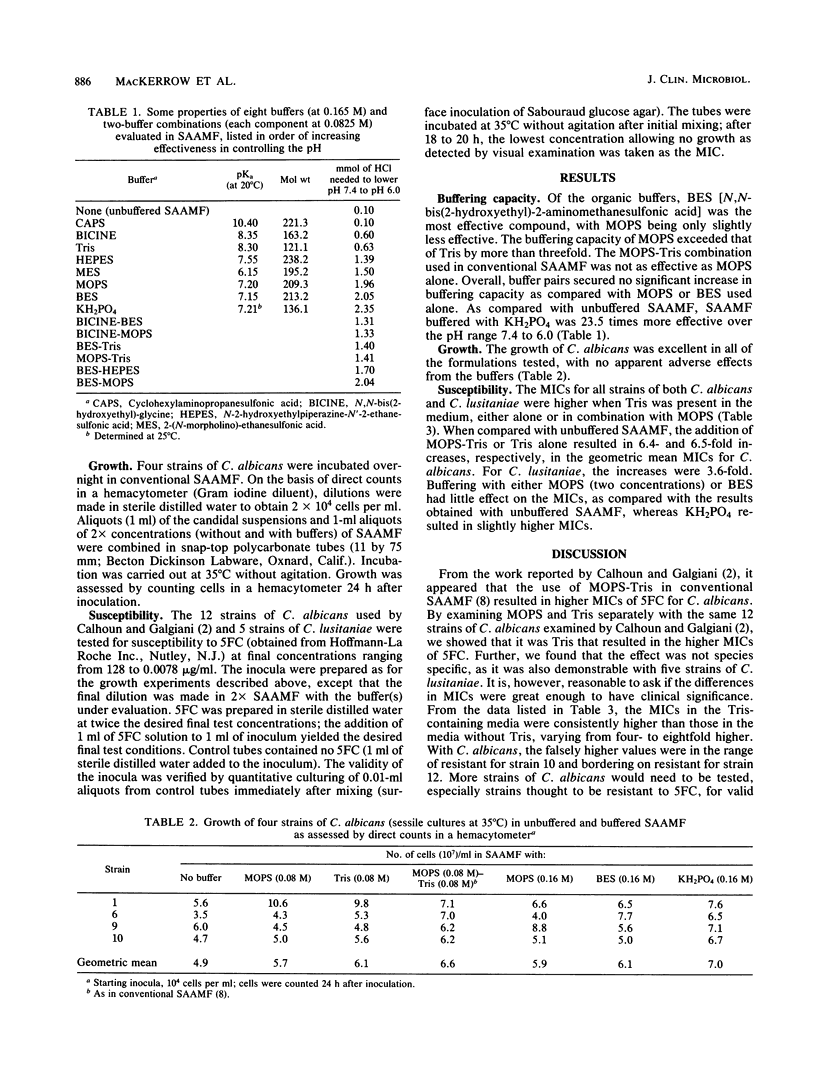
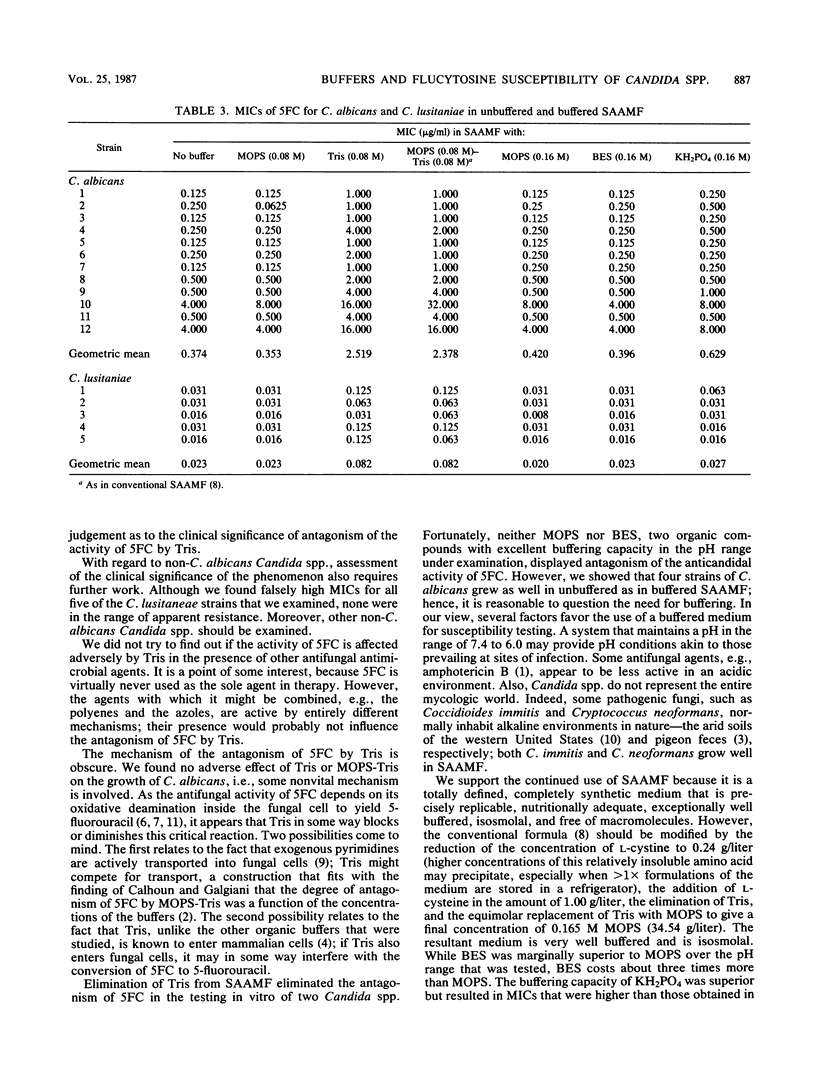
Abstract
Synthetic amino acid medium for fungi (SAAMF) is a totally defined, nutritionally adequate, macromolecule-free culture medium for fungi that is buffered with an organic weak acid-weak base pair: 2-(N-morpholino)-propanesulfonic acid (MOPS) and 2-amino-2-(hydroxymethyl)-1,3-propanediol (Tris). In 1984, it was reported that MOPS-Tris in SAAMF antagonized the activity of flucytosine against Candida albicans (D. L. Calhoun and J. N. Galgiani, Antimicrob. Agents Chemother. 26:364-367, 1984). Accordingly, we evaluated the buffering capacity of seven synthetic organic buffers and monobasic potassium phosphate, both singly and in pairs, over the pH range 7.4 to 6.0. Of these buffers, MOPS, BES [N,N-bis(2-hydroxyethyl)-2-aminomethanesulfonic acid], a BES-MOPS combination, and KH2PO4 provided the best buffering. Growth of C. albicans, in unbuffered SAAMF was equivalent overall to that in SAAMF containing buffers, singly or in pairs. Twelve strains of C. albicans and five strains of Candida lusitaniae were tested for susceptibility to flucytosine in SAAMF, with and without buffers. In the presence of Tris, the geometric mean MICs were 6.5- and 3.6-fold higher, respectively, for C. albicans and C. lusitaniae. We recommend replacing Tris with the nonantagonistic MOPS.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calhoun D. L., Galgiani J. N. Analysis of pH and buffer effects on flucytosine activity in broth dilution susceptibility testing of Candida albicans in two synthetic media. Antimicrob Agents Chemother. 