Abstract
In stalk-eyed flies (Diopsidae), the eyes are positioned at the end of rigid peduncles protruding laterally from the head. Sexual selection for eye span in male Cyrtodiopsis dalmanni results in eye span that exceeds body length and exceeds the eye span of females. We studied whether the twofold higher moment of inertia (MOI) of the male head results in a reduced head rotation velocity during turning. We analysed films of flies performing walking turns and compared the head kinematics between the sexes. The significance of head rotation to turning was evaluated from the turning kinematics of flies with immobilized (glued) heads. Male and female C. dalmanni rotated their heads relative to the surrounding environment 1.55-fold (male) and 1.65-fold (female) faster than the angular velocity of the body by performing rapid head saccades. During the larger turns, flies with immobilized heads were unable to reorient gaze as fast as the control flies. Despite the larger MOI of the head, male C. dalmanni match the head saccade of females suggesting that eye span elongation is coupled by an adaptation of the neck apparatus to rotate the wider head.
Keywords: moment of inertia, saccade, sexual selection, Diopsidae, locomotion
1. Introduction
In flies, motions of the head result in the motion of the fixed eyes relative to the surrounding panorama and hence changes the image projected on the retina. While eye translations can provide information on the three-dimensional structure of the environment, rapid rotation of the eyes (or image on the retina) results in reduced image resolution (reviewed by: Land 1999; Land & Nilsson 2002). Insects rely extensively on processing visual information during locomotion (Krapp & Hengstenberg 1996; Mazokhin-Porshnyakov 1969; Srinivasan et al. 1999; Taylor & Krapp 2008), and flies, in particular, have been shown to change their free flight or walking behaviour depending on visual stimuli (Collett & Land 1975; Strauss et al. 1997; Wagner 1986a,b; Tammero & Dickinson 2002; Frye et al. 2003). To improve visual input during locomotion, flies attempt to minimize rotational optic flow by fixing the orientation of the eyes between rapid changes in gaze direction (Collett & Land 1975; Land 1999; Schilstra & van Hateren 1998). The rapid gaze shifts are called ‘saccades’ after the functional similarity to saccadic eye motions in humans (Land 1999). Hoverflies, with restricted head motions around the yaw axis, perform rapid body saccades to change the orientation of the eyes in space (Collett & Land 1975). Blowflies use extensive neck motions to compensate for the roll of the body during aerial manoeuvres (Hengstenberg 1993; van Hateren & Schilstra 1999) and to reduce the duration of head yaw when the body is turning during flying and walking (Schilstra & van Hateren 1998; Blaj & van Hateren 2004).
In stalk-eyed flies (Diopsidae), the eyes are positioned at the end of rigid peduncles protruding laterally from the head (Shillito 1971). The stalks' length can exceed body length in some species. Both males and females possess eye stalks, but in Cyrtodiopsis dalmanni eye stalk length in males is sexually selected: female mate choice favours males with longer eye stalks (Burkhardt & de la Motte 1988; Wilkinson & Reillo 1994; Wilkinson et al. 1998), and males with shorter eye stalks retreat faster from territorial confrontations with males with longer eye stalks (Panhuis & Wilkinson 1999). As a result, eye span (distance between the eyes) in males exceeds the eye span of equally sized females (Baker & Wilkinson 2001; figure 1). As an emerging model organism for evolution by sexual selection, there is a growing literature on the development and expression of eye stalks (e.g. Knell et al. 1999; David et al. 2000; Baker & Wilkinson 2001; Cotton et al. 2004; see review by Warren & Smith 2007). However, the consequences of eye stalk elaboration outside the context of reproduction and sexual selection have rarely been addressed. Buschbeck & Hoy (1998) described the neural reorganization of the visual system in Cyrtodiopsis quinqueguttata (in particular, a reduced number of wide-field vertical cells of the lobula plate). The eyes of C. dalmanni have good temporal resolution and are sensitive to low light intensities (Burkhardt 1972). Burkhardt & de la Motte (1983) pointed out that the eye stalk length should increase spatial resolution for binocular vision.
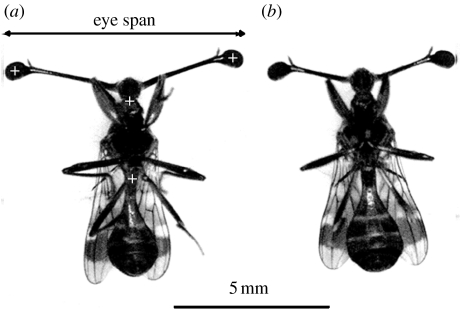
Figure 1.
(a) Male and (b) female C. dalmanni lying on their dorsal side. White pluses marked on the image of the male show the positions on the body that were digitized in the films for the kinematic analysis.
