Abstract
Evolutionary and plastic responses by males to the level of sperm competition (SC) are reported across widespread taxa, but direct tests of the consequences for male reproductive success in a competitive context are lacking. We varied male perception of SC to examine the effect on male competitive reproductive success and to test whether the outcomes were as predicted by theory. Exposure to rival males prior to mating increased a male's ejaculate investment (measured as mating duration); by contrast, exposure to rival males in the mating arena decreased mating duration. The results therefore suggested that SC intensity is important in shaping male responses to SC in this system, although the patterns were not strictly in accord with existing theory. We then tested whether males that responded to the level of SC had higher reproductive fitness in a competitive context. We found that males kept with rivals prior to mating again mated for longer; furthermore, they achieved significantly higher paternity share regardless of whether they were the first or second males to mate with a female. The plastic strategies employed by males therefore resulted in significantly increased reproductive success in a competitive context, even following subsequent rematings in which the majority of sperm were displaced.
Keywords: sexual selection, accessory gland proteins, Acps, ejaculate allocation, mating latency, mating duration
1. Introduction
There is clear evidence across many taxa that sperm competition (SC) is a potent driver of evolutionary change in male morphology, physiology and behaviour (Birkhead & Møller 1998; Simmons 2001). For example, males from polyandrous species invest more heavily in gonads, relative to somatic tissue (Harcourt et al. 1981; Jennions & Passmore 1993; Gage 1994; Karlsson 1995; Hosken 1997; Stockley et al. 1997; Møller & Ninni 1998). Theory provides clear predictions of how males should respond to SC (Parker et al. 1996, 1997). This includes the prediction that, when ejaculates are limiting (see Hihara (1981) and Linklater et al. (2007) for such evidence in Drosophila melanogaster), males can be selected to evolve plasticity in their ejaculate allocation (Gage & Baker 1991; Wedell et al. 2002). Consistent with this idea, there is good evidence that males that experience high levels of SC ejaculate more sperm (Gage & Baker 1991; Gage & Barnard 1996; Wedell & Cook 1999; Martin & Hosken 2002; Neff et al. 2003; Siva-Jothy & Stutt 2003; Pound & Gage 2004; Reichard et al. 2004; Friberg 2006; Carazo et al. 2007). In order to assess the level of potential competition, males may use cues from females that indicate female mating status and/or cues from males that indicate the number of actual or potential rival males. For example, male D. melanogaster mate for longer and stimulate higher egg laying when mating with virgin females that are perceived to be mated, compared with virgin females perceived as virgin (Friberg 2006). However, to date, no study has directly tested the hypothesis that plastic responses by males to the level of SC increase the share of paternity in a competitive context.
Here, we use the fruit fly model organism (D. melanogaster) to investigate the responses by males to number of rival competitors, both prior to and during mating. We focused on male-derived signals that communicate the level of SC. We tested whether the responses seen were as predicted by SC intensity and risk theory, and whether any such responses resulted in direct effects on a male's share of paternity in a competitive context. Altering the number of rival males potentially alters two parameters of SC: (i) intensity (the average number of males competing for a given set of eggs) and (ii) risk (the probability that females in the population have mated or will mate again; Parker 1990, 1993; Parker et al. 1996, 1997; Wedell et al. 2002; Engqvist & Reinhold 2005). Both intensity and risk can vary with respect to their average levels in a population or species, or to their current levels in a mating bout. Recent work has emphasized the importance of distinguishing between these components (Engqvist & Reinhold 2005). This is because increases in both average intensity and risk predict increased investment in ejaculates, whereas increases in current risk predict increased investment, but increases in current intensity predict the opposite, i.e. decreased investment, owing to diminishing fitness returns (Parker et al. 1997; Engqvist & Reinhold 2005). The theory is thought to be widely applicable (Wedell et al. 2002; Engqvist & Reinhold 2005, p. 1292), even in species such as D. melanogaster, which have high second male sperm precedence (Williams et al. 2005; Engqvist & Reinhold 2006).
To manipulate the perceived levels of SC, we altered a male's exposure to rival males for the 5 days prior to mating (‘previous’ male treatments), and to rival males in the mating arena (‘current’ male treatments), with the aim of varying the average and immediate levels of SC, respectively. In our first experiment, we used a fully factorial design to identify whether males respond predominately to SC risk (i.e. longer mating duration as both previous and current SC increase) or to SC intensity (i.e. longer matings with increasing previous SC, but shorter matings with increasing current SC), in line with the theoretical predictions (Parker et al. 1997; Engqvist & Reinhold 2005). In our second set of experiments, we tested whether any such male responses resulted in increased male reproductive success in a competitive environment.
