Abstract
Substantial evidence indicates that brain neurons containing and secreting noradrenaline and corticotropin-releasing factor (CRF) are activated during stress, and that physiological and behavioural responses observed during stress can be induced by exogenous administration of CRF and adrenoceptor agonists. This review focusses on the evidence for the involvement of these two factors in stress-related responses, and the inter-relationships between them. The possible abnormalities of these two systems in depressive illness are also discussed.
Index Words: Corticotropin-releasing factor (CRF), Noradrenaline, Stress, Feed-Forward, Locus coeruleus, Depression
1. Introduction
There is substantial evidence that neurons in the brain containing and secreting noradrenaline and corticotropin-releasing factor (CRF) are activated during stress, and that many of the physiological and behavioural responses observed during stress can be induced by exogenous administration of CRF and adrenoceptor agonists (Dunn and Berridge, 1990; Owens and Nemeroff, 1991). This suggests that endogenous secretion of CRF and noradrenaline may both be involved in the induction of responses observed during stress. This suggestion is supported by evidence that antagonists of CRF-receptors can attenuate or prevent certain responses observed during stress. The evidence for this is substantial and has been reviewed extensively so we will not review it here (Dunn and Berridge, 1990; Owens and Nemeroff, 1991; Smagin et al. 2001; Heinrichs and Koob, 2004). Instead, this review will focus on the differences between the physiological responses to noradrenergic and CRF agonists, and the inter-relationships between these two neuromediators.
2. Interactions between noradrenaline and corticotropin-releasing factor (CRF)
2.1 CRF and the Hypothalamo-Pituitary-Adrenocortical (HPA) Axis
CRF is a critical factor in the activation of the hypothalamo-pituitary-adrenocortical (HPA) axis, which has long been regarded as the characteristic, if not the defining, physiological manifestation of stress. CRF is secreted by hypothalamic hypophysiotropic neurons whose cell bodies are located in the hypothalamic paraventricular nucleus with terminals in the median eminence region of the hypothalamus (Figure 1). The CRF released is then carried in the portal veins to the anterior pituitary where it acts on CRF receptors (specifically CRF1 receptors) on corticotrophic cells stimulating the secretion of adrenocorticotrophic hormone (ACTH) (and β-endorphin) into the peripheral blood circulation. ACTH in turn binds to receptors on cells of the adrenal cortex stimulating the synthesis and secretion of the adrenal glucocorticoids, cortisol and corticosterone, the relative amounts of which vary with the species (cortisol predominates in man and most other animal species, whereas corticosterone is the major corticosteroid in rodents). CRF-containing neurons are not confined to the hypothalamic paraventricular nucleus, but are to be found in many other parts of the brain (see Smagin et al., 2001).
Fig. 1.
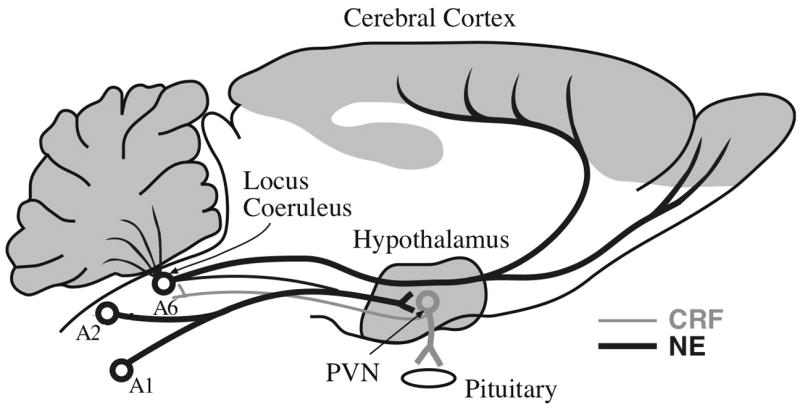
A simplified diagram indicating the two major ascending noradrenergic projection systems, the dorsal locus coeruleus (LC, A6) system, and the ventral A1/A2 system and their interactions with hypothalamic paraventricular nucleus (PVN), corticotropin-releasing factor (CRF)-containing neurons. Although the ascending axons are intermingled in the upper brain stem, the dorsal system largely innervates the telencephalon and the cerebellum, whereas the ventral A1/A2 system innervates the diencephalon and basal forebrain. CRF-containing cells in the hypothalamic paraventricular nucleus provide the principal regulation of the HPA axis by secreting CRF in the median eminence region which stimulates pituitary ACTH release, thus activating the HPA axis. Nevertheless there is a small noradrenergic projection from A6 to the hypothalamic paraventricular nucleus, and a reciprocal small CRF projection from the hypothalamic paraventricular nucleus to the area of the locus coeruleus. These two small projections may form a loop that could constitute a mutual excitation (feed-forward loop) of the major noradrenaline and CRF projection systems.
