Abstract
Global biodiversity loss and disease emergence are two of the most challenging issues confronting science and society. Recently, observed linkages between species-loss and vector-borne infections suggest that biodiversity may help reduce pathogenic infections in humans and wildlife, but the mechanisms underlying this relationship and its applicability to a broader range of pathogens have remained speculative. Here, we experimentally evaluated the effects of host community structure on transmission of the human pathogen, Schistosoma mansoni, which alternates between snail intermediate hosts and vertebrate definitive hosts. By manipulating parasite exposure and community diversity, we show that heterospecific communities cause a 25–50 per cent reduction in infection among snail hosts (Biomphalaria glabrata). Infected snails raised alongside non-host snails (Lymnaea or Helisoma sp.) also produced 60–80 per cent fewer cercariae, suggesting that diverse communities could reduce human infection risk. Because focal host density was held constant during experiments, decreases in transmission resulted entirely from diversity-mediated pathways. Finally, the decrease in infection in mixed-species communities led to an increase in reproductive output by hosts, representing a novel example of parasite-mediated facilitation. Our results underscore the significance of community structure on transmission of complex life-cycle pathogens, and we emphasize enhanced integration between ecological and parasitological research on the diversity–disease relationship.
Keywords: schistosomiasis, emerging disease, dilution effect, decoy effect, epidemiology, community ecology
1. Introduction
Global increases in population extirpations and species extinctions underscore the urgency of understanding the ecological and societal importance of biodiversity (Loreau et al. 2001; Duffy et al. 2007). Recently, growing attention has focused on the potential role of diversity in influencing parasite transmission and disease risk (Ostfeld & Keesing 2000; Dobson et al. 2006; Swaddle & Calos 2008). While host–parasite interactions are often explored in isolation (single host and parasite species), natural systems support a broad diversity of both hosts and pathogens, and interactions among these can have important implications for transmission (e.g. Collinge & Ray 2006). Empirical and theoretical evidence increasingly support the notion that higher levels of host diversity can reduce transmission of certain pathogens (i.e. the ‘dilution effect’ sensu Keesing et al. 2006). For vector-borne pathogens transmitted by blood-feeding arthropods, this can occur when vectors feed upon hosts that are resistant or refractory to infection, resulting in ‘wasted’ transmission events (LoGuidice et al. 2003; Ezenwa et al. 2006; Begon 2008; Swaddle & Calos 2008).
Despite a growing list of disease systems that correlate negatively with biodiversity, the ecological mechanisms underlying these patterns can be difficult to determine from field data alone. Two pressing questions in the ongoing study of the dilution effect are (i) how does diversity influence transmission of non-vector-borne pathogens, including those of human health significance, and (ii) what are the ecological mechanisms responsible for observed relationships between community structure and infection? We suggest that parasites with complex life cycles also respond strongly to patterns of community diversity (e.g. Thieltges et al. 2008b). These parasites, which include many trematodes, cestodes, nematodes, chytridiomycetes and myxozoans, collectively cause numerous diseases of medical, veterinary and conservation significance, including schistosomiasis in humans, whirling disease in fishes, chytridiomycosis in amphibians and fascioliasis in ruminants. Because such parasites move among hosts using free-living infectious stages (e.g. cercariae, zoospores, TAMs), which are often short-lived and host specific, changes in community structure can interfere with transmission. In diverse communities, the abundance of free-living infectious stages can be reduced by predation, the release of toxic substances, or by attempts to infect the ‘wrong’ host, collectively leading to what parasitologists term the ‘decoy effect’ (e.g. Chernin 1968; Thieltges et al. 2008b). In contemporary discussions of the dilution effect, this mechanism represents one form of what Keesing et al. (2006) called ‘encounter reduction’, in which an added species reduces the contact rate between susceptible hosts and a parasite source (e.g. infected vector, infected host, parasite propagules, etc.). Unlike many vector-borne infections, however, complex life-cycle parasites offer a tractable model for experimental research on the ecological mechanisms underlying the diversity–disease relationship. Despite this and the rich history of parasitological research on the decoy effect, complex life-cycle parasites have been largely omitted from recent ecological discussions of how community diversity influences infectious diseases.
