Abstract
Jaws and dentition closely resembling those of the extant tuatara (Sphenodon) are described from the Manuherikia Group (Early Miocene; 19–16 million years ago, Mya) of Central Otago, New Zealand. This material is significant in bridging a gap of nearly 70 million years in the rhynchocephalian fossil record between the Late Pleistocene of New Zealand and the Late Cretaceous of Argentina. It provides the first pre-Pleistocene record of Rhynchocephalia in New Zealand, a finding consistent with the view that the ancestors of Sphenodon have been on the landmass since it separated from the rest of Gondwana 82–60 Mya. However, if New Zealand was completely submerged near the Oligo-Miocene boundary (25–22 Mya), as recently suggested, an ancestral sphenodontine would need to have colonized the re-emergent landmass via ocean rafting from a currently unrecorded and now extinct Miocene population. Although an Early Miocene record does not preclude that possibility, it substantially reduces the temporal window of opportunity. Irrespective of pre-Miocene biogeographic history, this material also provides the first direct evidence that the ancestors of the tuatara, an animal often perceived as unsophisticated, survived in New Zealand despite substantial local climatic and environmental changes.
Keywords: biogeography, fossil, Gondwana, Miocene, Rhynchocephalia, Sphenodontinae
1. Introduction
The New Zealand tuatara (Sphenodon) is the only living member of the Rhynchocephalia (sensu Gauthier et al. 1988), the sister taxon to the Squamata (snakes, amphisbaenians and lizards). Sphenodon is often mistakenly described as a ‘living fossil’ (e.g. Robb 1977). However, fossil rhynchocephalians were surprisingly diverse in their morphology, diet and lifestyle (e.g. Reynoso 2000; Jones 2006a,b, 2008), and many features of the living genus, previously thought primitive, are now known to be derived (e.g. Gans 1983; Whiteside 1986; Evans 2003; Jones 2008). Furthermore, although squamates and rhynchocephalians separated 240–250 Mya, the latter are the more common in early Mesozoic assemblages and are found worldwide (figure 1), including England and Wales where their fossil remains occur in substantial quantities (e.g. Fraser 1988). However, the geographical range of rhynchocephalians seems to contract after the Early Jurassic, first in Laurasia and later in Gondwana (Evans et al. 2001, Apesteguía & Novas 2003; Apesteguía 2005a; Jones 2006c), possibly as the result of the competition with derived lizards (Saint-Girons 1985; Milner et al. 2000) and/or mammals (Gorniak et al. 1982; Whiteside 1986; Jones 2006b).
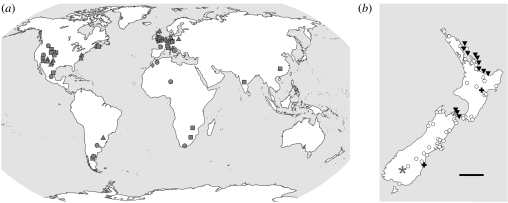
Figure 1.
Rhynchocephalian localities. (a) Global map showing fossil localities (see the electronic supplementary material). (b) New Zealand, redrawn from Hay et al. (2003). Triangles, Triassic; squares, Jurassic; filled circles, Cretaceous; diamonds, Palaeocene?; asterisk, Miocene; pluses, Pleistocene; open circles, Holocene; down triangles, extant populations. Scale bar, 200 km. 175×73 mm (600×600 dpi).
In marked contrast to its Early Mesozoic relatives, Sphenodon is restricted to approximately 35 islands off the coast of New Zealand (MacAvoy et al. 2007), mainland populations having become extinct with the arrival of humans (and associated animals, such as rats) ca 750 years ago (Towns & Daugherty 1994, Higham et al. 1999). Sphenodon bones are known from several Holocene localities (e.g. Crook 1975, Worthy & Holdaway 1996) but the oldest known rhynchocephalian material from New Zealand is currently Late Pleistocene in age, between 27 000 and 34 000 years old (Worthy & Grant-Mackie 2003). Previous reports of Miocene Sphenodon material (e.g. Crook 1975, p. 338; Rich 1975, p. 50; Robb 1977, p. 4) were based on a misidentified Holocene prey accumulation of the laughing owl Sceloglaux albifacies (Worthy & Holdaway 1996). Outside New Zealand, apart from a problematic lepidosaur specimen from the Palaeocene of Morocco (Augé & Rage 2006), the next most recent records of rhynchocephalians are from the Late Cretaceous Allen (Martinelli & Forasiepi 2004; Apesteguía & Rougier 2007) and Los Alamitos formations (Apesteguía 2005b) of Argentina (ca 70 Mya).
Here, we describe the first unambiguous pre-Pleistocene and post-Mesozoic rhynchocephalian material from the Miocene of New Zealand. This makes a significant addition to both the poorly known fossil record of New Zealand and the scant post-Mesozoic history of Rhynchocephalia. It also impacts on an ongoing debate as to the history of New Zealand's unique fauna and flora. It has recently been proposed that New Zealand was totally submerged sometime around the Oligocene–Miocene boundary (25–22 Mya), requiring that New Zealand's entire fauna, previously considered partially vicariant and archaic, is actually secondary (e.g. Campbell & Landis 2001; Waters & Craw 2006; Trewick et al. 2007; Campbell & Hutching 2008; Landis et al. 2008). An Early Miocene rhynchocephalian clearly has implications for this hypothesis.
