Abstract
Early Cambrian tommotiids are problematic fossil metazoans with external organophosphatic sclerites that have been considered to be basal members of the lophophorate stem group. Tommotiids are almost exclusively known from isolated or rarely fused individual sclerites, which made previous reconstructions of the actual organism highly conjectural. However, the recent discovery of the first articulated specimens of the tommotiid Eccentrotheca revealed a tubular sclerite arrangement (scleritome) that limited the possible life habit to sessile filter feeding and thus further supported a lophophorate affinity. Here, we report the first articulated specimens of a second tommotiid taxon, Paterimitra from the Early Cambrian of the Arrowie Basin, South Australia. Articulated specimens of Paterimitra are composed of two bilaterally symmetrical sclerite types and an unresolved number of small, asymmetrical and irregular crescent-shaped sclerites that attached to the anterior margin of the symmetrical sclerites. Together, the sclerites form an open cone in which the symmetrical sclerites are joined together and form a small posterior opening near the base of the scleritome, while the irregular crescent-shaped sclerites defined a broad anterior opening. The coniform scleritome of Paterimitra is interpreted to have attached to hard substrates via a pedicle that emerged through the small posterior opening (sometimes forming a tube) and was probably a sessile filter feeder. The scleritome of Paterimitra can be derived from the tubular scleritome of Eccentrotheca by modification of basal sclerites and reduction in tube height, and probably represents a more derived member of the brachiopod stem group with the paired symmetrical sclerites possibly homologous to brachiopod valves.
Keywords: Brachiopoda, Tommotiida, Paterimitra, scleritome construction, Early Cambrian, stem groups
1. Introduction
Brachiopods are marine, bivalved animals with an extensive fossil record. The group is insignificant in most modern faunas, but, during the Early Palaeozoic, brachiopods were one of the dominant groups of sessile, bottom-dwelling filter feeders. Molecular evidence shows that brachiopods are members of the Lophotrochozoa (Halanych 2004; Dunn et al. 2008), but their exact relationship to other lophotrochozoan phyla such as molluscs and annelids is still the focus of ongoing debate (Passamaneck & Halanych 2006; Helmkampf et al. 2008; Yokobori et al. 2008). However, the vastly disparate body plans of lophophorates and other lophotrochozoan phyla are difficult to reconcile with their proposed common ancestry (e.g. Lüter & Bartholomaeus 1997). The only means of tracing the morphological evolution of body plans past the origin of individual phyla is through the study of stem groups. Stem groups phylogenetically originate before the last common ancestor of a given phylum and have no extant descendents, which confines them to the fossil record and explains their often bizarre morphologies (Budd & Jensen 2000; Budd 2001). Evidence from shell composition and ultrastructure points to the problematic tommotiids (extinct Lower Cambrian metazoans that secreted multiple phosphatic sclerites; Bengtson 2004) as a potential brachiopod stem group (Holmer et al. 2002; Williams & Holmer 2002). However, until recently, the structure of the tommotiid scleritome was unknown, and most reconstructions (see summary in Li & Xiao 2004) were based on Halkieria, a coeval, slug-like bilaterian that secreted several rows of dorsal calcareous sclerites and possessed anterior and posterior shells (Conway Morris & Peel 1995).
The tommotiid–brachiopod relationship was recently strengthened by the discovery that the tommotiid Eccentrotheca secreted a tubular scleritome and was probably a sessile filter-feeding animal (Skovsted et al. 2008). The phylogenetic hypothesis advocated by Skovsted et al. (2008) placed Eccentrotheca in the stem lineage of phoronids, a group of marine animals considered to be closely related to brachiopods (Williams et al. 1996; Cohen & Weydmann 2005; Helmkampf et al. 2008; but see Yokobori et al. (2008) for an alternative view). However, the general tubular morphology exhibited by Eccentrotheca and the early appearance of this genus in the fossil record (Landing et al. 1989) suggest that a tube-dwelling habit might have been characteristic for a wider range of Early Cambrian problematica and that the same model may serve as a template for reconstructions of other tommotiids. Following this line of argument, Holmer et al. (2008) recently reinterpreted the tommotiid Micrina as a reduced tubular scleritome consisting of two opposing sclerites homologous to brachiopod valves.
