Abstract
Long-term depression (LTD) at striatal synapses is mediated by postsynaptic endocannabinoid (eCB) release and presynaptic cannabinoid 1 receptor (CB1R) activation. Previous studies have indicated that eCB mobilization at excitatory synapses might be regulated by afferent activation. To further address the role of neuronal activity in synaptic plasticity we examined changes in synaptic strength induced by the L-type calcium channel activator 2,5-dimethyl-4-[2-(phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylic acid methyl ester (FPL 64176, FPL) at glutamatergic and γ-aminobutyric acid (GABA)ergic synapses in the striatum. We found that the basic mechanisms for FPL-mediated eCB signaling are the same at glutamatergic and GABAergic synapses. FPL-induced LTD (FPL-LTD) was blocked in slices treated with the CB1R antagonist AM251 (2 µm), but established depression was not reversed by AM251. FPL-LTD was temperature dependent, blocked by protein translation inhibitors and prevented by intracellular loading of the anandamide transporter inhibitor VDM11 (10 µm) at both glutamatergic and GABAergic synapses. FPL-LTD at glutamatergic synapses required paired-pulse afferent stimulation, while FPL-LTD at GABAergic synapses could be induced even in the absence of explicit afferent activation. By evaluating tetrodotoxin-insensitive spontaneous inhibitory postsynaptic currents we found that neuronal firing is vital for eCB release and LTD induction at GABAergic synapses, but not for short-term depression induced by CB1R agonist. The data presented here suggest that the level of neuronal firing regulates eCB signaling by modulating release from the postsynaptic cell, as well as interacting with presynaptic mechanisms to induce LTD at both glutamatergic and GABAergic synapses in the striatum.
Keywords: anandamide, basal ganglia, LTD, L-type calcium channel, synaptic plasticity
Introduction
The basal ganglia are a collection of interconnected nuclei that are implicated in the planning and execution of controlled movement (Graybiel et al., 1994; Balleine et al., 2007). The striatum, which consists of both the caudate and putamen subnuclei, is the biggest nucleus of the basal ganglia and receives intrinsic glutamatergic inputs from nearly all regions of the cortex. Glutamatergic thalamic projections to the striatum arise from the lateral parafascicular nucleus and the central lateral nucleus. The majority of neurons in the striatum (> 90%) are γ-aminobutyric acid (GABA)ergic medium spiny neurons (MSNs; Tepper et al., 2004), while the majority of synapses (about 80%) are asymmetric glutamatergic synapses originating from the cortex and thalamus (Wilson, 2007; Tepper et al., 2007).
Synaptic transmission at both glutamatergic and GABAergic synapses can be depressed through activation of cannabinoid 1 receptors (CB1R; Szabo et al., 1998; Gerdeman & Lovinger, 2001; Adermark & Lovinger, 2007b). The CB1R are activated via retrograde transsynaptic endocannabinoid (eCB) signaling that appears to involve the putative anandamide transporter (AMT; Gerdeman et al., 2002; Ligresti et al., 2004; Ronesi et al., 2004; Moore et al., 2005; Lovinger, 2007; Adermark & Lovinger, 2007b). The basal ganglia are among the brain regions that express the highest density of CB1R both at the mRNA (Mailleux & Vanderhaeghen, 1992; Hohmann & Herkenham, 2000) and protein levels (Tsou et al., 1998; Egertova & Elphick, 2000; Fusco et al., 2004), suggesting that eCBs are a major neurochemical system involved in basal ganglia function. A recent study by Hilário et al. (2007) showed that mice carrying either a heterozygous or homozygous null mutation of the CB1R show reduced habit formation, further supporting a role for eCB-dependent synaptic plasticity in striatal function and learning and memory.
The firing patterns of MSNs are strongly affected by modulation of voltage-dependent calcium currents (Hernández-López et al., 1997; Carter & Sabatini, 2004). L-type calcium channels are voltage-gated, regulated by multiple G-protein-coupled receptors and facilitated by spike waveforms, suggesting that this process could enhance Ca2+ entry during naturally occurring high-frequency activity (Olson et al., 2005). Postsynaptic activation of L-type calcium channels also appears to be vital for formation of long-term depression (LTD) at glutamatergic synapses in the striatum (Calabresi et al., 1994; Kreitzer & Malenka, 2005; Wang et al. 2006), and recruitment of L-type calcium channels to synaptic signaling complexes by Shank proteins has been suggested to be a critical factor in determining how afferent synaptic activity is translated into long-term alterations in neuronal function (Calabresi et al., 1994; Olson et al., 2005). In line with these findings, we showed that strong activation of L-type calcium channels with 2,5-dimethyl-4-[2-(phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylic acid methyl ester (FPL 64176, FPL), paired with a slight depolarization (−50 mV) of clamped MSNs, is sufficient to induce LTD at glutamatergic synapses in the striatum (FPL-eLTD). These findings suggest that activation of postsynaptic L-type calcium channels and the subsequent postsynaptic [Ca2+]i increase is a crucial molecular switch for LTD induction in the striatum (Adermark & Lovinger, 2007a). However, FPL-eLTD requires afferent activation, and cannot be induced in the absence of afferent stimulation (Adermark & Lovinger, 2007a), indicating that FPL-eLTD also involves some presynaptic process as previously described for LTD formation at glutamatergic synapses in the striatum (Singla et al., 2007).
The goal of this study was to further characterize the basic features of FPL-eLTD, and to determine the level of afferent activation required in order for eCB signaling and FPL-eLTD to occur. We also wanted to compare the role of synaptic activity in FPL-mediated eCB signaling and LTD induction at glutamatergic and GABAergic synapses in the striatum. We found that the basic properties of FPL-LTD were similar at glutamatergic and GABAergic synapses. However, the level of neuronal activity required for both eCB release and LTD induction is greater at glutamatergic than at GABAergic striatal synapses.
Materials and methods
Experiments were carried out in accordance with the guidelines laid down by the NIH regarding the care and use of animals for experimental procedures (LIN-DL-11). Coronal brain slices containing the striatum were prepared from male and female p16–20 Sprague–Dawley rats as previously described (Charles River, Wilmington, MA, USA; Adermark & Lovinger, 2007a). Briefly, animals were deeply anesthetized with halothane (Sigma, St. louis, MO), and decapitated. Brains were rapidly removed and placed in ice-cold modified artificial cerebrospinal fluid (aCSF) containing (in mM); 194 sucrose, 30 Nacl, 4.5 KCL, 1 MgCL2, 26 NaHCO3, 1.2 NaH2PO4 and 10 dextrose, continuously bubbled with a mixture of 95% O2 / 5% CO2 gas. Coronal brain slices (350 µm thick) containing striatum and cortex were sectioned with a VIBRATOME® series 1000 sectioning system (Technical products International Inc, O’Fallon, MO). Slices were allowed to equilibrate for at least 1 h in artificial cerebrospinal fluid (aCSF) containing (in mm): NaCl, 124; KCl, 4.5; CaCl2, 2; MgCl2, 1; NaHCO3, 26; NaH2PO4, 1.2; D-glucose, 10; continuously bubbled with a mixture of 95% O2 / 5% CO2 gas. One hemisphere of a slice was transferred to a recording chamber and the slice electrophysiological system was set up as previously described (Adermark & Lovinger, 2007a). Internal solutions consisted of (in mm): CsMeSO3, 120; NaCl, 5; TEA-Cl, 10; HEPES, 10; QX-314, 5; EGTA, 1.1; Mg-ATP, 4; Na-GTP, 0.3 for experiments examining excitatory postsynaptic currents (EPSCs); and CsCl, 150; HEPES, 10; MgCl2, 2; Na-GTP, 0.3; Mg-ATP, 3; BAPTA, 0.2 for experiments examining inhibitory postsynaptic currents (IPSCs). pH was set at 7.2 with CsOH, and osmolarity was set to 300 mmol / kg with sucrose. For EPSC recordings 50 µm picrotoxin was added to the aCSF. Slices were perfused with 50 µm 5 d,l-2-amino-5 phosphonovaleric acid (AP-5) in some experiments, as AP-5 does not influence LTD induction or miniature (m)EPSC measures (Adermark & Lovinger, 2007a). For IPSC measurements, 5 µm 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX) and 50 µm AP-5 were added to the aCSF.