In the theory, mating ‘investment’ is an arbitrary value that is often referred to as ‘sperm number’ (e.g. Parker et al. 1996, 1997). However, such investment could equally well refer to and include the non-sperm ejaculate (Wedell et al. 2002). The transfer of sperm in D. melanogaster occurs quickly between 6 and 8 min after start of mating (Gilchrist & Partridge 2000), which itself normally lasts for approximately 15–20 min. Hence, as sperm transfer occurs during such a short part of the total mating duration, a male's investment in mating, and allocation of total ejaculate, is likely to involve more than just variation in sperm transfer. Here, we use mating duration as a proxy for ejaculate investment, and this is particularly appropriate given that mating duration is positively related to both seminal fluid transfer in this species (S. Wigby, L. Sirot, J. R. Linklater, A. Bretman, N. Buehner, M. F. Wolfner, & T. Chapman 2009 in review) and the induction of phenotypes known to be influenced by seminal fluid proteins, e.g. female refractoriness (Gilchrist & Partridge 2000).
2. Material and methods
Experiments were conducted in a 25°C humidified room, with a 12 L : 12 D cycle, using standard sugar–yeast (SY) medium (Bass et al. 2007), supplemented with live yeast granules. Wild-type flies were from the Dahomey strain (Bass et al. 2007). Marker flies for assessing paternity were from a sepia stock recently backcrossed for three generations into the Dahomey genetic background. Larvae were raised at a standard density of 100 per vial. At eclosion, flies were collected and sexes separated using ice anaesthesia. Virgin females and sepia males were kept 10 per vial for 5 days. Experimental males were randomly assigned to treatments. Flies were mated at 5 days post-eclosion.
(a) Responses of males to SC: mating duration and latency
To test whether males could respond to variation in SC we manipulated a male's perception of SC from low to high by keeping males in groups of 1, 2 or 4. To vary previous levels of SC prior to mating, males were kept from eclosion for 5 days up until mating in groups of 1, 2 or 4. To vary current levels of SC, males from each of the three previous treatments were randomly assigned to groups of 1, 2 or 4 males immediately before mating. This resulted in a fully factorial set of nine treatments with final sample sizes of 31–39. On the day of the matings, males were aspirated into vials each containing one virgin wild-type female. Females had been placed singly into experimental vials 24 hours prior to mating using ice anaesthesia. Vials that contained dead males were discarded before transfer. Mating latency and duration to the nearest minute were recorded for the first matings that occurred, without disturbing or moving vials. Flies were discarded if they did not mate within 2 hours.
(b) Fitness consequences for males of responding to variation in SC
To test the hypothesis that male responses to variation in SC were adaptive and resulted in increased male fitness, we measured the competitive reproductive success of males experiencing low and high levels of previous and current SC. To test the reproductive success of males in a first mating position (P1 experiment), virgin sepia females were mated to low and high SC treatment males, and then 24 hours later given the opportunity to mate with one sepia male. To test the reproductive success of males in a second mating position (P2 experiment), the same protocol was followed but the mating order was reversed. We used the most extreme (1 and 4) previous and current male treatments from the first experiment (i.e. four experimental treatments, sample sizes for P1 experiment=49–61, P2 experiment=42–50). As before, experimental males were kept in their previous treatment groups for 5 days until mating, when males from each group were randomly assigned to one of the two current male treatments. In all experiments, flies that did not mate within 2 hours were discarded, and flies were separated within 1 hour after mating to avoid any rematings on the same day. For all matings, mating latency and duration were recorded. The eggs laid by P1 experiment females between the first and second matings were counted. After the second matings in both the P1 and P2 experiments, females were aspirated into fresh vials every 24 hours for 4 days. The vacated vials were retained for 12 days until all offspring had eclosed. These cultures were then frozen before scoring the number and paternity of the emerging offspring.
(c) Statistical analysis
Statistical analysis was performed using R v. 2.6.1 (Ihaka & Gentleman 1996) and SPSS v. 14 (SPSS, Inc., Chicago, IL, USA). All data (both raw and residuals) were tested for normality using Kolmogorov–Smirnov tests and for homogeneity of variance using Bartlett tests, and data were transformed to normality where possible. Normally distributed data were analysed by ANOVA, with previous and current number of males as fixed factors. Where data could not be normalized, we used the Scheirer–Ray–Hare extension of the Kruskal–Wallis test (Dytham 1999). P1 and P2 data were analysed using general linear models (GLMs) based on a quasibinomial error distribution to account for overdispersion. Significance of factors was tested in an analysis of deviance through subtraction from the full model.
