas workers have a derived shorter developmental course (Cnaani & Hefetz 2001). Accordingly, workers but not queens were selected to facultatively express Dufour's octyl esters.
The question of whether pheromones play a coercive role in inhibiting worker reproduction, or act as fertility signals that advertise the quality of the reproducing individuals remains unsolved. Our data show that workers can produce esters to advertise their reproductive status and that the ensemble of these compounds decreases when the potential to reproduce arises. These facts support the active role of workers in restraining their own reproduction. Moreover, we were unable to detect any differences in Dufour's glands of queens under PCP conditions compared with CP conditions. The accumulation of the secretion, in particular its ester components, in the isolated, socially deprived workers provides further support for the use of the glandular exudates in social interactions. Notwithstanding, as it is still unclear as to which is the main recipient of the signal, queen or other workers, we cannot preclude the possibility of other queen-produced compounds in other glands that may inhibit worker reproduction. This must await direct experiments using natural secretions as well as the synthetic esters.
Although advertising sterility or fertility are two sides of the same coin, it is intriguing (but may not be unusual) that B. terrestris workers actively invest in producing sterility-specific compounds. We suggest that in primitively social, small-size colonies that exhibit high aggression towards workers with developed ovaries (Van Doorn & Heringa 1986; Duchateau 1989; Röseler & Honk van 1990), unambiguous signalling of ‘I am out of the reproductive race’ confers a high advantage to all partners, as well as minimizing errors in assessment. Dual signalling may thus have evolved with respect to reproductive competition. Fertile workers mimic the queen and exert dominance over other workers, but also attract aggression from the queen and competing workers; whereas sterile workers emit a pacifying signal that enables them to carry out their nest duties unmolested.
Acknowledgments
Part of this research was supported by a grant from The Israel Science Foundation founded by the Israel Academy of Sciences (ISF grant no. 535/08). We thank Naomi Paz for editorial assistance and Gaby Nudelman for the statistical advice.
References
- Alaux C., Jaisson P., Hefetz A. Queen influence on worker reproduction in bumblebees (Bombus terrestris) colonies. Insect. Soc. 2004a;51:287–293. doi:10.1007/s00040-004-0741-5 [Google Scholar]
- Alaux C., Savarit F., Jaisson P., Hefetz A. Does the queen win it all? Queen–worker conflict over male production in the bumblebee, Bombus terrestris. Naturwissenschaften. 2004b;91:400–403. doi: 10.1007/s00114-004-0547-3. doi:10.1007/s00114-004-0547-3 [DOI] [PubMed] [Google Scholar]
- Alaux C., Jaisson P., Hefetz A. Reproductive decision-making in semelparous colonies of the bumblebee Bombus terrestris. Behav. Ecol. Sociobiol. 2005;59:270–277. doi:10.1007/s00265-005-0035-6 [Google Scholar]
- Ayasse M., Marlovits T., Tengö J., Taghizadeh T., Francke W. Are there pheromonal dominance signals in the bumblebee Bombus hypnorum L. (Hymenoptera, Apidae)? Apidologie. 1995;26:163–180. doi:10.1051/apido:19950301 [Google Scholar]
- Bergström G., Svensson B.G. Studies on natural odoriferous compounds VIII. Characteristic marking secretions of the forms lapponicus and scandinavicus of Bombus lapponicus Fabr. (Hymenoptera, Apidae) Chimica Scripta. 1973;4:231–238. [Google Scholar]
