Abstract
The traditional assumption that vector-borne pathogens should evolve towards a benign relationship with their arthropod vectors has been challenged on theoretical grounds and empirical evidence. However, in the case of arboviruses (arthropod-borne viruses), although a number of investigators have reported experimental evidence for virus-induced vector mortality, others have failed to detect any significant impact. Whether this variation in the observed level of arbovirus virulence depends on biological traits or experimental design is unclear. Here, we perform a meta-analysis of studies across a range of mosquito–virus systems to show that, overall, arboviruses do reduce the survival of their mosquito vectors, but that the magnitude of the effect depends on the vector/virus taxonomic groups and the mode of virus transmission. Alphaviruses were associated with highest virulence levels in mosquitoes. Horizontal transmission (intrathoracic inoculation or oral infection) was correlated with significant virus-induced mortality, whereas a lack of adverse effect was found for Aedes mosquitoes infected transovarially by bunyaviruses—a group of viruses characterized by high natural rates of vertical transmission in their enzootic vectors. Our findings are consistent with the general prediction that vertically transmitted pathogens should be less virulent than those transmitted horizontally. We conclude that varying degrees of virulence observed among vector–virus systems probably reflect different selective pressures imposed on arboviruses that are primarily transmitted horizontally versus vertically.
Keywords: host–pathogen evolution, arbovirus, mosquito–virus interactions, virulence, vertical transmission, meta-analysis
1. Introduction
Understanding variation in the virulence of infectious diseases, defined as the reduction of host fitness following infection (i.e. disease severity), has considerable implications for human health, animal welfare and agriculture. Considering virulence as a product of evolution has provided valuable insights into the processes governing the severity of an infection (Read 1994; Ebert & Herre 1996). Traditionally, pathogens were predicted to always evolve towards a benign relationship with their hosts based on the belief that a pathogen that is harmful to its host is harmful to itself (Burnet & White 1972). This view has been challenged on theoretical grounds by the suggestion that an increased level of virulence can be evolutionarily advantageous if it concomitantly enhances pathogen transmission (Anderson & May 1982; Frank 1996; Levin 1996). In fact, there are numerous examples of host fitness reduction due to infection (Read 1994) and the existence of selective pressure for higher virulence has been repeatedly demonstrated, for example, in experimental evolution studies (Ebert 1998).
In this context, at least two evolutionary scenarios have been predicted to lead to low levels of pathogen virulence: (i) vertical transmission (that is, from parent hosts to their offspring) and (ii) interactions between vector-borne pathogens and their arthropod vectors (Ewald 1994). Underlying the first prediction is the fact that the fitness of vertically transmitted pathogens is explicitly linked to their host's own reproductive success, so that any cost to host fitness also reduces pathogen transmission (Ebert & Herre 1996). The predicted relative low virulence of vertically transmitted infectious agents was supported by a number of empirical studies. Horizontally transmitting ectoparasites of rock doves (Clayton & Tompkins 1994) or microsporidian parasites of mosquitoes (Agnew & Koella 1997) are more virulent than their vertically transmitted counterparts. Likewise, phages selected for vertical transmission are less virulent to their bacterial hosts than the original population selected for horizontal transmission (Bull et al. 1991).
The second prediction is based on the assumption that, if vector-borne pathogens rely on their principal hosts for amplification and use their vectors as agents for dispersal, they may reduce the fecundity of their vectors but should not kill them (Ewald 1994). This intuitive prediction has not always been supported by empirical observations. With varying levels of virulence observed among different vector–pathogen systems, the body of evidence indicates that there is no consistent universal pattern, as, for example, among mosquito–Plasmodium systems (Ferguson & Read 2002). Although it has been suggested that virulence may depend on the relative mobility of the vertebrate host and arthropod vector (Elliot et al. 2003), the degree of virulence to a vector and the advantage that it confers to the pathogen remain unclear.
This inconsistency is true for arthropod-borne viruses (arboviruses), which are responsible for some of the most devastating diseases of humans, domestic animals and wildlife, including dengue, West Nile fever, yellow fever, Rift Valley fever, Japanese encephalitis and various other encephalidities of medical and veterinary importance (Gubler 2001, 2002). Although some studies have demonstrated a negative effect of arbovirus infection on traits associated with vector fitness (Grimstad et al. 1980; Platt et al. 1997; Scott & Lorenz 1998; Lee et al. 2000; Moncayo et al. 2000; Mahmood et al. 2004), others have failed to detect any significant impact (Patrican & DeFoliart 1985; Berry et al. 1987; Putnam & Scott 1995). When they have been investigated, factors such as initial viral dose (Cooper et al. 2000; Mahmood et al. 2004), virus strain (Scott & Lorenz 1998) or environmental quality (Patrican & DeFoliart 1985; Dohm et al. 1991) did not significantly explain the extent of the fitness cost of infection. Thus, despite the importance of vector–virus relationships on arbovirus emergence and maintenance in nature (Kuno & Chang 2005; Weaver 2006) and the key role of vector fitness on vector-borne disease dynamics (Dye 1992; Kramer & Ebel 2003), the causes underlying variation in observed levels of arboviral virulence to vectors have not been elucidated. Here, we use a meta-analysis to disentangle the relative contributions of several biological and experimental factors to the effect of arbovirus infection on survival of their mosquito vectors. We focus on studies on the survival of adult female mosquitoes because they constitute the major portion of the published literature. Other components of vector fitness (e.g. fecundity) can be affected by arbovirus infection, and it will be valuable to include these traits in future studies when more data are available. Nevertheless, virus-induced mortality is a common measure of virulence effect (Anderson & May 1982; Read 1994) and vector survival probability has the greatest impact on the basic reproductive number of an arbovirus, i.e. the number of new virus-infected vertebrate hosts in a totally susceptible population (Dye 1992).
2. Material and methods
(a) Literature search
We conducted a literature survey on the effect of arboviral infection on mosquito fitness through the ISI Web of Science, NCBI PubMed and Armed Forces Pest Management Board Literature Retrieval System. We considered only those viruses that infect both vertebrate and invertebrate hosts (i.e. arboviruses) and, therefore, excluded studies on viruses that only infect arthropods. Criteria for inclusion in the meta-analysis were that the studies (i) had experimentally tested (as opposed to anecdotally observed) the effect of arbovirus infection on mosquito survival and (ii) provided either summary statistics (at least sample size and p-value) or sufficient raw data to allow us to perform an appropriate statistical analysis.
Our analysis was conducted at the level of the individual experiment. We considered separate experiments from the same study as individual units. Because we did not want to artificially increase the weight of a particular experiment, we assigned a single effect size (i.e. standardized measure of the magnitude of the effect; Rosenberg et al. 2000) to each experiment. When it consisted of several experimental groups, we pooled the data from different experimental treatments. When several control groups were used (see below), we included only the most stringent control group, i.e. testing direct effects of infection.