Currents were measured in conventional ruptured-patch whole-cell mode in MSNs voltage-clamped at −50 mV. The holding potential of −50 mV was chosen so that activation of L-type calcium channels with FPL would induce a robust LTD (Adermark & Lovinger, 2007a). In a subset of experiments we loaded MSNs with the eCB arachidonoyl ethanolamide (AEA) (50 µm), which previously has been shown to induce depression of EPSC and IPSC amplitudes (Gerdeman et al., 2002; Ronesi et al., 2004; Adermark & Lovinger, 2007b). Unless otherwise stated, baseline synaptic currents were evoked by single or paired (50-ms or 1-s interpulse interval) stimuli delivered every 20 s through a bipolar electrode placed at the border of the dorsolateral striatum and the overlying white matter. Stimulus parameters were adjusted to elicit baseline EPSC or IPSC amplitudes between 200 and 400 pA. The amplitude of baseline EPSCs or IPSCs (t = 0–5 min) was compared with EPSC or IPSC amplitude at t = 20–25 min and presented as mean value ± 95% confidence interval unless otherwise stated. Clampex 9.2 was used for data acquisition (Molecular Devices, Foster City, CA, USA), and graphs were assembled in GraphPad Prism (GraphPad Software, San Diego, CA, USA).
In a subset of recordings, spontaneous (s)IPSCs / sEPSCs were measured in the absence or presence of tetrodotoxin (TTX; 1 µm) or lidocaine (500 µm; mIPSCs / mEPSCs). Currents were recorded over a 3-min baseline period (5 min after establishing the whole cell configuration), and following 10 min treatment of FPL (500 nm) or WIN 55,212-2 (1 µm), or after postsynaptic loading with the eCB anandamide (50 µm), which previously has been shown to depress the event frequency of recorded sIPSCs (Adermark & Lovinger, 2007b). We also evaluated the sensitivity of FPL-LTD to altered levels of [K+]o by changing KCl to 1 or 10 mm in the aCSF. Data were analysed using the Mini Analysis program version 6.0.3 (Synaptosoft, Decatur, GA, USA). Amplitude and area thresholds were set manually for every data set, and the accuracy of the detected sIPSCs / mIPSCs / sEPSCs was manually verified. Event frequency, amplitude, rise time and decay time for each given experiment were compared with baseline values using the paired t-test (GraphPad Prism). Time course figures for evoked and spontaneous activity are plotted as mean amplitude compared with baseline, with standard error of the mean (SEM), and paired or unpaired t-tests were used for statistical analysis where appropriate.
Most chemicals were purchased from Sigma (St Louis, MO, USA) or Tocris (Ellisville, MO, USA). AEA was dissolved in ethanol to 25 mm, or dimethylsulfoxide (DMSO) to 50 mm, and used at 50 µm in the intracellular solution. NBQX was dissolved in H2O to 50 mm and used at 5 µm, and AP-5 was dissolved in aCSF and used at 50 µm. Picrotoxin was dissolved in aCSF to 50 µm. FPL was dissolved in ethanol to 25 mm and used at 500 nm. The AMT blocker VDM11 was diluted from DMSO stock solutions (50 mm) and used at 10 µm. The CB1 receptor blocker AM251 and the CB1 agonist WIN 55,212-2 were dissolved in DMSO to 50 mm and 10 mm, respectively, stock solutions were diluted in aCSF containing bovine serum albumin (0.5 g / L) and used at 2 µm (AM251) and 1 µm (WIN 55,212-2). Lidocaine was dissolved in aCSF to 500 µm, and TTX was dissolved in H2O to 1mm and used at 1 µm. Protein synthesis blockers anisomycin and cycloheximide were dissolved in aCSF to 20 µm and 80 µm, respectively. Experiments were performed at 29–32°C, with the temperature not varying more than 1°C throughout a given experiment. Experiments were discontinued if the series resistance varied by more than 20% or increased over 30 MΩ.
Results
The basic mechanisms for FPL-LTD are similar at glutamatergic and GABAergic synapses
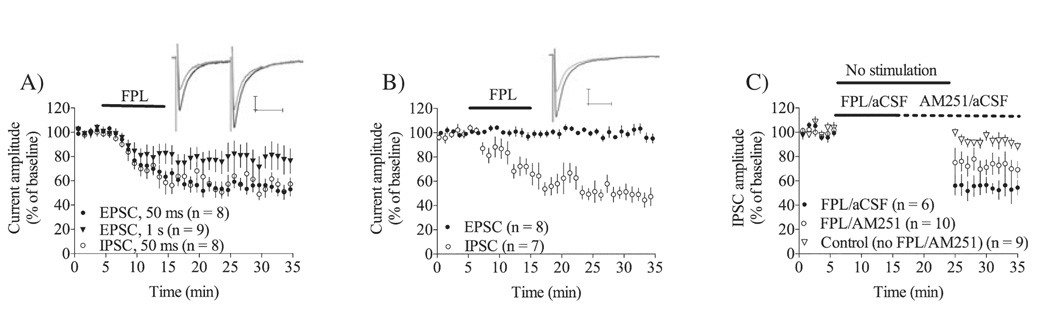
Treatment with the L-type calcium channel activator FPL (0.5 µm) combined with paired-pulse stimulation (50-ms interpulse interval) at 50 mHz induced a robust depression of transmission at both glutamatergic and GABAergic synapses in MSNs clamped at −50 mV. The CB1R antagonist AM251 (2 µm) completely blocked FPL-induced depression of EPSC and IPSC amplitudes when the antagonist was present throughout the experiment (EPSC amplitude = 111 ± 4.5% of baseline, n = 7, t = 6.74, df = 6, P < 0.001; IPSC amplitude = 108 ± 8.7% of baseline, n = 6, t = 1.69, df = 5, P > 0.05; Fig. 1A), but did not reverse established depression within the 15-min application time employed here (EPSC amplitude = 49 ± 5.3% of baseline, n = 5, t = 12.5, df = 4, P < 0.001; IPSC amplitude = 49 ± 18% of baseline, n = 6, t = 5.32, df = 5, P < 0.001; Fig. 1A), indicating that eCB-dependent LTD is induced using this protocol at both glutamatergic (FPL-eLTD) and GABAergic synapses (FPL-iLTD).
Fig. 1.