Stalk-eyed flies rotate their heads around the three major axes (Wickler & Seibt 1972; Burkhardt & de la Motte 1983). Optomotor experiments with tethered flies showed that, in the horizontal plane (yaw; rotation about the vertical axis), a sexually dimorphic stalk-eyed fly, Cyrtodiopsis whitei, responded to a rotating vertically striped pattern (18° stripe width) by moving their heads in the same direction as the rotating stripes at average yaw angles of 25° and 19° in females and males, respectively. By artificially applying pressure to one side of the head, it was determined that both sexes are free to yaw the head on average 29° on either side of the body. However, spontaneous head rotations during turning were only approximately 15° for both males and females (table 2 in Burkhardt & de la Motte 1983). We were interested to test whether the difference in eye span between the sexes results in changes in head rotation dynamics. Such changes would have sensory and physiological implications that, when placed in an ecological context, could constrain the evolution of eye span elongation.
In C. dalmanni, males and females of equal body mass have similar head mass, but as a result of eye stalk elongation in males the mass moment of inertia (hereafter ‘MOI’) of the head for yaw and roll is more than twofold higher than that in females (Ribak & Swallow 2007). MOI is a measure of the resistance a body offers to any change in its angular velocity. It is determined by the body's mass (m) and the distribution of the mass relative to an axis of rotation (Schaum & van der Merwe 1961).
| (1.1) |
Equation (1.1) denotes that the MOI of an object about some axis is the sum of all mass particles in the body multiplied by the square of their distances (r) from that axis. Owing to the second power of the distance in equation (1.1), small changes in distribution of mass away from the axis of rotation would have an amplified effect on the change in MOI of the body.
In the absence of significant friction, an unbalanced torque (L) acting on a body of given MOI produces in it an angular acceleration (α) according to the relationship
| (1.2) |
It follows that the twofold higher MOI of the head of a male C. dalmanni would imply a lower angular acceleration of the head relative to the thorax than that in a female when both sexes produce the same torque (L=const.) in their necks. Alternatively, to achieve the same angular acceleration as females, males would need to produce a twofold higher torque in their necks.
Here we investigate the saccadic head turns of male and female C. dalmanni from high-speed films of walking flies. We first describe the head turn dynamics and compare them between the sexes. The relevance of head motions to shifting the direction of gaze during turning is then studied from films of male flies turning after their heads were immobilized with a drop of glue.
2. Material and methods
(a) Experiments
Flies from a laboratory-raised population of C. dalmanni were used in the experiments. Rearing conditions have been described elsewhere (Ribak & Swallow 2007). Stalk-eyed flies reach peak sexual maturity four weeks after eclosion (Reguera et al. 2004) while lifespan in captivity can exceed two months (G. Ribak 2006–2008, personal observation). In the experiments, we used flies four to five weeks after eclosion. We used a high-speed video camera (Fastcam-512 PCI, Photron) to individually film flies from above as they were free to walk inside a circular arena (for additional details see the electronic supplementary material 1). Each time a fly entered the camera's field of view and made a turn, the sequence was captured at 1000 frames s−1. Because we were interested in rapid head motions, only large (more than 15°) and abrupt turns were saved for analysis in this experiment. These larger turns compose 62 per cent of all turns, according to the data from the control group of the second experiment described below.
The second experiment was designed to evaluate the significance of head rotations to walking turns. Male flies were anaesthetized using an 80 per cent N2 and 20 per cent CO2 gas mixture and the head was glued to the thorax with a single droplet of cyanoacrylate glue (‘super glue’) applied to the gap in the dorsal neck surface with a 30 gauge hypodermic needle mounted on a micromanipulator. Because flies lost mass from water evaporation during the anaesthesia (5.3%, s.d.=4.62, n=5), the added mass of the glue could not be measured directly from the flies after the glue dried. However, a series of trials where a drop of glue was applied in the same process on a small piece of pre-weighted aluminium foil showed that the dry mass of the glue drop was only 0.024 mg (s.d.=0.013, n=5), which is negligible (less than 1%) compared with the average body mass of male C. dalmanni (7.12±1.033 mg, Ribak & Swallow 2007). We successfully immobilized the head for five males and filmed them as described above. Filming was done 12 hours after treatment to allow the flies to recover from the anaesthesia and adjust to the new condition. Another five males served as a control group. They received the same treatment, only the glue drop was applied dorsally to the centre of the thorax instead of between the thorax and head. In this experiment, we filmed the flies each time they passed inside the field of view of the camera regardless of whether they were turning. This small distinction in sampling method allowed us to measure all turns, no matter how small, as long as they fulfilled our turn-defining criteria (see §2b). This was important to test statistically whether the treatment resulted in reduced turn size.