1984 Sep;26(3):364–367. doi: 10.1128/aac.26.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMONS C. W. Saprophytic sources of Cryptococcus neoformans associated with the pigeon (Columba livia). Am J Hyg. 1955 Nov;62(3):227–232. doi: 10.1093/oxfordjournals.aje.a119775. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Hoeprich P. D., Barry A. L., Fay G. D. Synthetic medium for susceptibility testing. Antimicrob Agents Chemother (Bethesda) 1970;10:494–497. [PubMed] [Google Scholar]
- Hoeprich P. D., Finn P. D. Obfuscation of the activity of antifungal antimicrobics by culture media. J Infect Dis. 1972 Oct;126(4):353–361. doi: 10.1093/infdis/126.4.353. [DOI] [PubMed] [Google Scholar]
- Hoeprich P. D., Ingraham J. L., Kleker E., Winship M. J. Development of resistance to 5-fluorocytosine in Candida parapsilosis during therapy. J Infect Dis. 1974 Aug;130(2):112–118. doi: 10.1093/infdis/130.2.112. [DOI] [PubMed] [Google Scholar]
- Jordan G. W., Hoeprich P. D. Susceptibility of three groups of Staphylococcus aureus to newer antimicrobial agents. Antimicrob Agents Chemother. 1977 Jan;11(1):7–12. doi: 10.1128/aac.11.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jund R., Lacroute F. Genetic and physiological aspects of resistance to 5-fluoropyrimidines in Saccharomyces cerevisiae. J Bacteriol. 1970 Jun;102(3):607–615. doi: 10.1128/jb.102.3.607-615.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak A., Scholer H. J. Mode of action of 5-fluorocytosine and mechanisms of resistance. Chemotherapy. 1975;21(3-4):113–130. doi: 10.1159/000221854. [DOI] [PubMed] [Google Scholar]