Basic properties of 2,5-dimethyl-4-[2-(phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylic acid methyl ester (FPL)-LTD are similar at glutamatergic (FPL-eLTD) and GABAergic synapses (FPL-iLTD). (A) FPL (500 nm) induced a robust depression in MSNs clamped at −50 mV that was prevented, but not reversed, by CB1R antagonist (AM251, 2 µm). (B) FPL-LTD was blocked by intracellular loading of the AMT inhibitor VDM11 (10 µm), indicating that eCB signaling involves a postsynaptic release step at both glutamatergic and GABAergic synapses. Example traces show excitatory postsynaptic currents (EPSCs) in a VDM11-loaded MSN at baseline (black) and post FPL treatment at t = 20–25 min (gray). (C) FPL-eLTD was significantly reduced in slices perfused with the protein translation inhibitor cycloheximide (80 µm). Bath application (filled circles) was more successful in inhibiting FPL-eLTD compared with intracellular loading (open triangles), suggesting that protein synthesis is required outside the postsynaptic cell. Example traces show EPSCs in a cycloheximide-loaded MSN clamped at baseline (black) and post FPL treatment at t = 20–25 min (gray). FPL-iLTD was also dependent on protein translation, and completely prevented in slices pretreated with cycloheximide (80 µm). The graph shows the mean inhibitory postsynaptic current (IPSC) amplitude with SEM in MSNs after 10 min treatment with 500 nm FPL (*P < 0.05). (D) Treatment with another blocker of protein synthesis, anisomycin (20 µm; filled circles) also successfully inhibited FPL-eLTD. EPSC / IPSC amplitude data are mean ± SEM. Scale bars: 100 pA and 25 ms for all traces. aCSF, artificial cerebrospinal fluid.
The magnitude of FPL-eLTD was reduced at room temperature (20–22°C, RT; EPSC amplitude = 82 ± 9.3% of baseline, n = 6, t = 3.77, df = 5, P < 0.05). Induction of FPL-LTD was prevented by postsynaptic loading of the AEA transporter inhibitor VDM11 (10 µm) at both glutamatergic and GABAergic synapses (EPSC amplitude = 96 ± 7.0% of baseline, n = 6, t = 0.73, df = 5, P > 0.05; IPSC amplitude = 101 ± 14% of baseline, n = 6, t = 0.23, df = 5, P > 0.05; Fig. 1B). These findings are consistent with the idea that FPL-LTD is dependent on a postsynaptic transport or mobilization step that is similar at glutamatergic and GABAergic synapses.
Protein translation has previously been shown to be critical for the expression of striatal LTD induced by high-frequency stimulation (Yin et al., 2006), and pretreatment with the protein translator inhibitor cycloheximide (80 µm) significantly reduced FPL-eLTD (EPSC amplitude = 92 ± 7.3% of baseline, n = 9, t = 2.36, df = 8, P < 0.05; Fig. 1C). Intracellular loading of cycloheximide was less effective in preventing FPL-eLTD (EPSC amplitude = 68 ± 9.2% of baseline, n = 11, t = 6.87, df = 10, P < 0.001; intracellular vs. extracellular treatment, unpaired t-test, t = 3.77, df = 18, P < 0.01; Fig. 1C), indicating that the majority of required protein synthesis occurs outside of the postsynaptic cell (Yin et al., 2006). FPL-eLTD was also reduced to a similar extent in slices treated extracellularly with the protein synthesis inhibitor anisomycin (20 µm; EPSC amplitude = 91 ± 6.4% of baseline, n = 9, t = 2.02, df = 8, P > 0.05; Fig. 1D). Protein synthesis was also required for FPL-iLTD (Fig. 1C). IPSC amplitude was not affected following 10 min FPL treatment in cycloheximide-treated slices (IPSC amplitude in cycloheximide-treated slices at t = 15 min = 99 ± 38% of baseline, n = 5, t = 0.11, df = 4, P > 0.05; IPSC amplitude in control slices at t = 15 min = 68 ± 14% of baseline, n = 6, t = 4.81, df = 5, P < 0.01), further supporting a role for protein synthesis in striatal plasticity (Yin et al., 2006). The average stimulation intensity needed to evoke IPSCs was not affected by cycloheximide treatment, indicating that cycloheximide blockade of LTD is not secondary to a general decrease in transmission.
Afferent activation is required for eCB signaling at glutamatergic but not GABAergic synapses in the striatum
We have previously shown that FPL-eLTD is not induced if afferent activation is not given during FPL treatment (Adermark & Lovinger, 2007a). To determine if a crucial level of afferent activation was required for FPL-eLTD, we increased the interpulse interval of the paired-pulse stimulation protocol. FPL-eLTD was significantly reduced when the interpulse interval was increased from 50 ms to 1 s (EPSC amplitude = 77 ± 17% of baseline, n = 9, t = 2.68, df = 8, P < 0.05; 50 ms vs. 1 s, t = 2.26, df = 16, P < 0.05; Fig. 2A), and completely prevented when a single stimulus was delivered at the same frequency (50 mHz; EPSC amplitude = 102 ± 2.8% of baseline,n = 7, t = 1.28, df = 6, P > 0.05; Fig. 2B).
Fig. 2.
Different requirement for afferent activation in 2,5-dimethyl-4-[2-(phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylic acid methyl ester (FPL)-eLTD and FPL-iLTD. (A) FPL treatment combined with paired-pulse stimulation induced a robust depression with a similar time course and amplitude at glutamatergic and GABAergic synapses in the striatum. The depression in excitatory postsynaptic current (EPSC) amplitude was significantly reduced when the interpulse interval was increased from 50 ms to 1 s. (B) When afferents were activated by a single pulse delivered every 20 s FPL treatment was sufficient to induce FPL-iLTD, while the EPSC amplitude remained unaffected. (C) Synaptic depression of GABAergic transmission that was resistant to AM251 reversal (FPL-iLTD) could be induced even when afferent activation was suspended, but this depression was significantly smaller compared with FPL-iLTD induced during continuous afferent activation. EPSC / inhibitory postsynaptic current (IPSC) amplitude data are mean ± SEM. Example traces in (A) and (B) show IPSCs at baseline (black) and at t = 20–25 min (gray). Scale bar: 100 pA and 25 ms. aCSF, artificial cerebrospinal fluid.
The time course and amplitude of FPL-LTD during paired-pulse stimulation was similar at glutamatergic and GABAergic synapses (EPSC amplitude = 54 ± 9.2% of baseline, n = 9, t = 9.21, df = 8, P < 0.001; IPSC amplitude = 54 ± 9.1% of baseline, n = 10, t = 9.94, df = 9, P < 0.001; Fig. 2A). In contrast to FPL-eLTD, induction of FPL-iLTD was independent of the stimulation protocol used (IPSC amplitude during single pulse stimulation = 58 ± 13% of baseline, n = 6, t = 5.12, df = 5, P < 0.01; paired- vs. single-pulse stimulation, unpaired t-test, t = 0.52, df = 14, P > 0.05; Fig. 2B). We observed that depression could be induced at GABAergic synapses even when stimulation was suspended during FPL treatment (IPSC amplitude when stimulation resumed = 55 ± 15% of baseline, n = 6, t = 6.15, df = 5, P < 0.001; Fig. 2C), which contrasts with previously published data for glutamatergic synapses (Adermark & Lovinger, 2007a; Singla et al., 2007), but agrees with data from GABAergic synapses during AEA loading (Adermark & Lovinger, 2007b). A small rundown was detected in control cells when stimulation resumed, but this was significantly smaller than depression induced by FPL (IPSC amplitude in control cells at t = 25–30 min = 94 ± 4.7% of baseline, n = 9, t = 2.56, df = 8,P < 0.05, control vs. FPL-treated, unpaired t-test, t = 6.25, df = 13, P < 0.01).
