Abstract
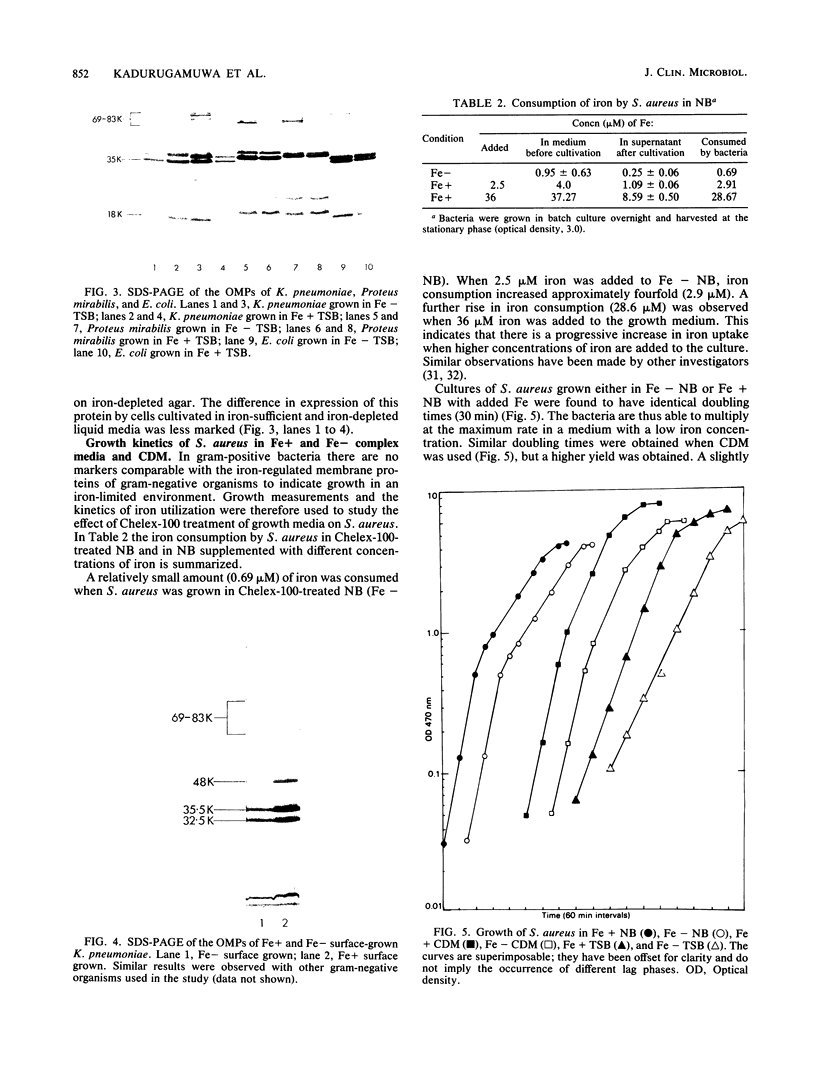
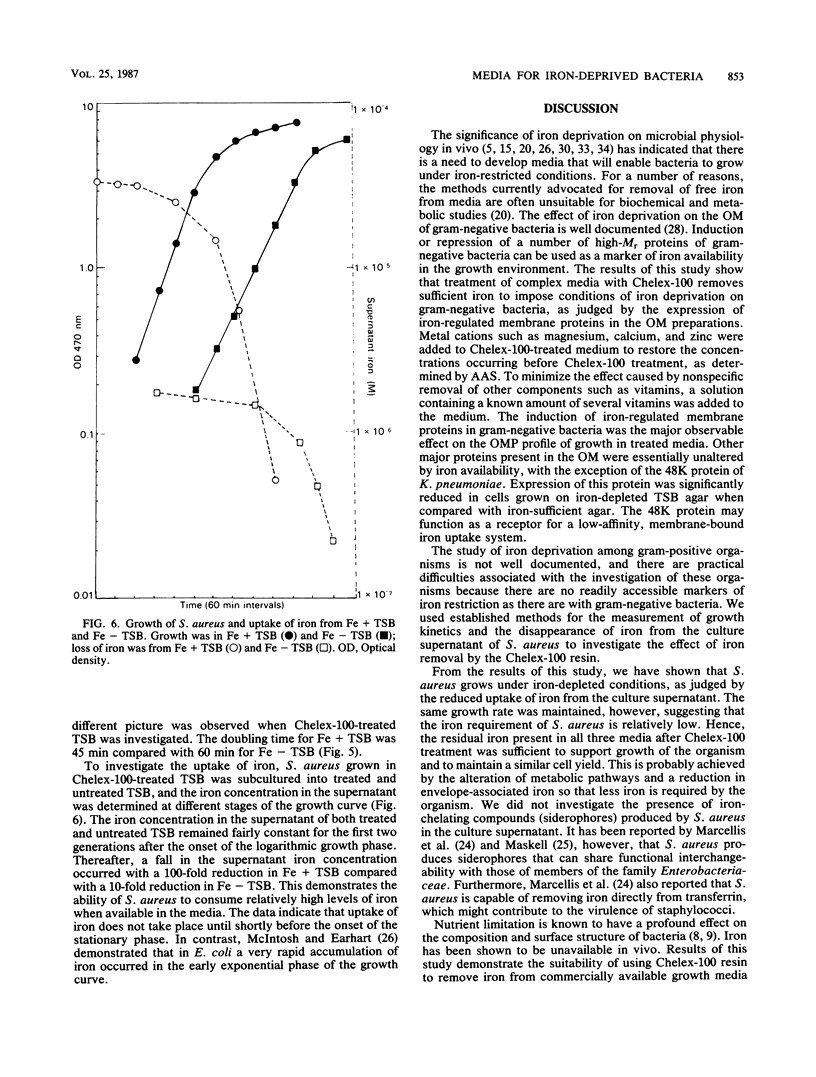
Ion-exchange chromatography was used to remove iron from complex and chemically defined laboratory media. The kinetics of metal cation removal from the media was investigated by using atomic absorption spectrophotometry, and the results indicated that over 90% of the iron could be eliminated from certain complex media by this treatment. The treated medium was used for growth studies in a gram-positive and a number of gram-negative organisms that were isolated from infections in humans. High-molecular-weight outer membrane proteins that are known to be induced under iron-depleted growth conditions (iron-regulated membrane proteins) were observed when a number of gram-negative pathogens were cultivated in the treated media. Iron uptake by Staphylococcus aureus varied, depending on the iron content of the medium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar H., Brown M. R., Lambert P. A. Effect of nutrient depletion on sensitivity of Pseudomonas cepacia to phagocytosis and serum bactericidal activity at different temperatures. J Gen Microbiol. 1983 Jul;129(7):2021–2027. doi: 10.1099/00221287-129-7-2021. [DOI] [PubMed] [Google Scholar]
- Anwar H., Lambert P. A., Brown M. R. Influence of sodium dodecyl sulphate quality on the electrophoretic mobility of the outer membrane proteins of mucoid and non-mucoid Pseudomonas aeruginosa. Biochim Biophys Acta. 1983 Dec 13;761(2):119–125. doi: 10.1016/0304-4165(83)90220-9. [DOI] [PubMed] [Google Scholar]
- Boggis W., Kenward M. A., Brown M. R. Effects of divalent metal cations in the growth medium upon sensitivity of batch-grown Pseudomonas aeruginosa to EDTA or polymyxin B. J Appl Bacteriol. 1979 Dec;47(3):477–488. doi: 10.1111/j.1365-2672.1979.tb01209.x. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Melling J. Loss of sensitivity to EDTA by Pseudomonas aeruginosa grown under conditions of Mg-limitation. J Gen Microbiol. 1968 Dec;54(3):439–444. doi: 10.1099/00221287-54-3-439. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Melling J. Role of divalent cations in the action of polymyxin B and EDTA on Pseudomonas aeruginosa. J Gen Microbiol. 1969 Dec;59(2):263–274. doi: 10.1099/00221287-59-2-263. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Williams P. Influence of substrate limitation and growth phase on sensitivity to antimicrobial agents. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):7–14. doi: 10.1093/jac/15.suppl_a.7. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Williams P. The influence of environment on envelope properties affecting survival of bacteria in infections. Annu Rev Microbiol. 1985;39:527–556. doi: 10.1146/annurev.mi.39.100185.002523. [DOI] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- DONALD C., PASSEY B. I., SWABY R. J. A comparison of methods for removing trace metals from microbiological media. J Gen Microbiol. 1952 Nov;7(3-4):211–220. doi: 10.1099/00221287-7-3-4-211. [DOI] [PubMed] [Google Scholar]
- Finch J. E., Brown M. R. Effect of growth environment on Pseudomonas aeruginosa killing by rabbit polymorphonuclear leudocytes and cationic proteins. Infect Immun. 1978 May;20(2):340–346. doi: 10.1128/iai.20.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. E., Brown M. R. The influence of nutrient limitation in a chemostat on the sensitivity of Pseudomonas aeruginosa to polymyxin and to EDTA. J Antimicrob Chemother. 1975 Dec;1(4):379–386. doi: 10.1093/jac/1.4.379. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Raffle V. J., Nicas T. I. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981 May;19(5):777–785. doi: 10.1128/aac.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. I., Coleman J. E. The biosynthesis of apo- and metalloalkaline phosphatases of Escherichia coli. J Biol Chem. 1968 Oct 10;243(19):5063–5073. [PubMed] [Google Scholar]
- Kenward M. A., Brown M. R., Fryer J. J. The influence of calcium or manganese on the resistance to EDTA, polymyxin B or cold shock, and the composition of Pseudomonas aeruginosa grown in glucose- or magnesium-depleted batch cultures. J Appl Bacteriol. 1979 Dec;47(3):489–503. doi: 10.1111/j.1365-2672.1979.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Klebba P. E., McIntosh M. A., Neilands J. B. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol. 1982 Mar;149(3):880–888. doi: 10.1128/jb.149.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Marcelis J. H., den Daas-Slagt H. J., Hoogkamp-Korstanje J. A. Iron requirement and chelator production of staphylococci, Streptococcus faecalis and enterobacteriaceae. Antonie Van Leeuwenhoek. 1978;44(3-4):257–267. doi: 10.1007/BF00394304. [DOI] [PubMed] [Google Scholar]
- Maskell J. P. The functional interchangeability of enterobacterial and staphylococcal iron chelators. Antonie Van Leeuwenhoek. 1980;46(4):343–351. doi: 10.1007/BF00421981. [DOI] [PubMed] [Google Scholar]