Experimental studies also provide an opportunity to explore the net ecological consequences of the dilution effect on host fitness. If non-host taxa reduce parasite transmission into focal hosts, they may indirectly ‘protect’ such hosts from parasite-induced pathology, enhancing host fitness in heterospecific communities relative to monospecific (or low diversity) communities (Thieltges et al. 2008a). Johnson et al. (2008) classified this relationship as ‘parasite-mediated facilitation’. While parasite-mediated and apparent competition, in which the strength or outcome of competitive interactions is altered in the presence of parasites, have received theoretical and empirical support (Hudson & Greenman 1998), the concept of parasite-mediated facilitation has received little attention. Given the severe pathology caused by certain infections in nature, the infection-reducing benefits conferred to sensitive hosts by low competency taxa could be substantial. However, because more diverse communities also have higher levels of interspecific competition, the net benefit of community-mediated changes in infection will depend on the costs of interspecific competition relative to parasitism.
Here, we evaluated the joint effects of parasitism and community structure on transmission of the human pathogen Schistosoma mansoni, a complex life-cycle parasite that alternates between freshwater snails (intermediate host) and humans (definitive hosts) via free-living infectious stages (e.g. miracidia and cercariae). We used an experimental approach to explore how community structure and species richness affected (i) parasite transmission into snails, (ii) the subsequent release of cercariae infectious to humans, and (iii) intermediate host pathology and fitness. By experimentally manipulating host community structure and parasite exposure, we evaluated the role of diversity while holding focal host density constant. We further evaluated the ecological trade-offs between interspecific competition, which was expected to be greater in heterospecific communities, and the costs of parasite exposure, which were hypothesized to be maximized in the monospecific condition. In this manner, we assessed the importance of community composition from two perspectives: infection risk in humans, as reflected by the release of cercariae from snails, and intermediate host fitness, as indicated by the survival, growth and reproduction of snail hosts. Considering that schistosomiasis continues to afflict more than 200 million people worldwide (Steinmann et al. 2006), sometimes causing serious morbidity, understanding the role of biodiversity for schistosome transmission has significant implications for human health and economic viability. Finally, we sought to unite the disparate literatures on the decoy effect (studied by parasitologists) and the dilution effect (studied by ecologists), which, despite their similarities, have remained largely isolated from one another.
2. Material and methods
To identify the effects of host community on parasite transmission, we conducted a 2×2×2 factorial experiment in which we manipulated parasite exposure and the presence of two non-host species (Helisoma trivolvis and Lymnaea stagnalis). All treatments included an individual focal host, Biomphalaria glabrata (NMRI strain), which is the obligate first intermediate host for S. mansoni. Experimental conditions were thus either monospecific (an individual Biomphalaria only), heterospecific with two species (Biomphalaria and either one Helisoma or one Lymnaea) or heterospecific with one individual of all three species. To avoid concurrent effects resulting from changes in focal host density, we held the density of Biomphalaria (1 l−1) constant among treatments and varied only species richness. Using this additive design was essential for disentangling the dilution mechanism of encounter reduction from susceptible host regulation (see Keesing et al. 2006). We replicated these four community structures 50 times, with parasite addition occurring in half of replicates (200 total containers). We selected Lymnaea and Helisoma because both genera (but not these exact species) commonly co-occur with Biomphalaria in Africa and South America.
Following Carter et al. (1982), we maintained snails in transmission arenas filled with 1.0 l of artificial pond water (see Nolan & Carriker 1946), and randomly assigned individual snails to treatments and containers; initial sizes ±1 s.e. of each species were: Biomphalaria (13.24±0.08 mm); Helisoma (12.34±0.20 mm); and Lymnaea (15.44±0.18 mm). One week after establishing communities, we added eight S. mansoni miracidia to half of the containers in each treatment based on the results of previous pilot experiments. We obtained S. mansoni eggs from the homogenized livers of experimentally infected mice (Yoshino & Laursen 1995), induced hatching via exposure to light and added free-swimming miracidia to each container using a pipette. We maintained snails on a Romaine lettuce diet and changed water and containers weekly. Experimental treatments were maintained on a 12 L : 12 D cycle at 28±1.0°C.
We monitored snails daily for mortality and measured the shell of each individual weekly. In the event of mortality of a non-host snail, we replaced the individual with a comparably sized snail from stock colonies. Mortality of a host (=Biomphalaria) snail required that the replicate be terminated. To obtain estimates of reproductive output by host snails, we recorded the number of egg masses laid weekly and counted the number of eggs per egg mass in 10 randomly selected egg masses per container. However, because Biomphalaria and Helisoma produce indistinguishable egg masses (both species are in the family Planorbidae), we isolated a subset of containers (n=10) each week from treatments that supported both species together and allowed eggs to hatch. Resulting offspring were examined under a stereomicroscope to assign them to genus, and we used the mean proportion of hatching snails in each genus as a correction factor to back-calculate the number of eggs produced by Biomphalaria in remaining containers.