Perfusion of AM251 beginning after FPL treatment and continuing for 10 min before stimulation resumed was insufficient to prevent FPL-induced depression, suggesting that FPL-iLTD can be induced in the absence of afferent activation at GABAergic synapses (IPSC amplitude when stimulation resumed after 10 min AM251 treatment = 74 ± 16% of baseline, n = 10, t = 3.26, df = 9, P < 0.05; Fig. 2C). However, the depression seen in these slices was significantly smaller than in slices in which afferents were continuously stimulated during FPL and AM251 treatment (IPSC at t = 25–30 min = 40 ± 10% of baseline, n = 5, t = 11.7, df = 4, P < 0.001, suspended stimulation vs. continuous stimulation, unpaired t-test, t = 2.79, df = 13, P < 0.05).
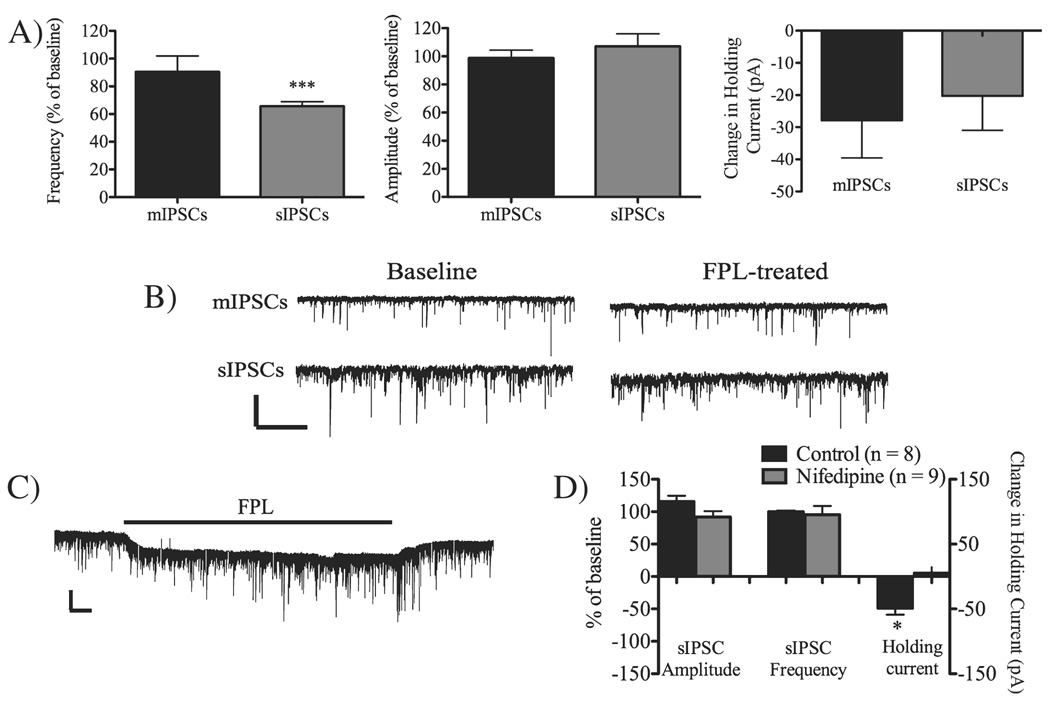
Reduced neuronal firing prevents eCB signaling at GABAergic synapses
The contrasting requirements for afferent activation in FPL-LTD induction at GABAergic and glutamatergic striatal synapses led us to evaluate if neuronal firing was even necessary for eCB-induced depression at GABAergic synapses. We thus measured sIPSCs and lidocaine-insensitive sIPSCs (mIPSCs) in MSNs treated with FPL (Fig. 3). For sIPSCs measured in the absence of lidocaine, event frequency but not amplitude was significantly depressed after 10 min of FPL treatment, further supporting the finding that FPL-induced depression is independent of afferent activation (event frequency = 69 ± 9.3% of baseline, n = 11, t = 6.53, df = 10, P < 0.001; amplitude = 109 ± 18% of baseline, n = 11, t = 0.95, df = 10, P > 0.05). However, FPL treatment did not affect mIPSCs, showing that neuronal firing is vital for eCB signaling at GABAergic synapses (event frequency = 91 ± 28% of baseline, n = 7, t = 0.64, df = 6, P > 0.05; amplitude = 97 ± 11% of baseline, n = 7, t = 0.06, df = 6, P > 0.05).
Fig. 3.
2,5-Dimethyl-4-[2-(phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylic acid methyl ester (FPL)-induced depression requires presynaptic activity. (A) FPL (10 min) treatment reduced the event frequency of spontaneous inhibitory postsynaptic currents (sIPSCs), but not miniature inhibitory postsynaptic currents (mIPSCs), showing that neuronal firing is vital for FPL-induced depression. sIPSC / mIPSC amplitude was not affected by FPL treatment. A small inward current was induced by FPL in both control and TTX-treated slices. Data are based on six cells in each group and presented as mean values compared with baseline with SEM. Statistics are paired t-test. ***P < 0.001. (B) Example traces shows mIPSCs and sIPSCs at baseline and following 10 min of FPL treatment. Note the slight inward current in the presence of FPL. Scale bar: 50 pA and 5 s. (C) Example trace showing mIPSCs at baseline, during FPL treatment, and washout. Note that the inward current reverses upon FPL washout. Scale bar: 25 pA and 1 min. (D) sIPSC frequency and amplitude were not affected by FPL treatment if the postsynaptic cell was clamped at −70 mV. The FPL-induced change in holding current remained in MSNs voltage-clamped at −70 mV, but was prevented by the L-type calcium channel blocker nifedipine (20 µm). *P < 0.05.
A small inward current (average 24.4 ± 6 pA) was observed upon FPL application in several neurons during experiments examining mIPSCs (Fig. 3). This current did not appear to affect mIPSC measurements as FPL had no effect on mIPSC frequency or amplitude, even when the inward current was present (see data above). Furthermore, the inward current reversed upon FPL washout, but the frequency and amplitude of mIPSCs were unchanged relative to control values, similar to the observations when FPL was present (event frequency = 85 ± 21% of baseline, amplitude = 105 ± 21% of baseline, n = 3). Interestingly, the FPL-mediated inward current was larger in MSNs voltage clamped at −70 mV than in cells clamped at −50 mV, even though sIPSC event frequency and amplitude remained unaffected after 10 min FPL treatment (sIPSC event frequency = 110 ± 22% of baseline, n = 7, t = 0.84, df = 6, P > 0.05; sIPSC amplitude = 105 ± 6.7% of baseline, n = 7, t = 1.43, df = 6, P > 0.05; compare with FPL-treated MSNs voltage-clamped at −50 mV: sIPSC event frequency = 67 ± 8.7% of baseline, n = 11, t = 7.58, df = 10, P < 0.001; sIPSC amplitude = 107 ± 18% of baseline, n = 11, t = 1.43, df = 6, P > 0.05; Fig. 3D). The lack of LTD in MSNs voltage-clamped at −70 mV is consistent with previously published data from glutamatergic synapses (Adermark & Lovinger, 2007a). The shift in holding current induced by FPL was blocked in slices pretreated with nifedipine (20 µm; Fig. 3D), indicating that the inward current is mediated through activation of L-type calcium channels, and not connected to non-specific effects caused by FPL.