2. Systematic palaeontology
Rhynchocephalia Günther (1867) sensu Gauthier et al. (1988).
Sphenodontinae Cope (1871) sensu Reynoso (1996)
cf. Sphenodon sp.
(a) Material
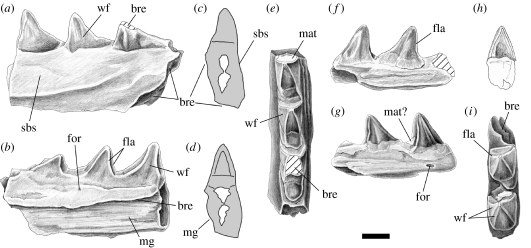
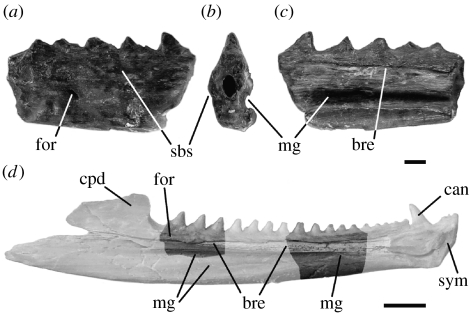
Three partial dentaries: a right bearing two posterior teeth (NMNZ S43075); a left bearing three posterior teeth (NMNZ S42282); and a left bearing five anterior teeth (NMNZ S50860) (figures 2 and 3).
Figure 2.
Partial sphenodontine dentaries from the Miocene Bannockburn Formation of New Zealand. (a,e) Specimen NMNZ S42282 and (f,i) specimen NMNZ S43075; (a,f) labial views, (b,g) lingual views, (c,h) anterior views, (d) posterior view, and (e,i) apical views. bre, breakage; fla, flange; for, foramen; mat, matrix; mg, meckelian groove; sbs, secondary bone skirt; wf, wear facet. Scale bar, 1 mm. 175×82 mm (600×600 dpi).
Figure 3.
Dentaries. Specimen NMNZ S50860 in (a) labial, (b) posterior and (c) lingual view. (d) A Holocene Sphenodon left dentary (CMC Rep35 from Marfell's Beach, Lake Grassmere, Marlborough, northern South Island) in lingual view indicating the parts of the dentary preserved by NMNZ S42282 and NMNZ S50860. bre, breakage; can, caniniform; cpd, coronoid process of the dentary; mg, Meckelian groove; sym, symphysis. Scale bar (a–c), 1 mm; scale bar (d), 5 mm. 83×57 mm (600×600 dpi).
(b) Locality
St Bathans, Central Otago, South Island, NZ; Bannockburn Formation of the Manuherikia Group, Early Miocene, 19–16 Mya, St Bathans Fauna (Worthy et al. 2006, 2007; see the electronic supplementary material).
(c) Palaeoenvironment
Sediments from the palaeo-Lake Manuherikia (more than 5600 km2) have yielded a diverse array of invertebrates, fishes, geckos, skinks, birds, a crocodilian, bats and an archaic terrestrial mammal (Molnar & Pole 1997; Worthy et al. 2002, 2006, 2007; Pole et al. 2003; Gibbs 2006; Worthy & Lee 2008). The climate was warm temperate and there was a wide range of vegetation including eucalypts, casuarinas, podocarps and palms (Pole et al. 2003; Pole 2008).
(d) Description
Although incomplete, the dentaries are well preserved. The teeth are acrodont (fused to the crest of the jawbone) and pyramidal with an anteriorly positioned apex (figure 2a,b,f,g). The anterior surface of each tooth is concave and bordered by weak anterolingual and anterolabial flanges (figure 2e,i), and the labial and lingual surfaces of all teeth are slightly worn (figure 2e,i). The bases of the teeth in NMNZ S42282 decrease in mesiodistal length from the posterior end (figure 2a,e).
The labial surface of NMNZ S42282 shows the posterior end of a secondary bone ‘skirt’ below the tooth row (figure 2a) whereas NMNZ S50860 shows the anterior end (figure 3). The lingual surfaces of NMNZ S42282 and NMNZ S50860 preserve part of the meckelian groove but the anterodorsal margin is broken away as is common in Holocene material (figures 2b,c and 3). The surface texture of the groove consists of very subtle longitudinal ridges (figure 2b). A small foramen is present (NMNZ S43075 and NMNZ S42282) on the lingual surface just ventral to the most posteriorly preserved tooth (figure 2b,g), and anteriorly on the lateral surface below the skirt of secondary bone in specimen NMNZ S50860. These probably correspond to similarly located nutrient foramina found in Sphenodon (e.g. specimens LDUCZ ×723, ×804).
