Abstract
Context
Coronary artery calcification is a subclinical predictor of coronary heart disease. Recent studies have found that sleep duration is correlated with established risk factors for calcification including glucose regulation, blood pressure, sex, age, education, and body mass index.
Objective
To determine whether objective and subjective measures of sleep duration and quality are associated with incidence of calcification over five years and whether calcification risk factors mediate the association.
Design
Observational cohort 2000–2006. Potential confounders (age, sex, race, education, apnea risk, smoking status) and mediators (lipids, blood pressure, body mass index, diabetes, inflammatory markers, alcohol, depression, hostility, self reported medical conditions) were measured at both baseline and follow-up. We examined sleep metrics (wrist actigraphy measured duration and fragmentation, daytime sleepiness, overall quality, self-reported duration) for association with incident calcification.
Setting
Home monitoring of healthy middle-aged population.
Participants
494 participants from the CARDIA cohort Chicago site (African American and white men and women aged 35–47 at baseline) with sleep, demographic and calcification measurements and no detectable baseline calcification.
Outcome
Coronary artery calcification was measured by CT in 2000–2001 and 2005–2006; incidence of new calcification over that time was the primary outcome.
Results
Five-year calcification incidence was 12.3% (n=61). Longer measured sleep duration was significantly associated with decreased adjusted odds of calcification (OR = 0.67 per hour, p=.011; 95% CI 0.49–0.91). No potential mediators appreciably altered the magnitude or significance of sleep (adjusted OR estimates ranged from 0.64 to 0.68 per sleep hour, maximum p-value=.020). Alternative sleep metrics were not significantly associated with calcification.
Conclusions
Longer measured sleep is associated with lower calcification incidence independently of examined potential mediators and confounders.
Introduction
Coronary artery calcification, the accumulation of calcified plaques visible by computed tomography [1], is a subclinical predictor of future coronary heart disease events [2, 3]. Risk factors for calcification include established heart disease risk factors such as male sex, older age, glucose intolerance, tobacco use, dyslipidemia, high blood pressure, obesity, raised inflammatory markers, and low educational attainment [4,5,6].
Recent experimental and epidemiological data implicate sleep quantity and quality as correlates of several of these risk factors, including glucose and appetite regulation [7], hypertension [8], inflammation [9], sex, age, education [10], and obesity [11]. However, some of these correlations have only been documented in studies where sleep is measured by self-report, which may be biased or insufficiently accurate [12,13,14]. Because of these associations, we set out to test whether objectively-measured sleep duration and other sleep characteristics predict calcification and if so whether calcification risk factors mediate this relationship.
Using sleep data collected in an ancillary study to the Coronary Artery Risk Development in Young Adults (CARDIA) study, we analyzed whether objective and subjective sleep measures predicted the development of incident calcification over five years of follow-up.
Materials and Methods
Study Sample
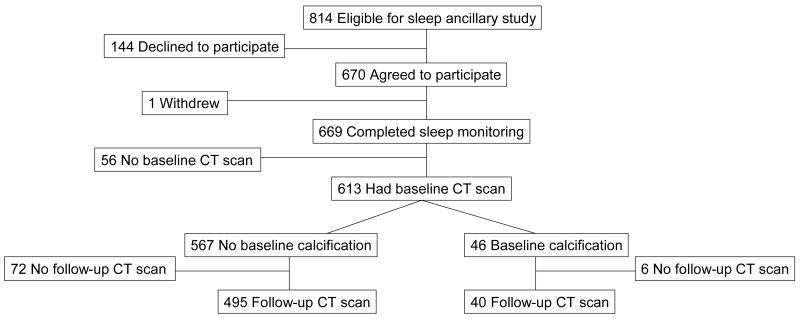
CARDIA is an ongoing, prospective, multicenter cohort study of the evolution of cardiovascular risk factors. The original CARDIA cohort was aged 18–30 years in 1985–1986 and was balanced by sex, race (black and white), and education. A detailed study description has been presented elsewhere [15]. The ancillary sleep study included participants from one of the four CARDIA sites: Chicago. Figure 1 illustrates the derivation of the sample used in the main analysis from the initial cohort. Non-pregnant participants in the clinical examination in year 15 of CARDIA (2000–2001) (n = 814) were invited to participate in the sleep study in 2003 and 2004; 670 (82%) agreed to do so. CARDIA participants were re-examined in 2005–2006, providing five-year follow-up. Data on sleep, described below, were collected between CARDIA years 15 (termed “baseline” in this report) and year 20 (termed “follow-up”), as described below. All participants gave informed written consent; the protocol was approved by the institutional review boards of Northwestern University and the University of Chicago and by the CARDIA Executive Committee. Participants were paid $50 for each wave of actigraphy, largely to encourage return of the monitors.