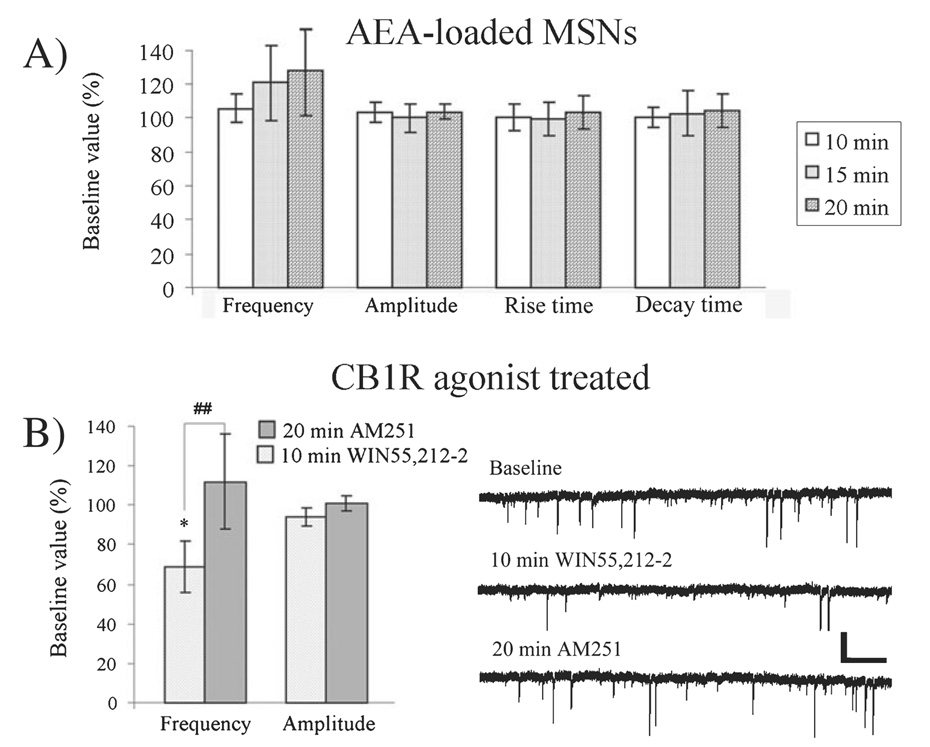
Neuronal firing modulates eCB release and LTD induction at GABAergic synapses
To determine if presynaptic activity was required for eCB production or release / mobilization, we loaded MSNs with the eCB AEA, which we have previously shown reduces sIPSC event frequency by ~25% within 20 min after establishing whole-cell recordings (Adermark & Lovinger, 2007b). AEA loading was insufficient to significantly affect event frequency, amplitude, rise time or decay time of mIPSCs recorded in slices perfused with 500 µm lidocaine or 1 µm TTX (Fig. 4A), indicating that neuronal firing is required for eCB release or CB1R activation. There was no difference in the data collected from slices perfused with lidocaine, compared with TTX-treated slices. mIPSCs were also not affected in vehicle-loaded cells 30 min after establishing the whole-cell recording configuration (event frequency = 98 ± 12% of baseline; amplitude = 108 ± 11% of baseline; rise time = 100 ± 10% of baseline; decay time = 106 ± 11% of baseline, n = 7).
Fig. 4.
eCB release and LTD induction at GABAergic synapses require presynaptic activity. (A) Postsynaptic loading of the eCB AEA (50 µm), which previously has been shown to decrease the event frequency of recorded sIPSCs (Adermark & Lovinger, 2007b), was insufficient to affect mIPSC event frequency, amplitude, rise time or decay time within a 20-min cell-loading period. (B) Treatment with the cannabinoid 1 receptor (CB1R) agonist WIN 55,212-2 (1 µm) significantly reduced mIPSC frequency, suggesting that neuronal firing is not required for CB1R activation of downstream events involved in transient synaptic depression. However, the WIN-induced decrease in mIPSC frequency recovered after post-agonist AM251 treatment, suggesting that iLTD is not induced by CB1R activation when neuronal firing is prevented. Example traces show mIPSCs at baseline, after 10 min WIN 55,212-2 treatment and after 20 min washout with AM251. Data are based on eight cells in each group and presented as mean values compared with baseline with 95% confidence intervals. Statistics are paired t-test. *P < 0.05, # #P < 0.01. Scale bar: 40 pA and 1.3 s. MSN, medium spiny neuron.
To establish if the required firing was related to eCB release, or presynaptic requirements, we measured TTX (1 µm)-insensitive spontaneous activity during treatment with the CB1R agonist WIN 55,212-2 (1 µm). CB1R activation caused a significant depression in the frequency of mIPSCs (66 ± 13% of baseline, n = 7, t = 2.95, df = 6, P < 0.05), but not amplitude (95 ± 5.7% of baseline, n = 7, t = 1.94, df = 6, P > 0.05), showing that activation of the CB1R, and resultant depression, is independent of neuronal firing at GABAergic synapses (Fig. 4B). However, the WIN 55,212-2-induced decrease in mIPSC event frequency was reversed by perfusion of AM251 (112 ± 24% of baseline, n = 7, t = 5.0, df = 6, P < 0.01 when compared with FPL-treated before AM251 perfusion), indicating that CB1R activation only produces short-lasting depression if neuronal firing is reduced (Fig. 4B).