3. Results
(a) Responses of males to SC: mating duration and latency
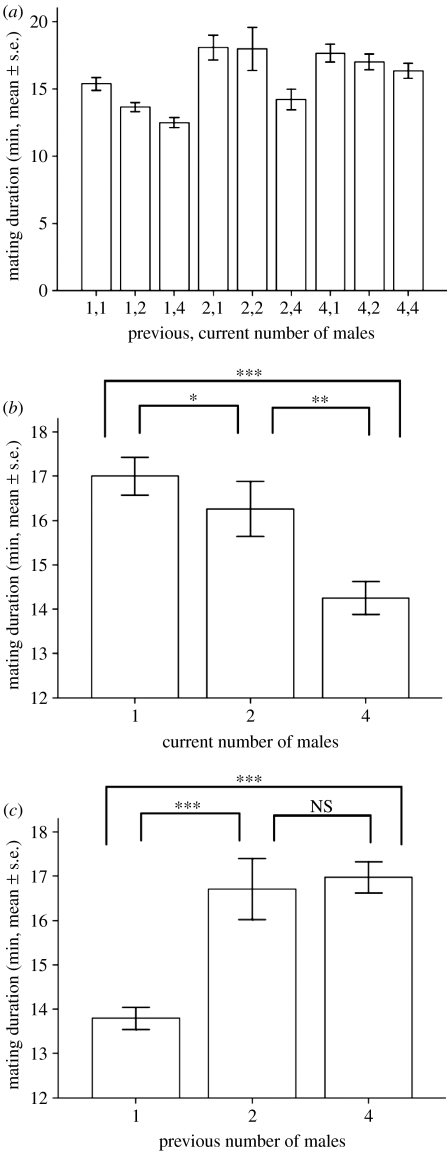
Mating duration was significantly longer when the previous level of SC was high (i.e. when males were kept with rivals prior to mating), but was significantly shorter when the current level of SC was high (when there were rival males present in the mating arena: Scheirer–Ray–Hare test previous treatment H2=14.70, p=0.0006; current treatment H2=6.90, p=0.03; interaction H4=2.30, p=0.68; figure 1a–c). Latency to mating was significantly shorter when rival males were present in the mating arena, but latency was not affected by a male's previous exposure to rivals (Scheirer–Ray–Hare test current treatment H2=6.74, p=0.03; previous treatment H2=4.41, p=0.11; interaction H4=2.05, p=0.73; see figure S1 in the electronic supplementary material).
Figure 1.
Effect of male responses to the previous or current level of SC on male investment in first mating duration. Males were kept for 5 days from eclosion in groups of 1, 2 or 4 males (previous treatments) and then placed 1, 2 or 4 males per vial in the mating arena (current treatments). (a) Mating duration (mean minutes±s.e.) and summary of the mating duration response (mean minutes±s.e.) to (b) current and (c) previous male treatments. Asterisks represent significant differences revealed by Mann–Whitney U-tests (*p<0.05, **p<0.01, ***p<0.0001; n.s., non-significant).
(b) Fitness consequences for males of responding to variation in SC
In both the P1 and P2 male reproductive success experiments, we found the same effects on mating duration as described above. A combined probability test (Sokal & Rohlf 1981) showed that mating duration increased significantly across all three experiments when males were kept with rivals for 5 days prior to mating (p<0.0001) and decreased when more than one male was present in the mating arena (p=0.0014). There was a non-significant trend for males to respond to variation in SC risk signified by female mating status, as seen by increased mating duration with once-mated females when compared with virgins (i.e. in the P2 experiment, Mann–Whitney U-test Z=−1.81, p=0.07).
The most striking result was that both first (P1) and second (P2) mating male paternity share was significantly higher for males that had been kept with rivals prior to mating (table 1), with males kept with rivals achieving either 14 (P1) or 9 (P2) per cent higher paternity (effect sizes expressed as the difference between the two mean proportions). An alternative analysis using instead the absolute progeny number fathered by males experiencing high or low SC also gave significantly higher absolute paternity for males experiencing high SC prior to mating when they were the first males to mate, but this was not significant when they were second males to mate (see table S1 in the electronic supplementary material). There was an additional effect of the current treatment, with males exposed to rivals in the mating arena having shorter mating duration and gaining lower paternity share in the P2 experiment (5% difference in mean proportions when mating with non-virgin females; table 1). These findings were robust to alternative analysis methods (see table S2 in the electronic supplementary material). None of the analyses gave a significant interaction effect (table 1; see table S2 in the electronic supplementary material) even though there was lower paternity share in the ‘previous 1×current 4’ treatment. There was also no interaction in the analysis of total progeny fathered (see table S1 in the electronic supplementary material). Interaction effects might have become apparent upon the use of larger sample sizes.
Table 1.
Analysis of paternity share for males responding to high and low levels of SC. (First (P1) and second (P2) male paternity share for low and high SC treatment males in the P1 and P2 experiments. Prior to mating, males were kept for 5 days in groups of one or four males (previous treatments) and then placed one or four males per vial in the mating arena (current treatments). In the P1 experiment, sepia females were first mated to low and high SC males and then 24 hours later to a sepia male. In the P2 experiment, the mating order was reversed. (a) Mean (±s.e.) P1 and P2 values calculated from progeny totals and sample size (n) per treatment and (b) results of a GLM with previous and current number of males as fixed factors. The dispersion parameter for the P1 model was 45.35 and P2 was 36.99.)