(b) Turn analysis
In both experiments, the criteria for defining a turn was the angular velocity of the head relative to the surrounding environment in excess of 300° s−1 consecutively for at least 5 ms (see the electronic supplementary material 2). The angular velocity threshold was chosen for rotation velocity that should result in some loss of image resolution (Land 1999). The minimum time threshold was added to allow sufficient time resolution in the analysis. The criteria set resulted in 70 and 75 normal voluntary turns from 20 males and 21 females, respectively. In the second experiment, we were able to measure 101 turns from five males with immobilized heads and 138 turns from five males in the control group.
The analysis of walking turns involves three primary rotations. The rotation of the thorax and the rotation of the head relative to the thorax (hereafter ‘head saccade’) result in a third rotation of the head relative to the surrounding environment (hereafter ‘head turn’). In each frame of each film, we digitized (Motion Tools, Photron) the position of the centre of the left and right eyes as well as the connection between the head and the thorax and the connection between the thorax and the abdomen (figure 1). The eye positions were used to calculate head orientation (angle relative to an arbitrary but fixed axis), and the body points were used to calculate the orientation of the long axis of the thorax. Subtracting the two orientations gave the angle of the head relative to the body. The instantaneous angle data were smoothed using a low-pass Gaussian filter with a cut-off frequency of 45 Hz (filter width=19 time steps) and time derivatives were calculated (using a four-point numerical parabola approximation; Rayner & Aldridge 1985) to yield the angular velocity and acceleration. For each turn, seven kinematic parameters were calculated. (i) Head turn duration was defined as the time difference between the first (t0) and last (t1) frames where the angular velocity of the head relative to the surrounding environment was more than 300° s−1 (e.g. see the electronic supplementary material 2). (ii) Head turn size was defined as the difference in orientation of the head between t0 and t1. Mean thorax (iii) and head (iv) angular velocity were averaged from the instantaneous angular velocity data between t0 and t1. To describe the saccade of the head relative to the body, we used the curve depicting the change in angle between the head and thorax with time (figure 2) to find the minimum and maximum points on this curve defining the head-saccade amplitude (v) in degrees. Mean angular velocity of the head saccade (vi) was averaged between these maximum and minimum time points. Finally, the time point where the angular velocity of the head relative to the thorax reached a maximum was used to define the point when the head stops its acceleration relative to the thorax, and angular acceleration of the head saccade (vii) was averaged from the initiation of motion of the head relative to the thorax to that time point. The analysis of turns in the head immobilization experiment followed the same conventions with the exception that for the flies with immobilized head there is only one rotation and hence only the eyes were digitized. We measured turn size, turn duration and angular velocity of the head as mentioned above. Angular velocity of the body was measured only in the control group. After the experiment was completed, the flies were killed in chloroform fumes and the immobility of the head relative to the thorax was verified mechanically under a dissecting microscope.
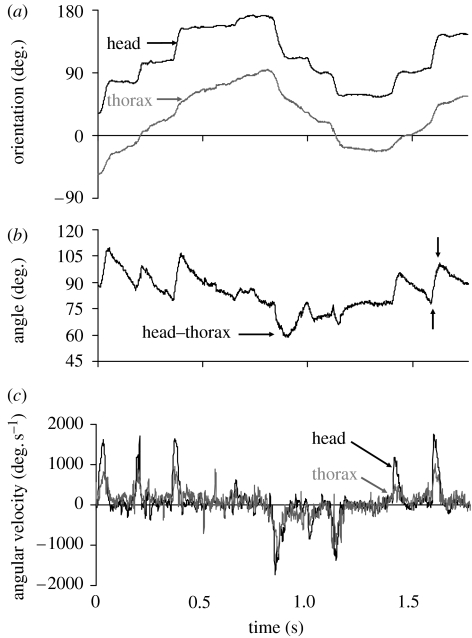
Figure 2.
The change in (a) orientation of the head and thorax and (b) the angle between the head and thorax in a walking male C. dalmanni. Vertical arrows denote the amplitude and duration of head rotation relative to the thorax in one turn. (c) The angular velocities at which the head and thorax rotate relative to the surrounding environment. The sharp spikes in the angular velocity curve of the head are examples of the turns analysed in this study.
(c) Statistical analysis
The sign of negative yaw turns was changed so that all turns were analysed as positive (anticlockwise). The dataset comprises sampled voluntary turns varying by head turn size where individual flies are represented more than once. We therefore used a mixed linear model (SYSTAT Software 2002) to test for differences in the parameters between males and females. Sex was used in the model as a fixed effect and individual fly number as a random effect. Head turn size was used as a covariate whenever the tested parameter was correlated with it. All data were log transformed before the analysis to improve linearity of the model. In the glue experiments, we replaced the ‘sex’ effect by ‘treatment’ (immobilized head versus control).