At four and six weeks following parasite exposure, we individually isolated all Biomphalaria into 24-well plates with 3 ml per well and exposed snails to light for 1 h to induce cercarial release. Released cercariae were killed with 80 per cent ethanol and counted to estimate per capita cercarial production. After eight weeks, we dissected any non-shedding Biomphalaria and examined them for signs of immature (prepatent) infection. While we also dissected any host snails that died prior to the end of the experiment, their tissues often became too decomposed to assess infection status with confidence.
We focused our analyses at two levels. In the first, we sought to identify how changes in community structure influenced infection success in host snails and their subsequent release of cercariae infectious to humans. We therefore calculated (i) the total number and percentage of infected host snails, (ii) the per capita daily release of cercariae among infected snails, and (iii) the total production of cercariae per treatment (product of the number of surviving infected snails and their daily release of cercariae). In the second level of analysis, we examined how interactions between community structure and parasite exposure affected the growth, survival and reproduction of Biomphalaria. This allowed us to evaluate the potential benefits of parasite-mediated facilitation relative to the costs of interspecific competition in mixed-species communities (see Johnson et al. 2008). Simply stated, do the infection-reducing benefits of non-host snails for Biomphalaria outweigh their added costs due to competition? Because Schistosoma infection causes reproduction castration in infected snails (Gerard & Theron 1997), we expected that the benefits of reduced infection would affect reproductive output.
We used logistic regression to analyse how community structure affected host infection status (infected versus uninfected) and ANOVA to detect differences in per capita cercarial production (cercariae released per infected snail) among treatments. Because we did not find statistically significant differences between Helisoma and Lymnaea on either response variable, we subsequently combined treatment data to analyse the effects of species richness (one, two or three species) on parasite transmission. Identical approaches were used for assessing the responses of focal hosts (Biomphalaria) to parasitism and interspecific competition, with logistic regression for snail survival (survived versus died) and ANOVA for individual egg production (sum of all eggs produced by surviving host snails during the experiment).
3. Results
(a) Community structure, parasite transmission and human infection risk
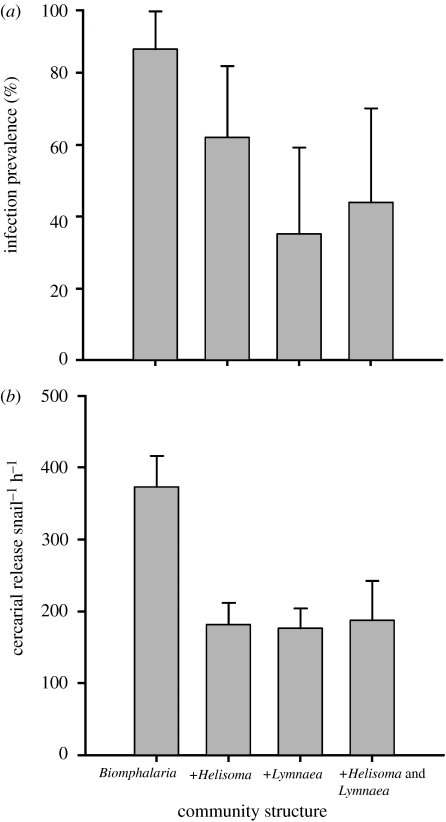
The addition of one or more non-host species substantially reduced the percentage of successful infections in Biomphalaria glabrata, with community richness negatively predicting host infection status (logistic regression, Χ2=10.98, p=0.004, n=105). While 80 per cent of hosts in the monospecific condition became infected with S. mansoni, less than 50 per cent of hosts in heterospecific communities supported the parasite (figure 1a). Variation in the heterospecific condition, from two to three species or from Lymnaea to Helisoma, did not significantly affect infection success, but the presence of Lymnaea was associated with a larger decrease in Biomphalaria infection prevalence (figure 1a). We did not find a significant interaction between the two non-host species (logistic regression, p=0.09, n=105), and no hosts were infected within the unparasitized condition.
Figure 1.
(a) Effects of snail community structure on the prevalence of Schistosoma mansoni infection within Biomphalaria glabrata. Bars represent the percentage of infected snails (+95% confidence interval) as determined through cercarial release and dissection. Experimental treatments included Biomphalaria alone, Biomphalaria with Helisoma, Biomphalaria with Lymnaea and all three species together. (b) Effects of community structure on cercarial production. Depicted is the average number of S. mansoni cercariae released infected snail−1 h−1 (+1 s.e.) in each community treatment.
