Abstract
Heterogeneities within disease hosts suggest that not all individuals have the same probability of transmitting disease or becoming infected. This heterogeneity is thought to be due to dissimilarity in susceptibility and exposure among hosts. As such, it has been proposed that many host–pathogen systems follow the general pattern whereby a small fraction of the population accounts for a large fraction of the pathogen transmission. This disparity in transmission dynamics is often referred to as ‘20/80 Rule’, i.e. approximately 20 per cent of the hosts are responsible for 80 per cent of pathogen transmission. We investigated the role of heterogeneity in contact rates among potential hosts of a directly transmitted pathogen by examining Sin Nombre virus (SNV) in deer mice (Peromyscus maniculatus). Using foraging arenas and powder marking, we documented contacts between wild deer mice in Great Basin Desert, central Utah. Our findings demonstrated heterogeneity among deer mice, both in frequency and in duration of contacts with other deer mice. Contact dynamics appear to follow the general pattern that a minority of the population accounts for a majority of the contacts. We found that 20 per cent of individuals in the population were responsible for roughly 80 per cent of the contacts observed. Larger-bodied individuals appear to be the functional group with the greatest SNV transmission potential. Contrary to our predictions, transmission potential was not influenced by breeding condition or sex.
Keywords: host heterogeneity, Sin Nombre virus, directly transmitted pathogen, deer mice, Peromyscus maniculatus, 20/80 Rule
1. Introduction
The rate of contact between hosts is a fundamental component of models describing the dynamics of host–pathogens systems (McCallum et al. 2001). However, heterogeneities among hosts indicate that not all individuals have the same probability of transmitting disease or becoming infected (Wilson et al. 2001; Lloyd-Smith et al. 2005). This heterogeneity is thought to be due to dissimilarity in susceptibility and exposure among hosts (Woolhouse et al. 1997; Dye & Gay 2003). A study of HIV/AIDS found that more than 90 per cent of virus transmission was derived from the most infectious 20 per cent of the population (Johnson et al. 1994). Similar heterogeneities have been reported for other human diseases such as severe acute respiratory syndrome (SARS), measles, monkeypox and smallpox (Lloyd-Smith et al. 2005). Woolhouse et al. (1997) proposed that many host–pathogen systems followed the 20/80 Rule, which proposes that approximately 20 per cent of the most infectious hosts are responsible for 80 per cent of transmission within a disease system. While this concept is often referred to as the 20/80 Rule, the precise ratios are not as important as the general concept that a minority of the population is responsible for the majority of the transmission in many host–pathogen systems.
Despite the role that host heterogeneity plays in influencing disease dynamics, few studies have addressed this parameter. Studies have examined heterogeneity in vector-borne zoonoses, such as West Nile fever (Kilpatrick et al. 2006) and tick-borne encephalitis (Perkins et al. 2003); however, very little is known about patterns of heterogeneity in directly transmitted pathogens, in part, because of the difficulty in measuring contacts between hosts. There are few techniques available to measure contacts, and those that are available can be costly or potentially interfere with the natural behaviour of the host, particularly for small-bodied species such as rodents (Mikesic & Drickamer 1992; McQuillen & Brewer 2000).
We investigated the role of heterogeneity in contact rates among potential hosts, and hence potential transmission of a directly transmitted zoonotic pathogen by examining Sin Nombre virus (SNV) in deer mice (Peromyscus maniculatus). SNV transmission between rodents occurs primarily through direct contact, most likely during aggressive behaviours such as biting and scratching, as evidenced by the strong correlation between SNV infection and scarring (Mills et al. 1997, 1999; Boone et al. 1998; Calisher et al. 1999, 2007). Males have higher rates of infection than females, most likely because they more frequently engage in aggressive behaviours thought to transmit the virus (Childs et al. 1994; Mills et al. 1997; Douglass et al. 2007). SNV infection in deer mice is chronic and, following infection, deer mice produce virus-specific antibodies for life (Botten et al. 2003). SNV can be transmitted to humans and can develop into hantavirus cardiopulmonary syndrome, a disease with high mortality (Hjelle et al. 1994; Kilpatrick et al. 2004). As such, SNV is a zoonotic agent of concern to human health.
The primary goal of our study was to determine whether contact rates differed among individual deer mice and to determine how host heterogeneities may influence potential SNV transmission. We documented contacts between wild deer mice in Great Basin Desert of central Utah using two distinct methods, one that measures contacts directly and one that approximates contacts between individuals. We examined contacts among deer mice to determine if individuals differed in contact frequency or duration; from this, we also assessed whether such disparities resulted in a differential probability of SNV infection. Additionally, we sought to characterize the functional group within the deer mouse population that was associated with the greatest proportion of contacts, and to determine if SNV dynamics could be explained in general by the 20/80 Rule, i.e. a minority of deer mice have a majority of the contacts. Since males exhibit a greater degree of scarring (Calisher et al. 2007), and are often found to have a greater proportion of the SNV infections, we predicted that males would have a greater number and/or longer contacts than female deer mice, and thereby higher potential of SNV transmission. Additionally, we predicted that deer mice in breeding condition would have a greater SNV transmission potential because reproductive condition can influence intraspecific interactions between deer mice (King 1957; Healey 1967) and SNV infection (Douglass et al. 2007). We also predicted that larger bodied individuals would have greater SNV transmission potential, since body mass has been correlated with wounding and aggressive interactions in other hantaviruses (Hinson et al. 2004) and with SNV prevalence in previous studies (Mills et al. 1997; Douglass et al. 2001; Calisher et al. 2007).