3. Phylogenetic affinity
The Manuherikia jaws reveal two diagnostic features of rhynchocephalians: acrodont teeth and an enlarged palatine tooth row near parallel to the maxillary row as inferred by the characteristic pattern of lingual wear on the dentary teeth (Reynoso 1996; Evans 2003). In addition, a secondary bone skirt is a character of derived rhynchocephalians, such as Clevosaurus and Sphenodon (Fraser 1988; Jones 2006a,c). The dentary teeth are unlike those of ‘basal taxa’, clevosaurs, pleurosaurs, sapheosaurs or eilenodontines; instead, they closely resemble those of Sphenodon (Robinson 1976; Gorniak et al. 1982; Jones 2006a,b) and of Mesozoic fossils, such as Cynosphenodon (Middle Jurassic of Mexico), that are allied with Sphenodon in Sphenodontinae (sensu Reynoso 1996). As well as characteristic pyramidal dentary teeth, sphenodontines are grouped by the possession of one or more caniniform teeth and smooth dental enamel (Reynoso 1996). The Miocene material is too incomplete to record the presence of a caniniform but the preserved enamel is smooth.
The Manuherikia specimens fall within the range of morphological variation exhibited by modern Sphenodon, but cannot be referred to it with confidence because our knowledge of other sphenodontines is so limited. Sphenodontines are now known from several localities worldwide but the material consists almost exclusively of partial jaws (e.g. Reynoso 1996). Interrelationships between representative taxa are poorly understood because comparisons are generally limited to caniniform number and minor differences in tooth morphology. Furthermore, some taxa cannot be directly compared with each other because they are each represented by different elements (see the electronic supplementary material). Moreover, characters currently used to distinguish sphenodontines from one another may not be reliable in small sample sizes because of the way rhynchocephalian teeth grow and are worn (Robinson 1976). For example, spacing of the dentary tooth cusps is considered smaller in Cynosphenodon, Sphenovipera and Theretairus than in Sphenodon (Reynoso 1996, 2005), but this feature is highly variable in the latter taxon (see the electronic supplementary material). We therefore refer the material to Sphenodontinae but not necessarily Sphenodon.
4. Fossil record and biogeography
This material helps to bridge a gap in the rhynchocephalian fossil record of nearly 70 Mya between the Late Pleistocene (Worthy & Grant-Mackie 2003) and the Late Cretaceous (Apesteguía & Novas 2003; Martinelli & Forasiepi 2004; Apesteguía 2005a,b). It also represents the first direct evidence that a rhynchocephalian was present on New Zealand prior to the Pleistocene. This is significant because it suggests that the ancestors of Sphenodon, an animal often considered to be primitive and unspecialized (e.g. Robb 1977; Dawbin 1982), survived in New Zealand despite substantial changes in climate and environment. These include not only a global temperature drop of approximately 8°C after the Mid-Miocene optimum, but also, more locally, mountain building, Pleistocene glacial–interglacial oscillations, floral overturn and faunal changes (Cooper & Millener 1993; Pole 1994; Cooper & Cooper 1995; Worthy et al. 2006; Pole 2008). This does not necessarily dispute recent suggestions that the living Sphenodon will be threatened by global warming because today's populations are geographically restricted and less extensive (Nelson et al. 2004; Mitchell et al. 2008).
The pre-Miocene history of rhynchocephalians on New Zealand remains unknown, as does the more general post-Mesozoic history of rhynchocephalians globally. The terrestrial Mesozoic fossil record of New Zealand vertebrates is limited to the non-avian dinosaurs and pterosaurs of the Late Cretaceous (75–80 Mya) Maungataniwha Sandstone (e.g. Molnar & Wiffen 1994; Molnar et al. 1996, 1998). However, most workers have inferred that rhynchocephalians ancestral to the modern Sphenodon were present on New Zealand when it separated from Antarctica and the rest of Gondwana 82–60 Mya (e.g. Fleming 1975; Robb 1977; Dawbin 1982; Cooper & Millener 1993; Cooper & Cooper 1995; Evans et al. 2001; Hay et al. 2003; Apesteguía 2005a,b; Gibbs 2006). At that time, the climate of Antarctica was warm to cool temperate (Dingle & Lavelle 1998).
Between the Late Oligocene and earliest Miocene (35–22 Mya) a eustatic sea-level rise submerged much of Zealandia (New Zealand and associated continental crust; Luyendyk 1995) and this may have caused the genetic bottleneck found in birds, skinks and flightless Orthoptera (Daugherty et al. 1993; Cooper & Cooper 1995; Trewick & Morgan-Richards 2005). Genetic divergence of living Sphenodon populations occurred fairly recently, perhaps related to climate change in the Pleistocene (Hay et al. 2003), and this unfortunately masks any evidence for or against an ‘Oligocene bottleneck’ in the genus.