Figure 1.
Study Participant Flow Diagram
Outcome: Coronary Artery Calcification
Two scans were obtained using electron beam computed tomography (Imatron C-150, GE Medical Systems, Milwaukee, Wisconsin) at baseline and follow-up for CARDIA participants, a methodology described previously [16]. Scans were read centrally; each participant’s scans were read independently blinded to all participant characteristics. The reader identified a region of interest for each potential focus of calcification, defined as 4 or more adjacent pixels (1.87 mm2) with a CT number >130 Hounsfield units (field of view = 35 cm). Agatston scores [17] were adjusted for between-center differences using a standard calcium phantom scanned underneath each participant, and summed across the four major coronary arteries to compute a total calcium score. Biweekly calibrations were conducted using a standard torso insert to guard against between center and temporal variability. The presence of calcification was defined as having a positive, non-zero Agatston score, using either of 2 scans [5]. Among sleep study participants, 535 have both baseline and follow-up scans.
Main Exposure: Sleep Measures
Sleep data were collected by the ancillary study in two waves, about one year apart. The first wave began approximately three years after the baseline examination (2003). All participants were asked to wear a wrist activity monitor (Actiwatch-16, Mini-Mitter Inc, Bend, OR) for Wednesday through Saturday in both waves, six total nights per participant. Wrist activity monitors contain highly sensitive omnidirectional accelerometers that count wrist movements in 30-second epochs [18]. For each night of actigraphy data collection, the time in bed when the participant was trying to sleep was also collected, using both an event marker button on the actigraph (which did not affect motion recording) and a sleep log which asked them to record the exact time that they began trying to fall asleep and when they got out of bed as a backup in case of missing event markers. The software only analyzed these specified periods for sleep. Wrist actigraphy has been validated against polysomnography, demonstrating a correlation over 0.9 in healthy subjects for total sleep duration [19]. Unlike polysomnography, actigraphy does not appear to alter sleep behavior, as there is no “first night effect” [20]. Using manufacturer-supplied software, we calculated total sleep duration and sleep fragmentation. Fragmentation, an index of restlessness, was calculated by summing the percentage of time spent “sleeping” when the subject is moving and the percentage of all immobile periods that last a minute or less. We used this as an objective measure of sleep quality. Further explanation of our actigraphy method has been reported elsewhere [10].
Self-reported habitual sleep duration was collected in the baseline CARDIA questionnaire. The ancillary study also included three validated sleep questionnaires: the Pittsburgh Sleep Quality Index [21], a 21-point scale of overall sleep quality and disturbance; the Epworth Sleepiness Scale, a 24-point scale of daytime sleepiness [22]; and the Berlin Questionnaire, an apnea risk measure that classifies an individual as high-risk for apnea if two of these three conditions are present: 1) loud or frequent snoring or frequent breathing pauses, 2) being frequently tired after sleeping or during wake time or having fallen asleep while driving, or 3) having high blood pressure or a body mass index of >30 kg/m2 [23].