(b) Meta-analysis factors
When information was available, we recorded several experimental and biological characteristics of experiments. We classified each mosquito–virus system according to whether it was primarily involved in virus transmission among a reservoir host population (enzoonotic or endemic cycles), in spillover transmission events out of the reservoir host population (epizootic or epidemic cycles), or in an interaction that does not occur in nature (unnatural). We assigned mosquito species and viruses to taxonomic groups at the genus level. We classified the experimental infection mode into three categories: oral feeding (on a live host or via membrane feeding); intrathoracic (IT) inoculation; and transovarial transmission. We recorded whether the control group consisted of mosquitoes that (i) fed on an uninfected blood meal (non-exposed), (ii) exposed to an infectious blood meal or had an infected mother but did not become infected (uninfected), or (iii) had a midgut infection but did not develop a disseminated infection to other tissues (non-disseminated). We noted whether mosquitoes were fed additional blood meals subsequent to an initial virus exposure. We distinguished experiments that were analysed by comparing mortality rates at particular time points (endpoint statistics) from experiments where analysis was based on entire survival curves (survival curve statistics). For each experiment, we also recorded the year of publication, sample size (total number of mosquitoes analysed), duration of the experiment (number of days elapsed between the start and the end of the experiment), infection prevalence (actual proportion of infected mosquitoes), final survival (proportion of mosquitoes alive at the end of the experiment) and incubation temperature.
(c) Meta-analysis statistics
All analyses were performed using the software MetaWin v. 2.0 (Rosenberg et al. 2000). The meta-analytic procedure consisted of three steps. First, we compiled a standardized effect size of the impact of arbovirus infection on vector survival for each separate experiment. Because different studies presented their summary statistics information in different forms, we converted one-tailed p-values into standard normal deviates, which can be transformed to correlation coefficients (Rosenberg et al. 2000). We then calculated estimates of effect sizes and their variances from the correlation coefficients using Fisher's z-transformation (Rosenberg et al. 2000). We arbitrarily assigned a positive value to the effect size if the mortality of infected mosquitoes was enhanced compared with that of controls. Second, we assumed no data structure to compile the cumulative effect size of the entire dataset, which is the average effect size weighted by sample size (Rosenberg et al. 2000). We also estimated the total heterogeneity (QT) of the dataset and determined its significance against a Χ2-distribution (Rosenberg et al. 2000). Third, we explored the influence of experimental and biological factors by incorporating data structure in the analysis. We used only one-way models because different explanatory variables were not necessarily independent. Importantly, we did not want to assume that there was a common true effect size shared by all experiments. We accounted for the fact that, in addition to sampling error, there was a true random component of variation in effect sizes between experiments by using mixed-effects models that include random variation among experiments and fixed effects of explanatory variables. Mixed-effects models have the advantage of allowing one to generalize the results beyond the studies included in the analysis (Sokal & Rohlf 1995). To test for significance of a variable, total heterogeneity (QT) was partitioned into the variation in effect sizes explained by the model (QM) and the residual error variance in effect sizes not explained by the model. The ratio QM/QT is analogous to the coefficient of determination of an ANOVA (R2). For categorical variables, the difference among groups was determined by testing QM against a Χ2-distribution with n−1 degrees of freedom (where n is the number of groups), whereas for continuous variables the significance level of QM was tested against a Χ2-distribution with one degree of freedom.
(d) Resampling methods
Because our dataset consisted of a relatively small number of experiments, we determined the accuracy of the meta-analytic metrics by using two types of method. First, we performed bootstrapping procedures (Rosenberg et al. 2000) to recalculate the confidence intervals of effect sizes by randomly choosing (with replacement) n experiments from a sample size of n, and calculating the desired statistics. The bootstrap confidence intervals assume that the distribution of bootstrap values is centred around the original value. Thus, when more than 50 per cent of the bootstrap replicates were above or below the original value, the bootstrap confidence interval was corrected for this bias. Second, we performed randomization tests (Rosenberg et al. 2000) based on 999 iterations to determine the significance of the model structure of the meta-analysis. For each iteration, the original effect size data were randomly assigned to groups such that the number of studies in each group was unchanged, and a test statistic was calculated from the randomly shuffled data. The actual test statistics were then compared with the frequency distribution of randomly generated statistics, and the proportion of randomly generated statistics more extreme than the actual statistic was taken to be the significance level for the dataset.
(e) Correspondence analysis
We evaluated the potential dependence of variables that had a significant effect in one-way meta-analytic models described above. Because these variables were categorical and thus standard correlation coefficients could not be measured, we used correspondence analysis (Benzécri 1973; Greenacre 1983) to explore the structure of categorical data. Correspondence analysis is a graphical technique conceptually similar to principal component analysis (PCA), which can help identify similar patterns of counts in a contingency table. Entries in tables of relative frequencies are plotted according to the distances between individual rows and columns in a two-dimensional space.
3. Results
We identified 12 studies published between 1960 and 2007 that met inclusion criteria for our meta-analysis, representing a total of 12 different mosquito–virus combinations (table 1). These studies consisted of 36 separate experiments (one to eight per study), of which 16 reported summary statistics and 17 provided sufficient raw data to allow us to perform an appropriate analysis. No effect size could be calculated for three experiments that lacked summary statistics and raw data. Meta-analysis was consequently conducted on a total of 33 individual experiments. A negative effect of arbovirus infection on mosquito survival was reported in 13 experiments, no effect was detected in 19 experiments, and a positive effect was detected in one experiment.
Table 1.
Studies included in the meta-analysis. (For each study that met the inclusion criteria of the meta-analysis, the virus and its genus, the mosquito species, their relationship in natural transmission cycles and the number of experiments for which sufficient data were provided to compute an effect size (no. of exp.) are indicated. References are ordered according to the publication year.)
| reference | virus (genus) | mosquito species | cycle | no. of exp. |
|---|---|---|---|---|
| Lamotte (1960) | Japanese B encephalitis (Flavivirus) | Culex pipiens | unnatural | 1 |
| Turell et al. (1982c) | California encephalitis (Bunyavirus) | Aedes dorsalis | enzoonotic | 2 |
| Aedes melanimon | enzoonotic | 2 | ||
| Patrican & DeFoliart (1985) | La Crosse (Bunyavirus) | Aedes triseriatus | enzoonotic | 8 |
| Faran et al. (1987) | Rift Valley fever (Phlebovirus) | Culex pipiens | epizootic | 3 |
| Dohm et al. (1991) | Rift Valley fever (Phlebovirus) | Culex pipiens | epizootic | 3 |
| Scott & Lorenz (1998) | eastern equine encephalitis (Alphavirus) | Culiseta melanura | enzoonotic | 4 |
| Cooper et al. (2000) | eastern equine encephalitis (Alphavirus) | Culiseta melanura | enzoonotic | 1 |
| Lee et al. (2000) | western equine encephalitis (Alphavirus) | Culex tarsalis | enzoonotic | 1 |
| Moncayo et al. (2000) | eastern equine encephalitis (Alphavirus) | Anopheles quadrimaculatus | epizootic | 2 |
| Coquillettidia perturbans | epizootic | 2 | ||
| Aedes albopictus | epizootic | 1 | ||
| Joshi et al. (2002) | dengue-3 (Flavivirus) | Aedes aegypti | endemic | 1 |
| Mahmood et al. (2004) | western equine encephalitis (Alphavirus) | Culex tarsalis | enzoonotic | 1 |
| Styer et al. (2007) | West Nile (Flavivirus) | Culex tarsalis | enzoonotic | 1 |
(a) Overall effect
Assuming no data structure, the cumulative effect size (the overall magnitude of the standardized effect across experiments) was 0.099. The bootstrapped, bias-corrected 95% confidence interval (0.037–0.155) did not bracket zero, indicating that this effect was statistically significant. Because we had arbitrarily assigned a positive value to the effect size of studies with decreased mortality of infected mosquitoes compared with controls, this result showed that, overall, arboviruses do reduce the survival of their mosquito vectors. The total heterogeneity of the sample was not statistically significant when tested against a Χ2-distribution (QT=31.8, d.f.=32, p=0.474), which was consistent with the absence of major data structure. However, the ratio between the square root of the estimate of the pooled variance (an estimate of between-experiment variation) and the mean variance of experiments (a measure of within-experiment variation) was relatively high (12.6), suggesting that the variance among effect sizes was greater than expected by sampling error or random variation between experiments.