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| previous male treatment | current male treatment | P1 mean (±s.e.) | n | P2 mean (±s.e.) | n | |||
| 1 | 1 | 0.51 (0.07) | 59 | 0.91 (0.04) | 49 | |||
| 1 | 4 | 0.35 (0.06) | 61 | 0.80 (0.06) | 42 | |||
| 4 | 1 | 0.58 (0.07) | 49 | 0.96 (0.03) | 42 | |||
| 4 | 4 | 0.58 (0.06) | 59 | 0.94 (0.03) | 50 | |||
| (b) | P1 experiment | P2 experiment | ||||||
|---|---|---|---|---|---|---|---|---|
| source | d.f. | deviance | F | p-value | d.f. | deviance | F | p-value |
| previous | 1 | 339.2 | 7.5 | 0.007 | 1 | 348.9 | 9.56 | 0.002 |
| current | 1 | 96.6 | 2.13 | 0.146 | 1 | 142.5 | 3.90 | 0.049 |
| previous×current | 1 | 101.6 | 2.24 | 0.136 | 1 | 11.0 | 0.30 | 0.608 |
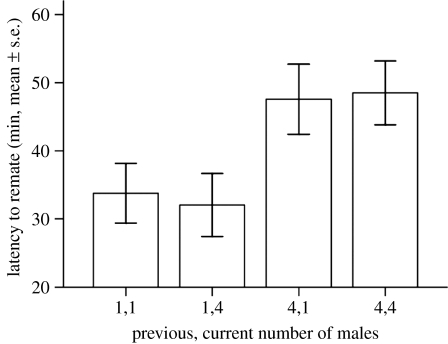
The remating latency of females was significantly affected by the SC treatment of their first mate. Females first mated to males kept together with rivals prior to mating took approximately 10 min longer to remate (ANOVA, previous treatment F1,224=10.45, p=0.001; current F1,224=0.01, p=0.92; interaction F1,224=0.08, p=0.78; figure 2), and there was also a trend for a lower frequency of remating in those females overall (Χ12=3.26, p=0.07). This suggests that longer first matings reduce subsequent female sexual receptivity, a finding that was also supported by the positive correlation between first mating duration and the remating latency (Spearman's r=0.200, p=0.002). We also found that the longer the second mating latency, the higher the P1 paternity share (r=0.566, p<0.001) and the shorter the second mating latency, the higher the P2 share (r=−0.580, p<0.001). This shows that males that could lengthen or shorten female time to remating in their favour achieved significantly higher reproductive success.
Figure 2.
Effect of male responses to the previous or current level of SC on female receptivity to a second mating. Mating latency (mean minutes±s.e.) of female remating 24 hours after a first mating with low or high SC treatment males. Females first mating with males kept with rival males prior to mating had significantly longer latencies to their second mating.
There were additional effects of variation in SC on female fecundity and fertility in the 24 hours following first matings to experimental males in the P1 experiment. Females mated to males kept with rivals prior to mating laid significantly more eggs (7% difference in mean), but the current number of males had no effect (ANOVA previous treatment F1,223=6.3, p=0.013; current treatment F1,223=0.01, p=0.93; interaction F1,223=1.54, p=0.21; see figure S2a in the electronic supplementary material). There were also significant effects on egg to adult survival. Females mated to males kept with rivals prior to mating laid eggs with an average 3 per cent higher survival to adulthood, but there was no effect of the current number of males in the mating arena (GLM dispersion parameter=29.8; previous treatment d.f.=1, deviance=224.1, F=7.6, p=0.006; current treatment d.f.=1, deviance=13.80, F=0.46, p=0.50; interaction d.f.=1, deviance=9.10, F=0.30, p=0.58; see figure S2b in the electronic supplementary material). We analysed these variables separately as we effectively had multiple dependent variables with significant collinearity between them. We analysed fecundity and egg fertility for experimental males in the P1 experiment only, as in the P2 experiment we could not tell which of the males had fertilized which of the newly laid eggs.
4. Discussion
The most important results from our study were that males altered their ejaculate investment, measured as mating duration, according to the level of SC, and that this resulted in significantly higher male reproductive success. Males invested more in mating duration when their exposure to males in the 5 days prior to mating was high and invested less when they were exposed to other males at the time of mating. This was consistent across all three experiments and was therefore true regardless of whether males were mating with virgin or once-mated females (e.g. in the P1 and P2 experiments, respectively). Our experiments show for the first time that male responses to increased levels of SC prior to mating resulted in a significantly increased paternity share in a competitive context, when males were the first or second to mate with a female. Hence, male responses to previous levels of SC were the important determinants in terms of male reproductive success.
In highly polyandrous species such as D. melanogaster, we expect the impact of SC intensity to be dominant, relative to SC risk (Simmons 2001). Risk is expected to be less important because females will have already mated and will regularly mate again, and hence the risk of SC is guaranteed and somewhat invariant (Engqvist & Reinhold 2005). By contrast, SC intensity, as represented by the number of males contributing to the stored sperm pool in females, varies more markedly. For example, in the wild female D. melanogaster have been observed to carry the sperm of between two and six males (Harshman & Clark 1998; Imhof et al. 1998), with an average of four males fathering the offspring of each female (Imhof et al. 1998). However, the D. melanogaster system differs from the theoretical assumptions in one key way. The characteristic high level of sperm displacement (Gromko et al. 1984; Civetta 1999), and potentially limited expansion of the female sperm storage organs in this species, may prevent additional sperm adding to an ever-increasing pool (Pitnick et al. 1999). This could mean that the number of contributors to the sperm stored by females (i.e. the intensity of SC) is less important than whether females will mate again (risk of SC). However, even if this were the case, the fitness-related effects of seminal fluid molecules, such as accessory gland proteins (Acps; e.g. Chapman et al. 2003), could themselves still be additive, increasing the importance of intensity relative to risk. There is a widely held view that recently mated D. melanogaster females typically have a long refractory period before remating, which would decrease the overlap in ejaculates and decrease the intensity of SC. However, although this is true for females that have time to develop a full refractory response over several hours, there is a surprisingly high probability of early, immediate remating (30–50% remating within 6 hours (Vanvianen & Bijlsma 1993); up to 80% remating within 4 hours, A. J. Bretman & T. Chapman 2008 unpublished data) and patterns of sperm displacement in such rapid rematings have not yet been investigated. Hence, these factors could increase still further variation in, and the importance of, SC intensity relative to risk.