An absence of Oligocene terrestrial sediments has prompted the suggestion that Zealandia was ‘totally’ submerged during the Waitakian Stage 25–22 Mya (Campbell & Landis 2001; Trewick et al. 2007; Campbell & Hutching 2008; Landis et al. 2008). If correct, this would require rhynchocephalians (and other taxa recorded from the Manuherikia Group) to have colonized New Zealand shortly after its re-emergence (Trewick et al. 2007). There is evidence that much of the New Zealand biota arrived post-Oligocene (including many plants; Pole 1994; Waters & Craw 2006). However, the biota of Madagascar has also undergone radical changes since the Mesozoic, and yet its complete submergence has never been required as an explanation (Krause et al. 1997). The age of the fossil material described here is compatible with both the ancient vicariance hypothesis and the post-Oligocene transoceanic dispersal hypothesis, but it does reduce the time available for a transoceanic dispersal from up to 25 Ma to perhaps as little as 3 Ma.
Waters & Craw (2006) stated that Sphenodon is ‘uninformative with regards to New Zealand's geological history’. They seem to accept a priori (without any reference to Sphenodon biology or a single citation on the rhynchocephalian fossil record) that a transoceanic dispersal was possible, and that a population of Sphenodon-like rhynchocephalians existed outside New Zealand until after the Oligocene. Oceanic dispersal of squamates probably occurred more frequently than previously appreciated (e.g. Carranza et al. 2000; Calsbeek & Smith 2003; De Queiroz 2005; Vidal et al. 2008), but the transoceanic capabilities of modern Sphenodon are questionable. It can swim, but only short distances (Newman 1878; C. H. Daugherty 2008, personal communication). It is able to survive without food for several months (Buller 1879), but dehydration would be a serious problem for a journey of several thousand kilometres (or several weeks) because Sphenodon demonstrates high rates of cutaneous water loss relative to its body mass (Cree & Daugherty 1991; A. Cree 2008, personal communication; C. H. Daugherty 2008, personal communication).
There is currently no evidence of a post-Oligocene source population outside New Zealand. Sanmartín & Ronquist (2004) showed that transoceanic dispersals to New Zealand are most likely to have come from the west but no rhynchocephalians have yet been recovered from the otherwise productive Oligo-Miocene sites at Riversleigh (over 200 localities) or in the Eyre Basin of Australia (Evans et al. 2001; Brace 2003; M. N. Hutchinson 2008, personal communication). A possible record from the Palaeocene of Morocco (Augé & Rage 2006) is based on an indeterminate partial dentary bearing a single tooth (not impossibly an acrodont squamate). Post-Mesozoic populations of rhynchocephalians may have persisted in South America for some time (Apesteguía 2005a), but there is currently no direct evidence of this. The record of Antarctica remains poorly known in general. Absence of evidence is not evidence of absence (Sagan 1996, p. 213), but this also applies to the absence in New Zealand of Cretaceous–Palaeogene rhynchocephalian fossil remains and of Oligocene terrestrial sediments. The sediments may have been eroded away in the 25 million years since their deposition, and known New Zealand Mesozoic localities (comprising shallow marine sediments with transported terrestrial components) have a low preservation potential for small animals; even the recovered pterosaur bone is fairly large (Molnar & Wiffen 1994). We urge further surveying and use of sieving techniques (e.g. Ward 1984).
It currently seems more likely that some local land surface persisted during the Oligocene and allowed the ancestors of Sphenodon, leiopelmatid frogs, Agathis (the kauri tree), certain birds, an archaic mammal, Hyridella (freshwater mussels) and numerous other invertebrates to survive the transgression (Graf & Foighil 2000; Gibbs 2006; Worthy et al. 2006; Knapp et al. 2007; Lee et al. 2007). The extent of the emergent land surface is open to speculation. Landis et al. (2008) have argued that it was much less than approximately 40 000 km2 (15% of the present-day land area), as estimated by Cooper & Cooper (1995), with no certainty that any land remained above water. However, even if Zealandia was reduced to only 1 per cent of today's surface area it would still represent over 2500 km2, well over 1000 times the surface area of Stephen's Island (1.5 km2), where over 30 000 Sphenodon currently live (MacAvoy et al. 2007).
Acknowledgments
We thank Dr David Gower (NHM, London, UK) and Mark Carnall (Grant Museum of Zoology, University College London, London, UK) for access to material, Dr Jennifer M. Hay (Massey University, NZ) who hosted M.E.H.J. in NZ, Rick Webber (Museum of New Zealand Te Papa Tongarewa, NZ) for the use of a binocular microscope and camera lucida, Profs Mike Archer (University of New South Wales, Australia), Charles Daugherty (Victoria University of Wellington, NZ) and Alison Cree (University of Otago, NZ) and Dr Mark N. Hutchinson (South Australia Museum, Adelaide, Australia) for correspondence, and Sebastián Apesteguía (Universidad Maimónides, Argentina), Dr Richard Butler (NHM, London, UK), Paul Upchurch (University College London, UK) and two anonymous referees for their feedback. We are grateful for the generous support of the landowners Jack Enright and Ann and Euan Johnstone, and to numerous people who helped with field excavations, in particular to Craig Jones (Institute of Geological and Nuclear Sciences, NZ) who introduced the HH1 site to T.H.W. and A.J.D.T. This research was supported by funding to T.H.W. from the Public Good Science Fund of the New Zealand Foundation for Research, Science and Technology (TWOX0201) and Australian Research Council grant (DP0770660) and to M.E.H.J. from the Palaeontological Association, UK (2006 Sylvester-Bradley Award).