2.2 Noradrenaline-CRF Interactions
Two major interactions have been studied between noradrenergic and CRF-containing neurons within the brain. Firstly, the hypothalamic paraventricular nucleus receives a major input from noradrenergic neurons in the nucleus tractus solitarius (A1/A2) and one of lesser magnitude from the locus coeruleus (A6). It has long been known that noradrenaline can excite CRF-containing cells in the hypothalamic paraventricular nucleus to activate the HPA axis. Noradrenaline and adrenoceptor agonists injected directly into the brain alter the electrophysiological activity of CRF-containing neurons (Saphier, 1993). This effect is thought to be mediated largely by α1-adrenoceptors, although a role for β-adrenoceptors has not been excluded (Al-Damluji, 1988, 1993; Plotsky et al., 1989; Saphier, 1989, 1993). It is believed that noradrenaline may activate CRF-containing neurons in extrahypothalamic regions, but this has not been clearly established. Secondly, intracerebroventricular (i.c.v.) injection of CRF into the brain increases the electrophysiological activity of noradrenergic neurons in the locus coeruleus (Valentino et al., 1983), and increases the metabolism of noradrenaline in the forebrain (increases in 3-methoxy,4-hydroxyphenylethylene glycol (MHPG: Dunn and Berridge, 1987; Emoto et al., 1993b), as well as apparent secretion of noradrenaline indicated by in vivo microdialysis (Lavicky and Dunn, 1993; Emoto et al., 1993a) clearly suggesting increased release of noradrenaline. Injection of CRF into the region of the locus coeruleus also results in increased noradrenaline release in the prefrontal cortex and hypothalamus assessed by microdialysis and voltammetry (see below).
A CRF-noradrenergic interaction has also been observed in the study of behaviour during stress, for example in the multicompartment chamber. The chamber is similar to a hole-board i.e., a square open field in which there are nine holes into which rats and mice can poke their noses. The differences are that there are arches separating the nine holes, and small coils of wire are fixed in the holes just below the floor, and the animals explore (lick, bite, and/or chew) on this wire. Several different stressful treatments (e.g., tail pinch, footshock and restraint) each decreased the mean times that rats and mice spend investigating the novel wire coils (Arnsten et al., 1985; Berridge and Dunn, 1986). In mice, this effect was mimicked by i.c.v. administration of CRF or the α1-adrenoreceptor agonist, phenylephrine (Berridge and Dunn, 1986). A CRF-receptor antagonist was able to prevent the behavioural changes induced by phenylephrine in the multicompartment chamber, whereas the responses to i.c.v. CRF were not prevented by the α1-adrenoceptor antagonist, prazosin (Dunn and Berridge, 1990). This suggested that noradrenergic neurons stimulate CRF neurons via α1-adrenoceptors, which in turn mediate the behavioural responses - the same arrangement involved in HPA activation. However, β1-adrenoceptor antagonists prevented the i.c.v. CRF-induced changes in behaviour in the multicompartment chamber (Dunn and Berridge, 1990), as well as in the defensive withdrawal response (Yang et al., 1990a, b) and the elevated plus-maze (Gorman and Dunn, 1993). These studies indicate that both α1- and β1-adrenoceptors are involved in the responses to stress in all three behavioural tasks. α1-Adrenoceptors appear to be involved in the activation of CRF neurons (directly or indirectly). The CRF neurons in turn appear to influence behaviour (directly or indirectly) via β1-adrenoceptors. Thus, α1-adrenoceptors are involved in the activation of CRF and hence of the HPA axis, and the behavioural responses, whereas β1-adrenoceptors are involved only in the behavioural responses whether to stressors or to exogenous administration of CRF. The locations of the specific α1- and β1-adrenoceptors involved in these behavioural responses have not been identified, and this interesting serial involvement of α1- and β1-adrenoceptors has not been further explored.
3. Noradrenergic Systems and the HPA axis in affective disorders
3.1 Noradrenergic Systems and the HPA axis in affective disorders
Longstanding evidence has indicated abnormalities in both noradrenaline and the HPA axis in anxiety and affective disorders, and specifically in depressive illness. It has long been known that many depressed patients exhibit elevated urinary and plasma concentrations of cortisol. Typically around 60–70% exhibit elevated plasma cortisol concentrations (e.g., Tichomirowa et al., 2005). Also, a large number of studies over 40 years has indicated that in patients with major depression, the urinary concentrations of the catabolites of noradrenaline (primarily 3-methoxy,4-hydroxyphenylethylene glycol, MHPG and its sulphated derivative) are elevated (Koslow et al. 1983), and similar findings have been made for cerebrospinal fluid (CSF) concentrations of MHPG, and, more recently, for noradrenaline itself (e.g., Wong et al., 2000). Many depressed patients also exhibit elevated CSF concentrations of CRF (Nemeroff et al., 1984; Arborelius et al., 1999, however, some depressed patients have lower or unchanged concentrations (Roy et al., 1987; France et al., 1988; Molchan et al., 1993). Despite the preponderance of the elevation of these biological measures: plasma and urinary cortisol, CSF noradrenaline and MHPG, and urinary MHPG, or increased CSF CRF, none of them is a reliable biological marker for depression.
