Abstract
Allometric equations are often used to extrapolate traits in animals for which only body mass estimates are known, such as dinosaurs. One important decision can be whether these equations should be based on mammal, bird or reptile data. To address whether this choice will have a relevant influence on reconstructions, we compared allometric equations for birds and mammals from the literature to those for reptiles derived from both published and hitherto unpublished data. Organs studied included the heart, kidneys, liver and gut, as well as gut contents. While the available data indicate that gut content mass does not differ between the clades, the organ masses for reptiles are generally lower than those for mammals and birds. In particular, gut tissue mass is significantly lower in reptiles. When applying the results in the reconstruction of a sauropod dinosaur, the estimated volume of the coelomic cavity greatly exceeds the estimated volume of the combined organ masses, irrespective of the allometric equation used. Therefore, substantial deviation of sauropod organ allometry from that of the extant vertebrates can be allowed conceptually. Extrapolations of retention times from estimated gut contents mass and food intake do not suggest digestive constraints on sauropod dinosaur body size.
Keywords: allometry, scaling, coelomic cavity, ingesta retention, digestion, gut
1. Introduction
Body mass (BM) is generally considered the most important predictor of morphological, physiological and ecological characteristics of animals, and a multitude of allometric correlations between BM and other measurements has been established in biology (Peters 1983; Schmidt-Nielsen 1984; Calder 1996). While mostly used for the investigation of fundamental laws determining the functions of certain animal groups, or of life in general, allometric equations are also often used for the reconstruction of morphological, physiological and ecological traits of animals for which BM, but few other biological parameters, can be estimated directly. Such equations have been applied especially in considerations about the characteristics and constraints of the extinct dinosaur megafauna (Alexander 1989; McGowan 1989).
One interesting approach in this respect is to test whether a specific set of predictions or estimates is really compatible with other aspects of anatomy or physiology. For example, Seymour & Lillywhite (2000) demonstrated in model calculations that an upright posture of the neck in sauropods is incompatible with the present understanding of cardiovascular function in vertebrates. Other examples for the use of allometry are the studies by Gunga et al. (2007, 2008), who used allometric equations on the organ size of mammals from Anderson et al. (1979), Schmidt-Nielsen (1984) and Calder (1996) to test whether reconstructions of the body size of a prosauropod and a sauropod (in particular, the volume of the coelomic cavity of these animals) match the calculated space requirement of the internal organs.
For such reconstructions, a concept is required: should physiological inferences in dinosaurs be based on mammals, birds or reptiles, and for which parameters does the choice of extant analogue make a difference? Dinosaurs are usually considered to have been endotherms (such as birds and mammals) rather than ectotherms (reptiles), but an ‘intermediate’ metabolism (Reid 1997) or even a distinct ontogenetic shift in metabolic rate has been hypothesized for them (Sander & Clauss 2008), which might be relevant for the size of metabolic organs.
In order to test whether the available data suggested a difference or a similarity of allometric correlations between BM and organ mass in reptiles, birds and mammals, we compared allometric equations for birds and mammals from the literature to allometric equations for reptiles derived from a collection of literature and hitherto unpublished data, and used the results for a plausibility test of a recent sauropod dinosaur reconstruction (Gunga et al. 2008) and a model calculation to assess whether digestive anatomy and physiology should be considered a limiting factor in sauropod body size.
2. Material and methods
A data collection on reptile organ mass was compiled using literature sources (Else & Hulbert 1981; Hailey 1997; Dohm et al. 1998), as well as unpublished data from personal observations (J. Hummel & M. Clauss 2007, unpublished data) and from three recent dissertation theses (Kopsch 2006; Eberle 2007; Schneemeier 2008). Data were available for the mass of the heart, kidneys, liver and empty gastrointestinal tract (GIT). Data on lung tissue mass were not available from these studies, and we could not locate other sources that provided sufficient data for inclusion of lung tissue in this study. Additionally, data on the wet content mass of the total GIT were compiled for herbivorous reptiles (Parra 1978; Karasov et al. 1986; Bjorndal & Bolten 1990; Foley et al. 1992; Barboza 1995; Hailey 1997; Mackie et al. 2004) and herbivorous birds (Herd & Dawson 1984; Dawson et al. 1989; Grajal 1995), and compared with the data collection for herbivorous mammals from Clauss et al. (2007a). If more than one set of data were available for a species, an average was calculated and used in the analyses, in order to avoid over-representation of any species. The data are given in the electronic supplementary material, appendix.
where Y is the organ mass correlated with BM (masses in kg). The exponent b is a scaling factor, which describes the scaling with body size. If b=0, body size has no effect; if b=1, Y shows a linear correlation to BM.