Covariates
Baseline questionnaires ascertained demographic information, alcohol consumption, smoking, and self reported diagnosis or treatment for the following conditions: gout, thyroid disease, HIV, liver disease, any heart condition, cancer, stroke, peripheral vascular disease, kidney disease, migraine, gallbladder disease, diabetes, dylipidemia, hypertension, gastrointestinal disease, depression, other mental or mood disorders, asthma. We categorized educational attainment into four levels: less than a high school degree, a high school degree or equivalent, some college, college graduate. Alcohol consumption was summarized as weekly mean milliliters of alcohol and categorized as non-drinkers, less than seven drinks per week, and seven drinks or more per week. Smoking was categorized as never-smokers, former smokers, and current smokers. Additional questionnaires measured the Center for Epidemiologic Studies Depression scale [24], the Cook-Medley Hostility subscale of the Minnesota Multiphasic Personality Inventory [25], and the Framingham Type A Scale [26]. Total physical activity was interrogated by the CARDIA physical activity history questionnaire described elsewhere [27]. These questionnaires were readministered at follow-up. Hostility was measured at an earlier examination (year 7).
Clinical examination results such as weight and height and most laboratory values were measured at both baseline and follow-up. Blood pressure was measured three times for each participant while seated. A Hawksley random-zero sphygmomanometer was used at baseline and an Omron HEM907XL at follow-up; we use calibrated systolic values and the mean of the second and third readings. For twelve hours prior to each examination, participants were asked to fast. For the two hours prior to each exam, participants were asked to avoid smoking and heavy physical activity. As reported elsewhere [5], plasma total cholesterol, high-density lipoprotein cholesterol, and triglycerides were determined using an enzymatic assay by Northwest Lipids Research Laboratory (Seattle, Washington); low-density lipoprotein cholesterol was calculated using the Friedewald equation [28]. Serum glucose was measured using hexokinase coupled to glucose-6-phosphate dehydrogenase by Linco Research (St. Louis, Missouri) [4]. C-reactive protein was measured using the BNII nephelometer from Dade Behring with a particle enhanced immunonepholometric assay [29]. These measures were all available at both the baseline and follow-up examinations. Interleukin-6 was measured by ultra-sensitive ELISA (R&D Systems, Minneapolis, MN) [29], and was only available at the follow-up examination. Plasma fibrinogen measurements were performed at the follow-up examination using an immunoassay. Total fibrinogen concentration for this assay was determined at the University of Vermont using immunonephelometry (BNII Nephelometer 100 Analyzer; Dade Behring, Deerfield, IL, USA). The amount of immuno-reactive fibrinogen present in the sample was quantitatively determined by light scatter intensity [30].
The Framingham Risk Score and 10-year estimated risk were calculated from baseline variables according to the recommendation of the National Cholesterol Education Program’s Adult Treatment Panel III recommendations [31].
Statistical Analysis
We dichotomized calcification as incident detectable calcification versus none because of the statistical distribution of the amount of calcification; there is a substantial floor effect. The prevalence of detectable calcification was low (40/535) at the baseline exam. We focus on incident calcification rather than increased calcification because, once developed, calcification has repeatedly been shown to expand exponentially [32], a feature that is duplicated in our dataset (39/40). Positive follow-up calcification occurred among less than one-fifth of the participants (101/535); among those the intensity was generally low (<20 AU in 59%). Sensitivity analyses were conducted using an alternative threshold for calcification of >10 AU rather than >0 AU. We used logistic regression to analyze the association of incident calcification with actigraph-measured sleep duration and four alternate sleep metrics: self-reported duration, daytime sleepiness and subjective and objective measures of sleep quality (Pittsburg Sleep Quality Index and fragmentation). These models were all adjusted for key potential confounders: age, sex-race group, educational attainment, smoking, and apnea risk. One participant missing educational attainment data was excluded from these regressions.