3. Results
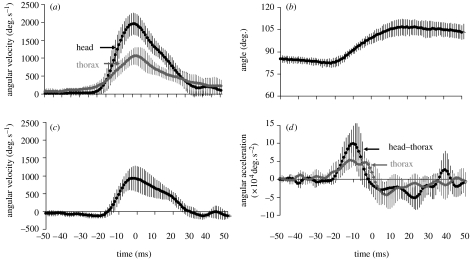
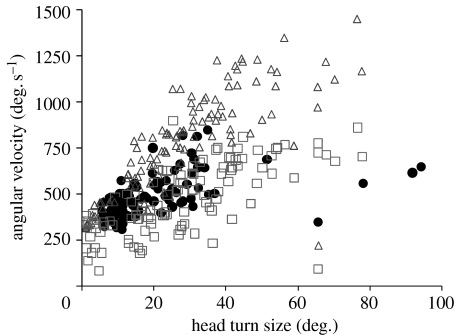
During voluntary walking, as the body makes relatively smooth changes in its orientation, the head undergoes a series of step-like changes (figure 2). These steep changes in head orientation relative to the background result from abrupt changes in the angle between the head and thorax. Figure 3 shows the kinematics of the head and body during a large (60°) turn. First the body starts to rotate and the head remains in its initial orientation (figure 3a) by a counter-rotation of the head relative to the thorax (figure 3b,c). Then, the head is yawed relative to the thorax in the same direction as the turning thorax (figure 3c). As a result, the head is turning, relative to the surrounding environment, at a turning velocity that is almost double the turning velocity of the thorax (figure 3a). During the first 20 ms after the head started to rotate in the same direction as the thorax, the head undergoes a large acceleration relative to the thorax (figure 3d). The kinematic data for the turns analysed are shown in table 1 and figure 4. By rotating their heads relative to the thorax, males and females increase the mean turn velocity of the head 1.55- and 1.65-fold, respectively, compared with the turning velocity of the body (table 1). The sexes did not differ in the size of the head turns analysed or the head turn duration when accounting for the head turn size (mixed model, p>0.52 and p>0.96, respectively). The mean turning velocity of the head relative to the background was also similar in both sexes (p>0.96) and increased with the size of the head turn (both sexes: r=0.85, p≪0.001, n=145). Mean angular velocity of the body did not differ between the sexes (p>0.067), but the mean turning velocity of the head saccade was marginally higher in females (p<0.051) and the difference became more significant when the analysis was repeated on the maximum angular velocity instead of the mean velocity (p<0.042). The difference between the sexes was small, however, amounting to less than 8 per cent in the mean and maximum angular velocities of the saccade.
Figure 3.
Head turn kinematics in C. dalmanni. The curves are averages (±1 s.d.) from five turns with the same head turn size=60°. The curves were averaged in a 100 ms time window centred (t=0) at the point of maximum angular velocity of the head relative to the surrounding environment. (a) The angular velocity at which the head and thorax are rotated relative to the surrounding background during the turn. (b) The change in the angle between the transverse axis of the head and the longitudinal axis of the thorax. (c) The angular velocity of the head rotation relative to the thorax, i.e. saccade angular velocity. (d) The angular acceleration of the thorax and the angular acceleration of the head relative to the thorax. Note that the turns shown are large relative to the mean turn size observed (table 1). The purpose here is to illustrate the rotational dynamics within a single turn.
Table 1.
Summary of turning kinematics (yaw) in male and female C. dalmanni. Values are the means per sex ±1 s.d. The p-values refer to the significance of sex effect in a mixed linear model (75 turns from 21 females and 70 turns from 20 males). All parameters were log transformed prior to the analysis. ω denotes angular velocity and the subscripts H, T and HT denote head, thorax and head relative to the thorax, respectively.
| kinematic parameter | male | female | p-value |
|---|---|---|---|
| turn size (deg.) | 42.0±11.93 | 39.5±14.46 | 0.523 |
| turn duration (ms) | 41.5±10.69 | 39.7±10.60 | 0.961 |
| Mean ωH (deg. s−1) | 996±168.6 | 961±190.6 | 0.964 |
| Mean ωT (deg. s−1) | 643±153.5 | 582±176.8 | 0.067 |
| Mean ωHT (deg. s−1) | 417±111.7 | 450±125.9 | 0.051 |
| head saccade—angular acceleration (×104deg. s−2) | 6.5±2.05 | 6.7±2.23 | 0.631 |
| head saccade—magnitude (deg.) | 15.4±5.28 | 16.0±6.03 | 0.074 |
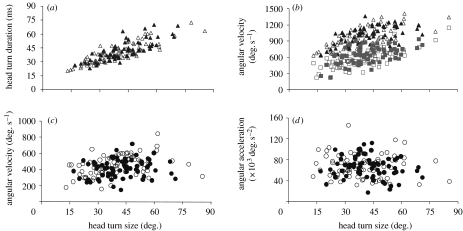
Figure 4.