Supplementary Material
Additonal text, tables and images for all sections of the primary publication. This includes a table of localities known to provide pre-Holocene rhynchocephalian fossils
References
- Apesteguía S. Post-Jurassic sphenodontids: identity of the last lineages. In: Kellner A.W.A., Henriques D.D.R., Rodrigues T., editors. Boletim de resumos, II congresso latino-americano de paleontologia de vertebrados. Museu Nacional, Rio de Janiero; Rio de Janiero, Brazil: 2005a. pp. 26–29. [Google Scholar]
- Apesteguía S. A late Campanian sphenodontid (Reptilia, Diapsida) from northern Patagonia. C. R. Palevol. 2005b;4:663–669. doi:10.1016/j.crpv.2005.06.003 [Google Scholar]
- Apesteguía S., Novas F.E. Large Cretaceous sphenodontian from Patagonia provides insight into lepidosaur evolution in Gondwana. Nature. 2003;425:609–612. doi: 10.1038/nature01995. doi:10.1038/nature01995 [DOI] [PubMed] [Google Scholar]
- Apesteguía S., Rougier G.W. A late Campanian sphenodontid maxilla from northern Patagonia. Am. Mus. Novit. 2007;3581:1–11. doi:10.1206/0003-0082(2007)3581[1:ALCSMF]2.0.CO;2 [Google Scholar]
- Augé M., Rage J.-C. Herpetofaunas from the upper Paleocene and lower Eocene of Morocco. Ann. Paléontol. 2006;92:235–253. doi:10.1016/j.annpal.2005.09.001 [Google Scholar]
- Brace, M. 2003 The stone menagerie. Geogr. Mag.75, July 2003, 17–21.
- Buller W.L. Further notes on the habits of the tuatara lizard. Trans. Proc. N. Z. Inst. 1879;11:349–351. [Google Scholar]
- Calsbeek R., Smith T.B. Ocean currents mediate evolution in island lizards. Nature. 2003;426:552–555. doi: 10.1038/nature02143. doi:10.1038/nature02143 [DOI] [PubMed] [Google Scholar]
- Campbell H.J., Hutching G. Penguin Books; Auckland, New Zealand: 2008. In search of ancient New Zealand. [Google Scholar]
- Campbell H.J., Landis C.A. New Zealand awash. N. Z. Geogr. 2001;51:6–7. [Google Scholar]
- Carranza S., Arnold E.N., Mateo J.A., López-Jurado L.F. Long-distance colonization and radiation in gekkonid lizards, Tarentola (Reptilia: Gekkonidae), revealed by mitochondrial DNA sequences. Proc. R. Soc. B. 2000;267:637–649. doi: 10.1098/rspb.2000.1050. doi:10.1098/rspb.2000.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A.J., Cooper R.A. The Oligocene bottleneck and the New Zealand biota: genetic record of a past environmental crisis. Proc. R. Soc. B. 1995;261:293–302. doi: 10.1098/rspb.1995.0150. doi:10.1098/rspb.1995.0150 [DOI] [PubMed] [Google Scholar]
- Cooper R.A., Millener P.R. The New Zealand biota: historical background and new research. Trends Ecol. Evol. 1993;8:429–433. doi: 10.1016/0169-5347(93)90004-9. doi:10.1016/0169-5347(93)90004-9 [DOI] [PubMed] [Google Scholar]
- Cope E.D. On the homologies of some of the cranial bones of the Reptilia, and on the systematic arrangement of the class. Proc. Am. Assoc. Advan. Sci. 1871;19:194–247. [Google Scholar]
- Cree, A. & Daugherty, C. H. 1991 High rates of cutaneous water loss in nocturnal New Zealand reptiles. Unpublished report to Director, Science and Research Directorate, Department of Conservation.
