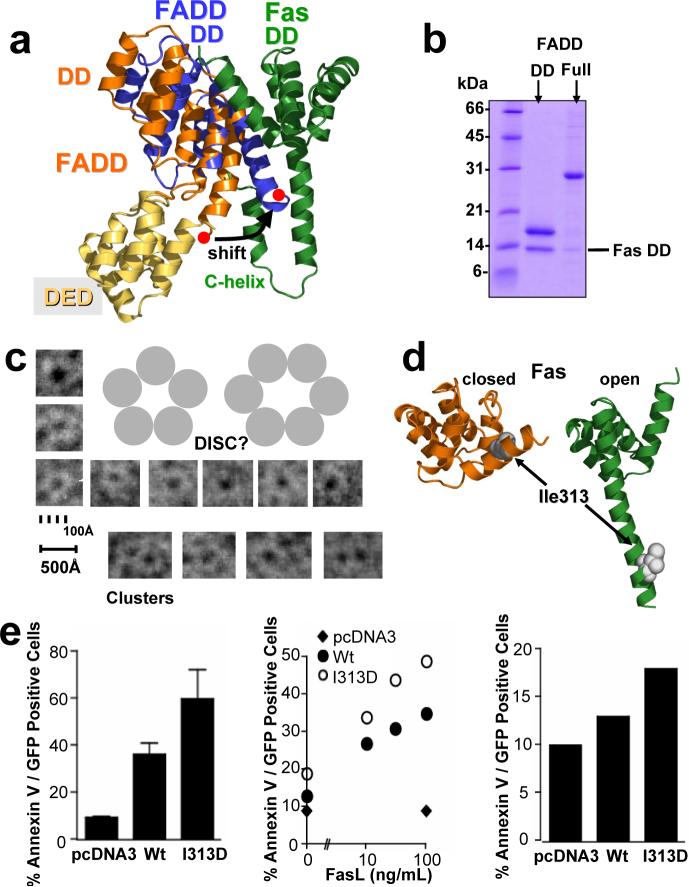
Figure 3. The Fas/FADD bridge in the DISC: binding of full length FADD and the key role of Fas opening in vivo.
a, Overlay of the structure of full length FADD* (pdb entry: 2gf5; orange) onto the Fas/FADD complex structure. The last helix of the DD of unbound FADD (red dot) shifts to avoid clashing with the newly formed Fas C-helix in the Fas/FADD complex. b, Conformational change in full length FADD. Proteins were expressed separately, and His-tagged versions of the DD of FADD, or full length FADD, were mixed with untagged Fas. Ni-NTA chromatography demonstrates that full length FADD shows reduced initial binding to the Fas DD, when compared to the FADD DD protein. c, While initial binding of full length FADD to Fas DD is reduced, prolonged incubation leads to the formation of DISC-like structures. Incubation of both proteins overnight led to the formation of ring-like structures with a strong tendency to form clusters as determined using electron microscopy. Displayed are single ring-like structures and clusters from several images. Due to their strong tendency to self-adhere no consistent monolayer for thorough evaluation could be generated to date. d/e, Propagating Fas opening results in hyperactive Fas. (d) Location of Ile313 in closed (unbound, orange) and open (complex, green) form of Fas. (e) Huh7 cells transfected with Fas I313D show elevated cell death, assessed by Annexin V reactivity, compared to Fas wt upon stimulation with Fas Antibody (left, standard deviations, n=3), FasL (middle), and also in the absence of a stimulus (right). Equal cell surface Fas expression was confirmed by FACS and immunoblot (data not shown). *Full length FADD refers to the well characterized FADD F25Y mutant (see Supplementary Methods)