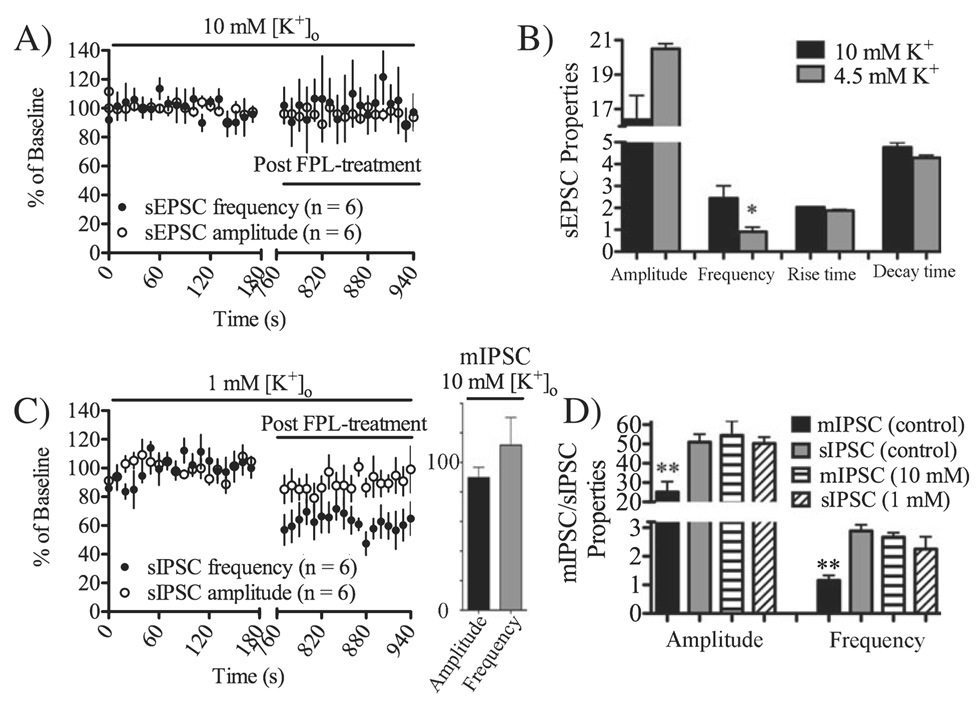
Extracellular potassium does not modulate FPL-LTD
The data presented above suggested a possible link between the degree of presynaptic activity and eCB signaling. However, it was still unclear if LTD induction was dependent on neuronal firing per se or if the level of presynaptic input was the important factor. It is known that altering [K+]o alters the frequency of spontaneous synaptic responses, and this appears to be secondary to changes in presynaptic calcium entry (Scanziani et al., 1995). Thus, we wished to determine if manipulating [K+]o would alter LTD induction at glutamatergic and GABAergic striatal synapses. Increasing potassium concentration from 4.5 to 10 mm significantly enhanced sEPSC frequency (192 ± 28% of baseline, n = 7, t = 3.31, df = 6, P < 0.05), while amplitude remained unaffected (101 ± 15% of baseline, n = 7, t = 1.80, df = 6, P > 0.05; Fig. 5B). Even though event frequency was enhanced, increased external K+ concentration did not facilitate FPL-eLTD induction (sEPSC frequency after 10 min FPL-treatment = 103 ± 30% of baseline, n = 6, t = 0.28, df = 5, P > 0.05; Fig. 5A). Increasing potassium concentration from 4.5 to 10 mm also increased mIPSC event frequency (117 ± 11%, n = 6, t = 2.73, df = 5, P < 0.05; mIPSC amplitude = 92 ± 6.9%, t = 2.17, df = 5, P > 0.05), but failed to facilitate FPL-iLTD in TTX-treated slices (mIPSC event frequency after 10 min FPL treatment = 117 ± 18.8%, n = 6, t = 0.62, df = 5, P > 0.05; mIPSC amplitude = 89.7 ± 7%, n = 6, t = 1.47, df = 5, P > 0.05). Furthermore, reducing external K+ concentration to 1 mm was also insufficient to prevent FPL-iLTD (sIPSC frequency after 10 min FPL treatment = 59 ± 17% of baseline, n = 6, t = 4.67, df = 5, P < 0.01; Fig. 5C), suggesting that changes in extracellular potassium, and the consequences of this treatment, do not alter FPL-LTD induction threshold.
Fig. 5.
2,5-Dimethyl-4-[2-(phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylic acid methyl ester (FPL)-LTD is not sensitive to altered external potassium. (A and B) Increasing the extracellular level of KCl to 10 mm increased the spontaneous excitatory postsynaptic current (sEPSC) frequency, but did not facilitate FPL-eLTD. (C) Reducing extracellular KCl to 1 mm did not prevent FPL-iLTD. Data show spontaneous inhibitory postsynaptic current (sIPSC) values compared with baseline in 10-s bins before and after 10 min FPL treatment. FPL-iLTD in TTX-treated slices was also not facilitated by 10 mm KCl. Graph showing miniature inhibitory postsynaptic current (mIPSC) amplitude and frequency compared with baseline level after 10 min FPL application, note the absence of any depression of mIPSC frequency. (D) The amplitude and frequency of postsynaptic currents were not affected when external potassium was reduced to 1 mm, but were significantly depressed in slices treated with TTX (1 µm). Increasing the extracellular level of KCl to 10 mm increased the mIPSC amplitude and frequency (*P < 0.05, **P < 0.01).
It is worth noting that the baseline frequency of sEPSCs in 4.5 mm KCl external solution was significantly lower that that of sIPSCs recorded under the same condition [0.91 ± 0.41 Hz (sEPSC) vs. 2.88 ± 0.43 Hz (sIPSC), t = 5.51, df = 15, P < 0.001]. However, in slices perfused with 10 mm KCl, sEPSC frequency was not significantly different compared with frequencies of sIPSCs or mIPSCs [2.46 ± 1.1 Hz (sEPSCs, 10 mm K+); 1.15 ± 0.18 Hz (mIPSCs); 2.25 ± 0.85 Hz (mIPSCs, 10 mm K+) Fig. 5]. Thus, it is not likely that FPL-LTD induction is simply a function of net presynaptic activity.
Discussion
The data presented here suggest that the basic features of eCB signaling at GABAergic, presumed inhibitory, and glutamatergic, presumed excitatory, synapses in the striatum are the same, but that the required level of presynaptic activation is lower at GABAergic synapses. The different requirements for afferent activation might be connected to the CB1Rs, which are present at high levels on both GABAergic axon terminals of MSNs and parvalbumin-positive interneurons, but only at low levels at glutamatergic synapses (Uchigashima et al., 2007). It is thus possible that a lower concentration of postsynaptically released eCBs is sufficient to induce CB1R-mediated depression at GABAergic synapses. The need for afferent activation might also involve different requirements for presynaptic activity for LTD induction at the two types of synapses (Sjostrom et al., 2003; Singla et al., 2007).
The role of L-type calcium channels in LTD
L-type channels play a role in generating somatic Ca2+ signals and modulating both action potential firing and EPSP amplitude in MSNs (Hernández-López et al., 1997; Carter & Sabatini, 2004). L-type calcium channels are gated by voltage, and opening is regulated through cAMP / PKA and PKC pathways. β-Adrenergic receptor stimulation or GABAA activation induces activation of L-type calcium channels, and there is a synergy between glutamatergic receptors and CaV1.3 channels in the generation of ‘upstates’, where the membrane potential of the MSN reaches approximately −50 mV (Wilson & Kawaguchi, 1996). Furthermore, nimodipine-sensitive Ca2+-influx makes a significant contribution to the calcium entry in dendritic spines caused by subthreshold depolarization and back-propagating action potentials at −50 mV (Carter & Sabatini, 2004).
Opening of L-type calcium channels is regulated by multiple G-protein-coupled receptors, and facilitated by spike waveforms, suggesting that this process could enhance Ca2+ entry during naturally occurring high-frequency activity (Olson et al., 2005). Activation of postsynaptic L-type calcium channels also appears to be vital for formation of LTD at glutamatergic synapses in the striatum (Calabresi et al., 1994; Wang et al. 2006). With respect to the membrane potential at which L-channels participate in LTD induction, Kreitzer & Malenka (2005) showed that striatal LTD can be induced by activation of group I mGluRs when the membrane potential is held at −50, but not −70 mV, and that this mGluR-LTD was prevented by a dihydropyridine L-channel blocker. These findings, along with our finding that FPL can induce LTD with a similar voltage dependence (Fig. 3; Adermark & Lovinger, 2007a), indicate that the threshold for LTD is probably at or near the threshold for action potential firing, within the range of voltage-dependence of CaV1.3, but probably not CaV1.2 activation. Furthermore, synaptic signaling complexes formed by CaV1.3 channels and Shank proteins have been suggested to be a critical factor in determining how afferent synaptic activity is translated into long-term alterations in neuronal function (Calabresi et al., 1994; Olson et al., 2005). Pharmacological manipulations aimed at altering the function of L-type channels might thus be attractive candidates for manipulation of striatum-based synaptic plasticity, learning and memory, as well as neurological disorders that involve an LTD-like process (Day et al., 2006; Adermark & Lovinger, 2007a).