Linear regressions were calculated using SPSS v. 16.0 (SPSS, Inc., Chicago, IL, USA) including the 95% confidence intervals (CIs) for both a and b. Because the original datasets of Calder (1996) were not available, we tested whether the 95% CIs for a and b in reptiles included the values given for the respective factors and exponents for birds and mammals by this author.
3. Results
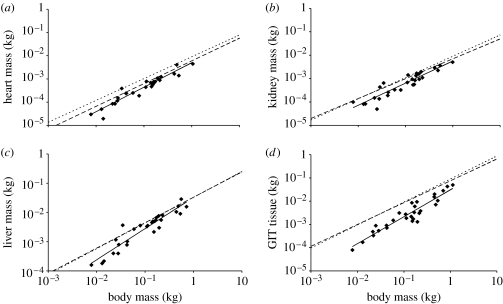
The 95% CI of the allometric exponent (b) included 1.0 for each of the four organs tested (table 1); in other words, all organs did not deviate significantly from a linear correlation with BM. The 95% CI of the allometric exponent also included the value given by Calder (1996) for birds and mammals for the heart and kidneys (table 1, figure 1), but not for the liver and not (though nearly) for the GIT. The 95% CI of the intercept of the ln-transformed equation (ln(a)) included values for birds and mammals in the case of the liver, indicating that, irrespective of the scaling pattern with BM, the actual mass of this organ is similar among the three vertebrate clades in the body size range studied (table 1, figure 1c). In the case of the heart, the mammalian value for a was just included in the upper 95% CI of reptiles, whereas that for birds was above the CI (table 1, figure 1a). Similarly, the 95% CI for the intercept of the kidney included the mammalian but not the avian value (figure 1b). The reptilian intercept was lower than both the mammalian and the avian values for the GIT. Thus, the data indicate that the GIT of reptiles, birds and mammals shows a similar scaling pattern with BM, but for reptiles it is at a generally lower level (figure 1d).
Table 1.
Statistics of regression analysis according to the equation organ mass=aBMb (masses in kg) for reptiles. (Allometric organ equations for birds and mammals are from Calder (1996); the data for gut contents of mammals from Clauss et al. (2007a) and for birds from Herd & Dawson (1984), Dawson et al. (1989) and Grajal (1995); n.a., not available.)
| organ | clade (species) | BM range (kg) | a | 95% CI | b | 95% CI | R2 | p |
|---|---|---|---|---|---|---|---|---|
| heart | reptile (28) | 0.008–1.052 | 0.005 | 0.0036–0.0070 | 1.055 | 0.929–1.181 | 0.919 | >0.001 |
| mammal (568) | — | 0.006 | — | 0.98 | — | — | — | |
| bird (n.a.) | — | 0.009 | — | 0.94 | — | — | — | |
| kidney | reptile (28) | 0.008–0.990 | 0.006 | 0.0037–0.0085 | 0.945 | 0.792–1.099 | 0.860 | >0.001 |
| mammal (138) | — | 0.007 | — | 0.85 | — | — | — | |
| bird (334) | — | 0.009 | — | 0.91 | — | — | — | |
| liver | reptile (29) | 0.008–0.715 | 0.033 | 0.0219–0.0484 | 1.066 | 0.917–1.216 | 0.888 | >0.001 |
| mammal (175) | — | 0.033 | — | 0.87 | — | — | — | |
| bird (n.a.) | — | 0.033 | — | 0.88 | — | — | — | |
| GIT | reptile (29) | 0.008–1.123 | 0.031 | 0.0207–0.0458 | 1.159 | 0.997–1.321 | 0.889 | >0.001 |
| mammal (41) | — | 0.075 | — | 0.94 | — | — | — | |
| bird (n.a.) | — | 0.090 | — | 0.99 | — | — | — | |
| GIT wet contents | reptile (12) | 0.059–3.150 | 0.080 | 0.0584–0.1104 | 1.389 | 1.195–1.583 | 0.962 | >0.001 |
| mammal (74) | 0.015–3140 | 0.107 | 0.094–0.121 | 1.062 | 1.029–1.095 | 0.983 | >0.001 | |
| bird (3) | 0.712–35.330 | 0.044 | 0.000–545.1 | 1.204 | −3.347 to 5.755 | 0.919 | 0.184 |
Figure 1.