Paterimitra is perhaps one of the least-known tommotiids, with a total of less than 30 specimens of a single, bilaterally symmetrical sclerite described from a small number of localities in South and Central Australia (Laurie 1986; Bengtson et al. 1990; Gravestock et al. 2001). In this paper, we show that sclerites of Paterimitra are, in fact, common constituents of the Early Cambrian fauna with specimens recovered from virtually every sampled section through the Wilkawillina, Wirrapowie and Ajax limestones throughout the Arrowie Basin of South Australia. The new collections (which amount to over 800 sclerites) include rare articulated scleritomes showing conclusively that Paterimitra consisted of a least three distinct sclerite types, each sharing a unique micro-ornament. Ontogenetic fusion of individual sclerites has previously been reported in the tommotiids Lapworthella and Tannuolina (Landing 1984; Qian & Bengtson 1989; Demidenko 2004; Li & Xiao 2004), and is relatively frequent in Eccentrotheca (Landing 1984; Bengtson et al. 1990), where the scleritome is a product of successive sclerite fusion into ring-like elements that eventually coalesce to form a tube (Skovsted et al. 2008). In Paterimitra, sclerite fusion is relatively rare and the articulated scleritomes described here (n=6) are all composed of sclerites that otherwise show signs of growth irregularities and/or deformation. Information on locality details for illustrated specimens are given in the electronic supplementary material.
2. Description of Paterimitra
(a) Isolated sclerites
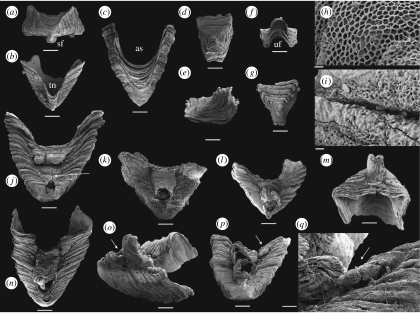
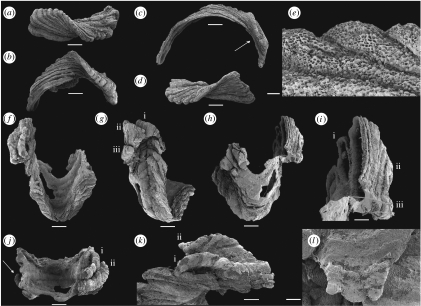
Three distinct types of sclerites are present in available collections: (i) large pyramidal, bilaterally symmetrical S1 sclerites (e.g. figure 1a–c); (ii) low, bilaterally symmetrical triangular or saddle-shaped S2 sclerites (e.g. figure 1d–g); (iii) high, laterally compressed, somewhat twisted and asymmetrical L sclerites (e.g. figure 2a–d). Only the pyramidal S1 sclerite has previously been described in the literature (Laurie 1986; Bengtson et al. 1990). The pyramidal S1 sclerites are high, and have a rectangular or trapezoidal cross section. The rounded apex is slightly displaced towards one of the long sides and the apex is commonly drawn out into a protruding flange (figure 1a) overhanging the subapical surface, here arbitrarily referred to as posterior, which exhibits a deep triangular notch (TN; figure 1b). The opposing anterior side of the sclerite possesses two, low radial ridges defining a central area with a deep semicircular sinus (figure 1c). Associated S2 sclerites are acutely triangular (figure 1d,g) with the narrow, posterior, tapering end raised and usually developed into an upturned flange (UF; figure 1e,f). L sclerites are highly variable, asymmetrical, laterally compressed and irregular ridge-shaped sclerites that are moderately to strongly twisted (figure 2a,d), and may have multiple apices reflecting ontogenetic fusion of originally separate sclerites arranged in a transverse file (figure 2l). The basal margin is moderately to strongly curved and the sclerites may be semicircular in lateral view (figure 2b,c). L sclerites are very similar to laterally compressed sclerites of Eccentrotheca, and one specimen was mistakenly identified as such by Skovsted et al. (2008; figure 2a,b). However, all sclerites of Paterimitra share an identical external reticulate ornament consisting of regular polygonal chambers (figure 1h), the walls of which may degenerate into small bubble-shaped vesicles (figures 1i and 2e). Although Eccentrotheca sclerites may exhibit reticulate patterns on internal surfaces, the sclerites are externally smooth or ornamented by simple wrinkles and irregular growth lines.