2. Material and methods
(a) Study sites and sampling
Deer mice were surveyed in a mark–recapture study at 12 sites near the West Tintic Mountains in the Great Basin Desert of central Utah (Juab County) on lands administered by the US Department of Agriculture and the Bureau of Land Management. To maintain independence and to avoid inter-site migration, all sites were located more than 700 m apart. Our data from 7 years of mark–recapture studies on these sites supports this claim as only 0.3 per cent (12 out of the 3615) deer mice have been recaptured on a different site. Study sites were dominated by big sagebrush (Artemisia tridentata) and Utah juniper (Juniperus osteosperma).
(b) Rodent sampling
Rodent sampling occurred in ‘spring’ (May, June) and ‘autumn’ (September, October) of 2005 during 15-day periods that coincided with the new moon. For three nights at each site, animals were live-trapped using 148 traps (H.B. Sherman Traps, Inc.) distributed in a ‘web’ configuration over 3.1 ha, following the methods of Mills et al. (1999). Upon capture, animals were identified to species and we documented sex, weight and breeding condition of each individual. Male deer mice were considered to be in breeding condition, if their testes were descended; females were considered to be in breeding condition if their vaginas were perforate, or if they were pregnant or lactating (Calisher et al. 2002). Deer mice were also marked with uniquely numbered ear tags (1005-1, National Band and Tag, Co., Newport, KY) and Passive Integrated Transponder tags (PIT; TX1400ST, BioMark, Inc., Boise, ID) injected subcutaneously between the scapulas. Approximately 0.2 ml of blood was drawn via the retro-orbital sinus from all deer mice at the time of initial capture for each season. Blood was stored immediately on dry ice until transferred to a −80°C freezer. After blood collection, all animals were released at the point of capture. All workers implemented precautions for handling animals potentially infected with hantavirus (Mills et al. 1995) and all techniques for capturing and handling animals were approved by the Institutional Animal Care and Use Committee of the University of Utah (IACUC nos. 0203011, 0503011).
(c) Sin Nombre virus antibody detection
Enzyme linked immunosorbent assays (ELISA) were used to screen deer mouse blood for IgG antibodies to SNV. The presence of antibodies is a reliable indicator of SNV infection because deer mice produce virus-specific IgG antibodies continuously after infection (Borucki et al. 2000; Botten et al. 2003; Safronetz et al. 2005). ELISAs were conducted in a BSL-3 facility at the University of Nevada following the methods described in Otteson et al. (1996).
(d) Powder marking to directly monitor contacts between deer mice
During the mark–recapture study, we powder marked five randomly selected male deer mice at all 12 sites for two nights of each sampling period. Deer mice were selected on the first morning after processing and maintained in traps with food and bedding until 1–2 hours before sunset. Immediately prior to their release, the mice were marked with a fluorescent powder by applying a unique powder colour to each mouse (Radiant Color Co., Richmond, CA) using a stiff toothbrush. The powder was combed backwards through the fur from tail to head over the entire animal. Although we could not standardize the amount of powder per gram of animal, each powdered animal was thoroughly covered with powder by the end of this procedure. Our preliminary investigations under both laboratory and field scenarios indicated that the powder needed to cover the entire animal in order for successful transfer during a brief encounter such as a fight. After being marked with powder, deer mice were released at the point of capture. We were limited to marking a total of five animals with powder per site because we could only distinguish 5 out of the 10 colours available for fluorescent powders, and we could not distinguish mixtures of powders from the original colours. Powder marking does not significantly alter animal behaviour (Stapp et al. 1994, Ebensperger & Tamarin 1997; Kalcounis-Ruppell et al. 2001).
The following morning, all deer mice captured were examined for powder. Each animal was placed in a clean zip-top bag, sealed with a small amount of air. We examined bagged animals under a UV light (Blak-Ray, Upland, CA; model ML-49) inside a large box (58×39×28 cm) covered with lightproof fabric. We checked for powder by brushing the fur backward over the entire body, paying careful attention to the head, ears, mouth, feet and tail, as we predicted those to be most likely areas of contact during aggressive interactions. The powder colour and location on the body were recorded for each deer mouse. If a deer mouse appeared to have a powder mark, but we were unable to distinguish between natural pelage colours and a powder mark, we rubbed a cotton swab over the coloured area. The swab was then examined under the UV light; if no colour appeared on the cotton swab, it was assumed to be natural colour of mouse pelage.
(e) Approximating contacts between deer mice using foraging arenas and passive integrated transponder tags
Contacts between deer mice were approximated at five of the study sites by using foraging arenas equipped with PIT antennae and data loggers (FS2001FT-ISO, Biomark, Inc., Boise, ID), powered by 14.1 V batteries. Time constraints and limited equipment meant that sites were sampled consecutively, not simultaneously, over the course of one month. Within one week after mark–recapture sampling, 12 foraging arenas were placed at a site for three nights. Arenas were placed throughout the site in locations where deer mice had been captured during the mark–recapture study. At sunset each night, foraging arenas (30 cm diameter) were filled with 2 L of sand plus 6 g of millet as an attractant to rodents. As millet comprises less than 1 per cent of the volume of the arena, the animals had to actively forage for the seed reward. PIT antennae were placed below the arenas with data loggers to continuously record the identity and time of each visiting individual. Foraging arenas were closed and leftover millet was sifted out at sunrise each morning. After recording rodent visits for three consecutive nights, data from loggers were downloaded onto a laptop computer. From these data, we determined which individuals visited arenas. Apparent contacts between individuals were defined as the presence of two individuals at a foraging arena within 15 s of one another. This length of time was appropriate given the 5 s lag in the readers coupled with our preliminary video analysis of deer mice at the foraging arenas. We also determined the duration of each apparent contact at each arena.