Protein synthesis inhibition and LTD
Data from our laboratory (Yin et al., 2006; Adermark & Lovinger, unpublished results) and the data presented in this report suggest that protein translation is critical for the expression of striatal LTD. Protein translation blockers prevent LTD induction, and do not appear to have non-specific effects that account for the loss of LTD. Observations in past and present studies indicate that translation inhibitors do not alter baseline synaptic responses (Yin et al., 2006). Cycloheximide does not interfere with mGluR function but blocks DHPG-induced LTD in the striatum (Yin et al., 2006). Blockade of protein translation does not prevent synaptic depression induced by postsynaptic eCB-loading at glutamatergic or GABAergic synapses, indicating that cycloheximide does not interfere with eCB release or synaptic depression induced by continuous CB1R activation (L. Adermark & D.M. Lovinger, unpublished results). Also, cycloheximide alone does not significantly reduce c-Fos expression, but blocks the enhancement induced by phorbol 12-myristate 13-acetate, further demonstrating the efficacy of cycloheximide as an inhibitor of protein synthesis in the brain slice (Yin et al., 2006).
In the present study we found that FPL-LTD was prevented during extracellular perfusion of cycloheximide or anisomycin, suggesting that protein synthesis also is involved in both FPL-eLTD and FPL-iLTD. It was surprising that only a small amount of short-term depression was observed during FPL treatment in the presence of translation inhibitors (Fig. 1C and D), as translation inhibitors appear to prevent LTD without affecting short-term depression (Huber et al., 2000; Yin et al., 2006). However, LTD develops gradually during combined FPL application and paired-pulse stimulation, and this gradual synaptic depression is completely blocked if a CB1 antagonist is present during FPL exposure (Adermark & Lovinger, 2007a). Thus, it is not clear that any short-term depression is induced by this treatment.
Postsynaptic intracellular loading of cycloheximide was less effective at inhibiting FPL-eLTD, which indicates that protein synthesis occurs somewhere outside of the postsynaptic cell. It should be noted that because the drug was loaded via the patch pipette in these experiments we cannot be sure that the amount of cycloheximide that diffused into the MSNs was high enough to block protein synthesis. However, postsynaptic loading of other drugs has been shown to effectively block LTD in our preparation (Yin et al., 2006; Adermark & Lovinger, 2007a), and postsynaptic loading of a protein translation inhibitor prevented mGluR agonist-induced LTD in hippocampal neurons (Yin et al., 2006). Thus, we do not believe that the lack of blockade of striatal LTD is due to the inability of the inhibitor to adequately fill the postsynaptic cell when delivered from the patch pipette.
Neuronal firing is vital for eCB-release and LTD induction
FPL treatment reduced the frequency of sIPSCs, and FPL-iLTD could be induced when stimulation was suspended. However, even though afferent activation appears to be unnecessary for eCB mobilization at GABAergic synapses, the magnitude of LTD produced under this condition (defined as established synaptic depression that is not reversed by AM251), was significantly smaller than LTD induced during continuous afferent stimulation, suggesting that afferent activation enhances FPL-LTD formation also at GABAergic synapses.
By treating slices with lidocaine or TTX we were able to show that a certain degree of neuronal firing is vital for eCB-induced depression even at highly eCB-sensitive GABAergic synapses. Blockade of sodium channels prevented FPL-iLTD, but also depression induced by postsynaptic loading of the eCB AEA, showing that neuronal firing is either required for eCB mobilization / release from the postsynaptic cell and / or for presynaptic CB1R activation or mechanisms down-stream from this receptor. WIN 55,212-2 treatment induced a robust depression of event frequency in TTX-treated slices, showing that activation of the CB1R and subsequent synaptic inhibition is independent of the degree of neuronal firing, further supporting our hypothesis that synaptic activity is crucial for eCB release (Adermark & Lovinger, 2007b). This WIN 55,212-2-induced change in event frequency of mIPSCs is different from what has been observed at glutamatergic synapses in the striatum (Singla et al., 2007) and at GABAergic synapses from the hippocampus (Hajos et al., 2000). However, data from this laboratory indicate that the CB1R agonist HU-210 reduces the sEPSC frequency in striatal MSNs in strontium-containing aCSF (Gerdeman & Lovinger, 2001), and the frequency of mIPSCs is reduced by WIN 55,212-2 in both striatum (Freiman et al., 2006), hippocampus (Heifets et al., 2008) and the cerebellum (Takahashi & Linden, 2000; Yamasaki et al., 2006).
Intriguingly, WIN 55,212-2 treatment only induced an antagonist-reversible decline in mIPSC frequency, which is different from the robust LTD that we observe when WIN 55,212-2-treatment is combined with afferent activation at GABAergic synapses (L. Adermark & D.M. Lovinger, unpublished results). This is in line with recently published data from the hippocampus (Heifets et al., 2008), and suggests that a certain degree of synaptic activity is not only required for eCB mobilization from the postsynaptic cell but also for some presynaptic process involved in LTD formation, as previously described at glutamatergic synapses (Sjostrom et al., 2003; Singla et al., 2007). Our findings are most consistent with the idea that afferent activity is necessary for both eCB mobilization and LTD induction at glutamatergic synapses, while at GABAergic synapses these processes require action potential firing in some neuronal population other than the postsynaptic neuron. In light of the Heifets et al. (2008) findings, we speculate that it is firing of the presynaptic GABAergic neuron that is most important in this context, but clarification of this point will have to await further studies.
The role of presynaptic activity in LTD
Elevating the extracellular potassium concentration increased the frequency, but not amplitude, of sEPSCs. This is consistent with findings in previous studies from the hippocampus, and is most likely attributable to increased activation of voltage-gated calcium channels (Scanziani et al., 1995). In our experiments, increasing extracellular potassium levels to 10 mm was not sufficient to lower the threshold for FPL-eLTD (Fig. 5). Furthermore, reducing K+ to 1 mm also failed to prevent FPL-iLTD at GABAergic synapses, indicating that FPL-LTD is not highly sensitive to membrane potential or presynaptic calcium influx. Still, our data indicate a critical role for action potential firing in FPL-LTD induction, and one possibility is that phasic calcium entry triggered by action potential firing participates in LTD induction, while tonic increases in calcium entry produced by changes in membrane potential are insufficient for plasticity induction. This fits well with the findings of Heifets et al. (2008) as regards GABAergic synapses in the hippocampus, and is also consistent with data of Singla et al. (2007), suggesting that presynaptic Ca2+ entry produced by afferent stimulation is vital for LTD induction at glutamatergic synapses in the striatum.
When recording spontaneous postsynaptic currents, we observed that both frequency and amplitude were significantly smaller at glutamatergic synapses. It is thus possible that a similar level of presynaptic firing is required for eCB signaling and LTD induction at both types of synapses, but that the low baseline activity at the glutamatergic synapses calls for additional afferent activation in order for this threshold level to be reached. The lack of LTD might thus be directly connected to a low level of action potential firing or event frequency. Increasing the activity in the terminal by elevating extracellular potassium concentration (10 mm), which might counteract the loss of action potentials, increased the frequency of sEPSCs and mIPSCs to a level similar to that of sIPSCs recorded in normal extracellular KCl, but did not facilitate FPL-eLTD or FPL-iLTD (Fig. 5A). Thus, our data suggest that it is action potential firing and not presynaptic activation / release that is important for LTD formation at glutamatergic and GABAergic synapses in the striatum.