Figure 1.
Paterimitra sclerites and articulated specimens from the Early Cambrian of the Arrowie Basin, Flinders Ranges, South Australia. Scale bars, (a–g, j–p) 200 μm, (h,i) 10 μm and (q) 50 μm. (a) SAMP 43302, from MMF/0.0, pyramidal S1 sclerite, apical view, posterior side down, showing a rectangular cross section and position of SF. (b) SAMP 43303, from MMF/0.0, pyramidal S1 sclerite in posterior view, apex down, showing TN. (c) SAMP 43304, from MMF/0.0, pyramidal S1 sclerite in anterior view, apex down, showing semicircular anterior sinus (AS). (d–f) SAMP 43305, from AJX-M/256, triangular S2 sclerite: (d) dorsal view with (posterior) UF down; (e) lateral view; and (f) view from UF. (g–i) SAMP 43306, from WILK/I, triangular S2 sclerite: (g) dorsal view with UF down; (h) detail of ornament with regular polygonal chambers; and (i) detail of ornament showing walls of chambers degenerated into bubble-shaped vesicles close to sclerite margin. (j) SAMP 43307, from MMF/0.0, articulated specimen with fused S1 and S2 sclerites in posterior view. The posterior flange of the S1 sclerite is damaged and the S2 sclerite is cracked and deformed. Pedicle opening is partly constricted by callous (indicated by an arrow) growing out from margins of the S1 sclerite. (k) SAMP 43308, from AJX-M/262.7, articulated specimen with fused S1 and S2 sclerites in posterior view, exhibiting circular pedicle opening. The surface of the sclerites is poorly preserved and the S2 sclerite is exfoliated, preserving no discernable growth increments. (l,m) SAMP 43309, from spot locality, upper Wirrapowie Limestone, Druid Range, articulated specimen with fused S1 and S2 sclerites: (l) in posterior view showing SF of S1 and UF of S2 forming elongated ‘pedicle’ tube and (m) oblique anterior view showing S2 sclerite fused at an oblique angle relative to S1 sclerite. Note that growth increments are continuous across the anterior border after sclerite fusion relatively early in ontogeny. (n–q) SAMP 43310, from MMF/0.0, articulated specimen with fused S1 and S2 sclerites and a single probable L sclerite (indicated by arrows in (p,q)): (n) in posterior view; (o) in lateral view showing well-developed ‘pedicle tube’ (indicated by an arrow); (p) in apical view showing S2 sclerite fused at an angle relative to S1 sclerite and the position of probable L sclerite behind and lateral to S2 sclerite; and (q) detail showing small probable L sclerite fused to the margin of the S1 sclerite.
Figure 2.
Paterimitra sclerites and articulated specimen from the Early Cambrian of the Arrowie Basin, Flinders Ranges, South Australia. Scale bars, (a–d,f–h,j) 200 μm, (e) 10 μm, (i,k) 100 μm and (l) 50 μm. (a,b) SAMP 43311, from WILK/Q, L sclerite: (a) in apical view showing helical twist and (b) in lateral view showing curved base. (c,e) SAMP 43312, from WILK/R, L sclerite: (c) in lateral view showing long, semicircular base and asymmetrically placed apex (indicated by an arrow) and (e) detail of sclerite surface showing polygonal chambers and walls with bubble-shaped vesicles. (d) SAMP 43313, from WILK/R, L sclerite in apical view showing helical twist. (f–l) SAMP 43314, from AJX-N/213, articulated specimen with fused S1 and four L sclerites: (f) posterior view showing deep TN and composite of L sclerites on the left lateral flank; (g) oblique lateral view showing relative position of L sclerites (indicated as (i)–(iii)) on the left lateral flank, note that sclerites ((ii),(iii)) are positioned in a transverse file and partly overlap sclerite (i); (h) anterior view showing swollen S1 sclerite with damaged anterior margin and base of L sclerites coinciding with curvature of anterior sinus; (i) detail of interior surface showing connecting walls between L sclerites and S1 sclerite partly draped by sheet-like shell layers, note that L sclerites ((ii),(iii)) fused relatively early and have a single internal cavity; (j) apertural view of the S1 sclerite showing L sclerites overlapping each other and margin of the S1 sclerite on the right and a single smaller L sclerite attached in a similar position of the left side (indicated by an arrow); (k) detail of the margin of the S1 sclerite with L sclerites attached, note that sclerite (i) overlaps the margin of the S1 sclerite; and (l) detail of L sclerite (ii) showing reticulate ornament and threefold apex.