(f) Statistical analysis
We used logistic regression to determine whether there was sampling bias between individuals marked with a PIT tag in the mark–recapture study and those that visited the foraging arenas. Presence or absence at the arena was the dependent variable, while sex and breeding condition were categorical independent variables, and mass was a continuous independent variable.
We examined the degree of heterogeneity in contact rate in the deer mouse population. The degree of heterogeneity or aggregation in the frequency distribution for number of unique contacts per individual was estimated from the data collected using the powder marking and PIT tag data, independently. We also estimated heterogeneity in the frequency distribution of the duration of contacts (in intervals of 5 s) measured by PIT tags only. We used the mean duration of contact per individual to estimate the typical length of a contact per individual and to account for differences in the number of encounters among individuals. We could not evaluate the distribution of the data for each individual because of limited data for some individuals (i.e. individuals with three to four observations). However, examination of the data indicated that individuals with long average durations tended to have multiple interactions of long duration and that the average was not driven by a single extreme value. Aggregation was quantified by the parameter k, the corrected moment estimate (Elliot 1977). When k is large (more than 20) the distribution is said to be random; when k approaches zero, the distribution is highly skewed and said to be aggregated, following a negative binomial distribution (Fisher et al. 1943; Elliot 1977; Wilson et al. 2001). If heterogeneity existed in the deer mouse population, as measured by the number of unique contacts or the average duration of contact per individual, we expected k to approach zero for one or both of these measures.
We used a generalized linear mixed model (GLMM) to examine the relationship between being SNV positive and the number of contacts using data collected with the powder marking method. The number of unique contacts per individual was considered a fixed effect. We accounted for spatial and temporal variations in the study by including site and sampling period (spring versus autumn) as random effects in the model.
We also used GLMM to examine the relationship between being SNV positive and the number of contacts estimated for PIT tagged animals. In this model, the number of unique contacts per individual, the average duration of contacts per individual and the interaction were entered as fixed effects. We accounted for spatial and temporal variations in the study by including site and sampling period as random effects in the model.
We created an index, ‘contacts-by-duration,’ by multiplying the number of unique contacts per individual by the average duration of contacts per individual; this index served as an aggregate measure of the number interactions deer mice had with other individuals as well as the length of time spent in contact with others. We used GLMM to examine the relationship between the contacts-by-duration index and SNV serostatus; site and sampling period were entered as random effects in this model.
To identify the functional group responsible for the greatest SNV transmission potential, we evaluated the relationship between contacts, as measured by the contacts-by-duration index, and host demographic factors using GLMM. In these models, host sex and breeding condition were categorical fixed effects, whereas mass was entered as a continuous fixed effect. Site and sampling period were entered as random effects to account for spatio-temporal variation.
All statistical analyses were conducted using PROC GLIMMIX or PROC GENMOD in SAS/STAT software (v. 9.1.3, SAS Institute Inc., Cary, NC, USA). All differences were considered statistically significant if p ≤0.05.
3. Results
(a) Differences in contact among deer mice
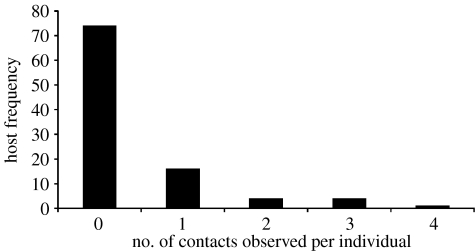
Using the powder marking methods, we estimated the number of direct contacts of 99 male deer mice at 12 sites in spring and autumn, 2005. Of those marked, 25 mice had at least one contact with another deer mouse during the two nights observed, with a maximum of four unique contacts observed for an individual deer mouse. The frequency distribution of unique contacts per individual deer mouse was non-normal and highly aggregated (figure 1, corrected moment estimate (k)=0.54), indicating that a small number of deer mice in the population were responsible for a large proportion of the contacts. Of all the deer mice observed for contacts, 25.3 per cent of the individuals had 100 per cent of the contacts we documented using this technique.
Figure 1.
Host frequency of the number of unique contacts per individual deer mouse, as estimated by powder marking. (During the mark–recapture study, five randomly selected male deer mice were powder marked at all 12 sites for two nights of each sampling period, with a total of 99 male mice being observed in spring and autumn, 2005. Of those marked, 25 mice had at least one contact with another deer mouse during the two nights observed, with a maximum of four unique contacts observed for an individual deer mouse. The frequency distribution of unique contacts per individual deer mouse, as estimated by powder marking, was non-normal and highly aggregated, k=0.54.)
We marked 300 individual deer mice with PIT tags at five sites during the spring and autumn, 2005 sampling periods (three nights per period). Of those marked, 171 mice were observed at the foraging arenas. Of arena visitors, 55 per cent were males and 45 per cent were females. There was no significant sampling bias by sex (t=−0.11, p=0.91), mass (t=1.38, p=0.17) or breeding condition (t=0.45, p=0.66) for individuals that visited arenas compared with the rest of the animals that were marked with PIT tags. Of the deer mice that visited arenas, 73 (42.7%) had at least one contact with another deer mouse while at an arena, with a maximum of 11 unique contacts observed for an individual deer mouse.