Turns performed by male (filled symbols) and female (open symbols) C. dalmanni during walking. Shown are turns (n=145) observed from 20 males and 21 females. (a) Head turn duration did not differ between the sexes (p>0.96). (b) Mean angular velocity of the head (triangles) and thorax (squares) relative to the surrounding environment. (c) Mean angular velocity of the head relative to the thorax as the head yaw in the direction of the turn. (d) Mean angular acceleration of the head relative to the thorax. This acceleration is measured from the time the head starts to rotate in the direction of turning until it reaches the maximum speed (relative to the thorax; circles).
During the turn, the (yaw) angle of the head relative to the thorax changed by 15.7±5.67° on average but ranged between 2.4 and 34.7°. The actual value for each saccade was positively correlated with the head turn size (r=0.65, p≪0.001, n=145). The angle did not differ significantly between the sexes (p>0.074).
The sexes did not differ in the acceleration of the head relative to the thorax (p>0.63) and this acceleration did not correlate with the head turn size (r=0.09, p>0.26, n=145). It seemed that the higher angular velocity of the head relative to the thorax in females could be explained by the fact that females allow the head to accelerate 4.5 per cent longer (female: 13.7±4.34, male: 13.1±3.83 ms; p=0.076) by moving the head through a 4 per cent larger angle (table 1, p=0.074). However, these differences were minor and not significant at the 95 per cent confidence level.
Mean turn size of the male flies with immobilized heads was 31 per cent smaller than that in the control group (19.1±15.32 and 27.8±17.66°, respectively), but did not differ significantly at the 95 per cent confidence level (p=0.057). Head turn duration did not differ between the treatments after accounting for head turn size (p>0.44), but the interaction between treatment (immobilized versus control) and turn size was significant (p<0.001), implying that the flies with immobilized heads took longer time to make the larger turns. Immobilizing the head did not result in a difference in the angular velocity of the head relative to the surrounding environment (p>0.52), but the interaction between the treatment and head turn size (p<0.001) again suggested that at larger turns the immobilized flies turned slower than the control flies (figure 5). The angular velocity at which the flies with immobilized heads turned did not differ significantly from that of the body in the control flies (p>0.13, no interaction with turn size p>0.61).
Figure 5.
Mean angular velocity of rotation relative to the surrounding environment in the control group (open symbols: triangles, head; squares, thorax) and the experiment group in which the head was immobilized with glue (filled circles). For an example of the altered turning behaviour, see the electronic supplementary material 3).
4. Discussion
Herein, we describe the head motions of male and female stalk-eyed flies during turning. Although reports on head motion as an optomotor response in tethered flies are common in the literature (McCann & MacGinitie 1965; Land 1973; Hengstenberg 1993), few studies have measured head motions, in good detail, during unimpeded (non-tethered) locomotion. Stalk-eyed flies are ideally suited for such a study because their characteristic head morphology (eye stalks) provides natural markers for head roll and yaw. Our observations are consistent with the reports made on head rotations during turning in freely walking and flying blowflies (Schilstra & van Hateren 1998; Blaj & van Hateren 2004). As in blowflies, our flies used head saccades during walking to reduce the duration of head (eyes) rotation relative to the stationary background. During larger turns, when motions of the head were artificially restricted, our flies were not capable of achieving the same rotation velocity of the head relative to the background as in the control flies. This experiment showed that body rotation velocity is a limiting factor for the head turn duration of larger turns. Therefore, the flies rely on head rotation relative to the thorax to produce rapid gaze shifts. It is this need for rapid head turns that allows us to test whether the higher MOI of the head becomes a limitation during head saccades in C. dalmanni.
Both sexes of C. dalmanni rotated their heads with remarkable similarity with regard to the velocity at which the eyes rotated relative to the surrounding environment (figure 4; table 1). This finding has two implications. First, head rotation velocity in the two sexes appears to be driven by the same, probably visual, constraint. Second, male flies are capable of compensating for the higher MOI of their heads. Our research paradigm assumed that, during turning, both sexes will attempt to rotate their heads relative to the surrounding environment as fast as possible to reduce the duration of blurred image (Land 1999). The fact that male flies compensate for higher head inertia to turn their heads as fast as female flies supports this assumption.
Our head immobilization experiment documents the need for rapid head rotations as part of turning in C. dalmanni, but further studies focusing on sensory input are needed to describe the neurological link between visual feedback and turning dynamics in walking stalk-eyed flies. It should be noted that the visual consequences of head immobilization should be dramatic. Aside from a reduced gaze shift speed, the flies are no longer capable of stabilizing gaze direction by counter-turning their head as the body starts to rotate.