(b) Articulated scleritomes
The majority (n=5) of articulated specimens preserve the saddle-shaped S2 sclerite nested within the TN on the subapical posterior surface of the large pyramidal S1 sclerite (figure 1j–l,n). The subapical flange (SF) on the pyramidal S1 sclerite and the UF of the S2 sclerite are opposed, and together define a more or less circular opening between the sclerites (figure 1j,k). In two specimens, the fusion between the S1 and S2 sclerites results in a tube extending at a high angle from the posterior margin of the S1 sclerite (figure 1l,n,o). The triangular S2 sclerite does not completely fill the TN of the S1 sclerite and a shallow posterior sinus is developed behind it. In one specimen, what appears to be a small additional sclerite is fused to the margin of the S1 sclerite directly behind and lateral to the triangular S2 sclerite (figure 1p,q). The sclerite is relatively high and narrow, and probably represents a small L sclerite.
The spatial relationship between pyramidal S1 sclerites and the asymmetrical L sclerites is preserved in a single specimen (figure 2f–l). In this specimen, the pyramidal S1 sclerite is deformed with the SF missing, the subapical surface slightly depressed and possessing an unusually wide TN (figure 2f,g). The opposing supra-apical surface is swollen, partly exfoliated and the anterior sinus is difficult to delineate (figure 2h). However, three distinct asymmetrical L sclerites are fused to the left lateral flank (when viewed from the subapical surface) of the pyramidal S1 sclerite (figure 2g,j) and a single small asymmetrical L sclerite is attached to its right lateral flank (figure 2j). On the left side, the first of the L sclerites (labelled (i); figure 2g,i) is attached to, and overlaps, the S1 sclerite along its lateral flank (figure 2k). The two additional L sclerites (labelled (ii) and (iii); figure 2g,i) are fused along their longitudinal axes and, together, overlap the margins of the pyramidal sclerite and the first L sclerite (figure 2g). The curved basal margin of the L sclerites is apparently aligned in continuation with the curvature of the anterior sinus (figure 2h). The first row (only represented by sclerite (i)) abuts directly onto the S1 sclerite, but the second row (sclerites (ii) and (iii)) are attached at a plane slightly above the margin of the S1 sclerite (figure 2g). Internally, the central cavity of both the first (i) and the combined second and third (ii, iii) L sclerites are clearly identifiable and show that sheet-like, internally secreted shell layers fused the sclerites by partly draping the walls between them, suggesting that the shell secreting epithelia merged during growth (figure 2i).
3. Scleritome reconstruction
We reconstruct the Paterimitra organism as a cone, attached to a hard substrate by an organic structure emerging from the subapical opening between the pyramidal S1 and triangular S2 sclerites. Although the exact sclerite configuration of the remaining parts of the scleritome is unknown, the area immediately posterior to the combined pyramidal S1 and triangular S2 sclerites was probably covered by a mosaic of L sclerites. The alignment of L sclerites in continuation of the semicircular anterior sinus of the S1 sclerite suggests that the scleritome may have exhibited an anterior opening defined by the sinus and an unresolved number of asymmetrical L sclerites. The position of the second row of fused L sclerites in a plane above the margin of the S1 sclerite sinus may indicate the presence of a tubular extension of the scleritome vertically above the pyramidal S1 sclerite. The more elongate, strongly curved L sclerites (figure 2c) may have filled positions lining this tubular extension.
The scleritome of Paterimitra can be derived from the tubular scleritome of Eccentrotheca by modification of the basal sclerites and reduction in the number of sclerite rings. Skovsted et al. (2008) described a spiral arrangement of sclerites in Eccentrotheca, but a close examination of available scleritomes reveal that the sclerites are actually arranged in discrete ring-like elements. The tubular shape of the Eccentrotheca scleritome results from the vertical stacking of such rings that are often oriented obliquely to the long axis of the scleritome, thus giving a superficial impression of spiral arrangement (Skovsted et al. 2008, figure 1a,b). Individual sclerite rings can contain a highly variable number of sclerites, sometimes simultaneously including both low cap-shaped sclerites and high, laterally compressed sclerites.