The presence of one or more non-host species also significantly reduced cercarial production among infected B. glabrata (ANOVA, four weeks post-exposure: F1,40=11.37, p=0.002; six weeks post-exposure: F1,39=7.55, p=0.009; figure 1b). Infected hosts in the monospecific community released, on average, between 70 and 170 per cent more cercariae than did infected hosts in heterospecific treatments. The degree of cercarial reduction did not differ between the Lymnaea and Helisoma treatments or between the two and three species treatments (figure 1b).
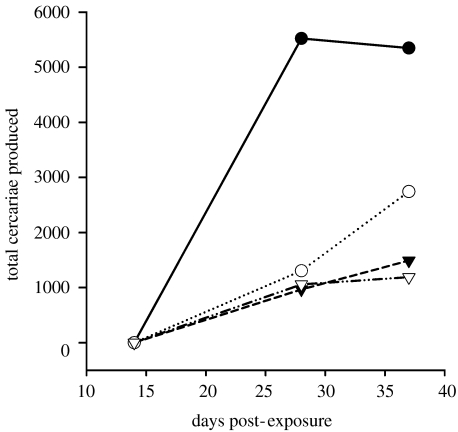
When the differences in infection prevalence (percentage of infected hosts) and in cercarial release (number of parasites per infected host) were combined, the treatment-level variation in parasite production and human infection risk was considerable. Because monospecific communities had more infected Biomphalaria, each of which released more cercariae, we estimate that this treatment produced between two and five times more cercariae per 24 h than that observed in heterospecific treatments (figure 2). As cercariae of S. mansoni are directly infectious to humans, this represents a potential increase in human infection risk.
Figure 2.
Effects of treatment manipulations on the total number of cercariae produced per treatment. The total number of cercariae produced was calculated as the mean number of cercariae produced per infected snail multiplied by the number of infected snails on a given sampling date. Filled circles, Biomphalaria; open circles, Biomphalaria+Helisoma; filled down triangles, Biomphalaria+Lymnaea; open down triangles, Biomphalaria+Helisoma+Lymnaea.
(b) Combined effects of community structure and parasitism on focal hosts
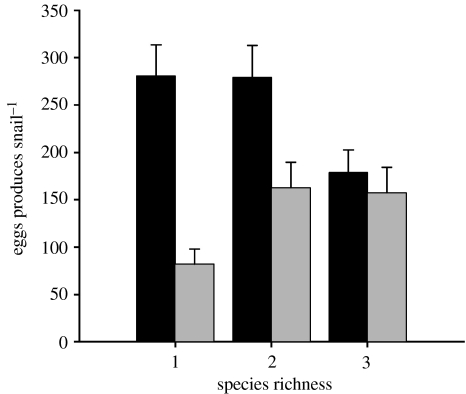
While we found no effect of parasite exposure or community structure on host survival or growth, both independent variables had pronounced effects on host reproduction. As expected, exposure to S. mansoni—which is a parasitic castrator (Gerard & Theron 1997)—significantly reduced fecundity among Biomphalaria in the monospecific treatment (ANOVA, F1,38=25.07, p<0.0001, n=40; figure 3). On average (±1 s.e.), individual hosts in the unparasitized and parasitized treatments produced 280 (±34.9) and 81 (±16.1) total eggs, respectively, during the experiment. Most infected snails completely halted reproduction after 23 days. However, parasite exposure interacted significantly with community structure (ANOVA, parasite×species richness, F2,139=4.47, p=0.013; figure 3): in the absence of parasitism, the addition of one or more non-host species had negative (Lymnaea) or neutral (Helisoma) effects on Biomphalaria egg production (ANOVA, species richness, F2,74=3.43, p=0.04); in the presence of parasitism, however, the addition of either non-host species significantly increased host egg production relative to the monospecific treatment (ANOVA, species richness, F2,65=4.16, p=0.02; figure 3). Within parasitized treatments, hosts in heterospecific treatments produced twice as many eggs during the experiment than did hosts in monospecific conditions. Indeed, the overall negative effect of parasite exposure on host reproduction was eliminated in the three-species condition (ANOVA, parasite, F1,30=0.351, p=0.59).
Figure 3.
Joint effects of parasite exposure and community structure on reproduction by host snails (Biomphalaria glabrata). Shown is the total number of eggs (+1 s.e.) produced per surviving B. glabrata as a function of whether the snail was raised alone (one species), with a second species (two species, either Helisoma or Lymnaea) or with two other species (three species in total). Results from parasite-exposed treatments are depicted in light grey while unexposed treatments are in black.