4. The Feed-Forward Loop
When we reviewed the CNS effects of CRF (Dunn and Berridge, 1990), we noted that because noradrenergic neurons in the brain can excite CRF-containing neurons in the hypothalamic paraventricular nucleus to secrete CRF, and CRF can excite noradrenergic neurons this arrangement could create a positive feedback loop which could result in a vicious cycle which we speculated might underlie panic. More recently, Koob (1999) suggested that the noradrenergic excitation of paraventricular nucleus CRF neurons and the projection of some of these neurons to the locus coeruleus could create a “feed-forward” loop that could lead to anxiety disorders and result in depressive illness. In our view, if such a feed-forward system existed, it would need to be carefully controlled, otherwise any stressful stimulus, however innocuous, would activate the feed-forward system and thus trigger depression (Dunn et al., 2004).
The anatomical relationships between the CRF and noradrenergic systems in the brain are complex and incompletely understood. In particular, the site(s) at which i.c.v. CRF acts to activate the locus coeruleus-noradrenergic neurons has/have not been unequivocally identified, and there may well be multiple sites. It is likely that the brain stem A1 and A2 nuclei are also activated by i.c.v. CRF because metabolite studies and in vivo microdialysis indicate pronounced activation of noradrenergic terminals in the hypothalamus (Dunn and Berridge, 1987; Shimizu and Bray, 1989; Emoto et al., 1993b; Lavicky and Dunn, 1993; Smagin et al., 1994), although there is a limited projection from the locus coeruleus to the hypothalamic paraventricular nucleus.
The known relationships between the hypothalamic paraventricular nucleus CRF-containing neurons and the brain stem noradrenergic neurons are depicted in Figure 1. Noradrenergic neurons in the A1 and A2 nuclei constitute the major projection to the hypothalamic paraventricular nucleus, but there is a smaller projection from the locus coeruleus (A6, Fig. 1) both projections activating α1-receptors (Aston-Jones et al., 1991). It is not known whether CRF-containing cells elsewhere in the brain receive specific noradrenergic inputs, although noradrenergic terminals are found in almost all parts of the brain, so it is certainly possible. The principal projection for the CRF-containing neurons of the hypothalamic paraventricular nucleus is the median eminence of the hypothalamus, the major pathway for HPA axis activation. Nevertheless, there is a small projection from the hypothalamic paraventricular nucleus to the brain stem in various parts of the pons, including areas close to the locus coeruleus (Aston-Jones et al., 1986, 1991; Valentino et al., 1992). This projection could provide the descending branch of a locus coeruleus-paraventricular nucleus-locus coeruleus feedback loop (see Figure 1).
As indicated by Koob (1999), the hypothalamic paraventricular nucleus is only one of several possible sites that could provide links between noradrenaline and CRF release. Other potential sources of CRF in the locus coeruleus and the pericoerulear region are Barrington’s nucleus, the nucleus paragigantocellularis, and the central nucleus of the amygdala (Valentino et al., 1992; Van Bockstaele et al., 1998). In the regulation of behaviour, the more interesting possibilities are the links between the locus coeruleus and several structures innervated by the dorsal noradrenergic bundle, including the cerebral cortex, hippocampus, lateral septum, central nucleus of the amygdala, and the bed nucleus of the stria terminalis, structures that could form noradrenaline-CRF loops to the locus coeruleus. Several of these structures are likely to be involved in stress-related behavioural responses. For example, there are substantial locus coeruleus-noradrenergic projections to the amygdala, and CRF-containing cells in the central amygdaloid nucleus project caudally to the brain stem in the general region of the locus coeruleus (Koegler-Muly et al., 1993). A similar case can be made for the lateral septum and the bed nucleus of the stria terminalis.
4.3 Electrophysiological studies with CRF
Valentino’s group has focussed on a potential direct action of CRF on locus coeruleus noradrenergic neurons. As indicated above, their initial study indicated electrophysiological activation of locus coeruleus noradrenergic neurons following i.c.v. application of CRF (Valentino et al., 1983). However, only 9 of 14 cells recorded showed such an effect, and this result was not statistically significant (Valentino et al., 1983). Subsequent studies have shown electrophysiological activation of locus coeruleus neurons by direct local application of CRF into the locus coeruleus (Curtis et al., 1997; Page and Abercrombie, 1999). Curtis et al. (1997) demonstrated increases in electrophysiological activity following direct application of CRF into the locus coeruleus, and indicated that the dose necessary to activate locus coeruleus neurons locally was 200-fold lower than that effective intracerebroventricularly. Anatomical studies have indicated apposition of CRF-containing terminals with tyrosine hydroxylase-containing neurons in the locus coeruleus (indicating that they are likely to be noradrenergic cells), and retrograde tracing studies from the locus coeruleus indicated neurons immunoreactive for CRF in the nucleus gigantocellularis and the hypothalamic paraventricular nucleus (Valentino et al., 1992; Van Bockstaele et al., 1996).
Limited binding of radiolabelled CRF was observed in the region of the locus coeruleus in primates (Millan et al., 1986), and of radiolabelled sauvagine in primates (Sánchez et al., 1999) and rats (Primus et al., 1997), suggesting the presence of CRF1 receptors. An in vitro study with horizontal slices from rat brain stem indicated that CRF appears to exert a direct effect on locus coeruleus neurons decreasing K+ conductance via CRF1 receptors (Jedema and Grace, 2004).