We conclude that while the number of males in the mating environment will give a focal male an accurate idea of the likely intensity of SC if he mates, the degree and pattern of both polyandry and sperm displacement can decrease the ability to predict whether males will respond primarily to SC risk or intensity. Perhaps consistent with this reasoning, our findings were not strictly in accord with either SC risk or intensity theory, but did, in general, support the idea that SC intensity was the more important force in shaping male responses to SC. Specifically, if males were responding primarily to increased SC risk, then we would expect to see increased mating duration when the previous level of risk was trebled (i.e. in the comparison between the previous two versus four male treatments, where each individual male was exposed to one or three other competitors, respectively; Parker et al. 1996, 1997; Engqvist & Reinhold 2005); however, we did not observe this (figure 1c). Risk models also predict increased investment when both previous and current risk increase; but, by contrast, we observed a decline in mating duration with increased current SC levels. We did, however, see a non-significant trend for males to respond to variation in risk as signified by female mating status, with increased mating duration with once-mated females when compared with virgins, consistent with a previous report (Friberg 2006).
If, on the other hand, males were responding primarily to SC intensity, we would expect to see increased mating duration with increased previous SC intensity (which we did observe) and decreased mating duration when more than one competitor was present in the mating arena (i.e. the peak of investment should be with one rival present; Parker et al. 1996, 1997; Engqvist & Reinhold 2005). The latter pattern was only partially seen; we observed a decrease in mating duration with increased numbers of current males at mating, but the longest duration was with no competitors rather than one competitor (i.e. two males in the mating arena; figure 1b). This departure from expectation could be explained if mating pairs were harassed by single males causing early termination of mating, or may indicate that females play a role in shortening matings when there is the potential for remating. However, previous work in D. melanogaster suggests that shifts in mating duration are under male control (Friberg 2006), and our data also support this idea. Overall, the fit of our data was closer to the predictions of models of SC intensity rather than risk, in line with our general expectations. Hence, SC intensity may be of greater importance in shaping a male's responses to SC than risk, under the conditions tested. An important result from our study, therefore, was that the benefits for males of responding to the presence of rival males persisted after female remating and despite the strong sperm precedence patterns observed.
There was no support for the idea that precopulatory sexual selection (i.e. where the ‘best’ male mates for longest) could explain the observed patterns of mating duration. For example, if males were winning precopulatory contests (through female choice or male–male competition for access to females) then mating duration would be maximized when four were present in the mating arena. This is because the best (longest duration) male sampled from four males should, on average, have a longer mating duration than the best male sampled from two or single males. However, this did not occur (figure 1c). Likewise, variation in mating duration times would be lower when more males were present at mating (with the variability of individual males capturing that of the population as a whole, but with the variability of the best one male from four being much less), but this also was not the case (figure 1c). However, it is possible that precopulatory sexual selection could be followed by harassment of the mating pair by the non-mating males present, an effect that could reduce mating duration.
We also observed significant effects of variation in SC on the latency to mating. The presence of rivals in the mating arena decreased mating latency, and just one competitor was enough to achieve this effect, which is consistent with a previous report (Crossley & Wallace 1987). This finding cannot wholly be explained by higher encounter rates in the presence of more males in the mating arena, otherwise matings in the presence of three rival males would have the shortest latencies, which we did not observe (see figure S1 in the electronic supplementary material).
Turning to mechanism, our results suggest that prolonged matings serve to increase sperm and/or seminal fluid transfer resulting in higher fertilization success (Michiels 1992; Hadrys et al. 1993; Arnqvist & Danielsson 1999; Friberg 2006). Previous studies of SC have almost exclusively focused on the number of sperm ejaculated (e.g. Gage 1991; Wedell & Cook 1999; Candolin & Reynolds 2002; Pizzari et al. 2003; Delbarco-Trillo & Ferkin 2004; Engqvist 2007; Ramm & Stockley 2007) and increased sperm transfer could be a part of the explanation for our results. However, the changes we observed in the post-mating responses of females in the P1 experiment, such as decreased sexual receptivity and increased egg production, are processes known to be controlled by male seminal fluid accessory gland proteins (Acps; Chapman et al. 2001, 2003; Gillott 2003), which suggests that longer matings serve to increase Acp transfer in this species. This is supported by direct evidence for correlations between mating duration and Acp transfer into the female reproductive tract (S. Wigby, L. Sirot, J. R. Linklater, A. Bretman, N. Buehner, M. F. Wolfner, & T. Chapman 2009 in review). Further support for the idea that longer matings permit more Acp transfer is the positive correlation we observed in this study between first mating duration to experimental males and a female's latency to a second mating, which suggests that males influence female mating behaviour by the amount of ejaculate they transfer. Sperm transfer is less likely to be a major explanation because, unlike many insects where sperm transfer shows a linear correlation with mating duration (Simmons & Siva-Jothy 1998; Simmons 2001), as detailed earlier, sperm transfer in D. melanogaster does not, and occurs quickly between 6 and 10 min from the start of mating (Gilchrist & Partridge 2000). Matings curtailed after sperm transfer but before their natural end result in no loss of fertility, but instead an increased likelihood of remating by females (Gilchrist & Partridge 2000), supporting further the idea that longer matings serve to transfer more Acps such as those that reduce female receptivity.