4. Discussion
In the light of ongoing biodiversity losses and increased emergence of human and wildlife diseases, ecologists have become increasingly interested in how changes in community composition influence pathogen transmission (Dobson et al. 2006; Keesing et al. 2006; Swaddle & Calos 2008). While the idea that greater biodiversity can indirectly reduce disease in particular focal hosts is not new (e.g. Elton 1958), recent efforts in ecology have sought to broadly understand the ecological mechanisms that control the diversity–disease relationship and identify when and for what types of diseases it will apply (see Keesing et al. 2006). Thus far, most efforts have focused on vector-borne pathogens with frequency-dependent transmission, leaving open the question of how the dilution effect applies to other types of pathogens. Experimental studies have also been limited, raising questions about the relative contributions of different mechanisms in driving field-based patterns of disease (Begon 2008).
Here, we experimentally examined how community structure affected transmission of S. mansoni, a complex life-cycle parasite that causes human schistosomiasis in Africa and South America. Our results revealed that increases in community diversity substantially reduced parasite transmission into snail hosts, B. glabrata, consistent with previous work on the decoy effect and contemporary research on the dilution effect (see Upatham 1972; Christensen 1980; LoGuidice et al. 2003; Ezenwa et al. 2006). Heterospecific communities with one or more non-host species alongside the target host supported 25–50 per cent fewer infections relative to monospecific communities. Importantly, our study design maintained a constant density of focal hosts, regardless of the number and identity of non-hosts, such that our results were due entirely to changes in community structure rather than in host density (see also Thieltges et al. 2008a, b). Over the time scale of our experiments, infected hosts that co-occurred with non-hosts also produced 60–80 per cent fewer cercariae. This pattern probably stemmed from either (i) reduced miracidial penetration rate into focal snails within mixed-species communities, leading to lower or delayed cercarial output, or (ii) competition-mediated reductions in the resources obtained by focal hosts and thus available to produce cercariae. Combining the results for infection prevalence and cercarial production, mixed-species treatments supported fewer infected snails and each of these produced fewer infectious parasites, translating into a 70–90 per cent decrease in the total number of cercariae released per day. Whether this effect equates to a significant reduction in human infection risk will depend on whether the decrease in snail infections persists at time scales beyond the scope of this study.
Our results are broadly consistent with previous research on what parasitologists term the decoy effect, which represents one mechanism of encounter reduction under the dilution effect (Keesing et al. 2006). Although research on the decoy effect has been largely omitted from contemporary discussions of biodiversity and disease, it could provide important and complementary insights on the ecological mechanisms and applicability of the dilution effect to a broader range of host–parasite systems. Beginning with the pioneering work of Eli Chernin (e.g. Chernin 1968; Chernin & Perlstein 1971), parasitologists have found that (i) the addition of a broad range of non-hosts (but not shells or inanimate objects) interferes with the ability of free-living parasites (trematode miracidia and cercariae) to locate and infect target hosts, (ii) the extent of interference varies among non-host species and with the ratio of hosts to non-hosts, and (iii) the addition of non-hosts can cause an increase or decrease in cercarial production among infected hosts, probably as a function of miracidial exposure level (e.g. owing to intra-snail competition by trematodes; Upatham 1972; Upatham & Sturrock 1973; Frandsen & Christensen 1977; Christensen 1980; Moné et al. 1986; Combes & Moné 1987; Moné 1991; Chipev 1993; Kopp & Jokela 2007; Thieltges et al. 2008a,b). The ecological mechanisms underlying the decoy effect involve diversion of infectious parasites away from the target host by non-hosts. Combes & Moné (1987) suggested that non-host species interfere with host infection through four pathways: parasite exhaustion; wrongful penetration; stimulus of host immune defences; and production of toxins. Observational and experimental studies have established that non-hosts can cause exhaustion and morbidity in trematode miracidia; when miracidia do penetrate the wrong host, they are either unable to complete normal development (physiological incompatibility) or are eliminated by the immune system (Combes & Moné 1987). Non-hosts can also interfere with the transmission of free-living parasite stages via filtration (e.g. clams, some snails), release of toxic substances (e.g. planarians) and, especially, through active predation (e.g. fishes, hydras, zooplanktons and macroinvertebrates) (see Chernin & Perlstein 1971; Christensen 1980; Combes & Moné 1987; Thieltges et al. 2008a,b).