(b) Publication bias
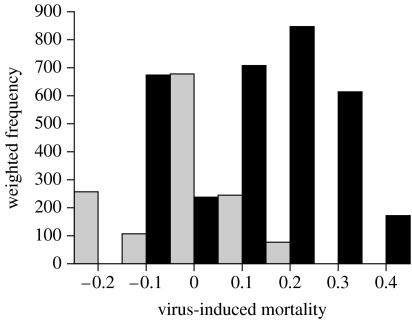
Publication bias—the selective publication of articles showing certain types of results over those showing other types of results—is a major concern in the meta-analysis (Rosenberg et al. 2000). Simple graphical methods did not display any visual indication of publication bias in our dataset. The weighted histogram of effect sizes (figure 1) showed no depression around zero, which would be indicative of publication bias against reporting studies with no or little effect. The funnel plot (scatter plot of effect size versus sample size; data not shown) was, as expected, shaped as a funnel with more variation in effect sizes at smaller than at larger sample sizes. The normal quantile plot (quantile plot of effect size versus normal distribution; data not shown) fell within the confidence bands of the line x=y, indicating no significant deviation from normality. The absence of publication bias was quantitatively confirmed by common rank correlation tests, Kendall's θ and Spearman's ρ (Sokal & Rohlf 1995). Both tests showed no significant correlation between the standardized effect size and sample size across studies (Kendall's θ=−0.104, Z=−0.853, p=0.394; Spearman's ρ=−0.129, p=0.473). Rosenthal's fail-safe number calculation (Rosenthal 1979) indicated that approximately 107 non-significant, unpublished or missing experiments would need to be added to the meta-analysis in order to change the results from significance to non-significance. Although this number was smaller than the value 5n+10=175 (where n=33 is the actual number of experiments), which gives a conservative critical value against which to test a fail-safe calculation (Rosenthal 1979), it seemed reasonable to be confident that the observed result, even with some minor publication bias, could be treated as a reliable estimate of the true effect.
Figure 1.
Relative contribution of transmission mode to arbovirus-induced mortality in mosquitoes. The weighted distribution of the standardized magnitude of virus-induced mortality (effect size) is shown as a function of the mode of viral transmission. The height of the bar in each class is made up of the combined weight of experiments falling in this class (weight=1/variance). Black bars represent experiments in which viruses were horizontally transmitted (n=21) and grey bars correspond to experiments based on vertical transmission (n=12). On the x-axis, positive values represent higher mortality of infected compared with control mosquitoes, whereas negative values correspond to a positive effect of viral infection on mosquito survival.
(c) Effect of experimental and biological factors
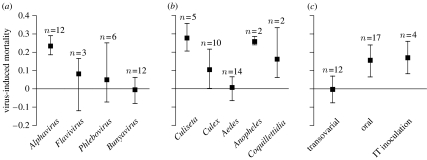
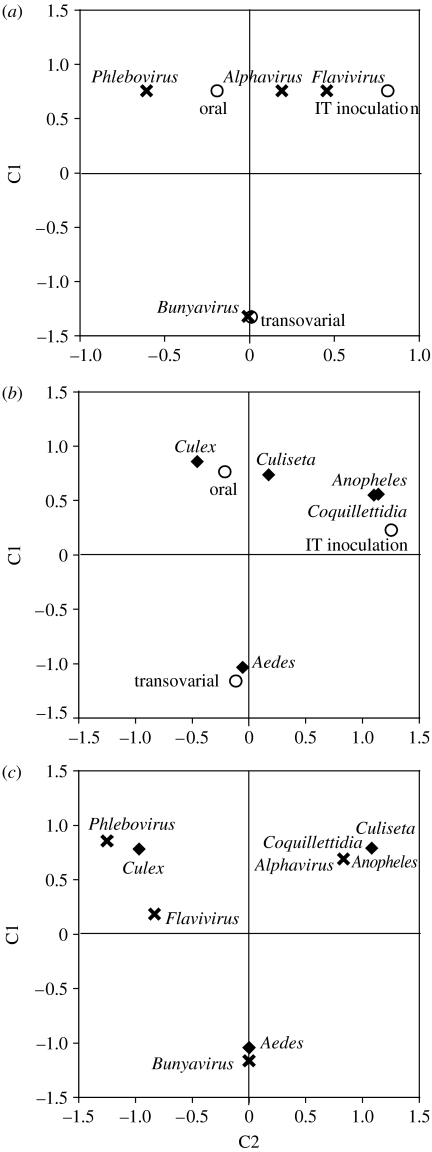
Of the seven categorical and six continuous variables that were tested as predictors of the standardized virus-induced mortality, only three explained a statistically significant portion of the heterogeneity in effect size (tables 2 and 3). First, virus genus explained 37 per cent of the total heterogeneity in effect sizes (table 2; figure 2a). Alphavirus was the only genus associated with a virus-induced mortality significantly different from zero. Pairwise comparison showed that the cumulative effect size associated with Alphavirus was significantly higher than that of every other genus (Alphavirus versus Flavivirus: QM/QT=0.359, p=0.006; Alphavirus versus Bunyavirus: QM/QT=0.541, p<0.0001; Alphavirus versus Phlebovirus: QM/QT=0.280, p=0.010), whereas all other genera were not significantly different from one another (Flavivirus versus Bunyavirus: QM/QT=0.074, p=0.301; Flavivirus versus Phlebovirus: QM/QT<0.001, p=0.964; Phlebovirus versus Bunyavirus: QM/QT=0.041, p=0.404). Second, mosquito genus explained 34 per cent of the total heterogeneity in effect sizes (table 2; figure 2b). All mosquito genera were associated with a positive cumulative effect size with the exception of the genus Aedes. Pairwise comparison revealed that the cumulative effect size associated with Aedes was significantly lower than that of Culiseta and Anopheles (Aedes versus Culiseta: QM/QT=0.508, p<0.0001; Aedes versus Anopheles: QM/QT=0.339, p=0.009), whereas all other combinations were not significantly different (Culiseta versus Culex: QM/QT=0.192, p=0.101; Culiseta versus Anopheles: QM/QT=0.050, p=0.682; Culiseta versus Coquillettidia: QM/QT=0.308, p=0.139; Culex versus Aedes: QM/QT=0.095, p=0.147; Culex versus Anopheles: QM/QT=0.099, p=0.318; Culex versus Coquillettidia: QM/QT=0.015, p=0.704; Aedes versus Coquillettidia: QM/QT=0.101, p=0.215; Anopheles versus Coquillettidia: QM/QT=0.370, p=0.286). Third, the experimental mode of infection explained 22 per cent of the total heterogeneity in effect sizes (table 2; figure 2c). Pairwise comparison indicated that experiments based on transovarial transmission had a significantly lower effect size than experiments in which mosquitoes were infected by IT inoculation (QM/QT=0.316, p=0.011) or were orally challenged (QM/QT=0.203, p=0.016), whereas there was no detectable difference in the effect of IT inoculation versus oral infection (QM/QT=0.001, p=0.901). Oral infection and IT inoculation were, therefore, combined under the category ‘horizontal transmission’ (as opposed to ‘vertical transmission’) in subsequent analyses. The effect of transmission mode (vertical versus horizontal) on virus-induced mortality was reflected in the bimodal distribution of the weighted effect sizes (figure 1). Importantly, correspondence analysis graphically showed that transovarial transmission, mosquitoes of the genus Aedes and bunyaviruses were strongly associated in the tables of relative frequencies (figure 3), indicating that these categories were confounded throughout the dataset.