In summary, our results demonstrate a plastic mating strategy by males that is not strictly in accord with existing SC game theory. What is clear is that males obtain information on the potential level of SC from the surrounding number of rivals, but how this information is obtained remains to be investigated. Importantly, the strategies employed by males result in significantly increased reproductive success in a competitive context, even following subsequent rematings in which the majority of a male's sperm are displaced.
Acknowledgments
We thank James Boone, Dave Gerrard and Jo Redler for their help with experiments, Andrew Bourke, Matt Gage, Lorenzo Zanette and Tom Tregenza for their valuable discussions, and the editor and three anonymous referees whose comments greatly improved the manuscript. We thank the BBSRC, the NERC and the Royal Society for funding (research grants and University Research Fellowship to T.C.).
Supplementary Material
Additional figures and tables
References
- Arnqvist G., Danielsson I. Postmating sexual selection: the effects of male body size and recovery period on paternity and egg production rate in a water strider. Behav. Ecol. 1999;10:358–365. doi:10.1093/beheco/10.4.358 [Google Scholar]
- Bass T.M., Grandison R.C., Wong R., Martinez P., Partridge L., Piper M.D.W. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2007;62:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhead T.R., Møller A.P. Academic Press; London, UK: 1998. Sperm competition and sexual selection. [Google Scholar]
- Candolin U., Reynolds J.D. Adjustments of ejaculation rates in response to risk of sperm competition in a fish, the bitterling (Rhodeus sericeus) Proc. R. Soc. B. 2002;269:1549–1553. doi: 10.1098/rspb.2002.2055. doi:10.1098/rspb.2002.2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo P., Font E., Alfthan B. Chemosensory assessment of sperm competition levels and the evolution of internal spermatophore guarding. Proc. R. Soc. B. 2007;274:261–267. doi: 10.1098/rspb.2006.3714. doi:10.1098/rspb.2006.3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T., Herndon L.A., Heifetz Y., Partridge L., Wolfner M.F. The Acp26Aa seminal fluid protein is a modulator of early egg hatchability in Drosophila melanogaster. Proc. R. Soc. B. 2001;268:1647–1654. doi: 10.1098/rspb.2001.1684. doi:10.1098/rspb.2001.1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T., Bangham J., Vinti G., Seifried B., Lung O., Wolfner M.F., Smith H.K., Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl Acad. Sci. USA. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. doi:10.1073/pnas.1631635100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civetta A. Direct visualization of sperm competition and sperm storage in Drosophila. Curr. Biol. 1999;9:841–844. doi: 10.1016/s0960-9822(99)80370-4. doi:10.1016/S0960-9822(99)80370-4 [DOI] [PubMed] [Google Scholar]
- Crossley S., Wallace B. The effects of crowding on courtship and mating success in Drosophila melanogaster. Behav. Genet. 1987;17:513–522. doi: 10.1007/BF01073118. doi:10.1007/BF01073118 [DOI] [PubMed] [Google Scholar]
- Delbarco-Trillo J., Ferkin M.H. Male mammals respond to a risk of sperm competition conveyed by odours of conspecific males. Nature. 2004;431:446–449. doi: 10.1038/nature02845. doi:10.1038/nature02845 [DOI] [PubMed] [Google Scholar]
- Dytham C. Blackwell Science Ltd; Oxford, UK: 1999. Choosing and using statistics. [Google Scholar]
- Engqvist L. Male scorpionflies assess the amount of rival sperm transferred by females' previous mates. Evolution. 2007;61:1489–1494. doi: 10.1111/j.1558-5646.2007.00107.x. doi:10.1111/j.1558-5646.2007.00107.x [DOI] [PubMed] [Google Scholar]
- Engqvist L., Reinhold K. Pitfalls in experiments testing predictions from sperm competition theory. J. Evol. Biol. 2005;18:116–123. doi: 10.1111/j.1420-9101.2004.00792.x. doi:10.1111/j.1420-9101.2004.00792.x [DOI] [PubMed] [Google Scholar]
- Engqvist L., Reinhold K. Theoretical influence of female mating status and remating propensity on male sperm allocation patterns. J. Evol. Biol. 2006;19:1448–1458. doi: 10.1111/j.1420-9101.2006.01134.x. doi:10.1111/j.1420-9101.2006.01134.x [DOI] [PubMed] [Google Scholar]