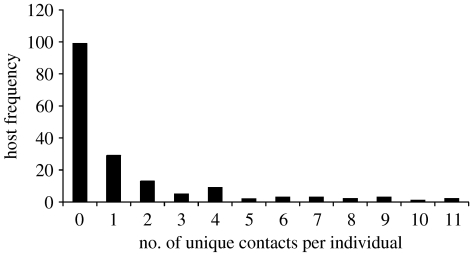
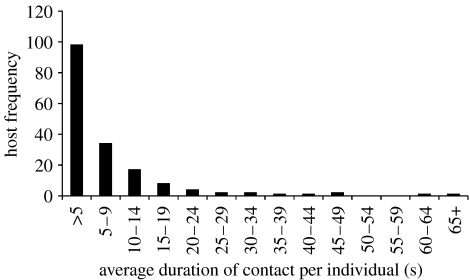
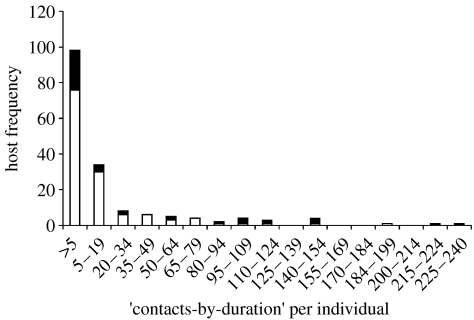
Using the PIT tag method, the frequency distribution of number of unique contacts per individual deer mouse was found to be non-normal and highly aggregated (figure 2, k=0.41). Of all deer mice observed at arenas, 17.5 per cent of the individuals accounted for 75.4 per cent of the total contacts, suggesting that a small number of deer mice in the population were responsible for a large proportion of the potential contacts. The frequency distribution of the average duration of contacts was also highly aggregated (figure 3, k=0.29). About 22.8 per cent of individuals had contacts that averaged 10 s or longer. The frequency distribution of the contacts-by-duration index was also non-normal and highly aggregated (figure 4, k=0.25). The top 20 per cent of individuals measured by the contacts-by-duration index were responsible for 77 per cent of all contacts; contacts ranged from 10 to 68 s in duration.
Figure 2.
Host frequency of the number of unique contacts per individual, as estimated by PIT tag and foraging arenas. (Deer mice (n=300) were marked with PIT tags at five sites during spring and autumn, 2005 (three nights per period). Contacts between deer mice were approximated using foraging arenas equipped with PIT antennae and data loggers. The frequency distribution of unique contacts per individual deer mouse, as estimated by PIT tag, was non-normal and highly aggregated, k=0.41.)
Figure 3.
Host frequency of the average duration of contacts per individual (by 5 s intervals). (Deer mice (n=300) were marked with PIT tags at five sites during spring and autumn, 2005 sampling periods (three nights per period). Duration of each contact between deer mice was recorded using foraging arenas equipped with PIT antennae and data loggers. For each individual, the average duration of contact was estimated as the mean of all contacts observed in which that mouse was involved. The frequency distribution of average duration of contacts per individual deer mouse was non-normal and highly aggregated, k=0.29.)
Figure 4.
Host frequency of contacts-by-duration per deer mouse by SNV infection (by 15 unit intervals). (Contacts-by-duration was estimated by multiplying the number of unique contacts per individual by the average duration of contacts per individual; this index served as an aggregate measure of the number interactions deer mice had with other individuals as well as the length of time spent in contact with others. The frequency distribution of contacts-by-duration per individual deer mouse was non-normal and highly aggregated, k=0.25. As such, the top 20 per cent of individuals have 80 per cent of all contacts, all of which were longer than 10 s in duration. Black, SNV seropositive; White, SNV seronegative.)
(b) The relationship between contacts and Sin Nombre virus
When we compared the number of unique contacts per individual as estimated by powder marking, contacts were not predictive of individuals being SNV seropositive (GLMM, t=0.61, d.f.=85, p=0.54).
When contacts were compared using the PIT tag method, the number of unique contacts was also not predictive of individuals being SNV seropositive (GLMM, t=−1.13, d.f.=162, p=0.26), and average duration of contacts was not statistically significant (GLMM, coefficient estimate (logit scale)=−0.08, s.e.±0.04, t=−1.70, d.f.=162, p=0.08); however, the interaction term (number of unique contacts×average duration) was a significant predictor of individuals being SNV seropositive (GLMM, coefficient estimate (logit scale)=0.03, s.e.±0.01, t=2.14, d.f.=162, p=0.03). Of the random terms, the variance component for site was 0.43 (s.e.±0.47) and sampling period was 0.17 (s.e.±0.39).
We created the contacts-by-duration index in order to further investigate the ‘behaviour’ of the interaction between number of unique contacts and average duration. We found that contacts-by-duration was significantly correlated with individuals being SNV seropositive (GLMM, coefficient estimate (logit scale)=0.008, s.e.±0.004, t=1.93, d.f.=164, p=0.05), suggesting that the index was an accurate representation of the interaction. Of the random terms in the model, the variance component was 0.44 (s.e.±0.49) for site and 0.1 (s.e.±0.27) for sampling period.
(c) Characteristics of deer mice responsible for the majority of contacts
Body mass was an important factor in explaining variability in the contacts-by-duration index, suggesting that animals with large body mass are more likely to be the most connected individuals in the population (GLMM, coefficient estimate=0.15, s.e.±0.06, t=2.42, d.f.=167, p=0.01). The average mass of the most connected individuals (21.5 g±0.76) was 11 per cent more than the mass of individuals in the rest of the distribution (19.0 g±0.43). Neither sex nor breeding condition, nor any interaction in host characteristics, contributed to the variation in contacts-by-duration. Of the random terms, the variance component was 1.48 (s.e.±1.48) for site and 0.84 (s.e.±1.37) for sampling period.