Our research provides ecological insights about the consequences of community-mediated changes in parasitism for host fitness. Unlike many previous parasitological studies focused on the decoy effect, our study (i) maintained community structures throughout the experiment rather than only during parasite exposure, (ii) measured host growth, reproduction and survival, and (iii) included a ‘no-parasite’ treatment to test for interactive effects between community structure and parasitism on host fitness. As expected, infection caused the castration of Biomphalaria, leading to a 70 per cent reduction in host egg production. However, because the presence of one or more non-host species indirectly ‘protected’ hosts from infection, heterospecific communities exhibited an increase in fecundity relative to their monospecific counterparts. Parasite-exposed hosts raised with non-hosts produced twice as many eggs as hosts raised alone. In the absence of parasites, the addition of non-hosts caused negative or neutral effects on host fecundity, possibly reflecting interspecific competition. Although food was not limiting in our study, snails can also interact indirectly through chemicals (e.g. Cope & Winterbourn 2004). Thus, our results exemplify parasite-mediated facilitation: the addition of a less suitable or resistant host species—by interfering with parasite transmission—causes a net benefit for more-susceptible host species (see also Kopp & Jokela 2007; Thieltges et al. 2008a). Similarly, Johnson et al. (2008) found that the addition of a less susceptible amphibian species diverted trematode cercariae away from more-susceptible hosts, leading to a decrease in total transmission and host pathology. This same effect was not observed when a second individual of the susceptible species was added. The net significance of parasite-mediated facilitation will depend on the relative costs of interspecific competition and parasitism. Here, parasite-induced castration probably represents a more significant cost to host fitness than competition, but we acknowledge the limitations of addressing this question through laboratory studies alone.
In nature, the importance of the decoy effect for parasite transmission and host fitness is probably dynamic, varying with changes in host density, non-host density and identity, and the force of infection, all of which change through time and across space. At extremely high levels of parasite abundance, it is possible to saturate the decoy effect, such that target hosts become infected even in the presence of non-hosts (e.g. Laracuente et al. 1979). Moreover, the efficacy of the community in reducing encounters between hosts and parasites depends strongly on the specific composition of the community and on interactions among those species (see Ostfeld & LoGuidice 2003). Some species are more effective decoys than others (e.g. Christensen 1980; Moné et al. 1986), and the effects of multiple decoy species may be non-additive, as seen in the current study. For example, Upatham & Sturrock (1973) found that while guppies and non-host snails were each effective decoys alone, their combined effects were less than additive. The authors suggested that fishes caused non-host snails to retreat into their shells, reducing their efficacy as decoys. In a more recent study, however, Thieltges et al. (2008a) showed a clear relationship between the number of filter-feeding decoy hosts added and the percentage reduction in focal host infections. Why the two decoy species used here failed to exhibit additive effects is unclear. While it is possible that these species had antagonistic effects upon each other, thereby reducing the sum of their diluting influence on transmission, it is also conceivable that the constrained volume (1 l) used in transmission trials obscured what might have been stronger effects in larger systems. To address this point, and to develop a broader understanding of the relationship between community assembly and parasite transmission, we highlight the need for subsequent experiments that incorporate simultaneous manipulation of species richness and evenness following the substitutive experimental designs used in biodiversity–ecosystem functioning research (e.g. Cardinale et al. 2006).
While our study was conducted under artificial conditions to maximize mechanistic understanding of the interactions between infection and community structure, available evidence suggests that the decoy effect also applies to more natural settings, including outdoor mesocosms, agricultural ditches, intertidal communities and whole wetlands (e.g. Upatham 1972; Upatham & Sturrock 1973; Laracuente et al. 1979; Thieltges et al. 2008a). Laracuente et al. (1979), for example, reported that, by adding the non-host snail Marisa cornuarietis to outdoor ponds, they completely blocked transmission of experimentally administered S. mansoni miracidia (relative to 8 per cent infection in control wetlands). In a marine intertidal experiment, Thieltges et al. (2008a) showed that non-native oysters reduced trematode infection in focal mussel hosts threefold. Taken together, results of previous work on the decoy effect and findings of the current study, which contribute a broader ecological perspective to the issue, highlight the importance of community structure for transmission of complex life-cycle parasites. Given the medical and veterinary importance of many such parasites, and the current and forecasted changes in community diversity in regions where they are endemic, we emphasize the theoretical and applied benefits that will emerge from integrating ecological research on the dilution effect and parasitological research on the decoy effect.
Acknowledgements
We thank K. Dosch and S. Orlofske and three anonymous reviewers for their helpful comments in revising the manuscript. This work was supported in part by NIH schistosomiasis supply contract AI30026 (Dr F. Lewis, Biomedical Research Institute, Rockville, MD). P. Johnson gratefully acknowledges the support of a fellowship from the David and Lucille Packard Foundation.