- Friberg U. Male perception of female mating status: its effect on copulation duration, sperm defence and female fitness. Anim. Behav. 2006;72:1259–1268. doi:10.1016/j.anbehav.2006.03.021 [Google Scholar]
- Gage M.J.G. Risk of sperm competition directly affects ejaculate size in the Mediterranean fruit-fly. Anim. Behav. 1991;42:1036–1037. doi:10.1016/S0003-3472(05)80162-9 [Google Scholar]
- Gage M.J.G. Associations between body size, mating patterns, testis size and sperm length across butterflies. Proc. R. Soc. Lond. B. 1994;258:247–254. doi:10.1098/rspb.1994.0169 [Google Scholar]
- Gage M.J.G., Baker R.R. Ejaculate size varies with sociosexual situation in an insect. Ecol. Entomol. 1991;16:331–337. doi:10.1111/j.1365-2311.1991.tb00224.x [Google Scholar]
- Gage A.R., Barnard C.J. Male crickets increase sperm number in relation to competition and female size. Behav. Ecol. Sociobiol. 1996;38:349–353. doi:10.1007/s002650050251 [Google Scholar]
- Gilchrist A.S., Partridge L. Why it is difficult to model sperm displacement in Drosophila melanogaster: the relation between sperm transfer and copulation duration. Evolution. 2000;54:534–542. doi: 10.1111/j.0014-3820.2000.tb00056.x. doi:10.1111/j.0014-3820.2000.tb00056.x [DOI] [PubMed] [Google Scholar]
- Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. doi:10.1146/annurev.ento.48.091801.112657 [DOI] [PubMed] [Google Scholar]
- Gromko M.H., Gilbert D.G., Richmond R.C. Sperm transfer and use in the multiple mating system of Drosophila. In: Smith R.L., editor. Sperm competition and the evolution of animal mating systems. Academic Press, Inc; San Diego, CA: 1984. pp. 371–426. [Google Scholar]
- Hadrys H., Schierwater B., Dellaporta S.L., Desalle R., Buss L.W. Determination of paternity in dragonflies by random amplified polymorphic DNA fingerprinting. Mol. Ecol. 1993;2:79–87. doi: 10.1111/j.1365-294x.1993.tb00002.x. doi:10.1111/j.1365-294X.1993.tb00002.x [DOI] [PubMed] [Google Scholar]
- Harcourt A.H., Harvey P.H., Larson S.G., Short R.V. Testis weight, body weight and breeding system in primates. Nature. 1981;293:55–57. doi: 10.1038/293055a0. doi:10.1038/293055a0 [DOI] [PubMed] [Google Scholar]
- Harshman L.G., Clark A.G. Inference of sperm competition from broods of field-caught Drosophila. Evolution. 1998;52:1334–1341. doi: 10.1111/j.1558-5646.1998.tb02015.x. doi:10.2307/2411303 [DOI] [PubMed] [Google Scholar]
- Hihara F. Effects of the male accessory gland secretion on oviposition and remating in females of Drosophila melanogaster. Zool. Mag. 1981;90:307–316. [Google Scholar]
- Hosken D.J. Sperm competition in bats. Proc. R. Soc. B. 1997;264:385–392. doi: 10.1098/rspb.1997.0055. doi:10.1098/rspb.1997.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R., Gentleman R. R: a language for data analysis and graphics. J. Comp. Graph. Stat. 1996;5:299–314. doi:10.2307/1390807 [Google Scholar]
- Imhof M., Harr B., Brem G., Schlotterer C. Multiple mating in wild Drosophila melanogaster revisited by microsatellite analysis. Mol. Ecol. 1998;7:915–917. doi: 10.1046/j.1365-294x.1998.00382.x. doi:10.1046/j.1365-294x.1998.00382.x [DOI] [PubMed] [Google Scholar]
- Jennions M.D., Passmore N.I. Sperm competition in frogs: testis size and a ‘sterile male’ experiment on Chiromantis xerampelina (Rhacophoridae) Biol. J. Linn. Soc. 1993;50:211–220. doi:10.1006/bijl.1993.1055 [Google Scholar]
- Karlsson B. Resource allocation and mating systems in butterflies. Evolution. 1995;49:955–961. doi: 10.1111/j.1558-5646.1995.tb02330.x. doi:10.2307/2410417 [DOI] [PubMed] [Google Scholar]
- Linklater J.R., Wertheim B., Wigby S., Chapman T. Ejaculate depletion patterns evolve in response to experimental manipulation of sex ratio in Drosophila melanogaster. Evolution. 2007;61:2027–2034. doi: 10.1111/j.1558-5646.2007.00157.x. doi:10.1111/j.1558-5646.2007.00157.x [DOI] [PubMed] [Google Scholar]
- Martin O.Y., Hosken D.J. Strategic ejaculation in the common dung fly Sepsis cynipsea. Anim. Behav. 2002;63:541–546. doi:10.1006/anbe.2001.1929 [Google Scholar]
- Michiels N.K. Consequences and adaptive significance of variation in copulation duration in the dragonfly Sympetrum danae. Behav. Ecol. Sociobiol. 1992;29:429–435. doi:10.1007/BF00170173 [Google Scholar]