- McIntosh M. A., Earhart C. F. Coordinate regulation by iron of the synthesis of phenolate compounds and three outer membrane proteins in Escherichia coli. J Bacteriol. 1977 Jul;131(1):331–339. doi: 10.1128/jb.131.1.331-339.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Fung D. Y. Amino acid requirements for the production of enterotoxin B by Staphylococcus aureus S-6 in a chemically defined medium. Appl Microbiol. 1973 May;25(5):800–806. doi: 10.1128/am.25.5.800-806.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980 Aug;143(2):872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M. Synthesis and utilization of siderophores by Shigella flexneri. J Bacteriol. 1980 Sep;143(3):1420–1424. doi: 10.1128/jb.143.3.1420-1424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M., Harada R. N. Effect of staphylococcal iron content on the killing of Staphylococcus aureus by polymorphonuclear leukocytes. Infect Immun. 1981 Apr;32(1):407–410. doi: 10.1128/iai.32.1.407-410.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Sciortino C. V., Finkelstein R. A. Vibrio cholerae expresses iron-regulated outer membrane proteins in vivo. Infect Immun. 1983 Dec;42(3):990–996. doi: 10.1128/iai.42.3.990-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand G. H., Anwar H., Kadurugamuwa J., Brown M. R., Silverman S. H., Melling J. In vivo evidence that bacteria in urinary tract infection grow under iron-restricted conditions. Infect Immun. 1985 Apr;48(1):35–39. doi: 10.1128/iai.48.1.35-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti P., De Stasio A., Seganti L., Mastromarino P., Sinibaldi L., Orsi N. Capacity of staphylococci to grow in the presence of ovotransferrin or CrCl3 as a character of potential pathogenicity. J Clin Microbiol. 1980 May;11(5):445–447. doi: 10.1128/jcm.11.5.445-447.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. H., Bergdoll M. S. Stimulation of Enterotoxin B Production II. Synthetic Medium for Staphylococcal Growth and Enterotoxin B Production. Infect Immun. 1971 Jun;3(6):784–792. doi: 10.1128/iai.3.6.784-792.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asbeck B. S., Marcelis J. H., Marx J. J., Struyvenberg A., van Kats J. H., Verhoef J. Inhibition of bacterial multiplication by the iron chelator deferoxamine: potentiating effect of ascorbic acid. Eur J Clin Microbiol. 1983 Oct;2(5):426–431. doi: 10.1007/BF02013899. [DOI] [PubMed] [Google Scholar]