References
- Begon M. Effects of host diversity on disease dynamics. In: Ostfeld R.S., Keesing F., Eviner V.T., editors. Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems. Princeton University Press; Princeton, NJ: 2008. pp. 12–29. [Google Scholar]
- Carter N.P., Anderson R.M., Wilson R.A. Transmission of Schistosoma mansoni from man to snail: laboratory studies on the influence of snail and miracidial densities on transmission success. Parasitology. 1982;85:361–372. doi: 10.1017/s0031182000055335. [DOI] [PubMed] [Google Scholar]
- Cardinale B.J., Duffy D., Wright J.E., Downing J.P., Sankaran A.L., Srivastava M., Jouseau C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443:989–992. doi: 10.1038/nature05202. doi:10.1038/nature05202 [DOI] [PubMed] [Google Scholar]
- Chernin E. Interference with the capacity of Schistosoma mansoni miracidia to infect the molluscan host. J. Parasitol. 1968;54:509–516. doi:10.2307/3277073 [PubMed] [Google Scholar]
- Chernin E., Perlstein J.M. Protection of snails against miracidia of Schistosoma mansoni by various aquatic invertebrates. J. Parasitol. 1971;57:217–219. doi:10.2307/3278017 [PubMed] [Google Scholar]
- Chipev N.H. Decoy effect and host infection by miracidia within snail communities. Parasitology. 1993;106:265–276. doi: 10.1017/s0031182000075089. [DOI] [PubMed] [Google Scholar]
- Christensen N.Ø. A review of the influence of host- and parasite-related factors and environmental conditions on the host-finding capacity of the trematode miracidium. Acta Tropica. 1980;37:303–318. [PubMed] [Google Scholar]
- Collinge S.K., Ray C. Disease ecology: community structure and pathogen dynamics. Oxford University Press; Oxford, UK: 2006. [Google Scholar]
- Combes C., Moné H. Possible mechanisms of the decoy effect in Schistosoma mansoni transmission. Int. J. Parasitol. 1987;17:971–975. doi: 10.1016/0020-7519(87)90017-8. doi:10.1016/0020-7519(87)90017-8 [DOI] [PubMed] [Google Scholar]
- Cope N.J., Winterbourn M.J. Competitive interactions between two successful molluscan invaders of freshwaters: an experimental study. Aquat. Ecol. 2004;38:83–91. doi:10.1023/B:AECO.0000021018.20945.9d [Google Scholar]
- Dobson A., Cattadori I., Holt R.D., Ostfeld R.S., Keesing F., Krichbaum K., Rohr J.R., Perkins S.E., Hudson P.J. Sacred cows and sympathetic squirrels: the importance of biological diversity to human health. PLoS Med. 2006;3:714–718. doi: 10.1371/journal.pmed.0030231. doi:10.1371/journal.pmed.0030231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J.E., Cardinale B.J., France K.E., McIntyre P.B., Thébault E., Loreau M. The functional role of biodiversity in food webs: incorporating trophic complexity. Ecol. Lett. 2007;10:522–538. doi: 10.1111/j.1461-0248.2007.01037.x. doi:10.1111/j.1461-0248.2007.01037.x [DOI] [PubMed] [Google Scholar]
- Elton C.S. Muehuen; London, UK: 1958. The ecology of invasions by animals and plants. [Google Scholar]
- Ezenwa V.O., Godsey M.S., King R.J., Guptill S.C. Avian diversity and West Nile virus: testing associations between biodiversity and infectious disease risk. Proc. R. Soc. B. 2006;273:109–117. doi: 10.1098/rspb.2005.3284. doi:10.1098/rspb.2005.3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen F., Christensen N.Ø. Effect of Helisoma duryi on the survival, growth and cercarial production of Schistosoma mansoni-infected Biomphalaria glabrata. B. World Health Organ. 1977;55:577–580. [PMC free article] [PubMed] [Google Scholar]
- Gerard C., Theron A. Age/size- and time-specific effects of Schistosoma mansoni on energy allocation patterns of its snail host Biomphalaria glabrata. Oecologia. 1997;112:447–452. doi: 10.1007/s004420050331. doi:10.1007/s004420050331 [DOI] [PubMed] [Google Scholar]
- Hudson P.J., Greenman J.V. Parasite mediated competition. Biological and theoretical progress. Trends Ecol. Evol. 1998;13:387–390. doi: 10.1016/s0169-5347(98)01475-x. doi:10.1016/S0169-5347(98)01475-X [DOI] [PubMed] [Google Scholar]