Correlations of BM and organ mass in reptiles (solid line with diamonds), mammals (dashed line) and birds (dotted line) for the (a) heart (reptile, y=0.005x1.06; mammal, y=0.006x0.98; bird, y=0.009x0.94), (b) kidneys (reptile, y=0.006x0.95; mammal, y=0.007x0.85; bird, y=0.009x0.91), (c) liver (reptile, y=0.033x1.07; mammal, y=0.033x0.87; bird, y=0.033x0.88) and (d) gastrointestinal tissue (reptile, y=0.03x1.16; mammal, y=0.08x0.94; bird, y=0.09x0.99). Reptile data from this study (see the electronic supplementary material, appendix), mammal and bird regression lines from Calder (1996).
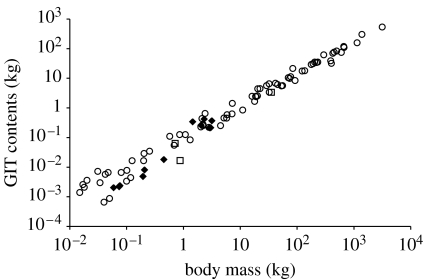
A visual comparison of data on the mass of the wet contents of the whole GIT (figure 2) indicates that systematic differences between herbivorous reptiles, birds and mammals are unlikely. The calculated difference in the allometric exponent between reptiles and mammals (table 1) should therefore be viewed with caution; using the calculated equation, a reptile-like herbivore would consist of nothing but gut contents at a BM of approximately 670 kg.
Figure 2.
Wet contents mass of the total GIT in mammals (circles; data from Clauss et al. 2007a), birds (squares; data from Herd & Dawson 1984; Dawson et al. 1989; Grajal 1995) and reptiles (diamonds; data in the electronic supplementary material, appendix) in relation to BM.
4. Discussion
The findings of this study suggest that, while there appear to be no relevant differences in the allometry of the liver mass and the mass of the gastrointestinal contents, differences do exist between mammals, birds and reptiles with respect to the allometry of the heart, kidney and gastrointestinal tissue mass. When compared with the allometric equations found by Else & Hulbert (1985) for reptiles, the animals in our study generally achieved higher organ weights for their BMs.
Given the variety of mammal, bird and reptile species, and the limited selection of species available for the derivation of allometric equations, such results need to be considered with caution. Organ masses in reptiles as well as other clades can be influenced by sex, reproductive status and hibernation status (Telford 1970; Beuchat & Braun 1988) or food availability and quality (Relyea & Auld 2004; Naya et al. 2005; Naya & Bozinovic 2006). However, in the collection of allometric equations of Calder (1996), which was used as a reference here, there is no evident separation of data for such factors; therefore, the undifferentiated inclusion of data appeared justified for a comparison between clades here.
In correspondence with the expectations linked to the differences in metabolism, with low metabolic rates in reptiles and higher rates in birds when compared with mammals (McNab 2002), the organ masses for heart and kidney showed higher values for a in the same sequence (table 1). Similarly, birds exceed mammals in the capacity and the weight of their respiratory system (Lasiewski & Calder 1971; Calder 1996; Maina 2006), but lung masses of mammals and reptiles are similar at similar BMs (Else & Hulbert 1985). The most impressive difference in organ mass between reptiles on the one hand, and mammals and birds on the other hand, is in the tissue of the GIT. Whereas the contents of the GIT appear to be similar in herbivorous mammals, reptiles and birds (Parra 1978; Bjorndal 1997), the endothermic clades have significantly higher gut tissue masses. Although intestinal microvilli area does probably not differ significantly between herbivorous reptiles and mammals (Ferraris et al. 1989), there is a significant difference in the intestinal surface area between the two clades, mainly owing to the differences in intestinal length (Karasov & Diamond 1985; Karasov et al. 1985, 1986; Ferraris et al. 1989). Birds and mammals have distinctively longer small intestines than reptiles (Stevens & Hume 1995), and in birds, the muscular gizzard additionally increases gut tissue mass.
