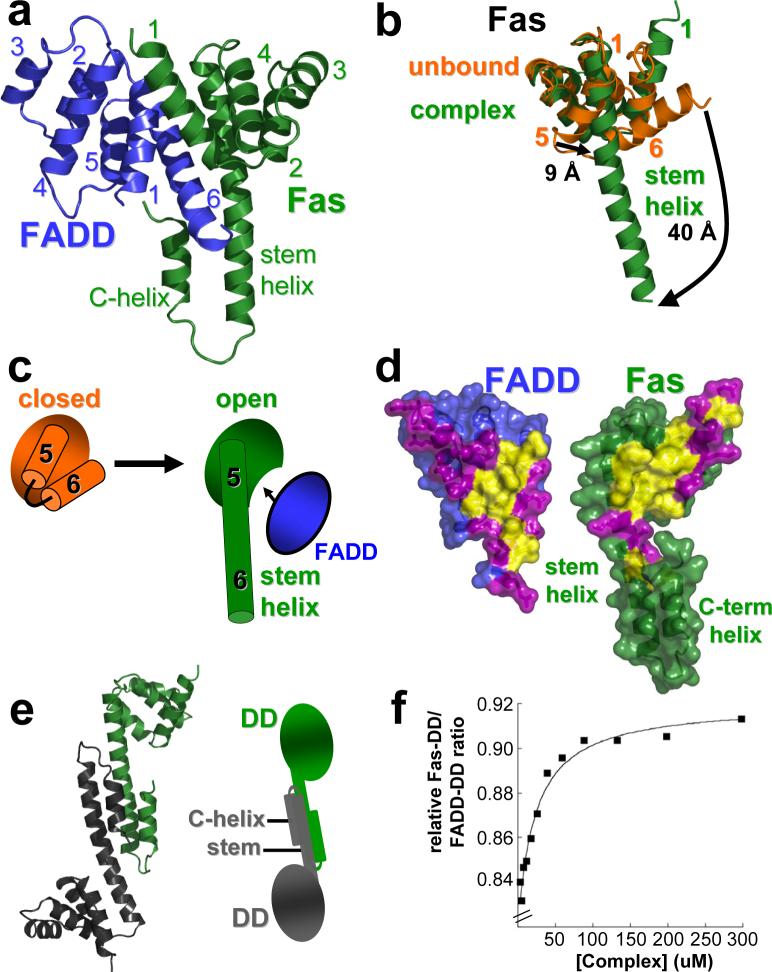
Figure 2. Fas/FADD DD complex: Fas/FADD and Fas/Fas interactions are dependent on Fas opening.
a, Primary Fas/FADD DD complex. FADD (blue) adopts the characteristic death domain fold with helices one and six participating in the main interaction site. In Fas (green) only helices one to four approximately adopt a death domain-like fold, while a long helix (stem helix) is found in place of helices five and six, which together with helix one provides the main interaction residues for FADD binding. Additionally a helix at the C-terminus of Fas (C-helix) is observed. b, Conformational change in Fas. Comparison of the structure of unbound Fas DD (closed form in orange; pdb entry: 1DDF) and Fas in the Fas/FADD complex. Due to formation of the stem helix residues of helix five and six shift significantly. Additionally, the rearrangement of helix six exposes part of the hydrophobic core of Fas. c, Cartoon illustration of Fas opening. d, Primary Fas/FADD interface. View onto interfaces governing primary complex formation. Surface representation shows complementary hydrophobic patches (yellow) on FADD and Fas surrounded by polar residues (magenta). The hydrophobic interface on Fas becomes exposed upon Fas opening. e, Fas-Dimer unit. Another consequence of Fas opening is the formation of Fas-dimer units, which interact via the stem and C-helix. (right) Cartoon representation of the dimer. f, The Fas/FADD DD complex is weak. Dilution experiment of the isolated Fas/FADD complex shows cooperative dissociation of the complex below concentrations as high as 50 μM (plot derived from quantitative SDS-PAGE analysis of Fas-DD retained on Ni-NTA resin from various Fas-DD/FADD-DD-His6 complex dilutions).