- Crook I.G. The tuatara. In: Kuschel G., editor. Biogeography and ecology in New Zealand. Junk; The Hague, The Netherlands: 1975. pp. 331–352. [Google Scholar]
- Daugherty C.H., Hitchmough R.A., Patterson G.B. A taxonomic review of the New Zealand herpetofauna: new data challenge some old ideas. N. Z. J. Zool. 1993;20:128. [Google Scholar]
- Dawbin W.H. The tuatara Sphenodon punctatus (Reptilia: Rhynchocephalia): a review. In: Newman D.G., editor. New Zealand herpetology. Wildlife Service Occasional Publication; Wellington, New Zealand: 1982. pp. 149–181. [Google Scholar]
- De Queiroz A. The resurrection of oceanic dispersal in historical biogeography. Trends Ecol. Evol. 2005;20:68–73. doi: 10.1016/j.tree.2004.11.006. doi:10.1016/j.tree.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Dingle R.V., Lavelle M. Late Cretaceous-Cenozoic climatic variations of the northern Antarctic Peninsula: new geochemical evidence and review. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1998;141:215–232. doi:10.1016/S0031-0182(98)00056-X [Google Scholar]
- Evans S.E. At the feet of the dinosaurs: the early history and radiation of lizards. Biol. Rev. 2003;78:513–551. doi: 10.1017/s1464793103006134. doi:10.1017/S1464793103006134 [DOI] [PubMed] [Google Scholar]
- Evans S.E., Prasad G.V.R., Manhas B.K. Rhynchocephalians (Diapsida: Lepidosauria) from the Jurassic Kota Formation of India. Zool. J. Linn. Soc. 2001;133:309–334. doi:10.1111/j.1096-3642.2001.tb00629.x [Google Scholar]
- Fleming C.A. The geological history of New Zealand. In: Kuschel G., editor. Biogeography and ecology in New Zealand. Junk; The Hague, The Netherlands: 1975. pp. 1–87. [Google Scholar]
- Fraser N.C. The osteology and relationships of Clevosaurus (Reptilia: Sphenodontida) Phil. Trans. R. Soc. B. 1988;321:125–178. doi:10.1098/rstb.1988.0092 [Google Scholar]
- Gans C. Is Sphenodon punctatus a maladapted relic? In: Rhodin A.G.J., Miyata K., editors. Advances in herpetology and evolutionary biology. Harvard University; Cambridge MA: 1983. pp. 613–620. [Google Scholar]
- Gauthier J.A., Estes R., de Queiroz K. A phylogenetic analysis of the Lepidosauromorpha. In: Estes R., Pregill G., editors. Phylogenetic relationships of the lizard families: essays commemorating Charles L. Camp. Stanford University Press; Stanford, CA: 1988. pp. 15–98. [Google Scholar]
- Gibbs G. Craig Potton Publishing; Nelson, New Zealand: 2006. Ghosts of Gondwana. The history of life in New Zealand. [Google Scholar]
- Gorniak G.C., Rosenberg H.I., Gans C. Mastication in the tuatara, Sphenodon punctatus (Reptilia: Rhynchocephalia): structure and activity of the motor system. J. Morphol. 1982;171:321–353. doi: 10.1002/jmor.1051710307. doi:10.1002/jmor.1051710307 [DOI] [PubMed] [Google Scholar]
- Graf D.L., Foighil D.Ó. Molecular phylogenetic analysis of 28S rDNA supports a Gondwanan origin for Australasian Hyriidae (Mollusca: Bivalvia: Unionoida) Vie et Milieu. 2000;50:245–254. [Google Scholar]
- Günther A. Contribution to the anatomy of Hatteria (Rhynchocephalus, Owen) Phil. Trans. R. Soc. Lond. 1867;157:1–34. doi:10.1098/rstl.1867.0001 [Google Scholar]
- Hay J.M., Daugherty C.H., Cree A., Maxson L.R. Low genetic divergence obscures phylogeny among populations of Sphenodon, remnant of an ancient reptile lineage. Mol. Phylogenet. Evol. 2003;29:1–19. doi: 10.1016/s1055-7903(03)00091-5. doi:10.1016/S1055-7903(03)00091-5 [DOI] [PubMed] [Google Scholar]
- Higham T.F.G., Anderson A.J., Jacomb C. Dating the first New Zealanders: the chronology of Wairau Bar. Antiquity. 1999;73:420–427. [Google Scholar]
- Jones, M. E. H. 2006a Skull evolution and functional morphology in Sphenodon and other Rhynchocephalia (Diapsida: Lepidosauria). Unpublished PhD thesis. University of London, London, UK.