Table 2.
Influence of categorical factors on arbovirus-induced mortality in mosquitoes. (For each class of individual factors, the corresponding number of experiments (no. of exp.), mean effect size (E) and its bootstrapped, bias-corrected 95% confidence interval (95% CI) are indicated. No effect size could be calculated when the category consisted of less than two experiments (n.a.). The influence of each factor was characterized using separate one-way mixed-model analyses in MetaWin v. 2.0 (Rosenberg et al. 2000). Effects were quantified with the ratio between the heterogeneity in effect size explained by the factor (QM) and the total heterogeneity of the sample (QT). A significant QM implies that there are differences among mean effect sizes for the groups.)
| factor | class | no. of exp. | E | 95% CI | QM | QM/QT | d.f. | p-value |
|---|---|---|---|---|---|---|---|---|
| infection mode | oral | 17 | 0.156 | 0.065–0.240 | 7.174 | 0.216 | 2 | 0.028 |
| IT inoculation | 4 | 0.170 | 0.083–0.260 | |||||
| transovarial | 12 | −0.003 | −0.076–0.070 | |||||
| virus genus | Alphavirus | 12 | 0.234 | 0.186–0.290 | 18.29 | 0.374 | 3 | 0.0004 |
| Flavivirus | 3 | 0.082 | −0.120–0.166 | |||||
| Phlebovirus | 6 | 0.050 | −0.072–0.251 | |||||
| Bunyavirus | 12 | −0.005 | −0.080–0.063 | |||||
| mosquito genus | Culiseta | 5 | 0.277 | 0.206–0.357 | 13.01 | 0.343 | 4 | 0.011 |
| Culex | 10 | 0.104 | 0.002–0.216 | |||||
| Aedes | 14 | 0.007 | −0.065–0.065 | |||||
| Anopheles | 2 | 0.258 | 0.240–0.286 | |||||
| Coquillettidia | 2 | 0.162 | 0.062–0.334 | |||||
| cycle | enzoonotic/endemic | 21 | 0.086 | 0.015–0.155 | 0.191 | 0.006 | 1 | 0.662 |
| epizootic/epidemic | 11 | 0.115 | 0.018–0.239 | |||||
| unnatural | 1 | n.a. | n.a. | |||||
| control type | non-exposed | 23 | 0.068 | 0.001–0.136 | 4.259 | 0.129 | 2 | 0.119 |
| uninfected | 7 | 0.134 | 0.026–0.227 | |||||
| non-disseminated | 3 | 0.285 | 0.191–0.378 | |||||
| statistics | survival curve | 14 | 0.150 | 0.049–0.240 | 1.984 | 0.064 | 1 | 0.159 |
| endpoint | 19 | 0.063 | −0.012–0.123 | |||||
| extra blood meals | no | 21 | 0.143 | 0.061–0.205 | 3.534 | 0.113 | 1 | 0.060 |
| yes | 12 | 0.026 | −0.061–0.104 |
Table 3.
Influence of continuous variables on arbovirus-induced mortality in mosquitoes. (For each individual variable, the number of experiments included in the analysis (no. of exp.), mean value and range, mean effect size (E) and its bootstrapped, bias-corrected 95% confidence interval (95% CI) are given. Final survival refers to the percentage of mosquitoes alive at the end of the experiment. The influence of each variable was characterized using separate one-way mixed-model analyses in MetaWin v. 2.0 (Rosenberg et al. 2000). Effects were quantified with the ratio between the heterogeneity in effect size explained by the regression model (QM) and the total heterogeneity of the sample (QT). A significant QM indicates that the variable explains a significant amount of the variability in effect size.)
| variable | no. of exp. | mean (range) | E | 95% CI | QM | QM/QT | p-value |
|---|---|---|---|---|---|---|---|
| publication year | 33 | 1991 (1960–2007) | 0.099 | 0.039–0.153 | 2.419 | 0.069 | 0.120 |
| sample size | 33 | 143.1 (30–600) | 0.099 | 0.025–0.159 | 0.570 | 0.019 | 0.450 |
| exp. duration | 26 | 37.5 days (7–105) | 0.138 | 0.077–0.199 | 0.386 | 0.015 | 0.534 |
| final survival | 24 | 45.0% (0–100) | 0.078 | 0.012–0.146 | 2.408 | 0.105 | 0.121 |
| infection rate | 25 | 91.3% (56.4–100) | 0.059 | 0.008–0.122 | 2.798 | 0.111 | 0.094 |
| temperature | 33 | 25.3°C (22–27) | 0.099 | 0.040–0.160 | 0.632 | 0.020 | 0.427 |
Figure 2.
Effect of infection mode and vector/virus taxonomic groups on arbovirus-induced mortality in mosquitoes. The graphs show the weighted mean of the standardized magnitude of virus-induced mortality as a function of (a) virus genus, (b) mosquito genus and (c) infection mode. Vertical bars represent the bootstrapped, bias-corrected 95% confidence intervals of the cumulative effect sizes. The number of experiments in each category is indicated above each bar. Positive values represent higher mortality of infected compared with control mosquitoes, whereas negative values correspond to a positive effect of viral infection on mosquito survival.
Figure 3.
Correspondence analysis of infection mode and vector/virus taxonomic groups. The association between (a) virus genus and infection mode, (b) mosquito genus and infection mode and (c) mosquito and virus genera are displayed using correspondence analysis plots (Benzécri 1973; Greenacre 1983). Different symbols represent different variables (crosses, virus genus; diamonds, mosquito genus; circles, infection mode), and the categories of each variable are indicated. The plots show the entries in the tables of relative frequencies in terms of the distances between individual rows and columns in a two-dimensional space. The C1 axis tends to score response levels linearly, whereas the C2 scores indicate non-neutrality. Overall, spatial clustering indicates similar patterns of counts in the contingency table.