- Møller A.P., Ninni P. Sperm competition and sexual selection: a meta-analysis of paternity studies of birds. Behav. Ecol. Sociobiol. 1998;43:345–358. doi:10.1007/s002650050501 [Google Scholar]
- Neff B.D., Fu P., Gross M.R. Sperm investment and alternative mating tactics in bluegill sunfish (Lepomis macrochirus) Behav. Ecol. 2003;14:634–641. doi:10.1093/beheco/arg032 [Google Scholar]
- Parker G.A. Sperm competition games: sneaks and extra-pair copulations. Proc. R. Soc. Lond. B. 1990;242:127–133. doi:10.1098/rspb.1990.0115 [Google Scholar]
- Parker G.A. Sperm competition games: sperm size and sperm number under adult control. Proc. R. Soc. Lond. B. 1993;253:245–254. doi: 10.1098/rspb.1993.0110. doi:10.1098/rspb.1993.0110 [DOI] [PubMed] [Google Scholar]
- Parker G.A., Ball M.A., Stockley P., Gage M.J.G. Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. Lond. B. 1996;263:1291–1297. doi:10.1098/rspb.1996.0189 [Google Scholar]
- Parker G.A., Ball M.A., Stockley P., Gage M.J.G. Sperm competition games: a prospective analysis of risk assessment. Proc. R. Soc. Lond. B. 1997;264:1793–1802. doi: 10.1098/rspb.1997.0249. doi:10.1098/rspb.1997.0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S., Markow T., Spicer G.S. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution. 1999;53:1804–1822. doi: 10.1111/j.1558-5646.1999.tb04564.x. doi:10.2307/2640442 [DOI] [PubMed] [Google Scholar]
- Pizzari T., Cornwallis C.K., Løvlie H., Jakobsson S., Birkhead T.R. Sophisticated sperm allocation in male fowl. Nature. 2003;426:70–74. doi: 10.1038/nature02004. doi:10.1038/nature02004 [DOI] [PubMed] [Google Scholar]
- Pound N., Gage M.J.G. Prudent sperm allocation in Norway rats, Rattus norvegicus: a mammalian model of adaptive ejaculate adjustment. Anim. Behav. 2004;68:819–823. doi:10.1016/j.anbehav.2004.02.004 [Google Scholar]
- Ramm S.A., Stockley P. Ejaculate allocation under varying sperm competition risk in the house mouse, Mus musculus domesticus. Behav. Ecol. 2007;18:491–495. doi:10.1093/beheco/arm003 [Google Scholar]
- Reichard M., Smith C., Jordan W.C. Genetic evidence reveals density-dependent mediated success of alternative mating behaviours in the European bitterling (Rhodeus sericeus) Mol. Ecol. 2004;13:1569–1578. doi: 10.1111/j.1365-294X.2004.02151.x. doi:10.1111/j.1365-294X.2004.02151.x [DOI] [PubMed] [Google Scholar]
- Simmons L.W. Princeton University Press; Princeton, NJ: 2001. Sperm competition and its evolutionary consequences in the insects. [Google Scholar]
- Simmons L.W., Siva-Jothy M.T. Sperm competition in insects: mechanisms and the potential for selection. In: Birkhead T.R., Møller A.P., editors. Sperm competition and sexual selection. Academic Press; London, UK: 1998. pp. 341–494. [Google Scholar]
- Siva-Jothy M.T., Stutt A.D. A matter of taste: direct detection of female mating status in the bedbug. Proc. R. Soc. B. 2003;270:649–652. doi: 10.1098/rspb.2002.2260. doi:10.1098/rspb.2002.2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R.R., Rohlf F.J. W.H. Freeman; New York, NY: 1981. Biometry. [Google Scholar]
- Stockley P., Gage M.J.G., Parker G.A., Møller A.P. Sperm competition in fishes: the evolution of testis size and ejaculate characteristics. Am. Nat. 1997;149:933–954. doi: 10.1086/286031. doi:10.1086/286031 [DOI] [PubMed] [Google Scholar]
- Vanvianen A., Bijlsma R. The adult component of selection in Drosophila melanogaster—some aspects of early-remating activity of females. Heredity. 1993;71:269–276. doi: 10.1038/hdy.1993.135. doi:10.1038/hdy.1993.135 [DOI] [PubMed] [Google Scholar]
- Wedell N., Cook P.A. Butterflies tailor their ejaculate in response to sperm competition risk and intensity. Proc. R. Soc. Lond. B. 1999;266:1033–1039. doi:10.1098/rspb.1999.0740 [Google Scholar]
- Wedell N., Gage M.J.G., Parker G.A. Sperm competition, male prudence and sperm limited females. Trends Ecol. Evol. 2002;17:313–320. doi:10.1016/S0169-5347(02)02533-8 [Google Scholar]
- Williams P.D., Day T., Cameron E. The evolution of sperm-allocation strategies and the degree of sperm competition. Evolution. 2005;59:492–499. doi:10.1554/04-668 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional figures and tables