- Jones M.E.H. Tooth diversity and function in the Rhynchocephalia (Diapsida: Lepidosauria) In: Barrett P.M., Evans S.E., editors. Ninth international symposium on Mesozoic terrestrial ecosystems and biota. Natural History Museum; London, UK: 2006b. pp. 55–58. [Google Scholar]
- Jones M.E.H. The Early Jurassic clevosaurs from China (Diapsida: Lepidosauria) In: Harris J.D., Lucas S., Kirkland J., Milner A.R.C., editors. The Triassic/Jurassic terrestrial transition. New Mexico Museum of Natural History and Science Bulletin; Albuquerque, NM: 2006c. pp. 548–562. [Google Scholar]
- Jones M.E.H. The evolution of skull shape and feeding strategy in Rhynchocephalia (Diapsida: Lepidosauria) J. Morphol. 2008;269:945–966. doi: 10.1002/jmor.10634. doi:10.1002/jmor.10634 [DOI] [PubMed] [Google Scholar]
- Knapp M., Mudaliar R., Havell D., Wagstaff S.J., Lockhart P.J. The drowning of New Zealand and the problem of Agathis. J. Syst. Biol. 2007;56:862–870. doi: 10.1080/10635150701636412. doi:10.1080/10635150701636412 [DOI] [PubMed] [Google Scholar]
- Krause D.W., Hartman J.H., Wells N.A. Late Cretaceous vertebrates from Madagascar: implications for biotic change in deep time. In: Goodman S.D., Patterson B.D., editors. Natural change and human impact in Madagascar. Smithsonian Institution Press; Washington, DC: 1997. pp. 3–43. [Google Scholar]
- Landis C.A., Campbell H.J., Begg J.G., Mildenhall D.C., Paterson A.M., Trewick S.A. The Waipounamu erosion surface: questioning the antiquity of the New Zealand land surface and terrestrial fauna and flora. Geol. Mag. 2008;145:173–197. doi:10.1017/S0016756807004268 [Google Scholar]
- Lee D.E., Bannister J.M., Lindqvist J.K. Late Oligocene–Early Miocene leaf macrofossils confirm a long history of Agathis in New Zealand. N. Z. J. Bot. 2007;45:565–578. [Google Scholar]
- Luyendyk B.P. Hypothesis for Cretaceous rifting of East Gondwana caused by subducted slab capture. Geology. 1995;23:373–376. doi:10.1130/0091-7613(1995)023<0373:HFCROE>2.3.CO;2 [Google Scholar]
- MacAvoy E.S., McGibbon L.M., Sainsbury J.P., Lawrence H., Wilson C.A., Daugherty C.H., Chambers G.K. Genetic variation in island populations of tuatara (Sphenodon spp) inferred from microsatellite markers. Conserv. Gen. 2007;8:305–318. doi:10.1007/s10592-006-9170-5 [Google Scholar]
- Martinelli A.G., Forasiepi A.M. Late Cretaceous vertebrates from the Bajo de Santa Rosa (Allen formation), Río Negro, Argentina, with the description of a new sauropod dinosaur (Titanosauridae) Rev. Mus. Argentino Cienc. Nat. 2004;6:257–305. [Google Scholar]
- Milner A.C., Milner A.R., Evans S.E. Global changes and biota: amphibians, reptiles and birds. In: Culver S., Rawson P., editors. Biotic response to global change: the last 145 million years. Cambridge University Press; Cambridge, UK: 2000. pp. 316–332. [Google Scholar]
- Mitchell N.J., Kearney M.R., Nelson N.J., Porter W.P. Predicting the fate of a living fossil: how will global warming affect sex determination and hatching phenology in tuatara? Proc. R. Soc. B. 2008;275:2185–2193. doi: 10.1098/rspb.2008.0438. doi:10.1098/rspb.2008.0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar R.E., Pole M. A Miocene crocodilian from New Zealand. Alcheringa. 1997;21:65–70. doi:10.1080/03115519708619185 [Google Scholar]
- Molnar R.E., Wiffen J. A late Cretaceous polar dinosaur fauna from New Zealand. Cretac. Res. 1994;15:689–706. doi:10.1006/cres.1994.1038 [Google Scholar]
- Molnar R.E., Lopez Angriman A., Gasparini Z. An Antarctic Cretaceous theropod. In: Novas F.E., Molnar R.E., editors. Proceedings of the Gondwanan dinosaur symposium. vol. 39. Mem. Queensland Mus.; Brisbane, Australia: 1996. pp. 669–674. [Google Scholar]
- Molnar R.E., Wiffen J., Hayes B. A probable theropod bone from the latest Jurassic of New Zealand. N. Z. J. Geol. Geophys. 1998;41:145–148. [Google Scholar]
- Nelson N.J., Thompson M.B., Pledger S., Keall S.N., Daugherty C.H. Do TSD, sex ratios, and nest characteristics influence the vulnerability of tuatara to global warming? Int. Congr. Ser. 2004;1275:250–257. doi:10.1016/j.ics.2004.08.093 [Google Scholar]
- Newman A.K. Notes on the physiology and anatomy of the tuatara (Sphenodon guntheri) Trans. Proc. N. Z. Inst. 1878;10:222–239. [Google Scholar]
- Pole M.C. The New Zealand flora-entirely long-distance dispersal? J. Biogeogr. 1994;21:625–635. doi:10.2307/2846036 [Google Scholar]
- Pole M.S. Dispersed leaf cuticle from the Early Miocene of southern New Zealand. Palaeontol. Electronica. 2008;11.3.15A:1–117. doi:10.1017/S1477201908002605 [Google Scholar]
- Pole M., Douglas B., Mason G. The terrestrial Miocene biota of southern New Zealand. J. R. Soc. N. Z. 2003;33:415–426. [Google Scholar]