- Bloch G., Borst D.W., Huang Z.Y., Robinson G.E., Hefetz A. Effects of social conditions on juvenile hormone mediated reproductive development in Bombus terrestris workers. Physiol. Entomol. 1996;21:257–267. doi:10.1111/j.1365-3032.1996.tb00863.x [Google Scholar]
- Bloch G., Hefetz A. Reevaluation of the role of mandibular glands in regulation of reproduction in bumblebee colonies. J. Chem. Ecol. 1999a;25:881–896. doi:10.1023/A:1020805103379 [Google Scholar]
- Bloch G., Hefetz A. Regulation of reproduction by dominant workers in bumblebee (Bombus terrestris) queenright colonies. Behav. Ecol. Sociobiol. 1999b;45:125–135. doi:10.1007/s002650050546 [Google Scholar]
- Bonavita-Cougourdan A., Theraulaz G., Bagneres A.-G., Roux M., Pratte M., Provost E., Clement J.L. Cuticular hydrocarbons, social organization and ovarian development in a polistine wasp: Polistes dominulus Christ. Comp. Biochem. Physiol. B. 1991;100:667–680. doi:10.1016/0305-0491(91)90272-F [Google Scholar]
- Bourke A.F.G., Franks N.R. Princeton University Press; Princeton, NJ: 1995. Social evolution in ants. [Google Scholar]
- Bourke A.F.G., Ratnieks F.L.W. Kin-selected conflict in the bumble-bee Bombus terrestris (Hymenoptera: Apidae) Proc. R. Soc. B. 2001;268:347–355. doi: 10.1098/rspb.2000.1381. doi:10.1098/rspb.2000.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser H.R., Arn H., Guerin P., Rauscher S. Determination of double bond position in monounsaturated acetates by mass spectrometry of dimethyldisulfide adducts. Annal. Chem. 1983;55:818–822. doi:10.1021/ac00257a003 [Google Scholar]
- Butler C.G. The source of the substance produced by a queen honeybee (Apis mellifera) which inhibits development of the ovaries of the workers of her colony. Proc. R. Soc. B. 1959;34:137–138. [Google Scholar]
- Cnaani J., Robinson G.E., Bloch G., Borst D., Hefetz A. The effect of queen–worker conflict on caste determination in the bumblebee Bombus terrestris. Behav. Ecol. Sociobiol. 2000;47:346–352. doi:10.1007/s002650050675 [Google Scholar]
- Cnaani J., Hefetz A. Are queen Bombus terrestris giant workers or are workers dwarf queens? Solving the ‘chicken and egg’ problem in a bumblebee species. Naturwissenschaften. 2001;88:85–87. doi: 10.1007/s001140000202. doi:10.1007/s001140000202 [DOI] [PubMed] [Google Scholar]
- Crozier, R. H. & Pamilo, P. 1996 Evolution of social insect colonies: sex allocation and kin selection Oxford Series in Ecology and Evolution. Oxford, UK: Oxford University Press.
- D'Ettorre P., Heinze E., Schulz C., Francke W., Ayasse M. Does she smell like a queen? chemoreception of a cuticular hydrocarbon signal in the ant Pachycondyla inversa. J. Exp. Biol. 2004;207:1085–1091. doi: 10.1242/jeb.00865. doi:10.1242/jeb.00865 [DOI] [PubMed] [Google Scholar]
- Dietemann V., Peeters C., Liebig J., Thivet V., Hölldobler B. Cuticular hydrocarbons mediate discrimination of reproductives and nonreproductives in the ant Myrmecia gulosa. Proc. Natl Acad. Sci. USA. 2003;100:10 341–10 346. doi: 10.1073/pnas.1834281100. doi:10.1073/pnas.1834281100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor R., Katzav-Gozansky T., Hefetz A. Dufour's gland pheromone as a reliable fertility signal among honeybee (Apis mellifera) workers. Behav. Ecol. Sociobiol. 2005;58:270–276. doi:10.1007/s00265-005-0923-9 [Google Scholar]
- Duchateau M.J. Agonistic behaviors in colonies of the bumblebee Bombus terrestris. J. Ethol. 1989;7:141–152. doi:10.1007/BF02350036 [Google Scholar]