The choice of the allometric equation for the extrapolation of organ tissue masses thus can have relevance for the outcome of organismal reconstructions (table 2). Using organ allometries for ectothermic organisms (reptiles) should yield generally lower estimates. However, when extrapolating to gigantic BMs by the use of allometric equations, such as those derived in the present study, a conceptual problem arises (table 2). Any slight differences in the allometric exponent b will, at very large BMs, lead to very different results, which may, in their scope and ranking, even be different from the observed ranking (table 1) based on a. In table 2, it can be seen that when the exact equations from table 1 are used for the estimation of organ masses in a 38 tonne dinosaur in the ‘allometric approach’, the derived reptile equation would lead to dramatically higher estimates for the liver mass, although reptiles would be assumed to have similar (this study) or even slightly lower (Else & Hulbert 1985) liver masses than mammals. This paradoxical result is caused by the difference in the allometric exponent b (1.061 in reptiles as opposed to 0.87 in mammals). Evidently, at extrapolations to such gigantic masses, the error in the estimation of b inherent in the use of imperfect datasets is too large to yield realistic results. A potential solution to overcome this effect, especially when comparing different sets of calculations, is to assume a common exponent b for all clades. In our case, where the 95% CI for b always included 1.0 (linearity) in the reptiles, we suggest that, in the absence of information on 95% CIs in birds and mammals, all correlations can be assumed to be linear. This approach leads to a consistent ranking of extrapolated organ masses according to the reptile–mammal–bird sequence that can be observed in the original equations (table 1).
Table 2.
Extrapolation of organ masses (in kg) of a hypothetical 38 000 kg vertebrate (the estimated mass of Brachiosaurus, a sauropod dinosaur; Gunga et al. 2008) under different assumptions: ‘linear approach’, assuming linear scaling with BM for all clades, i.e. b=1.0, using values for a from table 1; ‘allometric approach’, using the exact equations as given in table 1. (Note that owing to small differences in the exponent b, extrapolations using the exact equations will yield fundamentally different results.)
| linear approach | allometric approach | |||||
|---|---|---|---|---|---|---|
| reptile | mammal | bird | reptile | mammal | bird | |
| heart | 190 | 228 | 342 | 339 | 185 | 182 |
| kidney | 228 | 266 | 342 | 128 | 55 | 132 |
| liver | 1254 | 1254 | 1254 | 2515 | 318 | 354 |
| GIT tissue | 1178 | 2850 | 3420 | 6300 | 1514 | 3078 |
Whether we assume that a reptile (ectotherm) or mammal/bird (endotherm) equation should be used for a 38 tonne sauropod dinosaur can lead to a difference in estimated gut tissue mass of more than 1670 kg (or 4.4% of the assumed BM). In the case of sauropods, it has been postulated that these animals underwent an ontogenetic shift in their metabolic rate, from juvenile endotherms to adult mass homeotherms (with low metabolic rates; Farlow 1990; Sander & Clauss 2008), and the intestinal length is usually considered to reflect metabolic rate (Williams et al. 2001). For example, owing to the apparent association of intestinal length and metabolism, this view of sauropod metabolism would imply that the growth of intestinal tissue mass was less during ontogeny in sauropods than that in mammals. This view would therefore justify the use of ‘reptile equations’ for adult sauropods, thus alleviating theoretical constraints on the capacity of the coelomic cavity. Gunga et al. (2008) had already concluded that the coelomic cavity of a 38 tonne sauropod dinosaur (Brachiosaurus brancai), which they assumed to harbour a volume of 32 m3 according to their body size reconstructions, provided more space than that necessary for most of the organs of this cavity (including a proportion of the skeleton, blood volume and muscle mass, but without accounting for mesenteries, coelomic fat and reproductive organs), which they estimated at 21 m3. Using our ‘linear’ approach and the reptile functions (table 2), and adopting a linear approach based on the mammal functions used by Gunga et al. (2008) for those organs that we could not include in our study, we arrive at a volume estimate of only 17.6 m3. Evidently, even when considering that mesenteries, fat and reproductive organs are not included in these calculations, the present data allow for a dramatic increase in organ masses in the reconstruction of sauropod dinosaurs. As sauropods are thought to have heterogeneous (avian-type) lungs with an air sac system (Sander & Clauss 2008), a part of the space in the coelomic cavity was probably filled with these air sacs. In birds, the lungs and air sacs may account for as much as 20 per cent of the total body volume (King 1966); in the 38 tonne sauropod of Gunga et al. (2008), with an estimated total volume of approximately 47.6 m3, this would represent a total lung and air sac volume of 9.5 m3. Even if we assume that the majority of this volume was placed within the coelomic cavity, the reconstruction would still allow for theoretical increases in any organ masses.