However, an extensive study by Borsody and Weiss (1996) found that the first few seconds of the response of locus coeruleus-noradrenergic neurons to the local application of CRF was inhibitory, with excitation occurring only much later (Borsody and Weiss, 1996). Another electrophysiological study found that stimulation of the central amygdaloid nucleus produced a transient (10–20 ms) excitation of locus coeruleus-noradrenergic neurons followed by an inhibition for more than 2 seconds (Bouret et al., 2003). The early response (but not the later one) was prevented by the local application into the locus coeruleus of the CRF antagonist, α-helical CRF9-41, suggesting that this response was induced by release of CRF. Interestingly, there appears to be a substantial peripheral component to the delayed activation of the locus coeruleus-noradrenergic neurons, because the electrophysiologic activation was sensitive to the peripherally acting ganglionic blocker, chlorisondamine, and the largely peripherally acting β-adrenoceptor blocker, timolol (Borsody and Weiss, 1996). This may reflect changes in blood pressure, and may indicate a role for CRF in regulating blood pressure (it is to be noted that CRF possesses vasodilatory properties on peripheral blood vessels: Lei et al., 1993).
4.4 Microdialysis and Chronoamperometric Studies
As indicated above, several studies have shown increases of brain noradrenaline metabolism in many brain regions following i.c.v. injection of CRF. Microdialysis studies have shown increases in cortical and hippocampal noradrenaline following application of CRF either i.c.v. (Lavicky and Dunn, 1993; Emoto et al., 1993a; Lee et al., 1994) or directly into the locus coeruleus (Smagin et al., 1994; Schulz and Lehnert, 1996). Smagin et al. (1997) showed that α-helical CRF9–41 injected into the region of the locus coeruleus of rats antagonized the increase in microdialysate concentrations of noradrenaline collected from the medial prefrontal cortex induced by acute immobilization.
Studies employing in vivo chronoamperometry have confirmed that i.c.v. CRF activates locus coeruleus-noradrenaline neurons as indicated by increased noradrenaline release in the hippocampus and frontal cortex (Zhang et al., 1998). However, our chronoamperometric studies have indicated substantial delays in the release of noradrenaline in the hippocampus in response to CRF injected locally into the locus coeruleus (Palamarchouk et al., 2000; 2002). In rats anesthetized with urethane, we found that injection of CRF (90–100 ng) into the region of the locus coeruleus stimulated apparent noradrenaline release in the hippocampus. This noradrenergic response occurred most frequently when the injection sites were in or very close to the locus coeruleus or in the medial part of the adjacent parabrachial nucleus. However, there was a mean delay in the response of around 6–7 minutes and the peak noradrenaline concentration was not reached until 13 minutes, slower than would be expected if CRF acted directly on receptors on noradrenergic neurons in the locus coeruleus (Palamarchouk et al., 2000). Significantly, glutamate injected into the same sites (through the same cannulae) induced responses within 30 seconds which peaked after about 1 minute.
The latter study was performed in rats anesthetized with urethane, and thus it is possible that the delay was an artifact of the anesthesia. To rule out this possibility, a procedure was developed for recording noradrenaline chronoamperometrically from the hippocampus in freely moving animals. The results of these technically difficult experiments resembled quite closely what we had observed in urethane-anesthetized rats. The increase in hippocampal noradrenaline did not start until around 7 minutes after CRF infusion, and peaked around 16 minutes. By contrast, the response to glutamate occurred within 1 minute and peaked around 7 minutes (Palamarchouk et al., 2002).
Thus the chronoamperometric results suggest that the functional connections between CRF-containing neurons in the region of the locus coeruleus and locus coeruleus- noradrenergic neurons resulting in their activation are indirect. An indirect input would permit local modulation of CRF input to locus coeruleus-noradrenergic neurons. An example of such local modulation would be inhibition of locus coeruleus neurons via a GABAergic projection from the nucleus prepositus hypoglossi acting on GABAA receptors within the locus coeruleus (Valentino et al., 1992). Indeed, the benzodiazepine, chlordiazepoxide, attenuated the effects of CRF administered into the locus coeruleus on the release of noradrenaline in the medial prefrontal cortex and hypothalamus (Swiergiel et al., in press). From a physiological perspective, it may not be critical whether the effects of CRF on noradrenergic neurons are direct or indirect. However, whether the net effect of CRF on locus coeruleus-noradrenergic activity is excitatory or inhibitory is important.