- Duchateau M.J., Velthuis H.H.W. Development and reproductive strategies in Bombus terrestris colonies. Behaviour. 1988;107:186–207. doi:10.1163/156853988X00340 [Google Scholar]
- Duchateau M.J., Velthuis H.H.W. Ovarian development and egg laying in workers of Bombus terrestris. Entomol. Exp. Appl. 1989;51:199–213. doi:10.1007/BF00347883 [Google Scholar]
- Duchateau M.J., Velthuis H.H.W., Boomsma J.J. Sex ratio variation in the bumblebee Bombus terrestris. Behav. Ecol. 2004;15:71–82. doi:10.1093/beheco/arg087 [Google Scholar]
- Endler A., Liebig J., Schmitt T., Parker J.E., Jones G.R., Schreier P., Holldobler B. Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc. Natl Acad. Sci. USA. 2004;101:2945–2950. doi: 10.1073/pnas.0308447101. doi:10.1073/pnas.0308447101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G.W., Veland K. Alkylthiolation for the determination of double bond position in linear alkenes. J. Chromatogr. 1981;219:379–384. doi:10.1016/S0021-9673(00)80381-7 [Google Scholar]
- Francke W., et al. Identification of oxygen containing volatiles in cephalic secretions of workers of Brazilian stingless bees. J. Braz. Chem. Soc. 2000;11:562–571. doi:10.1590/S0103-50532000000600003 [Google Scholar]
- Hamilton W.D. The genetical evolution of social behaviour. I, II. J. Theor. Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. doi:10.1016/0022-5193(64)90038-4 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D. Altruism and related phenomena mainly in the social insects. Annu. Rev. Ecol. Syst. 1972;2:193–232. doi:10.1146/annurev.es.03.110172.001205 [Google Scholar]
- Hammond R.L., Keller L. Conflict over male parentage in social insects. PLos Biol. 2004;2:1472–1482. doi: 10.1371/journal.pbio.0020248. doi:10.1371/journal.pbio.0020248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefetz A. The evolution of hydrocarbon pheromone parsimony in ants (Hymenoptera: Formicidae)—interplay of colony odor uniformity and odor idiosyncrasy. A review. Myrmecol. News. 2007;10:59–68. [Google Scholar]
- Hefetz A., Taghizadeh T., Francke W. The exocrinology of the queen bumble bee Bombus terrestris (Hymenoptera: Apidae, Bombini) Z. Naturforsch. 1996;51:409–422. [Google Scholar]
- Heinze J., Oberstadt B., Tentschert J., Hölldobler B., Bestmann H.J. Colony specificity of Dufour gland secretions in a functionally monogynous ant. Chemoecology. 1998;8:169–174. doi:10.1007/s000490050022 [Google Scholar]
- Heinze J., Stengl B., Sledge M.F. Worker rank, reproductive status and cuticular hydrocarbon signature in the ant, Pachycondyla cf. inversa. Behav. Ecol. Sociobiol. 2002;52:59–65. doi:10.1007/s00265-002-0491-1 [Google Scholar]
- Hillbur Y., Celander M., Baur R., Rauscher S., Haftmann J., Franke S., Francke W. Identification of the sex pheromone of the swede midge, Contarinia nasturtii. J. Chem. Ecol. 2005;31:1807–1828. doi: 10.1007/s10886-005-5928-3. doi:10.1007/s10886-005-5928-3 [DOI] [PubMed] [Google Scholar]
- Honk C.G.J.v., Hogeweg P. The ontogeny of the social structure in a captive Bombus terrestris colony. Behav. Ecol. Sociobiol. 1981;9:111–119. doi:10.1007/BF00293582 [Google Scholar]
- Honk van C.G.J., Velthuis H.H.W., Roseler P.F., Malotaux M.E. The mandibular glands of Bombus terrestris queens as a source of queen pheromone. Entomol. Exp. Appl. 1980;28:191–198. doi:10.1007/BF00287128 [Google Scholar]
- Hoover S.E.R., Keeling C.I., Winston M.L., Slessor K.N. The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften. 2003;90:477–480. doi: 10.1007/s00114-003-0462-z. doi:10.1007/s00114-003-0462-z [DOI] [PubMed] [Google Scholar]