Given that we must assume elevated metabolic rates in certain ontogenetic stages, and no mastication of ingesta (Farlow 1990; Sander & Clauss 2008), the gastrointestinal contents could be a plausible candidate for a mass above estimates based on regressions from extant animals—to allow a thorough digestion in spite of absent food comminution and without compromising intake (Farlow 1987; Clauss et al. 2007b). In order to roughly estimate whether gut capacity should be considered a limiting factor in sauropods, we extrapolated the dry matter intake for sauropods from Hummel et al. (2008) to a 38 tonne sauropod; these values are given at four assumed levels of metabolism. Assumptions were made for a medium- and a low-quality diet (with presumed apparent dry matter/energy digestibilities of 44 and 33%, respectively); additionally, we estimated the dry matter concentration in sauropod gut contents to be 15 per cent, a level similar to that of mammals (but probably lower than that in reptiles; M. Clauss 2008, personal observation). Using the equation by Holleman & White (1989), which links dry matter intake, digestibility, dry matter gut capacity and ingesta retention time, we can estimate the mean retention time (MRT) in hypothetical sauropods of varying metabolic level (table 3; see the electronic supplementary material, appendix, for details). At the normal, extrapolated gut capacity, retention times are between 4 and 8 days for a medium-quality food; a doubling of the gut content—which would still leave approximately 10 m3 of the presumed coelomic cavity unoccupied for mesenteries, fat and reproductive organs—would result in retention times between 8 and 16 days. Thus, estimated retention times fall within the range of 11 days measured in Galápagos tortoises (Geochelone nigra; Hatt et al. 2002), which—as extant reptiles—do not chew their food.
Table 3.
Estimation of ingesta MRT in a hypothetical 38 000 kg vertebrate (the estimated mass of Brachiosaurus, a sauropod dinosaur; Gunga et al. 2008) at different levels of metabolism and hence daily food intake (for ‘medium’- and ‘low’-quality food; Hummel et al. 2008) at the extrapolated gut capacity of 610 kg dry matter (from table 1, linear approach, assuming a dry matter concentration of 15% in gut contents) and at a doubled gut capacity; MRT estimated according to Holleman & White (1989). (DMI, dry matter intake; DFE, dry faecal excretion.)
| level of metabolism | DMI (kg d−1) | DFE (kg d−1) | MRT hours (days) | |
|---|---|---|---|---|
| gut capacity | ||||
| 610 kg DM | 1220 kg DM | |||
| medium-quality food | ||||
| reptile | 20 | 11 | 927 (39) | 1854 (77) |
| intermediate 1 | 96 | 53 | 197 (8) | 394 (16) |
| intermediate 2 | 140 | 78 | 135 (6) | 269 (11) |
| mammal | 188 | 104 | 100 (4) | 201 (8) |
| low-quality food | ||||
| reptile | 28 | 18 | 639 (27) | 1278 (53) |
| intermediate 1 | 127 | 84 | 139 (6) | 278 (12) |
| intermediate 2 | 186 | 124 | 94 (4) | 189 (8) |
| mammal | 250 | 166 | 70 (3) | 141 (6) |
In conclusion, this study, as well as that of Gunga et al. (2008), shows that, from the aspect of organismal reconstruction based on body volume and organ estimates, no restrictions are evident in the sauropod bauplan; on the contrary, given our present equations for organ allometry, the body cavity of sauropods as reconstructed allows leeway for any adjustments in organ size that one might deem necessary to fit their—potentially unique—lifestyle. In particular, digestive physiology is an unlikely candidate for a potential body size limitation in sauropods.
Acknowledgments
This is contribution no. 47 of the DFG research unit 533 ‘The Biology of Sauropod Dinosaurs’. We thank two anonymous referees for their comments.