4. Discussion
Our study demonstrates significant heterogeneity in contacts among deer mice and, as a result, significant heterogeneity in SNV transmission potential. Using two distinct methods, we have demonstrated that there is heterogeneity among deer mice, both in frequency and duration of contacts with other deer mice. This finding is important in understanding the dynamics of SNV, as it suggests that individuals do not have equal probability of transmitting or becoming infected with SNV. As estimated by both methods, contact between deer mice appears to follow the 20/80 Rule, as we found that 20 per cent of individuals in the population are responsible for roughly 80 per cent of the contacts observed.
(a) The relationship between contacts and Sin Nombre virus
The most important measure in predicting SNV transmission potential was the interaction between the number of contacts and the average duration of contacts for individual deer mice, as defined by the contacts-by-duration index. When SNV serostatus was modelled as a function of the number of unique contacts alone, estimated by the powder marking method or the PIT tag method, we found that it was not a significant predictor of SNV infection. The average duration of contacts was also not a statistically significant predictor of SNV serostatus at α=0.05; however, the p-value for this variable (0.08) suggests that duration should not be completely discounted as a predictor of transmission. The relationship of transmission potential and duration of contacts between hosts is a novel finding that has been previously overlooked in pathogen dynamics, as few studies document the duration of contact between infected hosts. In the laboratory, SNV was transmitted less frequently and less efficiently than other hantaviruses (Botten et al. 2002); perhaps the quality of interactions (i.e. contact-by-duration) between hosts plays an important role in successful transmission. These results suggest that understanding the nature of interactions, including frequency and especially duration, between hosts may be a critical component in understanding host–pathogen dynamics, particularly for directly transmitted diseases.
(b) Characteristics of deer mice responsible for the majority of contacts
The results of our study indicate that body mass was an important overall predictor of contact heterogeneity (p=0.01), regardless of deer mouse sex or breeding condition, suggesting that body mass plays an important role in intraspecific contact. Given that body mass increases with age, this result suggests that older individuals are more likely to be connected than younger deer mice and, thereby, have greater SNV transmission potential. Older or heavier animals may have greater home range to meet their energetic demands, creating more frequent opportunities for contact as they travel. Alternatively, owing to prior experience older animals may be more likely to engage in interactions with other deer mice as they defend territory, nest sites or food resources. Our study corroborates the findings of previous studies that have found a positive relationship between SNV antibody presence and body mass (Mills et al. 1997; Douglass et al. 2001; Calisher et al. 2007). Large, older animals are also thought to be the source for SNV infection after a population bottleneck (Abbott et al. 1999).
Males are more frequently infected with SNV and are thought to engage in more aggressive behaviour than females. As such, we predicted that sex would be an important characteristic in determining the functional group of deer mice responsible for the majority of contacts. However, our data suggest that males and females have similar SNV transmission potential, at least when measured by contact heterogeneities. Studies of rodent behaviour suggest that female deer mice may exhibit aggressive behaviour in an effort to protect their offspring from intra- and interspecific infanticide (Wolff et al. 1983; Wolff 1993). Perhaps this defensive behaviour results in a similar probability of females having aggressive contacts with other mice compared with males.
Contrary to our predictions, breeding condition was not predictive of contacts among deer mice. This finding contrasts with that of other hantavirus studies that have found a positive and time-lagged relationship between breeding condition and SNV seroconversion (Douglass et al. 2007). This pattern is assumed to be due to increased contact between deer mice during breeding. Our results suggest that, while there may be a correlation between breeding condition and increased incidence of SNV infection in deer mice, it is not necessarily due to increased contact. It has been proposed that differences in host infectiousness may also play role in host heterogeneity (Woolhouse et al. 1997; Dye & Gay 2003). Our previous work revealed that, when presented with artificial immune challenges, male deer mice in breeding condition exhibit a reduced inflammatory response compared with females, suggestive of depressed immunocompetence (Lehmer et al. 2007). Immunological differences, particularly during reproductive periods, may make individuals more or less likely to contract or transmit SNV, independent of differences in contact behaviour.
Our results are similar to other studies of heterogeneity in zoonotic pathogens, where a small fraction of hosts was responsible for the majority of potential disease transmission. For example, in tick-borne encephalitis, 20 per cent of the yellow-necked mouse (Apodemus flavicollis) population had 94 per cent of the disease transmission potential because this group predominantly had the co-feeding ticks required for transmission. Furthermore, that 20 per cent comprised large, male mice in breeding condition (Perkins et al. 2003). In a study of West Nile virus (WNV) in the Eastern US, American robins (Turdus migratorius) comprised 3.7 per cent of total avian abundance, yet they accounted for 43 per cent of mosquito feedings that potentially transmit WNV (Kilpatrick et al. 2006). Although, we had a substantial sample size (n=171), perhaps further exploration of important SNV host demographic factors would be aided with a larger study; other studies of host heterogeneity found distinct patterns with more than 700 individuals (Perkins et al. 2003).
Future studies could also explore the seasonal pattern of contact heterogeneity and its subsequent influence on SNV prevalence. A review by Altizer et al. (2006) found that prevalence in many host–pathogen systems demonstrates seasonal patterns driven by changes in host social behaviour and contact rates, variation in infective stages of the host, annual pulses of host births and deaths and changes in host immune defences. We found significant heterogeneity in contacts among deer mice in both spring and autumn; however, due to sample size constraints we examined the data in aggregate and did not determine whether different demographic factors influenced contact heterogeneity between the two seasons.