It is worth mentioning that 8 of the 12 experiments in which transovarial transmission was used as the infection mode came from a single study (Patrican & DeFoliart 1985). In that study, four successive generations of the same initial population of Ae. triseriatus reared under two different larval diets were used to test the effect of La Crosse (LAC) virus on adult vector survival. To ensure that our conclusion was not overly influenced by this particular study, we reanalysed the data using the mean sample size and correlation coefficient of all experiments from Patrican & DeFoliart (1985) to compute effect sizes. Despite the resulting low number of experiments in the vertical transmission category (5), the effect of transmission mode remained marginally insignificant (QM/QT=0.147, p=0.054). The mean effect size was significantly positive for horizontal transmission (E=0.160, 95% CI=0.081–0.231), whereas it did not differ from zero for vertical transmission (E=−0.006, 95% CI=−0.084–0.132). These results allowed us to rule out the potential caveat that the relatively large number of experiments from a single study (Patrican & DeFoliart 1985) had biased our analysis.
4. Discussion
Our meta-analysis showed that, across systems, arboviruses do reduce the survival of their adult mosquito vectors. However, the magnitude of the effect depended on mosquito/virus taxonomic groups (at the genus level) and the experimental mode of infection (horizontal versus vertical). The cumulative effect size was significantly higher for the Alphavirus genus, whereas Aedes was the only mosquito genus for which it was not different from zero. Virus-induced mortality was significantly positive when the virus was transmitted horizontally (by oral infection or IT inoculation), but no such effect was detected when the virus was transmitted vertically (transovarially). Correspondence analysis showed that transovarial transmission was strongly associated with mosquitoes of the genus Aedes and viruses of the genus Bunyavirus in our dataset.
That mosquito-borne arboviruses, overall, reduce the survival of their vectors supports the conclusions from an earlier meta-analysis on mosquito–Plasmodium systems (Ferguson & Read 2002). In that study, the authors concluded that malaria parasites do reduce the survival of their mosquito vectors, but that the effect was more likely to be detected in unnatural associations and in studies of longer duration. We did not detect an effect due to the nature of the mosquito–virus association or study duration, indicating that these features might be specific to mosquito–malaria systems. That the level of virulence did not depend on the type of transmission cycle indicates that the frequency of co-occurrence is not a major determinant of virulence in mosquito–virus systems. However, only a single experiment included in our meta-analysis represented an unnatural mosquito–virus combination, which prevented us from testing an effect of co-occurrence itself, regardless of its frequency. Together, our results and those from the meta-analysis of Ferguson & Read (2002) support the conclusion that the relationship between vector-borne pathogens and their arthropod vectors is not necessarily benign, in contrast to conventional wisdom (Burnet & White 1972) and earlier predictions based on evolutionary theory (Ewald 1994).
Further analysis showed that the overall positive virus-induced mortality of mosquitoes actually masked the influence of several explanatory variables. In particular, the cumulative effect size was highest for alphaviruses. Because this category was not evidently confounded by another factor in the analysis, this finding indicates that some aspects of the biology of alphaviruses may render them more virulent for their vectors. A unique feature of alphaviruses is their bipartite genome organization as opposed to the tripartite genome of bunyaviruses and phleboviruses and the single open reading frame of flaviviruses. Future research could explore the possible relationship between virus replication strategy and virulence for mosquito vectors.
A lack of adverse effects of viral infection on vector survival was associated with experiments where transovarial transmission was the means of infection. All of these experiments involved Aedes mosquitoes infected with bunyaviruses. Further study of a broader range of taxa is encouraged to confirm that the differential virulence we observed is not due to genus-specific effects independent of transmission mode. The limited amount of data available did not allow us to conduct a proper meta-analysis on the effect of arboviral infection on other components of mosquito fitness. However, it is noteworthy that, of the five studies of our analysis that compared the fecundity of infected and control mosquitoes, the four studies that used a virus transmitted horizontally reported a negative effect of infection on fecundity (Scott & Lorenz 1998; Joshi et al. 2002; Mahmood et al. 2004; Styer et al. 2007), whereas no adverse effect was detected in the study based on transovarial infection (Patrican & DeFoliart 1985). This indicates that our conclusions about survival may be applicable for other components of fitness.
One caveat of our analysis is that it does not directly allow us to disentangle the role of the natural mode of transmission (the evolutionary history of the virus) from the experimental means of infection. Evolutionary histories of viral isolates prior to their use in experimental infections are generally unknown. However, isolation and passage histories of the viruses included in our analysis indicated that we could reasonably assume that the means of infection was representative of their natural mode of transmission. Of the 21 experiments based on IT inoculation or oral infection, 9 used viruses that had been initially isolated from vertebrate host tissue, thus indicative of recent horizontal transmission. Of the 12 experiments based on transovarial infection, eight experiments used a virus originating from a pool of mosquito larvae, which thus had been vertically transmitted. With the exception of one virus that was passaged 21 times in mouse brain and one virus that was passaged 12 times in mouse brain and twice in monkey kidney cells, all of the studies used viruses that had been passaged less than four times prior to their use in experimental infections of mosquitoes. Finally, one of the studies included in the meta-analysis, in which two different experimental methods of infection were used with the same mosquito–virus combination (Joshi et al. 2002), helps tease apart the respective influences of the evolutionary history and the experimental means of infection. In that study, parental Ae. aegypti inoculated intrathoracically with dengue-3 virus had higher mortality than diluent-inoculated controls. In the subsequent seven generations, although adult mortality was not reported, transovarially infected progeny had higher larval mortality and lower fecundity than uninfected siblings (Joshi et al. 2002). Thus, in this instance, the level of virulence did not seem to depend on the experimental mode of infection but rather on biological features of this particular vector–virus association.
A more likely explanation is that varying degrees of virulence observed in different mosquito–virus systems may in fact be due to differing selective pressures on viruses that are primarily transmitted horizontally versus vertically in nature. Theory predicts that the optimal level of virulence depends on the relative frequencies of horizontal and vertical transmission (Ebert & Herre 1996). Although it can be complicated by spatial population structure (Ferdy 2009), phenotypic plasticity (Vizoso & Ebert 2005b) or multiplicity of infection (Vizoso & Ebert 2005a), the general prediction is that vertical transmission of pathogens selects for lower virulence. When viruses are primarily maintained by vertical transmission we would expect selection to favour reduced virulence in order to maximize the virus's basic reproductive number, which is directly dependent on the progeny of the host they are currently infecting and, thus, host fitness. Conversely, viruses that are primarily maintained horizontally can reduce fitness of their current host without reducing their basic reproductive number, as long as any adverse host effects do not reduce their transmission rate. In other words, we expect stronger selective pressure for attenuation of virulence in vertically than horizontally transmitted viruses.