Supplementary Material
Compilation of literature data
Calculation of retention time using the equation from Holleman & White (1989)
References
- Alexander R.M. Columbia University Press; New York, NY: 1989. Dynamics of dinosaurs and other extinct giants. [Google Scholar]
- Anderson J.F., Rahn H., Prange H.D. Scaling of supportive tissue mass. Q. Rev. Biol. 1979;54:139–148. doi:10.1086/411153 [Google Scholar]
- Barboza P. Digesta passage and functional anatomy of the digestive tract in the desert tortoise (Xerobates agassizii) J. Comp. Physiol. B. 1995;165:193–202. doi: 10.1007/BF00260810. doi:10.1007/BF00260810 [DOI] [PubMed] [Google Scholar]
- Beuchat C.A., Braun E.J. Allometry of the kidney: implications for the ontogeny of osmoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1988;255:R760–R767. doi: 10.1152/ajpregu.1988.255.5.R760. [DOI] [PubMed] [Google Scholar]
- Bjorndal, K. A. 1997 Fermentation in reptiles and amphibians. In Gastrointestinal microbiology. Vol. 1: gastrointestinal ecosystems and fermentations (eds R. I. Mackie & B. A. White), pp. 199–230. New York, NY: ITP.
- Bjorndal K., Bolten A. Digestive processing in a herbivorous freshwater turtle: consequences of small-intestine fermentation. Physiol. Zool. 1990;63:1232–1247. [Google Scholar]
- Calder W.A. Dover Publications, Inc; Mineola, NY: 1996. Size, function and life history. [Google Scholar]
- Clauss M., Schwarm A., Ortmann S., Streich W.J., Hummel J. A case of non-scaling in mammalian physiology? Body size, digestive capacity, food intake, and ingesta passage in mammalian herbivores. Comp. Biochem. Physiol. A. 2007a;148:249–265. doi: 10.1016/j.cbpa.2007.05.024. doi:10.1016/j.cbpa.2007.05.024 [DOI] [PubMed] [Google Scholar]
- Clauss M., Streich W.J., Schwarm A., Ortmann S., Hummel J. The relationship of food intake and ingesta passage predicts feeding ecology in two different megaherbivore groups. Oikos. 2007b;116:209–216. doi:10.1111/j.0030-1299.2007.15461.x [Google Scholar]
- Dawson T.J., Johns A.B., Beals A.M. Digestion in the Australian wood duck (Chenonetta jubata): a small avian herbivore showing selective digestion of the hemicellulose component of fiber. Physiol. Zool. 1989;62:522–540. [Google Scholar]
- Dohm M., Garland T., Jr, Cole C., Townsend C. Physiological variations and allometry in western whiptail lizards (Cnemidophorus tigris) from a transect across a persistent hybrid zone. Copeia. 1998;1:1–13. doi:10.2307/1447696 [Google Scholar]
- Eberle, A. 2007 Untersuchungen zur Körperzusammensetzung von Schlangen, pp. 191. Dissertation, Institut für Physiologie, Physiologische Chemie und Tierernährung der Tierärztlichen Fakultät der Ludwig-Maximilians-Universität München, Munich, Germany.
- Else P.L., Hulbert A.J. Comparison of the ‘mammal machine’ and the ‘reptile machine’: energy production. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1981;240:3–9. doi: 10.1152/ajpregu.1981.240.1.R3. [DOI] [PubMed] [Google Scholar]
- Else P.L., Hulbert A.J. An allometric comparison of the mitochondria of mammalian and reptilian tissues: the implications for the evolution of endothermy. J. Comp. Physiol. B. 1985;156:3–11. doi: 10.1007/BF00692920. doi:10.1007/BF00692920 [DOI] [PubMed] [Google Scholar]
- Farlow J.O. Speculations about the diet and digestive physiology of herbivorous dinosaurs. Paleobiology. 1987;13:60–72. [Google Scholar]
- Farlow, J. O. 1990 Dinosaur energetics and thermal biology. In The Dinosauria (eds D. B. Weishampel, P. Dodson & H. Osmólska), pp. 43–55. Berkeley, CA: University of California Press.