Our study suggests that adherence to the 20/80 Rule may be widespread, including pathogens that are directly transmitted. Identifying the pattern of heterogeneity in a host population provides a significant opportunity to develop control and prevention measures that are more effective than those that treat all members of a host population equally (Woolhouse et al. 1997; Perkins et al. 2003). In the case of SNV, our study indicates that the greatest risk of SNV infection is in populations with a large proportion of older, heavy individuals. Our study corroborates the findings of Calisher et al. (2001) that tested the hypothesis that populations with lower proportions of older, heavier deer mice (i.e. populations with high turnover rates) would not maintain SNV. Together, these studies suggest that low population turnover is often associated with high SNV transmission potential among hosts, and presumably from hosts to humans.
Estimating contacts between wild animals is difficult because the few techniques available can alter natural behaviour and are often prohibitively expensive. Previous studies have employed radio-telemetry (Ramsey et al. 2002; Gompper & Wright 2005), viewpoint scanning (Richomme et al. 2006) and proximity data loggers (Ji et al. 2005) to document interactions. However, it is unlikely that these techniques would be successful in monitoring rodent behaviour, since equipment can alter behaviour due to its weight (as in the case of radio telemetry and proximity loggers). Moreover, the presence of fieldworkers may alter rodent behaviour (as in the case of view scanning and radio telemetry). For this study, we employed two techniques to estimate contacts between deer mice; powder marking, which tracks contacts between individuals directly, and PIT tags, which approximates contacts between deer mice. While both methods have limitations, they are unlikely to alter natural rodent behaviour and their limitations may be reduced when used in conjunction with each other. Foraging arenas equipped with PIT antennae allow PIT tagged animals to be observed while they are moving freely. PIT tags are lightweight (0.06 g) and are unlikely to limit or alter animal movement. However, this technique may underestimate contacts, as it only records contacts between individuals if they come to the foraging arenas. Alternatively, it may exaggerate contacts because animals may have confrontations over the seed resource available at the arenas. However, foraging arenas were designed to be only slightly better resources that the background environment. Often more than half of the seeds remained in the trays by morning, suggesting that the availability of resources was higher outside of the arena and that the arenas were not likely to create additional confrontations.
Powder marking offers an alternative means to monitor contacts, which does not involve food resources. Studies have shown that it is an effective method to document social interactions such as mate choice and nesting behaviour, with minimal effects on animal health or behaviour (Stapp et al. 1994; Ebensperger & Tamarin 1997; Kalcounis-Ruppell et al. 2001). However, because there are a limited number of powder colours that can be distinguished from one another, few animals can be monitored simultaneously. Also, because animals have to be trapped for contacts to be observed, it probably underestimates contacts between individuals; once a powder-marked animal is trapped it can no longer have additional contacts.
Our study indicated that host heterogeneity has potential to play an important role in the dynamics of SNV in the wild. In particular, SNV transmission may follow the 20/80 Rule in that a small portion of the population is responsible for a large fraction of the SNV transmission potential, as measured by the frequency and duration of contacts. Males and females neither differ in SNV transmission potential, nor did animals in and out of breeding condition. However, large-bodied, and probably older individuals appear to be an important functional group that has high SNV transmission potential within the deer mouse population.
Acknowledgments
All techniques for capturing and handling animals were approved by the Institutional Animal Care and Use Committee of the University of Utah (IACUC nos. 0203011, 0503011)
Many thanks to S. Perkins at Penn State University and F. Adler at University of Utah for their lengthy discussion regarding this study. We thank Dr Paul Rainey and several anonymous reviewers for their comments that improved the manuscript. For invaluable assistance with fieldwork and many other aspects of the project we thank J. Allen, S. Appleby, J. Billy, G. Boeder, M. Braebec, D. Burrow, C. Davis, B. Goller, J. Griggs, S. Haley, A. Harrison, E. Heward, R. Holcomb, C. Johnson, J. Johnson, L. Kelly, J. Kendrick, J. Maddox, N. Malik, Dr A. Mangione, J. Matthews, C. Mower, N. Moyano, A. Nelson, K. Nelson, S. Newman, S. O'Grady, J. Pearce, L. Phillips, B. Puchner, K. Richins, M. Skopec, A-M. Torregrossa, A. Tweel, C. Votaw, R. Waddell, K. Young. Research support provided by NSF-NIH grant (EF 0326999) and University of Utah seed grant to M.D.D.; an NIH Training grant to E.M.L. (AI055434-01A1); a University of Utah Graduate Research Fellowship and American Society of Mammalogists grant-in-Aid of Research to C.A.C.