In our meta-analysis, low levels of virulence were associated with transovarial transmission in Aedes–Bunyavirus combinations that are characterized by high natural rates of vertical transmission. Vertical transmission rates of the majority of mosquito-borne viruses are low (generally less than 1% and often less than 0.1%) in the progeny of infected females (Turell 1988). Exceptions are members of the California serogroup of the genus Bunyavirus that are typically transmitted vertically at a frequency of approximately 20 per cent (Turell 1988). For example, vertical transmission rates of California encephalitis (CE) virus ranged from 13 to 26 per cent in Ae. melanimon and from 9 to 29 per cent in Ae. dorsalis (Turell et al. 1982b), and up to 71 per cent for Ae. triseriatus infected by LAC virus (Miller et al. 1977). In addition, when females were initially infected either orally or by IT inoculation, daughters that became infected transovarially (approx. 20%) allowed the establishment of ‘stabilized’ transovarial transmission at a high frequency (more than 90%), which persisted for several generations (Tesh & Shroyer 1980; Turell et al. 1982a). Mating experiments indicated that stabilized infections of CE virus in Ae. dorsalis did not result from selection for a mosquito genotype with high vertical transmission (Turell et al. 1982a). Rather, variation in vertical transmission rates among virus strains suggested that the mode of transmission was determined by the viral genotype (Turell et al. 1982b). Thus, it is more likely that the virus isolates initially used in these studies consisted of a mixture of genetic variants differing in their relative efficiency at horizontal versus vertical transmission, and that variants more efficient at vertical transmission were selected in a single generation in the laboratory. The analysis of a population dynamical model for the evolution of virulence with both vertical and horizontal transmission showed that two types of strains favoured by each mode of transmission can coexist (Lipsitch et al. 1996). When rates of horizontal and vertical transmission were allowed to evolve independently, the model predicted high vertical transmission and low horizontal transmission levels if vertical transmission was associated with low virulence, and vice versa if vertical transmission required high virulence (Lipsitch et al. 1996). This theoretical result is in agreement with the low virulence of vertically transmitted viruses revealed by our meta-analysis and the observed high rates of vertical transmission by California serogroup mosquito-borne viruses. It supports the hypothesis that although occasional horizontal transmission is required for their maintenance in nature (Fine 1975), these arboviruses have evolved to be maintained primarily by vertical transmission (Turell 1988). The results from another theoretical study predict that this situation may eventually lead to differentiation of avirulent, vertically transmitted symbionts and virulent, horizontally transmitted pathogens (Ferdy & Godelle 2005).
The larger view, however, may not be this simple. Indeed, negative effects on vector fitness by arboviruses in the California serogroup have been reported for fitness-related traits other than adult survival. For example, infection by LAC virus delayed larval development of Ae. dorsalis and Ae. melanimon (Turell et al. 1982c) and decreased the ability to blood feed (increased probing but reduced engorgement) by Ae. triseriatus (Grimstad et al. 1980). Reduced fecundity was reported for Ae. albopictus infected with San Angelo virus, another member of the California serogroup (Tesh 1980). Nevertheless, although these effects negatively impact the fitness of mosquitoes and may, therefore, act as a selective pressure on vector populations (Grimstad et al. 1977), the cost to the virus may be relatively small. Examination of the various mechanisms of arbovirus virulence in vectors (see below) can help explain why.
Several mechanisms have been proposed to explain deleterious effects of arboviral infection on vector fitness. Tissue damage due to viral replication may disrupt physiological functions such as digestion or salivation. Cytopathological effects of arbovirus infection have been found in mosquito midgut (Weaver et al. 1988, 1992; Vaidyanathan & Scott 2006) and salivary gland (Mims et al. 1966; Girard et al. 2005) tissues. Resource depletion due to direct competition with pathogen growth is thought to be responsible for a direct cost on mosquito reproduction (Hurd et al. 1995). Indirect effects such as alterations in the blood chemistry of an infected host (Ferguson et al. 2005), or the cost of immune activation (Ahmed & Hurd 2006), may also have substantial consequences on vector fitness. Finally, behavioural modifications that are advantageous for virus transmission, such as increased probing and feeding times (Platt et al. 1997), may contribute to increased feeding-associated mosquito mortality (Anderson et al. 2000). The relative contribution of these different virulence mechanisms is currently unknown. However, higher mosquito mortality was observed for disseminated infections (in legs) compared with non-disseminated infections in two of the studies included in our meta-analysis (Faran et al. 1987; Moncayo et al. 2000). This supports the idea that the cost of infection depends on the viral load and could be due to the direct impact of virus growth. This topic merits additional study.
We speculate that, although mosquitoes infected vertically probably suffer from the direct costs of infection, such as tissue damage and resource depletion, they may experience fewer indirect negative effects. In particular, because they do not become infected by feeding on an infected host, they do not undergo potentially negative effects associated with altered blood composition and digestion. Moreover, transovarial transmission may be achieved at lower titres than horizontal transmission (Tesh & Shroyer 1980), thus minimizing viral load and cytopathological effects. Because blood feeding is not required for arboviruses primarily maintained by vertical transmission, these viruses may not have evolved behavioural manipulation strategies associated with enhanced transmission and concomitant increased vector mortality (Hurd 2003).
Our results confirm that the relationship between vector-borne viruses and their arthropod vectors need not be benign. Our meta-analysis indicates that the overall higher virulence of horizontally transmitted arboviruses was probably confounded in the literature by lower virulence levels among vertically maintained viruses.
Acknowledgments
The authors thank Samuel Alizon and Aaron Brault for their helpful discussions and two anonymous reviewers for their comments, which that improved the manuscript. L.L. is supported by a postdoctoral Marie Curie Outgoing International Fellowship from the Sixth Framework Programme of the European Commission.