- Katzav-Gozansky T., Soroker V., Hefetz A., Cojocaru D., Erdmann H., Francke W. Plasticity of caste-specific Dufour's gland secretion in the honey bee (Apis mellifera L.) Naturwissenschaften. 1997;84:238–241. doi:10.1007/s001140050386 [Google Scholar]
- Katzav-Gozansky T., Boulay R., Soroker V., Hefetz A. Queen-signal modulation of worker pheromonal composition in honeybees. Proc. R. Soc. B. 2004;271:2065–2069. doi: 10.1098/rspb.2004.2839. doi:10.1098/rspb.2004.2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L., Nonacs P. The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 1993;45:787–794. doi:10.1006/anbe.1993.1092 [Google Scholar]
- Le Conte Y., Hefetz A. Primer pheromones in social Hymenoptera. Annu. Rev. Entomol. 2008;53:523–542. doi: 10.1146/annurev.ento.52.110405.091434. doi:10.1146/annurev.ento.52.110405.091434 [DOI] [PubMed] [Google Scholar]
- Lopez-Vaamonde C., Koning J.W., Brown R.M., Jordan W.C., Bourke A.F.G. Social parasitism by male-producing reproductive workers in a eusocial insect. Nature. 2004;430:557–560. doi: 10.1038/nature02769. doi:10.1038/nature02769 [DOI] [PubMed] [Google Scholar]
- Malka O., Snior S., Katzav-Gozansky T., Hefetz A. Aggressive reproductive-competition among hopelessly queenless honeybee workers triggered by pheromone signaling. Naturwissenschaften. 2008;95:553–559. doi: 10.1007/s00114-008-0358-z. doi:10.1007/s00114-008-0358-z [DOI] [PubMed] [Google Scholar]
- Maynard Smith J., Harper H. Oxford Series in Ecology and Evolution. Oxford University Press; Oxford, UK: 2003. Animal signals. [Google Scholar]
- McLafferty F.W., Stauffer D.B., editors. Wiley/NBS registry of mass spectral data. Wiley Interscience; New York, NY: 1989. [Google Scholar]
- Monnin T., Peeters C. Cannibalism of subordinates' eggs in the monogynous queenless ant Dinoponera quadriceps. Naturwissenschaften. 1997;84:499–502. doi:10.1007/s001140050433 [Google Scholar]
- Monnin T., Ratnieks F.L.W., Jones G.R., Beard R. Pretender punishment induced by chemical signalling in a queenless ant. Nature. 2002;419:61–65. doi: 10.1038/nature00932. doi:10.1038/nature00932 [DOI] [PubMed] [Google Scholar]
- Peeters C., Monnin T., Malosse C. Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc. R. Soc. B. 1999;266:1323–1327. doi:10.1098/rspb.1999.0782 [Google Scholar]
- Ratnieks F.L.W., Foster K.R., Wenseleers T. Conflict resolution in insect societies. Annu. Rev. Entomol. 2006;51:581–608. doi: 10.1146/annurev.ento.51.110104.151003. doi:10.1146/annurev.ento.51.110104.151003 [DOI] [PubMed] [Google Scholar]
- Röseler P.F., Honk van C.G.J. Caste and reproduction in bumblebees. In: Engels W., editor. Social insects: an evolutionary approach to castes and reproduction. Springer; Berlin, Germany: 1990. pp. 147–166. [Google Scholar]
- Röseler P.F., Röseler I., van Honk C.G.J. Evidence for inhibition of corpora allata in workers of Bombus terrestris by a pheromone from the queen's mandibular gland. Experientia. 1981;37:348–351. doi:10.1007/BF01959856 [Google Scholar]
- Trivers R.L., Hare H. Haplodiploidy and the evolution of the social insects. Science. 1976;191:249–263. doi: 10.1126/science.1108197. doi:10.1126/science.1108197 [DOI] [PubMed] [Google Scholar]
- Van Doorn A., Heringa J. The ontogeny of a dominance hierarchy in colonies of the bumblebee Bombus terrestris (Hymenoptera, Apidae) Insect. Soc. 1986;33:3–25. doi:10.1007/BF02224031 [Google Scholar]
- Visscher P.K., Dukas R. Honey-bees recognize development of nestmates ovaries. Anim. Behav. 1995;49:542–544. doi:10.1006/anbe.1995.0074 [Google Scholar]