References
- Abbott K.D., Ksiazek T.G., Mills J. Long-term hantavirus persistence in rodent populations in Central Arizona. Emerg. Infect. Dis. 1999;5:102–112. doi: 10.3201/eid0501.990112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S., Dobson D., Hosseini P., Hudson P., Rohani P. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 2006;9:467–484. doi: 10.1111/j.1461-0248.2005.00879.x. doi:10.1111/j.1461-0248.2005.00879.x [DOI] [PubMed] [Google Scholar]
- Boone J.D., Otteson E.W., McGwire K.C., Villard P., Rowe J.E., St Jeor S.C. Ecology and demographics of hantavirus infection in rodent populations in the Walker river basin of Nevada and California. Am. J. Trop. Med. Hyg. 1998;59:445–451. doi: 10.4269/ajtmh.1998.59.445. [DOI] [PubMed] [Google Scholar]
- Borucki M.K., Boone J.D., Rowe J.E., Bohlman M.C., Kuhn E.A., DeBacca R., St Jeor S.C. Role of maternal antibody in natural infection of Peromyscus maniculatus with Sin Nombre virus. J. Virol. 2000;74:2426–2429. doi: 10.1128/jvi.74.5.2426-2429.2000. doi:10.1128/JVI.74.5.2426-2429.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botten J., Mirowsky K., Ye C., Gottlieb K., Saavedra M., Ponce L., Hjelle B. Shedding and intracage transmission of Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus) model. J. Virol. 2002;76:7587–7594. doi: 10.1128/JVI.76.15.7587-7594.2002. doi:10.1128/JVI.76.15.7587-7594.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botten J., Mirowsky K., Kusewitt D., Ye C., Gottlieb K., Prescott J., Hjelle B. Persistent Sin Nombre virus infection in deer mice (Peromyscus maniculatus) model: sites of replication and strand-specific expression. J. Virol. 2003;77:1540–1550. doi: 10.1128/JVI.77.2.1540-1550.2002. doi:10.1128/JVI.77.2.1540-1550.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher C.H., Sweeney W., Mills J.N., Beaty B.J. Natural history of Sin Nombre Virus in Western Colorado. Emerg. Infect. Dis. 1999;5:126–134. doi: 10.3201/eid0501.990115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher C.H., Mills J.N., Sweeney W.P., Choate J.R., Sharp D.E., Canestorp K.M., Beaty B.J. Do unusual site-specific population dynamics of rodent reservoirs provide clues to the natural history of hantaviruses? J. Wildl. Dis. 2001;37:280–288. doi: 10.7589/0090-3558-37.2.280. [DOI] [PubMed] [Google Scholar]
- Calisher C.H., Root J.J., Mills J., Beaty B.J. Assessment of ecologic and biologic factors leading to hantavirus pulmonary syndrome, Colorado, USA. Croat. Med. J. 2002;43:330–337. [PubMed] [Google Scholar]
- Calisher C.H., et al. Demographic factors associated with the prevalence of antibody to Sin Nombre virus in deer mice in the western United States. J. Wildl. Dis. 2007;43:1–11. doi: 10.7589/0090-3558-43.1.1. [DOI] [PubMed] [Google Scholar]
- Childs J.E., et al. Serologic and genetic identification of Peromyscus maniculatus as the primary rodent reservoir for a new hantavirus in the southwestern United States. J. Infect. Dis. 1994;169:1271–1280. doi: 10.1093/infdis/169.6.1271. [DOI] [PubMed] [Google Scholar]
- Douglass R.J., Wilson T., Semmens W.J., Zanto S.N., Bond C.W., Van Horn R.C., Mills J.N. Longitudinal studies of Sin Nombre virus in deer-mouse dominated ecosystems of Montana. Am. J. Trop. Med. Hyg. 2001;65:33–41. doi: 10.4269/ajtmh.2001.65.33. [DOI] [PubMed] [Google Scholar]
- Douglass R.J., Calisher C.H., Wagoner K.D., Mills J. Sin Nombre infection of deer mice in Montana: characteristics of newly infected mice, incidence, and temporal pattern of infection. J. Wildl. Dis. 2007;43:12–22. doi: 10.7589/0090-3558-43.1.12. [DOI] [PubMed] [Google Scholar]
- Dye C., Gay N. Modeling the SARS epidemic. Science. 2003;300:1884–1885. doi: 10.1126/science.1086925. doi:10.1126/science.1086925 [DOI] [PubMed] [Google Scholar]
- Ebensperger L.A., Tamarin R.H. Use of fluorescent powder to infer mating activity of male rodents. J. Mammal. 1997;78:888–893. doi:10.2307/1382947 [Google Scholar]
- Elliot J.M. Freshwater Biological Association; Ambleside, UK: 1977. Statistical analysis of samples of benthic invertebrates. [Google Scholar]
- Fisher R.A., Corbet A.C., Williams C.B. The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 1943;12:42–58. doi:10.2307/1411 [Google Scholar]
- Gompper M.E., Wright A.N. Altered prevalence of raccoon roundworm (Baylisascaris procyonis) owing to manipulated contact rates of hosts. J. Zool. (Lond.) 2005;266:215–219. doi:10.1017/S0952836905006813 [Google Scholar]
- Healey M.C. Aggression and self-regulation of population size in deermice. Annu. Rev. Ecol. Syst. 1967;48:377–392. doi:10.2307/1932673 [Google Scholar]
- Hinson E.R., Shone S.M., Zink M.C., Glass G.E., Klein S.L. Wounding: the primary mode of Seoul virus transmission among male Norway rats. Am. J. Trop. Med. Hyg. 2004;70:310–317. [PubMed] [Google Scholar]
- Hjelle B., Jenison S., Torrez-Martinez N., Yamada T., Nolte K., Zumwalt R., MacInnes K., Myers G. A novel hantavirus associated with an outbreak of fatal respiratory disease in the southwestern United States: evolutionary relationships to known hantaviruses. J. Virol. 1994;68:592–596. doi: 10.1128/jvi.68.2.592-596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., White P.C.L., Clout M.N. Contact rates between possums revealed by proximity data loggers. J. Appl. Ecol. 2005;42:595–604. doi:10.1111/j.1365-2664.2005.01026.x [Google Scholar]