- Ferraris R., Lee P., Diamond J. Origin of regional and species differences in intestinal glucose uptake. Am. J. Physiol. Gastrointest. Liver Physiol. 1989;257:689–697. doi: 10.1152/ajpgi.1989.257.5.G689. [DOI] [PubMed] [Google Scholar]
- Foley W., Bouskila A., Shkolnik A., Choshniak I. Microbial digestion in the herbivorous lizard Uromastyx aegyptius (Agamidae) J. Zool. 1992;226:387–398. [Google Scholar]
- Grajal A. Structure and function of the digestive tract of the Hoatzin (Opisthocomus hoazin): a folivorous bird with foregut fermentation. Q. J. Ornithol. 1995;112:20–28. [Google Scholar]
- Gunga H., Suthau T., Bellmann T., Friedrich A., Schwanebeck T., Stoinski S., Trippel T., Kirsch K., Hellwich O. Body mass estimations for Plateosaurus engelhardti using laser scanning and 3D reconstruction methods. Naturwissenschaften. 2007;94:623–630. doi: 10.1007/s00114-007-0234-2. doi:10.1007/s00114-007-0234-2 [DOI] [PubMed] [Google Scholar]
- Gunga H., Suthau T., Bellmann A., Stoinski S., Friedrich A., Trippel T., Kirsch K., Hellwich O. A new body mass estimation of Brachiosaurus brancai Janensch, 1914 mounted and exhibited at the Museum of Natural History (Berlin, Germany) Fossil Rec. 2008;11:28–33. doi:10.1002/mmng.200700011 [Google Scholar]
- Hailey A. Digestive efficiency and gut morphology of omnivorous and herbivorous African tortoises. Can. J. Zool. 1997;75:787–794. doi:10.1139/z97-100 [Google Scholar]
- Hatt J.M., Gisler R., Mayes R., Lechner-Doll M., Clauss M., Liesegang A., Wanner M. The use of dosed and herbage n-alkanes as markers for the determination of intake, digestibility, mean retention time and diet selection in Galápagos tortoises. Herpetol. J. 2002;12:45–54. [Google Scholar]
- Herd R.M., Dawson T.J. Fibre digestion in the emu, Dromaius novaehollandiae, a large bird with a simple gut and high rates of passage. Physiol. Zool. 1984;57:70–84. [Google Scholar]
- Holleman D.F., White R.G. Determination of digesta fill and passage rate from non-absorbed particulate phase markers using the single dosing method. Can. J. Zool. 1989;67:488–494. [Google Scholar]
- Hummel J., Gee C.T., Südekum K.H., Sander P.M., Nogge G., Clauss M. In vitro digestibility of fern and gymnosperm foliage—implications for sauropod feeding ecology and diet selection. Proc. R. Soc. B. 2008;275:1015–1021. doi: 10.1098/rspb.2007.1728. doi:10.1098/rspb.2007.1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov W.H., Diamand J.M. Digestive adaptations for fueling the cost of endothermy. Science. 1985;228:202–204. doi: 10.1126/science.3975638. doi:10.1126/science.3975638 [DOI] [PubMed] [Google Scholar]
- Karasov W.H., Solberg D., Diamond J. What transport adaptations enable mammals to absorb sugars and amino acids faster than reptiles? Am. J. Physiol. Gastrointest. Liver Physiol. 1985;249:271–283. doi: 10.1152/ajpgi.1985.249.2.G271. [DOI] [PubMed] [Google Scholar]
- Karasov W.H., Petrossian E., Rosenberg L., Diamond J. How do food passage rate and assimilation differ between herbivorous lizards and nonruminant mammals? J. Comp. Physiol. B. 1986;156:599–609. doi: 10.1007/BF00691047. doi:10.1007/BF00691047 [DOI] [PubMed] [Google Scholar]
- King A.S. Structural and functional aspects of the avian lungs and air sacs. Int. Rev. Gen. Exp. Zool. 1966;2:171–267. [Google Scholar]
- Kopsch, G. 2006 Untersuchungen zur Körperzusammensetzung von Schildkröten, p. 185. Dissertation, Institut für Physiologie, Physiologische Chemie und Tierernährung der Tierärtzlichen Fakultät der Ludwig-Maximilians-Universität München, Munich, Germany.