- Reynoso V.H. A Middle Jurassic Sphenodon-like sphenodontian (Diapsida: Lepidosauria) from Huizachal Canyon, Tamaulipas, Mexico. J. Vertebr. Paleontol. 1996;16:210–221. [Google Scholar]
- Reynoso V.H. An unusual aquatic sphenodontian (Reptilia: Diapsida) from the Tlayua Formation (Albian), central Mexico. J. Paleontol. 2000;74:133–148. doi:10.1666/0022-3360(2000)074<0133:AUASRD>2.0.CO;2 [Google Scholar]
- Reynoso V.H. Possible evidence of a venom apparatus in a Middle Jurassic sphenodontian from the Huizachal red beds of Tamaulipas, México. J. Vertebr. Paleontol. 2005;25:646–654. doi:10.1671/0272-4634(2005)025[0646:PEOAVA]2.0.CO;2 [Google Scholar]
- Rich T.H. Potential pre-Pleistocene fossil sites in New Zealand. Mauri Ora. 1975;3:45–54. [Google Scholar]
- Robb J. Meadowfield Press Limited; Durham, UK: 1977. The tuatara. [Google Scholar]
- Robinson P.L. How Sphenodon and Uromastix grow their teeth and use them. In: Bellairs A.d'A., Cox C.B., editors. Morphology and biology of the reptiles. Academic Press; London, UK: 1976. pp. 43–64. [Google Scholar]
- Sagan C. Ballantine Books; New York, NY: 1996. The demon-haunted world: science as a candle in the dark. [Google Scholar]
- Saint-Girons H. The Sphenodon: ecological features and some hypotheses concerning its evolution. Bull. Chin. Herp. Soc. 1985;20:48–51. [Google Scholar]
- Sanmartín I., Ronquist F. Southern hemisphere biogeography inferred by event-based models: plant versus animal patterns. Syst. Biol. 2004;53:216–243. doi: 10.1080/10635150490423430. doi:10.1080/10635150490423430 [DOI] [PubMed] [Google Scholar]
- Towns D.R., Daugherty C.H. Patterns of range contractions and extinctions in the New Zealand herpetofauna following human colonisation. N. Z. J. Zool. 1994;21:325–339. [Google Scholar]
- Trewick S.A., Morgan-Richards M. After the deluge: mitochondrial DNA indicates Miocene radiation and Pliocene adaptation of tree and giant weta (Orthoptera: Anostostomatidae) J. Biogeogr. 2005;32:295–309. doi:10.1111/j.1365-2699.2004.01179.x [Google Scholar]
- Trewick S.A., Paterson A.M., Campbell H.J. Hello New Zealand. J. Biogeogr. 2007;34:1–6. doi:10.1111/j.1365-2699.2006.01643.x [Google Scholar]
- Vidal N., Azvolinsky A., Cruaud C., Hedges S.B. Origin of tropical American burrowing reptiles by transatlantic rafting. Biol. Lett. 2008;4:115–118. doi: 10.1098/rsbl.2007.0531. doi:10.1098/rsbl.2007.0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D.J. Collecting isolated microvertebrate fossils. Zool. J. Linn. Soc. 1984;82:245–259. doi:10.1111/j.1096-3642.1984.tb00546.x [Google Scholar]
- Waters J.M., Craw D. Goodbye Gondwana? New Zealand biogeography, geology, and the problem of circularity. Syst. Biol. 2006;55:351–356. doi: 10.1080/10635150600681659. doi:10.1080/10635150600681659 [DOI] [PubMed] [Google Scholar]
- Whiteside D.I. The head skeleton of the Rhaetian sphenodontid Diphydontosaurus avonis gen. et sp. nov., and the modernising of a living fossil. Phil. Trans. R. Soc. B. 1986;312:379–430. doi:10.1098/rstb.1986.0014 [Google Scholar]
- Worthy T.H., Grant-Mackie J.A. Late-Pleistocene avifaunas from Cape Wanbrow, Otago, South Island, New Zealand. J. R. Soc. N. Z. 2003;33:427–485. [Google Scholar]
- Worthy T.H., Holdaway R.N. Quaternary fossil faunas, overlapping taphonomies, and palaeofaunal reconstruction in North Canterbury, South Island, New Zealand. J. R. Soc. N. Z. 1996;26:275–361. [Google Scholar]
- Worthy T.H., Lee M.S.Y. Affinities of Miocene waterfowl (Anatidae: Manuherikia, Dunstanetta and Miotadorna) from the St Bathans Fauna, New Zealand. Palaeontology. 2008;51:677–708. doi:10.1111/j.1475-4983.2008.00778.x [Google Scholar]
- Worthy T.H., Tennyson A.J.D., Jones C., Mcnamara J.A. A diverse early-Miocene (15–20 Ma) terrestrial fauna from New Zealand reveals snakes and mammals. Geol. Soc. Aust. Abstr. 2002;68:174–175. [Google Scholar]
- Worthy T.H., Tennyson A.J.D., Archer M., Musser A.M., Hand S.J., Jones C., Douglas B.J., McNamara J.A., Beck R.M.D. Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific. Proc. Natl Acad. Sci. USA. 2006;103:19419–19423. doi: 10.1073/pnas.0605684103. doi:10.1073pnas.0605684103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthy T.H., Tennyson A.J.D., Jones C., McNamara J.A., Douglas B.J. Miocene waterfowl and other birds from central Otago, New Zealand. J. Syst. Palaeontol. 2007;5:1–39. doi:10.1017/S1477201906001957 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additonal text, tables and images for all sections of the primary publication. This includes a table of localities known to provide pre-Holocene rhynchocephalian fossils