The symmetrical sclerites of Paterimitra enclose the subapical perforation and are probably equivalent to the low, cap-shaped sclerites surrounding the apical aperture of Eccentrotheca (Skovsted et al. 2008, figure 1b,d–f), which presumably housed similar attachment structures. This would require a reduction in number and functional specialization of the multiple and highly variable low plates in Eccentrotheca, with a consequent introduction of bilateral symmetry. As noted above, L sclerites of Paterimitra are morphologically closely comparable to the high, triangular sclerites of Eccentrotheca that occupy antapical portions in its tubular scleritome. L sclerites and the high, laterally compressed sclerites in Eccentrotheca probably had a similar function by at least partially lining the apertural margins of the scleritome. The Paterimitra scleritome is thus a simplified version of the Eccentrotheca tube with a single basal ring of two modified, low, cap-shaped plates (S1 and S2) and a larger set of high, triangular sclerites (L).
4. Discussion
Paterimitra is only the second tommotiid for which articulated scleritomes are known. The general shape of the Paterimitra scleritome, which forms an inverted cone with a small circular opening close to the apex, is similar to the structure of Eccentrotheca (Skovsted et al. 2008) and indicates that both were related sessile filter feeders. Available evidence, in particular the presence of asymmetrical, laterally compressed L sclerites, suggests that the Paterimitra scleritome was derived from a tubular Eccentrotheca-like ancestor. Paterimitra also exhibits similarities to brachiopods, particularly to paterinids as reflected by the name. Paterinid characters of Paterimitra include: the supra-apical flange of the S1 sclerite, which is very reminiscent of a young adult paterinid homeodeltidium; the often straight planar posterior margin of the S1 sclerite, which in many specimens closely resembles the posterior margin of paterinid ventral valves; and the reticulate ornament of the paterinid Askepasma (Williams et al. 1998). In addition, we demonstrate that the S1 sclerite of Paterimitra is complemented by a bilaterally symmetrical, triangular S2 sclerite. Together, these sclerites define a circular posterior opening (equivalent to a foramen), a construction that is most reminiscent of the skeletal configuration of brachiopods. We hypothesize that the Paterimitra S1 and S2 sclerites are homologous to the valves of organophosphatic brachiopods, and that the attachment structure emerging through the posterior opening between the sclerites is homologous to the brachiopod pedicle. This scenario implies that the bivalved condition in organophosphatic brachiopods arose through the successive simplification of a tubular scleritome, and that Paterimitra represents a condition in which two valves had already evolved, but did not yet fully enclose the entire body of the animal.
Tannuolinid tommotiids represent another case where the scleritome is restricted to a small number of sclerites. Two sclerite types are present in tannuolinids, cap-shaped mitrals and laterally compressed sellates, both characterized by a laminar shell structure and shell-penetrating setae. In Tannuolina, only the sellate sclerites are bilaterally symmetrical while mitral sclerites form bilaterally symmetrical sinistral/dextral pairs (Li & Xiao 2004, fig. 5.2–4). In the tannuolinid genus, Micrina, both sclerite types are bilaterally symmetrical. Holmer et al. (2008; figure 2) reconstructed Micrina as a bivalved stem group brachiopod attached to the substrate via a pedicle emerging between the mitral sclerite and the duplicature of the sellate sclerite. As with Paterimitra, the sclerites in Micrina would not have completely enclosed the animal. Based on this interpretation, the Tannuolina scleritome may be reconstructed as a member of the brachiopod stem group consisting of three to four sclerites, whereas Micrina represents a more derived, fully bivalved member.
It is notable that tannuolinids share details of morphology and ultrastructure, particularly their shell-penetrating setal canals, with some problematic brachiopods (Holmer et al. 2002; Balthasar 2004). While some of these brachiopods share a close affinity with lingulids in terms of shell microstructure and overall morphology (e.g. Mickwitzia cf. occidens; Skovsted & Holmer 2003), others are more closely linked to paterinids (e.g. Mickwitzia muralensis; Balthasar 2004). In the light of the similarities between Paterimitra and paterinids, and in the absence of compelling intermediates between tannuolinids and Paterimitra, an independent origin of the bivalved condition, Paterimitra to paterinids and Micrina to linguloids, appears possible.