- Lasiewski R.C., Calder W.A.J. A preliminary allometric analysis of respiratory variables in resting birds. Respir. Physiol. 1971;11:152–166. doi: 10.1016/0034-5687(71)90020-x. doi:10.1016/0034-5687(71)90020-X [DOI] [PubMed] [Google Scholar]
- Mackie R., Rycyk M., Ruemmler R., Aminov R., Wikelski M. Biochemical and microbiological evidence for fermentative digestion in free-living land iguana (Conolophus pallidus) and marine iguana (Amblyrhynchus cristatus) on the Galápagos Archipelago. Physiol. Biochem. Zool. 2004;77:127–138. doi: 10.1086/383498. doi:10.1086/383498 [DOI] [PubMed] [Google Scholar]
- Maina J.N. Development, structure, and function of a novel respiratory organ, the lung-air sac system of birds: to go where no other vertebrate has gone. Biol. Rev. 2006;81:545–579. doi: 10.1017/S1464793106007111. doi:10.1017/S1464793106007111 [DOI] [PubMed] [Google Scholar]
- McGowan C. Harvard University Press; Cambridge, MA: 1989. Dinosaurs, spitfires, and sea dragons. [Google Scholar]
- McNab B.K. Cornell University Press; Ithaca, NY: 2002. The physiological ecology of vertebrates. A view from energetics. [Google Scholar]
- Naya D.E., Bozinovic F. The role of ecological interactions on the physiological flexibility of lizards. Funct. Ecol. 2006;20:601–608. doi:10.1111/j.1365-2435.2006.01137.x [Google Scholar]
- Naya D.E., Farfan G., Sabat P., Mendez M.A., Bozinovic F. Digestive morphology and enzyme activity in the Andean toad Bufo spinulosus: hard-wired or flexible physiology? Comp. Biochem. Physiol. 2005;140:165–170. doi: 10.1016/j.cbpb.2004.11.006. doi:10.1016/j.cbpb.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Parra R. Comparison of foregut and hindgut fermentation in herbivores. In: Montgomery G.G., editor. The ecology of arboreal folivores. Smithsonian Institution Press; Washington, DC: 1978. pp. 209–229. [Google Scholar]
- Peters R. Cambridge University Press; New York, NY: 1983. The ecological implications of body size. [Google Scholar]
- Reid, R. E. H. 1997 Dinosaurian physiology: the case for ‘intermediate’ dinosaurs. In The complete dinosaur (eds J. O. Farlow & M. K. Brett-Surman), pp. 449–473. Bloomington, IN: Indiana University Press.
- Relyea R., Auld J. Having the guts to compete: how intestinal plasticity explains costs of inducible defences. Ecol. Lett. 2004;7:869–875. doi:10.1111/j.1461-0248.2004.00645.x [Google Scholar]
- Sander P.M., Clauss M. Sauropod gigantism. Science. 2008;322:200–201. doi: 10.1126/science.1160904. doi:10.1126/science.1160904 [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Cambridge University Press; New York, NY: 1984. Scaling: why is animal size so important? [Google Scholar]
- Schneemeier, C. E. 2008 Untersuchungen zur Körperzusammensetzung von Echsen, p. 221. Dissertation, Institut für Physiologie, Physiologische Chemie und Tierernährung der Tierärztlichen Fakultät der Ludwig-Maximilians-Universität München, Munich, Germany.
- Seymour R.S., Lillywhite H.B. Hearts, neck posture and metabolic intensity of sauropod dinosaurs. Proc. R. Soc. B. 2000;267:1883–1887. doi: 10.1098/rspb.2000.1225. doi:10.1098/rspb.2000.1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C.E., Hume I.D. Cambridge University Press; Cambridge, UK: 1995. Comparative physiology of the vertebrate digestive system. [Google Scholar]
- Telford S.R., Jr Seasonal fluctuations in liver and fat body weights of the Japanese lacertid Takydromus tachydromoides Schlegel. Copeia. 1970;4:681–688. doi:10.2307/1442310 [Google Scholar]
- Williams T.M., Haun J., Davis R.W., Fuiman L.A., Kohin S. A killer appetite: metabolic consequences of carnivory in marine mammals. Comp. Biochem. Physiol. A. 2001;129:785–796. doi: 10.1016/s1095-6433(01)00347-6. doi:10.1016/S1095-6433(01)00347-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Compilation of literature data
Calculation of retention time using the equation from Holleman & White (1989)