In the present study, the existence of a sensory constraint on head rotation dynamics is useful to examine the evolutionary cost associated with sexual selection for long eye stalks. We anticipated the mechanical effect of the higher MOI of the head to manifest itself in the rotation of the head relative to the thorax. The average angular velocity of this rotation differed between the sexes, but only by 8 per cent. Furthermore, the acceleration of the head relative to the thorax, which should have been reduced to half in males owing to the higher MOI, showed no sign of difference between the sexes. The ability of male C. dalmanni to accelerate their heads in a comparable manner to females implies that they are capable of producing head turning moments that are twofold larger than those of females. However, a remaining question is to what extent head inertia represents a constraint for saccade dynamics. There is some evidence to support the significance of head inertia as a mechanical constraint on head turn dynamics. First, both the sexes of C. dalmanni possess long eye stalks, so inertia of the wide head is high in the female to begin with and then this inertia is doubled in males. Second, the acceleration of the head relative to the thorax did not increase with head turn size even though the flies were increasing the velocity at which the head was rotating relative to the thorax. The lack of relationship between saccade acceleration and head turn size may indicate that the flies are incapable of a larger acceleration of the head.
If male stalk-eyed flies have evolved the capacity to produce larger torques in their necks in order to retain a certain level of head rotation despite the higher MOI, then the change in neck structure represents the evolutionary cost associated with sexual selection. Examining the difference between the sexes in the functional anatomy of the neck can provide a mechanism to evaluate this cost. The neck anatomy may also limit further selection for eye span elongation. The dependence of the MOI on the squared distance from the axis of rotation means that the MOI of the head, and hence the maximum torque produced by the neck, would increase in proportion to the square power of the increase in eye span. For example, a 1.4-fold difference in eye span between males and females of similar body mass results in a doubled MOI of the head for yaw and roll (Ribak & Swallow 2007). To produce the larger torques, the relevant muscles in the neck structure would need to double in cross section or the skeletal leverage would need to double in length. These anatomical changes would need to occur in male C. dalmanni within a constraint of fixed body size, if only eye span is growing. Therefore, how much the neck structure can grow in proportion to the square of eye span elongation without significant growth of the body may place an upper limit to eye span elongation. Artificial selection for eye span length in male C. dalmanni over 57 generations seemed to level off after only an approximately 10 per cent increase in eye span compared with the control lines (Wilkinson et al. 2005). Whether it is the torque required for head movements that limits eye span elongation or some other factor (e.g. neural conduction time) is presently unknown.
The mechanical considerations of the saccade are somewhat more complex than a simple difference in MOI of the head. First, head rotation is not frictionless. Apart from the friction at neck joints, the head is rotated through the air and hence experiences some air resistance. Since both sexes rotated their heads relative to the air at similar angular velocities (table 1), the 1.4-fold longer eye stalks in males would imply a higher tangential velocity relative to the air for the eye moving through a larger arc. As a result, any restricting effect of air resistance would be felt more strongly in males and hence would add to the picture portrayed by the differences in MOI. Second, when the head starts to rotate relative to the thorax in the same direction as the body, the body is already accelerating (figure 3d). Owing to inertia, the acceleration of the head relative to the accelerating body calls for a larger torque in the neck muscles compared with the case when the head is turned relative to a stationary body. However, the angular acceleration of the body did not differ between the sexes (p>0.42), making the comparison valid. Clearly, although the mechanics of head saccade are somewhat more complicated than a simple rotation of the head relative to the thorax, eye span elongation should result in a significant effect on the head saccade of stalk-eyed flies. The fact that the male flies are capable of matching the head rotations of females despite the handicap from their longer eye stalks is evidence of the successful adaptation within male C. dalmanni to cope with an exaggerated sexual trait.
Acknowledgments
We thank Gerald S. Wilkinson from the University of Maryland for providing the initial population of flies used in this study. Donita Jolkowski, Heidi Moline, Kari Wolf and Kyla Feickert helped with maintaining the fly population. Two anonymous reviewers provided helpful comments that improved the manuscript. The study was funded by NSF-CAREER and NSF-REU grants to J.G.S. (no. IOB0448060).