5. The Feed-Forward Loop and Chronic Stress
5.1 Sensitization or Desensitization
A role for a feed-forward loop in depression or any other behavioural disorder, would require a sensitization somewhere in the circuit. Such a sensitization should be expressed as an augmentation in the behavioural response(s) to stress or any event precipitating the depression. But, most studies using chronic or repeated stress models in animals have shown desensitization. For example, immobilization repeated daily for 6 days decreased the basal concentrations of microdialysate noradrenaline in the hypothalamic paraventricular nucleus (Pacak et al., 1992), and in the central nucleus of the amygdala (Pacak et al., 1993). We have made similar observations using chronic footshock or restraint in rats, and chronic footshock in mice (Swiergiel et al., 2007a, 2007b, see below). However, the responses to acute immobilization were of similar magnitude in repeatedly immobilized and untreated rats in both the hypothalamic paraventricular nucleus (Pacak et al., 1992) and the central nucleus of the amygdala (Pacak et al., 1993). Consistent with this, Curtis et al. observed that daily footshock for five days desensitized the electrophysiologic activation of locus coeruleus-noradrenaline neurons by CRF (Curtis et al., 1995). Moreover, repeated forced swims decreased the electrophysiological responses of serotonergic cells in the dorsal raphe nucleus (Price and Lucki, 2001). However, other studies found that the effects of repeated stress on the responses to CRF to be dose-dependent, such that sensitization occurred to low doses, and desensitization only to very high doses (3 μg) (Curtis et al., 1995, 1999).
Basal locus coeruleus-noradrenergic activity may be elevated in depressed patients, consistent with the elevations of CSF MHPG and noradrenaline (Koslow et al., 1983; Maas et al., 1987; Gold and Chrousos, 1999). This would be consistent with the increased tyrosine hydroxylase activity reported to occur in the locus coeruleus of depressed suicide victims (Zhu et al., 1999). The only data suggesting that depression might be related to adaptations in locus coeruleus-noradrenergic neurons and its interactions with CRF are the results of chronic antidepressant treatments in rodents. For example, Grant and Weiss (2001) showed that several different antidepressant treatments (desipramine, imipramine, sertraline, phenelzine, and mianserin) reduced the basal electrophysiological activity of locus coeruleus-noradrenaline neurons, as well as the response to sensory stimulation. With regard to noradrenergic-CRF interactions, Curtis and Valentino (1994) reported that chronic imipramine treatment diminished the electrophysiological responses of locus coeruleus-noradrenergic neurons to CRF. However, the inferences of these results for the changes associated with depression and for the feed-forward model are indirect.
Whether behavioural responses are sensitized or desensitized may depend on the specific behavioural model studied. In the acoustic startle reflex, often used to study anxiety-like responses, behavioural sensitization can be observed. The acoustic startle reflex appears to be mediated by both CRF1 and CRF2 receptors (Risbrough et al., 2003). It has been reported that footshock treatment leads to a sensitized excitatory effect of CRF on the acoustic startle reflex on the following day. Also, olfactory bulbectomy enhanced sensitization of the acoustic startle reflex to acute or repeated footshock (McNish and Davis, 1997). This response could be mediated by a disinhibition of the bed nucleus of the stria terminalis or the central nucleus of the amygdala (Gewirtz et al., 1998). We can only conclude that persistent stress may lead to dysregulation of the subsequent stress response, but the mechanisms of such changes remain unknown.
We have recently studied the effects of chronic stress in both rats and mice, on the subsequent behavioural responses to CRF. In rats, acute footshock (20–25 1-s 0.5 mA shocks in 20 min) or restraint (4 h) decreased floating in the Porsolt forced swim test (see Table 1). However, chronic footshock (repeated daily for 15 days) consistently increased the time spent floating in the forced swim test, consistent with depression-like activity (Swiergiel et al., 2007a). However, chronic restraint (4 h per day for 14 days) decreased floating, although this effect fell short of statistical significance (Swiergiel et al., 2007a). These results are consistent with the existing literature, in particular the report of Platt and Stone (1982) who found that in rats chronic footshock increased, whereas chronic restraint decreased floating in the forced swim test. In our experiments, acute footshock also decreased floating in chronically footshocked rats. Of particular interest, i.c.v. CRF (100–300 ng) increased floating, mimicking the effect of footshock, but not that of restraint (Swiergiel et al., 2007a).
Table 1.
A summary of the behavioural results from rats and mice subjected to chronic and acute footshock or restraint and i.c.v. administration of CRF in the Porsolt forced swim test (rats and mice), and in the tail-suspension test (mice only). The arrows indicate the direction of the changes; parentheses indicate that the trends were not statistically significant. In most cases the results were obtained from multiple experiments. For full details see Swiergiel et al. (1997ab), and Dunn and Swiergiel (2008), from which this table is derived.