Acknowledgments
Ian and Di Fargher, owners of Angorichina Station, are thanked for access to the MMF field locality and for providing excellent field accommodation. Mr Graham Ragless, owner of Beltana Station, graciously provided access to the field area on his property and also provided accommodation. Jim Jago, Bob Morgan, Bo Jonak, Peter Cockle, Tom Bradley, Brett Pyemont, Thomas Brock, Joel Brock and Daniel Holmer are thanked for assistance in the field. Dennis Rice of the South Australian Museum (Adelaide) is thanked for making specimens from the Brian Daily collections available for study. Graham E. Budd and Michael Streng (both at Uppsala University) provided valuable discussions of tommotiids, and comments by two anonymous reviewers greatly improved the manuscript. Financial support from the National Geographic Committee for Research and Exploration (grant 7918-05 to Brock, Skovsted, and Paterson), a Macquarie University Development Research Grant to C.B.S. and G.A.B., grants from the Swedish Research Council (VR) to C.B.S. and U.B. and a grant from the Wenner Gren Foundation to G.A.B. and C.B.S. are gratefully acknowledged.
Supplementary Material
Summarised descriptions of localities in the Flinders Ranges yielding illustrated specimens of Paterimitra with references to relevant literature
References
- Balthasar U. Shell structure, ontogeny and affinities of the Lower Cambrian bivalved problematic fossil Mickwitzia muralensis Walcott, 1913. Lethaia. 2004;37:381–400. doi:10.1080/00241160410002090 [Google Scholar]
- Bengtson S. Early skeletal fossils. In: Lipps J.H., Waggoner B.M., editors. Neoproterozoic–Cambrian biotical revolutions. The Paleontological Society Papers. vol. 10. The Paleontological Society; Austin, TX: 2004. pp. 67–77. [Google Scholar]
- Bengtson S., Conway Morris S., Cooper B.J., Jell P.A., Runnegar B.N. Early Cambrian fossils from South Australia: Association of Australasian Palaeontologists. Memoir. 1990;9:1–364. [Google Scholar]
- Budd G.E. Why are arthropods segmented? Evol. Dev. 2001;3:332–342. doi: 10.1046/j.1525-142x.2001.01041.x. doi:10.1046/j.1525-142X.2001.01041.x [DOI] [PubMed] [Google Scholar]
- Budd G.E., Jensen S. A critical reappraisal of the fossil record of the bilaterian phyla. Biol. Rev. 2000;75:253–295. doi: 10.1017/s000632310000548x. doi:10.1017/S000632310000548X [DOI] [PubMed] [Google Scholar]
- Cohen B.L., Weydmann A. Molecular evidence that phoronids are a subtaxon of brachiopods (Brachiopoda: Phoronata) and that genetic divergence of metazoan phyla began long before the Early Cambrian. Organ. Divers. Evol. 2005;5:253–273. doi:10.1016/j.ode.2004.12.002 [Google Scholar]
- Conway Morris S., Peel J.S. Articulated halkieriids from the Lower Cambrian of North Greenland and their role in early protostome evolution. Phil. Trans. R. Soc. B. 1995;347:305–358. doi:10.1098/rstb.1995.0029 [Google Scholar]
- Demidenko Yu.E. New data on the sclerite morphology of the tommotiid species Lapworthella fasciculata. Paleontol. J. 2004;38:134–140. [Google Scholar]
- Dunn C.W., et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–749. doi: 10.1038/nature06614. doi:10.1038/nature06614 [DOI] [PubMed] [Google Scholar]
- Gravestock D.I., et al. The Cambrian biostratigraphy of the Stansbury Basin, South Australia. Russ. Acad. Sci. Trans. Palaeontol. Inst. 2001;282:1–344. [Google Scholar]
- Halanych K.M. The new view of animal phylogeny. Annu. Rev. Ecol. Evol. Syst. 2004;35:229–256. doi:10.1146/annurev.ecolsys.35.112202.130124 [Google Scholar]