Supplementary Material
Technical details of the experimental set-up
An example of the turn defining criteria
An example of the altered turning behaviour of flies with immobilized heads
References
- Baker R.H., Wilkinson G.S. Phylogenetic analysis of sexual dimorphism and eye-span allometry in stalk-eyed flies (Diopsidae) Evolution. 2001;55:1373–1385. doi: 10.1111/j.0014-3820.2001.tb00659.x. doi:10.1111/j.0014-3820.2001.tb00659.x [DOI] [PubMed] [Google Scholar]
- Blaj G., van Hateren J.H. Saccadic head and thorax movements in freely walking blowflies. J. Comp. Physiol. A. 2004;190:861–868. doi: 10.1007/s00359-004-0541-4. doi:10.1007/s00359-004-0541-4 [DOI] [PubMed] [Google Scholar]
- Burkhardt D. Electrophysiological studies on the compound eye of a stalk-eyed fly, Cyrtodiopsis dalmanni (Diopsidae, Diptera) J. Comp. Physiol. 1972;81:203–214. doi:10.1007/BF00696633 [Google Scholar]
- Burkhardt D., de la Motte I. How stalk-eyed flies eye stalk-eyed flies: observations and measurements of the eyes of Cyrtodiopsis whitei (Diopsidae, Diptera) J. Comp. Physiol. 1983;151:407–421. doi:10.1007/BF00605457 [Google Scholar]
- Burkhardt D., de la Motte I. Big ‘antlers’ are favoured: female choice in stalk eyed flies (Diptera, Insecta), field collected harems and laboratory experiments. J. Comp. Physiol. A. 1988;162:649–652. doi:10.1007/BF01342640 [Google Scholar]
- Buschbeck E.K., Hoy R.R. Visual system of the stalk-eyed fly, Cyrtodiopsis quinqueguttata (Diopsidae, Diptera): an anatomical investigation of unusual eyes. J. Neurobiol. 1998;37:449–468. doi: 10.1002/(sici)1097-4695(19981115)37:3<449::aid-neu10>3.0.co;2-5. doi:10.1002/(SICI)1097-4695(19981115)37:3<449::AID-NEU10>3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- Collett T.S., Land M.F. Visual control of flight behaviour in the hoverfly, Syritta pipiens L. J. Comp. Physiol. 1975;99:1–66. doi:10.1007/BF01464710 [Google Scholar]
- Cotton S., Fowler K., Pomiankowski A. Condition dependence of sexual ornament size and variation in the stalk-eyed flies Cyrtodiopsis dalmanni (Diptera: Diopsidae) Evolution. 2004;58:1038–1046. doi: 10.1111/j.0014-3820.2004.tb00437.x. doi:10.1554/03-514 [DOI] [PubMed] [Google Scholar]
- David P., Bjorksten T., Fowler K., Pomiankowski A. Condition dependent signaling of genetic variation in stalk-eyed flies. Nature. 2000;406:186–188. doi: 10.1038/35018079. doi:10.1038/35018079 [DOI] [PubMed] [Google Scholar]
- Frye M.A., Tarsitano M., Dickinson M.H. Odor localization requires visual feedback during free flight in Drosophila melanogaster. J. Exp. Biol. 2003;206:843–855. doi: 10.1242/jeb.00175. doi:10.1242/jeb.00175 [DOI] [PubMed] [Google Scholar]
- Hengstenberg R. Multisensory control in insect oculomotor systems. In: Miles F.A., Wallman J., editors. Visual motion and its role in stabilization of gaze. Elsevier Science Publishers; Amsterdam, The Netherlands: 1993. pp. 285–298. [PubMed] [Google Scholar]
- Knell R.J., Fruhauf N., Norris K.A. Conditional expression of a sexually selected trait in the stalk-eyed fly Diasemopsis aethiopica. Ecol. Entomol. 1999;24:323–328. doi:10.1046/j.1365-2311.1999.00200.x [Google Scholar]
- Krapp H.G., Hengstenberg R. Estimation of self motion by optic flow processing in single interneurons. Nature. 1996;384:463–466. doi: 10.1038/384463a0. doi:10.1038/384463a0 [DOI] [PubMed] [Google Scholar]
- Land M.F. Head movement of flies during visually guided flight. Nature. 1973;243:299–300. doi:10.1038/243299a0 [Google Scholar]
- Land M.F. Motion and vision: why animals move their eyes. J. Comp. Physiol. A. 1999;185:341–352. doi: 10.1007/s003590050393. doi:10.1007/s003590050393 [DOI] [PubMed] [Google Scholar]
- Land D.E., Nilsson M.F. Oxford University Press; Oxford, UK: 2002. Animal eyes. [Google Scholar]
- Mazokhin-Porshnyakov G.A. Plenum Press; New York, NY: 1969. Insect vision. [Google Scholar]
- McCann G.D., MacGinitie G.F. Optomotor response studies of insect vision. Proc. R. Soc. B. 1965;163:369–401. doi: 10.1098/rspb.1965.0074. doi:10.1098/rspb.1965.0074 [DOI] [PubMed] [Google Scholar]