| Mouse Forced Swim Test | ||||
|---|---|---|---|---|
| Acute Treatment | None | Acute Footshock | Acute Restraint | Icv CRF |
| Chronic Treatment | ||||
| None | (↓) | ↓ | ↓ | |
| Chronic Footshock | ↑ | ↓ | ↓ | 0 |
| Chronic Restraint | nd | nd | ||
| Mouse Tail Suspension Test | ||||
| Acute Treatment | None | Acute Footshock | Acute Restraint | Icv CRF |
| Chronic Treatment | ||||
| None | ↓ | ↓ | ↓ | |
| Chronic Footshock | (↓) | (↓) | 0 | 0 |
| Chronic Restraint | ↓ | nd | ↓ | nd |
| Rat Forced Swim Test | ||||
| Acute Treatment | None | Acute Footshock | Acute Restraint | Icv CRF |
| Chronic Treatment | ||||
| None | ↓ | ↓ | ↑ | |
| Chronic Footshock | ↑ | ↓ | 0 | 0 |
| Chronic Restraint | (↓) | nd | ↓ | nd |
Interestingly, the results in mice were similar (see Table 1), but there was one important difference (Dunn and Swiergiel, 2008). As in rats, acute footshock and acute restraint decreased floating, although the former effect was not statistically significant. Also, chronic footshock increased floating, whereas chronic restraint decreased floating, although the latter effect did not reach statistical significance in mice (Dunn and Swiergiel, 2008). The most important difference was that, whereas in rats i.c.v. CRF (100 ng) increased floating consistent with a depression-like activity and mimicking the effects of acute footshock and restraint (Swiergiel et al., 2007a), in mice, i.c.v. CRF decreased floating (Swiergiel et al., 2007b). The differences in the two species in their responses to CRF may be attributable to their innate responses to water and restraint (see Dunn and Swiergiel, 2008).
In mice, we also performed the tail-suspension test (Swiergiel et al., 2007b). The results were in most cases the same as for the forced swim test. However, chronic footshock decreased (nonsignificantly) the time spent immobile, whereas in the forced swim test chronic footshock increased floating (Swiergiel et al., 2007b). In both rats and mice, acute footshock in chronically footshocked animals induced smaller responses in brain noradrenaline, and in plasma ACTH and corticosterone (Swiergiel et al., 2007b; Dunn and Swiergiel, 2008).
6. Summary
It can be concluded that both noradrenaline and CRF are involved in behavioural responses in stress. However, CRF appears to be responsible for a wider spectrum of responses than noradrenaline, although in many cases, both molecules are involved. We have reviewed the interactions between neurons containing noradrenaline and CRF in the brain. Even with our present limited knowledge, these interactions appear to be extensive and complex. We also assessed the “feed-forward” model of CRF-noradrenergic interactions that has been proposed to underlie stress-related disorders, particularly depression. Currently there is relatively little evidence for a sensitized CRF-noradrenergic loop as the basis for depression. In most studies the noradrenergic responses to repeated stressful treatments exhibit desensitization. Typically, responses to CRF administration in chronically stressed animals also indicate a desensitization. However, results with the acoustic startle response appear to indicate sensitization to CRF. Such a sensitization may form the basis of some stress and anxiety-related disorders. However, the current evidence does not support sensitization as the major mechanism; desensitization is more frequent.
Acknowledgments
The authors research cited in this article were supported by grants from the U.S. National Institute of Mental Health (MH54387).
Footnotes
Proofs to be sent to: Dr. Adrian J. Dunn, Pacific Biosciences Research Center, University of Hawaii, 1993 East-West Road, Honolulu, HI 96822, Phone: (808) 956-5632, Fax: (808) 956-6984, E-mail: ajdunn@hawaii.edu
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Damluji S. Adrenergic mechanisms in the control of corticotrophin secretion. J Endocrinol. 1988;119:5–14. doi: 10.1677/joe.0.1190005. [DOI] [PubMed] [Google Scholar]
- Al-Damluji S. Adrenergic control of the secretion of anterior pituitary hormones, Baillière’s Clin. Endocrinol Metab. 1993;7:355–392. doi: 10.1016/s0950-351x(05)80180-6. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Berridge C, Segal DS. Stress produces opioid-like effects on investigatory behavior. Pharmacol Biochem Behav. 1985;22:803–809. doi: 10.1016/0091-3057(85)90531-3. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, Van Bockstaele Pieribone EV, Shiekhatter R, Akaoka H, Drolet G, Astier B, Charlety P, Valentino RJ, Williams RT. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Progr Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Dunn AJ. Corticotropin-releasing factor elicits naloxone-sensitive stress-like alterations in exploratory behavior in mice. Regulat Peptides. 1986;16:83–93. doi: 10.1016/0167-0115(86)90196-5. [DOI] [PubMed] [Google Scholar]
- Borsody MK, Weiss JM. Influence of corticotropin-releasing hormone on electrophysiological activity of locus coeruleus neurons. Brain Res. 1996;724:149–168. doi: 10.1016/0006-8993(96)00199-0. [DOI] [PubMed] [Google Scholar]