- Johnson P.T.J., Hartson R.B., Larson D.J., Sutherland D.R. Diversity and disease: community structure drives parasite transmission and host fitness. Ecol. Lett. 2008;11:1017–1026. doi: 10.1111/j.1461-0248.2008.01212.x. doi:10.1111/j.1461-0248.2008.01212.x [DOI] [PubMed] [Google Scholar]
- Keesing F., Holt R.D., Ostfeld R.S. Effects of species diversity on disease risk. Ecol. Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. doi:10.1111/j.1461-0248.2006.00885.x [DOI] [PubMed] [Google Scholar]
- Kopp K., Jokela J. Resistant invaders can convey benefits to native species. Oikos. 2007;116:295–301. doi:10.1111/j.0030-1299.2007.15290.x [Google Scholar]
- Laracunte A., Brown R.A., Jobin W. Comparison of four species of snails as potential decoys to intercept schistosome miracidia. Am. J. Trop. Med. Hyg. 1979;28:99–105. doi: 10.4269/ajtmh.1979.28.99. [DOI] [PubMed] [Google Scholar]
- LoGuidice K., Ostfeld R.S., Schmidt K.A., Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl Acad. Sci. USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. doi:10.1073/pnas.0233733100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreau M., et al. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science. 2001;294:804–808. doi: 10.1126/science.1064088. doi:10.1126/science.1064088 [DOI] [PubMed] [Google Scholar]
- Moné H. Influence of non-target molluscs on the growth of Biomphalaria glabrata infected with Schistosoma mansoni: correlation between growth and cercarial production. J. Mollus. Stud. 1991;57:1–10. doi:10.1093/mollus/57.1.1 [Google Scholar]
- Moné H., Theron A., Combes C. Interaction between the Biomphalaria glabrata–Schistosoma mansoni host–parasite system and the non-target molluscs: influence on cercarial production. J. Parasitol. 1986;72:410–416. doi:10.2307/3281681 [PubMed] [Google Scholar]
- Nolan L.E., Carriker J.P. Observations on the biology of the snail Lymnaea stagnalis appressa during twenty years of laboratory culture. Amer. Midl. Natl. 1946;36:467–493. doi:10.2307/2421516 [Google Scholar]
- Ostfeld R.S., Keesing F. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. 2000;78:2061–2078. doi:10.1139/cjz-78-12-2061 [Google Scholar]
- Ostfeld R.S., LoGuidice K. Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology. 2003;84:1421–1427. doi:10.1890/02-3125 [Google Scholar]
- Steinmann P., Keiser J., Bos R., Tanner M., Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006;6:411. doi: 10.1016/S1473-3099(06)70521-7. doi:10.1016/S1473-3099(06)70521-7 [DOI] [PubMed] [Google Scholar]
- Swaddle J.P., Calos S.E. Increased avian diversity is associated with lower incidence of human West Nile infection: observation of the dilution effect. PLoS One. 2008;3:e2488. doi: 10.1371/journal.pone.0002488. doi:10.1371/journal.pone.0002488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieltges D.W., Bordalo M.D., Hernández A.C., Prinz K., Jensen K.T. Ambient fauna impairs parasite transmission in a marine parasite–host system. Parasitology. 2008a;135:1111–1116. doi: 10.1017/S0031182008004526. doi:10.1017/S0031182008004526 [DOI] [PubMed] [Google Scholar]
- Thieltges D.W., Jensen K.T., Poulin R. The role of biotic factors in the transmission of free-living endohelminth stages. Parasitology. 2008b;135:407–426. doi: 10.1017/S0031182007000248. doi:10.1017/S0031182007000248 [DOI] [PubMed] [Google Scholar]
- Upatham E.S. Interference by unsusceptible aquatic animals with the capacity of the miracidia of Schistosoma mansoni Sambon to infect Biomphalaria glabrata (Say) under field-simulated conditions in St. Lucia, West Indies. J. Helminthol. 1972;46:277–288. doi: 10.1017/s0022149x00024421. [DOI] [PubMed] [Google Scholar]
- Upatham E.S., Sturrock R.F. Field investigations on the effect of other aquatic animals on the infection of Biomphalaria glabrata by Schistosoma mansoni miracidia. J. Parasitol. 1973;59:448–453. doi:10.2307/3278770 [PubMed] [Google Scholar]
- Yoshino T.P., Laursen J.R. Production of Schistosoma mansoni daughter sporocysts from mother sporocysts maintained in synxenic culture with Biomphalaria glabrata embryonic (BGE) cells. J. Parasitol. 1995;81:714–722. doi:10.2307/3283960 [PubMed] [Google Scholar]