References
- Agnew P., Koella J.C. Virulence, parasite mode of transmission, and host fluctuating asymmetry. Proc. R. Soc. B. 1997;264:9–15. doi: 10.1098/rspb.1997.0002. doi:10.1098/rspb.1997.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A.M., Hurd H. Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microbes Infect. 2006;8:308–315. doi: 10.1016/j.micinf.2005.06.026. doi:10.1016/j.micinf.2005.06.026 [DOI] [PubMed] [Google Scholar]
- Anderson R.M., May R.M. Coevolution of hosts and parasites. Parasitology. 1982;85:411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- Anderson R.A., Knols B.G.J., Koella J.C. Plasmodium falciparum sporozoites increase feeding-associated mortality of their mosquito hosts Anopheles gambiae s.l. Parasitology. 2000;120:329–333. doi: 10.1017/s0031182099005570. doi:10.1017/S0031182099005570 [DOI] [PubMed] [Google Scholar]
- Benzécri J.P. Dunod; Paris, France: 1973. L'Analyse des Données, v. II. L'Analyse des Correspondences. [Google Scholar]
- Berry W.J., Rowley W.A., Clarke J.L., 3rd, Swack N.S., Hausler W.J., Jr Spontaneous flight activity of Aedes trivittatus (Diptera: Culicidae) infected with trivittatus virus (Bunyaviridae: California serogroup) J. Med. Entomol. 1987;24:286–289. doi: 10.1093/jmedent/24.3.286. [DOI] [PubMed] [Google Scholar]
- Bull J.J., Molineux I.J., Rice W.R. Selection of benevolence in a host–parasite system. Evolution. 1991;45:875–882. doi: 10.1111/j.1558-5646.1991.tb04356.x. doi:10.2307/2409695 [DOI] [PubMed] [Google Scholar]
- Burnet F.M., White D.O. Cambridge University Press; Cambridge, UK: 1972. Natural history of infectious diseases. [Google Scholar]
- Clayton D.H., Tompkins D.M. Ectoparasite virulence is linked to mode of transmission. Proc. R. Soc. B. 1994;256:211–217. doi: 10.1098/rspb.1994.0072. doi:10.1098/rspb.1994.0072 [DOI] [PubMed] [Google Scholar]
- Cooper L.A., Sina B.J., Turell M.J., Scott T.W. Effects of initial dose on eastern equine encephalomyelitis virus dependent mortality in intrathoracically inoculated Culiseta melanura (Diptera: Culicidae) J. Med. Entomol. 2000;37:815–819. doi: 10.1603/0022-2585-37.6.815. [DOI] [PubMed] [Google Scholar]
- Dohm D.J., Romoser W.S., Turell M.J., Linthicum K.J. Impact of stressful conditions on the survival of Culex pipiens exposed to Rift Valley fever virus. J. Am. Mosq. Control Assoc. 1991;7:621–623. [PubMed] [Google Scholar]
- Dye C. The analysis of parasite transmission by bloodsucking insects. Annu. Rev. Entomol. 1992;37:1–19. doi: 10.1146/annurev.en.37.010192.000245. doi:10.1146/annurev.en.37.010192.000245 [DOI] [PubMed] [Google Scholar]
- Ebert D. Experimental evolution of parasites. Science. 1998;282:1432–1435. doi: 10.1126/science.282.5393.1432. doi:10.1126/science.282.5393.1432 [DOI] [PubMed] [Google Scholar]
- Ebert D., Herre E.A. The evolution of parasitic diseases. Parasitol. Today. 1996;12:96–101. doi: 10.1016/0169-4758(96)80668-5. doi:10.1016/0169-4758(96)80668-5 [DOI] [PubMed] [Google Scholar]
- Elliot S.L., Adler F.R., Sabelis M.W. How virulent should a parasite be to its vector? Ecology. 2003;84:2568–2574. doi:10.1890/02-8013 [Google Scholar]
- Ewald P.W. Evol. Infect. Dis. Oxford University Press; Oxford, UK: 1994. Vectors, vertical transmission, and the evolution of virulence; pp. 35–56. [Google Scholar]
- Faran M.E., Turell M.J., Romoser W.S., Routier R.G., Gibbs P.H., Cannon T.L., Bailey C.L. Reduced survival of adult Culex pipiens infected with Rift Valley fever virus. Am. J. Trop. Med. Hyg. 1987;37:403–409. doi: 10.4269/ajtmh.1987.37.403. [DOI] [PubMed] [Google Scholar]
- Ferdy J.-B. Virulence and transmission modes in metapopulations: when group selection increases virulence. J. Theor. Biol. 2009;256:286–296. doi: 10.1016/j.jtbi.2008.09.032. doi:10.1016/j.jtbi.2008.09.032 [DOI] [PubMed] [Google Scholar]
- Ferdy J.-B., Godelle B. Diversification of transmission modes and the evolution of mutualism. Am. Nat. 2005;166:613–627. doi: 10.1086/491799. doi:10.1086/491799 [DOI] [PubMed] [Google Scholar]
- Ferguson H.M., Read A.F. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 2002;18:256–261. doi: 10.1016/s1471-4922(02)02281-x. doi:10.1016/S1471-4922(02)02281-X [DOI] [PubMed] [Google Scholar]
- Ferguson H.M., Gouagna L.C., Obare P., Read A.F., Babiker H., Githure J., Beier J.C. The presence of Plasmodium falciparum gametocytes in human blood increases the gravidity of Anopheles gambiae mosquitoes. Am. J. Trop. Med. Hyg. 2005;73:312–320. [PubMed] [Google Scholar]
- Fine P.E. Vectors and vertical transmission: an epidemiologic perspective. Ann. N.Y. Acad. Sci. 1975;266:173–194. doi: 10.1111/j.1749-6632.1975.tb35099.x. doi:10.1111/j.1749-6632.1975.tb35099.x [DOI] [PubMed] [Google Scholar]
- Frank S.A. Models of parasite virulence. Q. Rev. Biol. 1996;71:37–78. doi: 10.1086/419267. doi:10.1086/419267 [DOI] [PubMed] [Google Scholar]
- Girard Y.A., Popov V., Wen J., Han V., Higgs S. Ultrastructural study of West Nile virus pathogenesis in Culex pipiens quinquefasciatus (Diptera: Culicidae) J. Med. Entomol. 2005;42:429–444. doi: 10.1093/jmedent/42.3.429. doi:10.1603/0022-2585(2005)042[0429:USOWNV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Greenacre M. Academic Press; London, UK: 1983. Theory and applications of correspondence analysis. [Google Scholar]
- Grimstad P.R., Craig G.B., Jr, Ross Q.E., Yuill T.M. Aedes triseriatus and La Crosse virus: geographic variation in vector susceptibility and ability to transmit. Am. J. Trop. Med. Hyg. 1977;26:990–996. doi: 10.4269/ajtmh.1977.26.990. [DOI] [PubMed] [Google Scholar]
- Grimstad P.R., Ross Q.E., Craig G.B., Jr Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. II. Modification of mosquito feeding behavior by virus infection. J. Med. Entomol. 1980;17:1–7. doi: 10.1093/jmedent/17.1.1. [DOI] [PubMed] [Google Scholar]
- Gubler D.J. Human arbovirus infections worldwide. Ann. N.Y. Acad. Sci. 2001;951:13–24. doi: 10.1111/j.1749-6632.2001.tb02681.x. [DOI] [PubMed] [Google Scholar]
- Gubler D.J. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. doi:10.1016/S0188-4409(02)00378-8 [DOI] [PubMed] [Google Scholar]
- Hurd H. Manipulation of medically important insect vectors by their parasites. Annu. Rev. Entomol. 2003;48:141–161. doi: 10.1146/annurev.ento.48.091801.112722. doi:10.1146/annurev.ento.48.091801.112722 [DOI] [PubMed] [Google Scholar]
- Hurd H., Hogg J.C., Renshaw M. Interactions between bloodfeeding, fecundity and infection in mosquitoes. Parasitol. Today. 1995;11:411–416. doi:10.1016/0169-4758(95)80021-2 [Google Scholar]
- Joshi V., Mourya D.T., Sharma R.C. Persistence of dengue-3 virus through transovarial transmission passage in successive generations of Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg. 2002;67:158–161. doi: 10.4269/ajtmh.2002.67.158. [DOI] [PubMed] [Google Scholar]
- Kramer L.D., Ebel G.D. Dynamics of Flavivirus infection in mosquitoes. Adv. Virus Res. 2003;60:187–232. doi: 10.1016/s0065-3527(03)60006-0. doi:10.1016/S0065-3527(03)60006-0 [DOI] [PubMed] [Google Scholar]