- Bouret S, Duvel A, Onat S, Sara SJ. Phasic activation of locus ceruleus neurons by the central nucleus of the amygdala. J Neurosci. 2003;23:3491–3497. doi: 10.1523/JNEUROSCI.23-08-03491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Valentino RJ. Corticotropin-releasing factor neurotransmission in locus coeruleus: a possible site of antidepressant action. Brain Res Bull. 1994;35:581–587. doi: 10.1016/0361-9230(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exptl Therap. 1997;281:163–172. [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Grigoriadis DE, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Corticotropin-releasing factor administration elicits a stress-like activation of cerebral catecholaminergic systems. Pharmacol Biochem Behav. 1987;27:685–691. doi: 10.1016/0091-3057(87)90195-x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann NY Acad Sci. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of acute and chronic stressors and CRF in rat and mouse tests for depression. Ann NY Acad Sci. 2008 doi: 10.1196/annals.1410.022. in press. [DOI] [PubMed] [Google Scholar]
- Emoto H, Yokoo H, Yoshida M, Tanaka M. Corticotropin-releasing factor enhances noradrenaline release in the rat hypothalamus assessed by intracerebral microdialysis. Brain Res. 1993a;601:286–288. doi: 10.1016/0006-8993(93)91722-5. [DOI] [PubMed] [Google Scholar]
- Emoto H, Tanaka M, Koga C, Yokoo H, Tsuda A, Yoshida M. Corticotropin-releasing factor activates the noradrenergic neuron system in the rat brain, Pharmacol. Biochem Behav. 1993b;45:419–422. doi: 10.1016/0091-3057(93)90259-v. [DOI] [PubMed] [Google Scholar]
- France RD, Urban B, Krishnan KR, Bissette G, Banki CM, Nemeroff CB, Speilman FJ. CSF corticotropin-rleasing factor-like immunoreactivity in chronic pain patients with and without major depression. Biol Psychiat. 1988;23:86–88. doi: 10.1016/0006-3223(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, McNish KA, Davis M. Lesions of the bed nucleus of the stria terminalis block sensitization of the acoustic startle reflex produced by repeated stress, but not fear-potentiated startle. Prog Neuropsychopharmacol Biol Psychiat. 1998;22:625–648. doi: 10.1016/s0278-5846(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assn Amer Physicians. 1999;111:22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- Gorman AL, Dunn AJ. β-Adrenergic receptors are involved in stress-related behavioral changes, Pharmacol. Biochem Behav. 1993;45:1–7. doi: 10.1016/0091-3057(93)90078-8. [DOI] [PubMed] [Google Scholar]
- Grant MM, Weiss JM. Effects of chronic antidepressant drug administration and electroconvulsive shock on locus coeruleus electrophysiologic activity. Biol Psychiat. 2001;49:117–129. doi: 10.1016/s0006-3223(00)00936-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exptl Therap. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24:9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegler-Muly SM, Owens MJ, Ervin GN, Kilts CD, Nemeroff CB. Potential corticotropin-releasing factor pathways in the rat brain as determined by bilateral electrolytic lesions of the central amygdaloid nucleus and the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 1993;5:95–98. doi: 10.1111/j.1365-2826.1993.tb00367.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiat. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koslow SH, Maas JW, Bowden CL, Davis JM, Hanin I, Javaid J. CSF and urinary biogenic amines and metabolites in depression and mania, Archs. Gen Psychiat. 1983;40:999–1010. doi: 10.1001/archpsyc.1983.01790080081011. [DOI] [PubMed] [Google Scholar]
- Lavicky J, Dunn AJ. Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. J Neurochem. 1993;60:602–612. doi: 10.1111/j.1471-4159.1993.tb03191.x. [DOI] [PubMed] [Google Scholar]
- Lee EHY, Chang SY, Chen AYJ. CRF facilitates NE release from the hippocampus: a microdialysis study. Neurosci Res. 1994;19:327–330. doi: 10.1016/0168-0102(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Lei S, Richter R, Bienert M, Mulvany MJ. Relaxing actions of corticotropin-releasing factor on rat resistance arteries. Brit J Pharmacol. 1993;108:941–947. doi: 10.1111/j.1476-5381.1993.tb13490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas JW, Koslow SH, Davis J, Katz M, Frazer A, Bowden CL, Berman N, Gibbons R, Stokes P, Landis DH. Catecholamine metabolism and disposition in healthy and depressed subjects. Archs Gen Psychiat. 1987;44:337–344. doi: 10.1001/archpsyc.1987.01800160041007. [DOI] [PubMed] [Google Scholar]
- McNish KA, Davis M. Olfactory bulbectomy enhances sensitization of the acoustic startle reflex produced by acute or repeated stress. Behav Neurosci. 1997;111:80–91. doi: 10.1037//0735-7044.111.1.80. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen P, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–474. [PubMed] [Google Scholar]
- Pacak K, Armando I, Fukuhara K, Kvetnansky R, Palkovits M, Kopin IJ, Goldstein DS. Noradrenergic activation in the paraventricular nucleus during acute and chronic immobilization stress in rats: an in vivo microdialysis study. Brain Res. 1992;589:91–96. doi: 10.1016/0006-8993(92)91165-b. [DOI] [PubMed] [Google Scholar]
- Pacak K, Kvetnansky R, Palkovits M, Fukuhara K, Yadid G, Kopin IJ, Goldstein DS. Adrenalectomy augments in vivo release of norepinephrine in the paraventricular nucleus during immobilization stress. Endocrinol. 1993;133:1404–1410. doi: 10.1210/endo.133.3.8396018. [DOI] [PubMed] [Google Scholar]