- Helmkampf M., Bruchhaus I., Hausdorf B. Phylogenomic analyses of lophophorates (brachiopods, phoronids and bryozoans) confirm the Lophotrochozoa concept. Proc. R. Soc. B. 2008;275:1927–1933. doi: 10.1098/rspb.2008.0372. doi:10.1098/rspb.2008.0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmer L.E., Skovsted C.B., Williams A. A stem group brachiopod from the Lower Cambrian—support for a Micrina (halkieriid) ancestry. Palaeontology. 2002;45:875–882. doi:10.1111/1475-4983.00265 [Google Scholar]
- Holmer L.E., Skovsted C.B., Brock G.A., Valentine J.L., Paterson J.R. The Early Cambrian tommotiid Micrina, a sessile bivalved stem group brachiopod. Biol. Lett. 2008;4:724–728. doi: 10.1098/rsbl.2008.0277. doi:10.1098/rsbl.2008.0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landing E. Skeleton of lapworthellids and the supragenetic classification of tommotiids (Early and Middle Cambrian phosphatic problematica) J. Paleontol. 1984;58:1380–1398. [Google Scholar]
- Landing E., Myrow P., Benus A.P., Narbonne G.M. The Placentian series: appearance of the oldest skeletalized faunas in southeastern Newfoundland. J. Paleontol. 1989;63:739–769. [Google Scholar]
- Laurie J.R. Phosphatic fauna of the early Cambrian Todd River Dolomite, Amadeus Basin, central Australia. Alcheringa. 1986;10:431–454. doi:10.1080/03115518608619151 [Google Scholar]
- Li G., Xiao S. Tannuolina and Micrina (Tannuolinidae) from the Lower Cambrian of eastern Yunnan, south China, and their scleritome reconstruction. J. Paleontol. 2004;78:900–913. doi:10.1666/0022-3360(2004)078<0900:TAMTFT>2.0.CO;2 [Google Scholar]
- Lüter C., Bartholomaeus T. The phylogenetic position of Brachiopoda—a comparison of morphological and molecular data. Zool. Scripta. 1997;26:245–253. doi:10.1111/j.1463-6409.1997.tb00414.x [Google Scholar]
- Passamaneck Y.J., Halanych K.M. Lophotrochozoan phylogeny assessed with LSU and SSU data: evidence of lophophorate polyphyly. Mol. Phylogenet. Evol. 2006;40:20–28. doi: 10.1016/j.ympev.2006.02.001. doi:10.1016/j.ympev.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Qian Y., Bengtson S. Palaeontology and biostratigraphy of the Early Cambrian Meishucunian Stage in Yunnan Province, South China. Fossils Strata. 1989;24:1–156. [Google Scholar]
- Skovsted C.B., Holmer L.E. The Early Cambrian stem group brachiopod Mickwitzia from Northeast Greenland. Acta Palaeontol. Polon. 2003;48:11–30. [Google Scholar]
- Skovsted C.B., Brock G.A., Paterson J.R., Holmer L.E., Budd G.E. The scleritome of Eccentrotheca from the Lower Cambrian of South Australia: lophophorate affinities and implications for tommotiid phylogeny. Geology. 2008;36:171–174. doi:10.1130/G24385A.1 [Google Scholar]
- Williams A., Holmer L.E. Shell structure and inferred growth, functions and affinities of the sclerites of the problematic Micrina. Palaeontology. 2002;45:845–873. doi:10.1111/1475-4983.00264 [Google Scholar]
- Williams A., Carlson S.J., Brunton C.H.C., Holmer L.E., Popov L.E. A supra-ordinal classification of the Brachiopoda. Phil. Trans. R. Soc. Lond. B. 1996;351:1171–1193. doi:10.1098/rstb.1996.0101 [Google Scholar]
- Williams A., Popov L.E., Holmer L.E., Cusack M. Diversity of paterinide brachiopods. Palaeontology. 1998;41:221–262. [Google Scholar]
- Yokobori S., Iseto T., Asakawa S., Sasaki T., Shimizu N., Yamagishi A., Oshima T., Hirose E. Complete nucleotide sequences of mitochondrial genomes of two solitary entoprocts, Loxocorone allax and Loxosomella aloxiata: implications for lophotrochozoan phylogeny. Mol. Phylogenet. Evol. 2008;47:612–628. doi: 10.1016/j.ympev.2008.02.013. doi:10.1016/j.ympev.2008.02.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summarised descriptions of localities in the Flinders Ranges yielding illustrated specimens of Paterimitra with references to relevant literature