- Kuno G., Chang G.J. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin. Microbiol. Rev. 2005;18:608–637. doi: 10.1128/CMR.18.4.608-637.2005. doi:10.1128/CMR.18.4.608-637.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte L.C., Jr Japanese B encephalitis virus in the organs of infected mosquitoes. Am. J. Hyg. 1960;72:73–87. doi: 10.1093/oxfordjournals.aje.a120136. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Rowley W.A., Platt K.B. Longevity and spontaneous flight activity of Culex tarsalis (Diptera: Culicidae) infected with western equine encephalomyelitis virus. J. Med. Entomol. 2000;37:187–193. doi: 10.1603/0022-2585-37.1.187. [DOI] [PubMed] [Google Scholar]
- Levin B.R. The evolution and maintenance of virulence in microparasites. Emerg. Infect. Dis. 1996;2:93–102. doi: 10.3201/eid0202.960203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M., Siller S., Nowak M.A. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution. 1996;50:1729–1741. doi: 10.1111/j.1558-5646.1996.tb03560.x. doi:10.2307/2410731 [DOI] [PubMed] [Google Scholar]
- Mahmood F., Reisen W.K., Chiles R.E., Fang Y. Western equine encephalomyelitis virus infection affects the life table characteristics of Culex tarsalis (Diptera: Culicidae) J. Med. Entomol. 2004;41:982–986. doi: 10.1603/0022-2585-41.5.982. [DOI] [PubMed] [Google Scholar]
- Miller B.R., DeFoliart G.R., Yuill T.M. Vertical transmission of La Crosse virus (California encephalitis group): transovarial and filial infection rates in Aedes triseriatus (Diptera: Culicidae) J. Med. Entomol. 1977;14:437–440. doi: 10.1093/jmedent/14.4.437. [DOI] [PubMed] [Google Scholar]
- Mims C.A., Day M.F., Marshall I.D. Cytopathic effect of Semliki Forest virus in the mosquito Aedes aegypti. Am. J. Trop. Med. Hyg. 1966;15:775–784. doi: 10.4269/ajtmh.1966.15.775. [DOI] [PubMed] [Google Scholar]
- Moncayo A.C., Edman J.D., Turell M.J. Effect of eastern equine encephalomyelitis virus on the survival of Aedes albopictus, Anopheles quadrimaculatus, and Coquillettidia perturbans (Diptera: Culicidae) J. Med. Entomol. 2000;37:701–706. doi: 10.1603/0022-2585-37.5.701. [DOI] [PubMed] [Google Scholar]
- Patrican L.A., DeFoliart G.R. Lack of adverse effect of transovarially acquired La Crosse virus infection on the reproductive capacity of Aedes triseriatus (Diptera: Culicidae) J. Med. Entomol. 1985;22:604–611. doi: 10.1093/jmedent/22.6.604. [DOI] [PubMed] [Google Scholar]
- Platt K.B., Linthicum K.J., Myint K.S., Innis B.L., Lerdthusnee K., Vaughn D.W. Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am. J. Trop. Med. Hyg. 1997;57:119–125. doi: 10.4269/ajtmh.1997.57.119. [DOI] [PubMed] [Google Scholar]
- Putnam J.L., Scott T.W. Blood-feeding behavior of dengue-2 virus-infected Aedes aegypti. Am. J. Trop. Med. Hyg. 1995;52:225–227. doi: 10.4269/ajtmh.1995.52.225. [DOI] [PubMed] [Google Scholar]
- Read A.F. The evolution of virulence. Trends Microbiol. 1994;2:73–76. doi: 10.1016/0966-842x(94)90537-1. doi:10.1016/0966-842X(94)90537-1 [DOI] [PubMed] [Google Scholar]
- Rosenberg M.S., Adams D.C., Gurevitch J. Sinauer Associates; Sunderland, MA: 2000. MetaWin: statistical software for meta-analysis, version 2. [Google Scholar]
- Rosenthal R. The ‘file drawer problem’ and tolerance for null results. Psychol. Bull. 1979;86:638–641. doi:10.1037/0033-2909.86.3.638 [Google Scholar]
- Scott T.W., Lorenz L.H. Reduction of Culiseta melanura fitness by eastern equine encephalomyelitis virus. Am. J. Trop. Med. Hyg. 1998;59:341–346. doi: 10.4269/ajtmh.1998.59.341. [DOI] [PubMed] [Google Scholar]
- Sokal R.R., Rohlf F.J. W. H. Freeman & Co; New York, NY: 1995. Biometry: the principles and practice of statistics in biological research. [Google Scholar]
- Styer L.M., Meola M.A., Kramer L.D. West Nile virus infection decreases fecundity of Culex tarsalis females. J. Med. Entomol. 2007;44:1074–1085. doi: 10.1603/0022-2585(2007)44[1074:wnvidf]2.0.co;2. doi:10.1603/0022-2585(2007)44[1074:WNVIDF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tesh R.B. Experimental studies on the transovarial transmission of Kunjin and San Angelo viruses in mosquitoes. Am. J. Trop. Med. Hyg. 1980;29:657–666. doi: 10.4269/ajtmh.1980.29.657. [DOI] [PubMed] [Google Scholar]
- Tesh R.B., Shroyer D.A. The mechanism of arbovirus transovarial transmission in mosquitoes: San Angelo virus in Aedes albopictus. Am. J. Trop. Med. Hyg. 1980;29:1394–1404. doi: 10.4269/ajtmh.1980.29.1394. [DOI] [PubMed] [Google Scholar]
- Turell M.J. Horizontal and vertical transmission of viruses by insect and tick vectors. In: Monath T.P., editor. The arboviruses: epidemiology and ecology. CRC Press; Boca Raton, FL: 1988. pp. 127–152. [Google Scholar]
- Turell M.J., Hardy J.L., Reeves W.C. Stabilized infection of California encephalitis virus in Aedes dorsalis, and its implications for viral maintenance in nature. Am. J. Trop. Med. Hyg. 1982a;31:1252–1259. doi: 10.4269/ajtmh.1982.31.1252. [DOI] [PubMed] [Google Scholar]
- Turell M.J., Reeves W.C., Hardy J.L. Evaluation of the efficiency of transovarial transmission of California encephalitis viral strains in Aedes dorsalis and Aedes melanimon. Am. J. Trop. Med. Hyg. 1982b;31:382–388. doi: 10.4269/ajtmh.1982.31.382. [DOI] [PubMed] [Google Scholar]
- Turell M.J., Reeves W.C., Hardy J.L. Transovarial and trans-stadial transmission of California encephalitis virus in Aedes dorsalis and Aedes melanimon. Am. J. Trop. Med. Hyg. 1982c;31:1021–1029. doi: 10.4269/ajtmh.1982.31.1021. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R., Scott T.W. Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis. 2006;11:1643–1651. doi: 10.1007/s10495-006-8783-y. doi:10.1007/s10495-006-8783-y [DOI] [PubMed] [Google Scholar]
- Vizoso D.B., Ebert D. Mixed inoculations of a microsporidian parasite with horizontal and vertical infections. Oecologia. 2005a;143:157–166. doi: 10.1007/s00442-004-1771-4. doi:10.1007/s00442-004-1771-4 [DOI] [PubMed] [Google Scholar]
- Vizoso D.B., Ebert D. Phenotypic plasticity of host–parasite interactions in response to the route of infection. J. Evol. Biol. 2005b;18:911–921. doi: 10.1111/j.1420-9101.2005.00920.x. doi:10.1111/j.1420-9101.2005.00920.x [DOI] [PubMed] [Google Scholar]
- Weaver S.C. Evolutionary influences in arboviral disease. Curr. Top. Microbiol. Immunol. 2006;299:285–314. doi: 10.1007/3-540-26397-7_10. doi:10.1007/3-540-26397-7_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C., Scott T.W., Lorenz L.H., Lerdthusnee K., Romoser W.S. Togavirus-associated pathologic changes in the midgut of a natural mosquito vector. J. Virol. 1988;62:2083–2090. doi: 10.1128/jvi.62.6.2083-2090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C., Lorenz L.H., Scott T.W. Pathologic changes in the midgut of Culex tarsalis following infection with Western equine encephalomyelitis virus. Am. J. Trop. Med. Hyg. 1992;47:691–701. doi: 10.4269/ajtmh.1992.47.691. [DOI] [PubMed] [Google Scholar]