- Page ME, Abercrombie ED. Discrete local application of corticotropin-releasing factor increases locus coeruleus discharge and extracellular norepinephrine in rat hippocampus. Synapse. 1999;33:304–313. doi: 10.1002/(SICI)1098-2396(19990915)33:4<304::AID-SYN7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Palamarchouk VS, Swiergiel AH, Dunn AJ. Hippocampal noradrenergic responses to CRF injected into the locus coeruleus of unanesthetized rats. Brain Res. 2002;950:31–38. doi: 10.1016/s0006-8993(02)02983-9. [DOI] [PubMed] [Google Scholar]
- Palamarchouk VS, Zhang JJ, Zhou G, Swiergiel AH, Dunn AJ. Hippocampal norepinephrine-like voltammetric responses to infusion of corticotropin-releasing factor into the locus coeruleus. Brain Res Bull. 2000;51:319–326. doi: 10.1016/s0361-9230(99)00241-5. [DOI] [PubMed] [Google Scholar]
- Platt JE, Stone EA. Chronic restraint stress elicits a positive antidepressant response on the forced swim test. Europ J Pharmacol. 1982;82:179–181. doi: 10.1016/0014-2999(82)90508-8. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Cunningham ET, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocrine Rev. 1989;10:437–458. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacol. 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Roy A, Pickar D, Paul S, Doran A, Chrousos GP, Gold PW. CSF corticotropin-releasing hormone in depressed patients and normal control subjects. Amer J Psychiat. 1987;144:641–645. doi: 10.1176/ajp.144.5.641. [DOI] [PubMed] [Google Scholar]
- Saphier D. Electrophysiology and neuropharmacology of noradrenergic projections to rat PVN magnocellular neurons. Amer J Physiol. 1993;264:R891–902. doi: 10.1152/ajpregu.1993.264.5.R891. [DOI] [PubMed] [Google Scholar]
- Schulz C, Lehnert H. Activation of noradrenergic neurons in the locus coeruleus by corticotropin-releasing factor. A microdialysis study. Neuroendocrinol. 1996;63:454–458. doi: 10.1159/000127071. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Bray GA. Modulation by corticotropin-releasing factor of monoamine metabolism in the lateral hypothalamus. Neurosci Letts. 1989;103:74–80. doi: 10.1016/0304-3940(89)90488-6. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Swiergiel AH, Dunn AJ. Corticotropin-releasing factor administered into the locus coeruleus, but not the parabrachial nucleus, stimulates norepinephrine release in the prefrontal cortex. Brain Res Bull. 1994;36:713–724. doi: 10.1016/0361-9230(94)00166-x. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Heinrichs SC, Dunn AJ. The role of CRH in behavioral responses to stress. Peptides. 2001;22:713–724. doi: 10.1016/s0196-9781(01)00384-9. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Zhou YP, Dunn AJ. Effects of chronic footshock, restraint and corticotropin-releasing factor on freezing, ultrasonic vocalization and forced swim behavior in rats. Behav Brain Res. 2007a;183:178–187. doi: 10.1016/j.bbr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Leskov IL, Dunn AJ. Effects of chronic and acute stressors and CRF on depression-like behavior in mice. Behav Brain Res. 2007b doi: 10.1016/j.bbr.2007.07.018. in press. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Li Y, Wei Z, Dunn AJ. Effects of chlordiazepoxide on footshock- and corticotropin-releasing factor-induced increases in cortical and hypothalamic norepinephrine secretion in rats. Neurochemistry International. doi: 10.1016/j.neuint.2008.01.002. in press. [DOI] [PubMed] [Google Scholar]
- Tichomirowa MA, Keck ME, Schneider HJ, Paez-Pereda M, Renner U, Holsboer F, Stalla GK. Endocrine disturbances in depression. J Endocrinol Invest. 2005;28:89–99. doi: 10.1007/BF03345535. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page M, Van Bockstaele E, Aston-Jones G. Corticotropin-releasing factor innervation of the locus coeruleus region: distribution of fibers and sources of input. Neurosci. 1992;48:689–705. doi: 10.1016/0306-4522(92)90412-u. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EEO, Valentino RJ. Corticotropin-releasing-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EEO, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Bajic D, Proudfit H, Valentino RJ. Topographic architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiol Behav. 2001;73:273–283. doi: 10.1016/s0031-9384(01)00448-6. [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TDJ, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci USA. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-M, Dunn AJ. Central β1-adrenergic receptors are involved in CRF-induced defensive-withdrawal. Pharmacol Biochem Behav. 1990;36:847–851. doi: 10.1016/0091-3057(90)90088-y. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ. The involvement of central noradrenergic systems and corticotropin-releasing factor in defensive-withdrawal in rats. J Pharmacol Exptl Therapeut. 1990;255:1064–1070. [PubMed] [Google Scholar]
- Zhang JJ, Swiergiel AH, Palamarchouk VS, Dunn AJ. Intracerebroventricular infusion of CRF increases extracellular concentrations of norepinephrine in the hippocampus and cortex as determined by in vivo voltammetry. Brain Res Bull. 1998;47:277–284. doi: 10.1016/s0361-9230(98)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Klimek V, Dilley GE, Haycock JW, Stockmeier C, Overholser JC, Meltzer HY, Ordway GA. Elevated levels of tyrosine hydroxylase in the locus coeruleus in. 1999 doi: 10.1016/s0006-3223(99)00135-3. [DOI] [PubMed] [Google Scholar]