Based on the need for presynaptic activity for eCB-release (Fig. 4A) and the high expression of presynaptic CB1Rs at GABAergic terminals (Uchigashima et al., 2007), it is possible that the requirement of paired-pulse activation for FPL-eLTD is connected to the amount of eCBs that have to be released for CB1R-mediated depression to occur. Greater sensitivity to eCBs may thus be what ultimately facilitates LTD induction at GABAergic synapses.
Both the MSNs and fast-spiking striatal interneurons express presynaptic CB1 receptors (Uchigashima et al., 2007), and WIN 55,212-2 has been shown to inhibit GABAergic transmission at synapses made onto MSNs by both neuronal subtypes (Freiman et al., 2006). Thus, we cannot be sure at this point which GABAergic synapses onto MSNs express FPL-induced iLTD. A recent study performed in our laboratory has shown that mGluR-dependent iLTD can be induced by low-frequency afferent stimulation (L. Adermark & D.M. Lovinger, unpublished results). This finding indicates that LTD induced in this manner most likely occurs at those GABAergic synapses located on the distal dendrites and dendritic spines of MSNs, as only these synapses would be close to the glutamatergic synapses that are present on the outer two-thirds of the dendritic arbor. These observations suggest that ‘feedback’ inhibitory inputs from other MSNs are among the GABAergic synapses that exhibit this form of mGluR-dependent iLTD. Further studies will be required to determine if FPL-LTD occurs at synapses made by the different subtypes of striatal interneurons.
Concluding remarks
The data presented here show that the level of neuronal firing regulates eCB signaling by modulating release from the postsynaptic cell, as well as interacting with presynaptic mechanisms to induce LTD at both glutamatergic and GABAergic synapses in the striatum. Furthermore, our data imply that there is a threshold level of neuronal activity that needs to be reached in order for eCB signaling and LTD induction to be activated. This threshold level appears to be different at glutamatergic and GABAergic synapses, and it is thus possible that net striatal output is regulated by the frequency of different synaptic inputs during eCB-dependent plasticity.
Acknowledgements
This work was supported by the Division of Intramural Clinical and Basic Research, NIAAA, NIH, The Swedish Research Council, the Swedish Society for Medical Research (2006-2425; 2006-4988; 2006-6385), Ake Wiberg Foundation (113300049) and The Swedish Society of Medicine (2008-21390).
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AEA
arachidonoyl ethanolamide
- AMT
anandamide transporter
- AP-5
5d,l-2-amino-5 phosphonovaleric acid
- CB1R
cannabinoid 1 receptor
- DMSO
dimethylsulfoxide
- eCB
endocannabinoid
- EPSC
excitatory postsynaptic current
- FPL
2,5-dimethyl-4-[2-(phenylmethyl) benzoyl]-1H-pyrrole-3-carboxylic acid methyl ester (FPL 64176)
- GABA
γ-aminobutyric acid
- IPSC
inhibitory postsynaptic current
- LTD
long-term depression
- mEPSC
miniature excitatory postsynaptic current
- mIPSC
miniature inhibitory postsynaptic current
- MSN
medium spiny neuron
- NBQX
1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium salt
- sEPSC
spontaneous excitatory postsynaptic current
- sIPSC
spontaneous inhibitory postsynaptic current
- TTX
tetrodotoxin
References
- Adermark L, Lovinger DM. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. J. Neurosci. 2007a;27:6781–6787. doi: 10.1523/JNEUROSCI.0280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Lovinger DM. Retrograde endocannabinoid signaling at striatal synapses requires a regulated postsynaptic release step. Proc. Natl Acad. Sci. USA. 2007b;104:20564–20569. doi: 10.1073/pnas.0706873104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Post-receptor mechanisms underlying striatal long-term depression. J. Neurosci. 1994;14:4871–4881. doi: 10.1523/JNEUROSCI.14-08-04871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J. Comp. Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Freiman I, Anton A, Monyer H, Urbanski MJ, Szabo B. Analysis of the effects of cannabinoids on identified synaptic connections in the caudate-putamen by paired recordings in transgenic mice. J. Physiol. 2006;15:789–806. doi: 10.1113/jphysiol.2006.114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco FR, Martorana A, Giampa C, De March Z, Farini D, D’Angelo V, Sancesario G, Bernardi G. Immunolocalization of CB1 receptor in rat striatal neurons: a confocal microscopy study. Synapse. 2004;53:159–167. doi: 10.1002/syn.20047. [DOI] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J. Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur. J. Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Chevaleyre V, Castillo PE. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc. Natl Acad. Sci. USA. 2008;105:10250–10255. doi: 10.1073/pnas.0711880105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-López S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J. Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilário MRF, Clouse E, Yin HH, Costa RM. Endocannabinoid signaling is critical for habit formation. Front. Integr. Neurosci. 2007;1:1–12. doi: 10.3389/neuro.07.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J. Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligresti A, Morera E, Van Der Stelt M, Monory K, Lutz B, Ortar G, Di Marzo V. Further evidence for the existence of a specific process for the membrane transport of anandamide. Biochem. J. 2004;380:265–272. doi: 10.1042/BJ20031812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Endocannabinoid liberation from neurons in transsynaptic signaling. J. Mol. Neurosci. 2007;33:87–93. doi: 10.1007/s12031-007-0043-2. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Moore SA, Nomikos GG, Dickason-Chesterfield AK, Schober DA, Schaus JM, Ying BP, Xu YC, Phebus L, Simmons RM, Li D, Iyengar S, Felder CC. Identification of a high-affinity binding site involved in the transport of endocannabinoids. Proc. Natl Acad. Sci. USA. 2005;102:17852–17857. doi: 10.1073/pnas.0507470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J. Neurosci. 2005;25:1050–1062. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J. Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Gahwiler BH, Thompson SM. Presynaptic inhibition of excitatory synaptic transmission by muscarinic and metabotropic glutamate receptor activation in the hippocampus: are Ca2+ channels involved? Neuropharmacology. 1995;34:1549–1557. doi: 10.1016/0028-3908(95)00119-q. [DOI] [PubMed] [Google Scholar]
- Singla S, Kreitzer AC, Malenka RC. Mechanisms for synapse specificity during striatal long-term depression. J. Neurosci. 2007;27:5260–5264. doi: 10.1523/JNEUROSCI.0018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Szabo B, Dörner L, Pfreundtner C, Nörenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Linden DJ. Cannabinoid receptor modulation of synapses received by cerebellar Purkinje cells. J. Neurophysiol. 2000;83:1167–1180. doi: 10.1152/jn.2000.83.3.1167. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Abercrombie ED, Bolam JP. Basal ganglia macrocircuits. Prog. Brain Res. 2007;160:3–7. doi: 10.1016/S0079-6123(06)60001-0. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachido-noyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J. Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. GABAergic inhibition in the neostriatum. Prog. Brain Res. 2007;160:91–110. doi: 10.1016/S0079-6123(06)60006-X. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Hashimoto K, Kano M. Miniature synaptic events elicited by presynaptic Ca2+ rise are selectively suppressed by cannabinoid receptor activation in cerebellar Purkinje cells. J. Neurosci. 2006;26:86–95. doi: 10.1523/JNEUROSCI.2258-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Davis MI, Ronesi JA, Lovinger DM. The role of protein synthesis in striatal long-term depression. J. Neurosci. 2006;26:11811–11820. doi: 10.1523/JNEUROSCI.3196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]