- Johnson A.M., Wadsworth J., Wellings K., Field J. Blackwell Scientific; Oxford, UK: 1994. Sexual attitudes and lifestyles. [Google Scholar]
- Kalcounis-Ruppell M.C., Patrick A., Millar J.S. Effect of fluorescent powder marking of females on mate choice by male white-footed mice (Peromyscus leucopus) Am. Midl. Nat. 2001;146:429–433. doi:10.1674/0003-0031(2001)146[0429:EOFPMO]2.0.CO;2 [Google Scholar]
- Kilpatrick E.D., Terajima M., Koster F.T., Catalina M.D., Cruz J., Ennis F.A. Role of specific CD8+T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J. Immunol. 2004;172:3297–3304. doi: 10.4049/jimmunol.172.5.3297. [DOI] [PubMed] [Google Scholar]
- Kilpatrick A.M., Daszak P., Jones M.J., Marra P.P., Kramer L.D. Host heterogeneity dominates West Nile virus transmission. Proc. R. Soc. B. 2006;273:2327–2333. doi: 10.1098/rspb.2006.3575. doi:10.1098/rspb.2006.3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.A. Intra and interspecific conflict of Mus and Peromyscus. Ecology. 1957;38:355–357. doi:10.2307/1931697 [Google Scholar]
- Lehmer E.M., Clay C.A., Wilson E., St Jeor S., Dearing M.D. Differential resource allocation in deer mice exposed to Sin Nombre hantavirus. Physiol. Biochem. Zool. 2007;80:514–521. doi: 10.1086/520128. doi:10.1086/520128 [DOI] [PubMed] [Google Scholar]
- Lloyd-Smith J.O., Schreiber S.J., Kopp P.E., Getz W.M. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. doi:10.1038/nature04153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum H., Barlow N., Hone J. How should pathogen transmission be modeled? Trends Ecol. Evol. 2001;16:295–300. doi: 10.1016/s0169-5347(01)02144-9. doi:10.1016/S0169-5347(01)02144-9 [DOI] [PubMed] [Google Scholar]
- McQuillen H.L., Brewer L.W. Methodological considerations for monitoring wild bird nests using video technology. J. Field Ornithol. 2000;71:167–172. [Google Scholar]
- Mikesic D.G., Drickamer L.C. Effects of radiotransmitters and fluorescent powders on activity of wild house mice (Mus musculus) J. Mammal. 1992;73:663–667. doi:10.2307/1382040 [Google Scholar]
- Mills J., Childs J., Ksiazek T., Peters C., Velleca W. Methods for trapping and sampling small mammals for virologic testing. Centers for Disease Control and Prevention; Atlanta, GA: 1995. [Google Scholar]
- Mills J.N., et al. Patterns of association with host and habitat: antibody reactive with Sin Nombre virus in small mammals in the major biotic communities of the southwestern United States. Am. J. Trop. Med. Hyg. 1997;56:273–284. doi: 10.4269/ajtmh.1997.56.273. [DOI] [PubMed] [Google Scholar]
- Mills J.N., Ksiazek T.G., Peters C.J., Childs J.E. Long-term studies of hantavirus reservoir populations in the Southwestern United States: a synthesis. Emerg. Infect. Dis. 1999;5:135–142. doi: 10.3201/eid0501.990116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteson E.W., Riolo J., Rowe J.E., Nichol S.T., Ksiazek T.G., Rollin P.E., St Jeor S.C. Occurrence of hantavirus within the rodent population of northeastern California and Nevada. Am. J. Trop. Med. Hyg. 1996;54:127–133. doi: 10.4269/ajtmh.1996.54.127. [DOI] [PubMed] [Google Scholar]
- Perkins S.E., Cattadori I.M., Tagliapietra V., Rizzolo A.P., Hudson P.J. Empirical evidence for key hosts in persistence of a tick-borne disease. Int. J. Parasitol. 2003;33:909–917. doi: 10.1016/s0020-7519(03)00128-0. doi:10.1016/S0020-7519(03)00128-0 [DOI] [PubMed] [Google Scholar]
- Ramsey D., Spencer N., Caley P., Efford M., Hansen K., Lam M., Cooper D. The effects of reducing population density on contact rates between brushtail possums: implications for transmission of bovine tuberculosis. J. Appl. Ecol. 2002;39:806–818. doi:10.1046/j.1365-2664.2002.00760.x [Google Scholar]
- Richomme C., Gauthier D., Fromont E. Contract rates and exposure to inter-species disease transmission in mountain ungulates. Epidemiol. Infect. 2006;134:21–30. doi: 10.1017/S0950268805004693. doi:10.1017/S0950268805004693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronetz D., Lindsay R., Dibernardo A., Hjelle B., Xiao R., Artsob H., Drebot M.A. A preliminary study of the patterns of Sin Nombre viral infection and shedding in naturally infected deer mice (Peromyscus maniculatus) Vector Borne Zoonotic Dis. 2005;5:127–132. doi: 10.1089/vbz.2005.5.127. doi:10.1089/vbz.2005.5.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapp P., Young J.K., Vandewoude S., Van Horne B. An evaluation of the pathological effects of fluorescent powder on deer mice (Peromyscus maniculatus) J. Mammal. 1994;75:704–709. doi:10.2307/1382519 [Google Scholar]
- Wilson K., Bjornstad O.N., Dobson A.P., Merler S., Poglayen G., Randolph S.E., Read A.F., Skorping A. Heterogeneities in macroparasite infections: patterns and processes. In: Hudson P.J., Rizzoli A.P., Grenfell B.J., Hessterbeek H., Dobson A.P., editors. The ecology of wildlife diseases. Oxford University Press; Oxford, UK: 2001. [Google Scholar]
- Wolff J.O. Why are female small mammals territorial? Oikos. 1993;68:364–370. doi:10.2307/3544853 [Google Scholar]
- Wolff J.O., Freeberf M.H., Dueser R.D. Interspecific territoriality in two sympatric species of Peromyscus (Rodentia: Cricetidea) Behav. Ecol. Sociobiol. 1983;12:237–242. doi:10.1007/BF00290776 [Google Scholar]
- Woolhouse M.E.J., et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl Acad. Sci. USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. doi:10.1073/pnas.94.1.338 [DOI] [PMC free article] [PubMed] [Google Scholar]