- Panhuis T.M., Wilkinson G.S. Exaggerated male eye span influences contest outcome in stalk-eyed flies (Diopsidae) Behav. Ecol. Sociobiol. 1999;46:221–227. doi:10.1007/s002650050613 [Google Scholar]
- Rayner J.M.V., Aldridge H.D.J. Three-dimensional reconstruction of animal flight paths and the turning flight of microchiropteran bats. J. Exp. Biol. 1985;118:247–265. [Google Scholar]
- Reguera P., Pomiankowski A., Fowler K., Chapman T. Low cost of reproduction in female stalk-eyed flies, Cyrtodiopsis dalmanni. J. Insect Physiol. 2004;50:103–108. doi: 10.1016/j.jinsphys.2003.10.004. doi:10.1016/j.jinsphys.2003.10.004 [DOI] [PubMed] [Google Scholar]
- Ribak G., Swallow J.G. Free flight maneuvers of stalk-eyed flies: do eye-stalks affect aerial turning behavior? J. Comp. Physiol. A. 2007;193:1065–1079. doi: 10.1007/s00359-007-0259-1. doi:10.1007/s00359-007-0259-1 [DOI] [PubMed] [Google Scholar]
- Schaum D., van der Merwe C.W. Schaum Publishing; New York, NY: 1961. Theory and problems of college physics. [Google Scholar]
- Schilstra C., van Hateren J.H. Stabilizing gaze in flying blowflies. Nature. 1998;395:654. doi: 10.1038/27114. doi:10.1038/27114 [DOI] [PubMed] [Google Scholar]
- Shillito F.J. Dimorphism in flies with stalked eyes. Zool. J. Linn. Soc. 1971;50:297–305. doi:10.1111/j.1096-3642.1971.tb00764.x [Google Scholar]
- Srinivasan M.V., Poteser M., Kral K. Motion detection in insect orientation and navigation. Vision Res. 1999;39:2749–2766. doi: 10.1016/s0042-6989(99)00002-4. doi:10.1016/S0042-6989(99)00002-4 [DOI] [PubMed] [Google Scholar]
- Strauss R., Schuster S., Götz K. Processing of artificial visual feedback in the walking fruit fly Drosophila melanogaster. J. Exp. Biol. 1997;200:1281–1296. doi: 10.1242/jeb.200.9.1281. [DOI] [PubMed] [Google Scholar]
- SYSTAT Software. SYSTAT Software, Inc; Richmond, CA: 2002. SYSTATTM 10.2 statistics II. [Google Scholar]
- Tammero L.F., Dickinson M.H. The influence of visual landscape on the free flight behavior of the fruit fly Drosophila melanogaster. J. Exp. Biol. 2002;205:327–343. doi: 10.1242/jeb.205.3.327. [DOI] [PubMed] [Google Scholar]
- Taylor G.K., Krapp G.H. Sensory systems and flight stability: what do insects measure and why? Adv. Insect Physiol. 2008;34:231–316. doi:10.1016/S0065-2806(07)34005-8 [Google Scholar]
- van Hateren J.H., Schilstra C. Blowfly flight and optic flow II. Head movements during flight. J. Exp. Biol. 1999;202:1491–1500. doi: 10.1242/jeb.202.11.1491. [DOI] [PubMed] [Google Scholar]
- Wagner H. Flight performance and visual control of flight of the free-flying housefly (Musca domestica L.) II. Pursuit of targets. Phil. Trans. R. Soc. Lond. B. 1986a;312:553–579. doi:10.1098/rstb.1986.0018 [Google Scholar]
- Wagner H. Flight performance and visual control of flight of the free-flying housefly (Musca domestica L.) III. Interactions between angular movement induced by wide- and smallfield stimuli. Phil. Trans. R. Soc. Lond. B. 1986b;312:581–595. doi:10.1098/rstb.1986.0019 [Google Scholar]
- Warren I., Smith H. Stalk-eyed flies (Diopsidae): modeling the evolution and development of an exaggerated sexual trait. BioEssays. 2007;29:300–307. doi: 10.1002/bies.20543. doi:10.1002/bies.20543 [DOI] [PubMed] [Google Scholar]
- Wickler V.W., Seibt U. Zur Ethologie Afrikanischer Stielaugenfliegen (Diptera, Diopsidae) Z. Tierphysiol. 1972;31:113–130. [Google Scholar]
- Wilkinson G.S., Reillo P.R. Female choice response to artificial selection on an exaggerated male trait in a stalk-eyed fly. Proc. R. Soc. Lond. B. 1994;255:1–6. doi:10.1098/rspb.1994.0001 [Google Scholar]
- Wilkinson G.S., Kahler H., Baker R.H. Evolution of female mating preference in stalk-eyed flies. Behav. Ecol. 1998;9:525–533. doi:10.1093/beheco/9.5.525 [Google Scholar]
- Wilkinson G.S., Amitin E.G., Johns P.M. Sex-linked correlated responses in female reproductive traits to selection on male eye span in stalk eyed flies. Integr. Comp. Biol. 2005;45:500–510. doi: 10.1093/icb/45.3.500. doi:10.1093/icb/45.3.500 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technical details of the experimental set-up
An example of the turn defining criteria
An example of the altered turning behaviour of flies with immobilized heads