To explore potential mediators of the calcification-sleep relationship, we added covariates to the adjusted model of sleep duration: body mass index, fasting glucose, serum C-reactive protein, low-density lipoprotein, high-density lipoprotein, triglycerides, fibrinogen, interleukin-6, depression scores, systolic blood pressure, alcohol consumption, Framingham Type A scores, physical activity, Framingham Risk, Cook-Medley hostility scores, and indicator variables for each of the self-reported disease diagnosis and treatment categories ascertained at baseline. If a variable mediates the sleep-calcification association, we would expect to see attenuation of the coefficient for sleep when the mediator is added. For covariates measured at both examinations, we included both baseline and five-year change as covariates. Each variable was standardized to a Z-score (mean=0, variance=1) using the baseline standard deviation. Because of correlations among the potential mediators, testing all of them simultaneously was not illuminating. We regressed the above confounder-adjusted model with the addition of each potential mediator (and its five-year change if available) one at a time. We also tested a model simultaneously adjusting for key heart disease risk factors: low-density lipoprotein, high-density lipoprotein, systolic blood pressure, body mass index, diabetes, age, sex, race, education, smoking, and apnea risk. Ratios of regression coefficients were calculated to quantify how much difference in an established risk factor (systolic blood pressure) was equivalent to a difference of one hour sleep.
Interaction terms between each covariate and sleep were tested for heterogeneity of the sleep effect. We conducted regressions within racial groups (retaining sex as a covariate), within sex groups (retaining race as a covariate) and within apnea risk groups to further identify heterogeneity among these groups. Regression diagnostics were performed, and data points examined for excessive influence. Additionally, we repeated the regression using only the participants who reported fasting for eight hours prior to both clinical examinations. We also repeated the main analysis using relative risk (Poisson) regression in place of logistic regression.
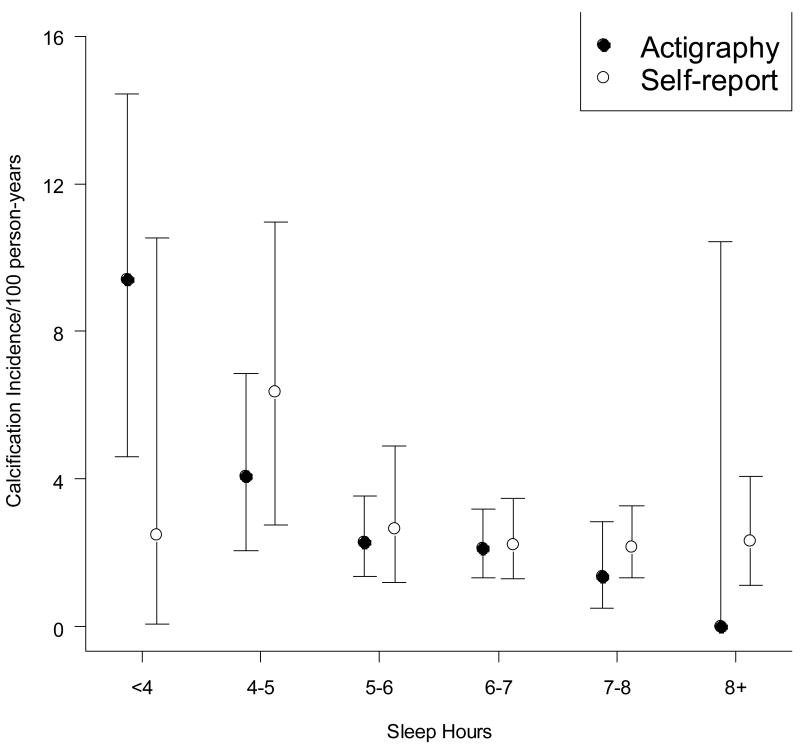
Participants, non-participants, and non-follow-up subjects were compared by two sided t-tests and Fisher’s exact test where appropriate using a.05 significance threshold (Table 1). Regression coefficients were tested for significance at the.05 level using two-sided tests. Unadjusted tests for trends among hour-groupings were calculated by logistic regression (Figure 2). 95% Confidence intervals for hour-group incidence (Figure 2) were computed by exact binomial methods.
Table 1.
Characteristics of study participants versus non-participants as well as at-risk participants (ie, baseline scan negative) with follow-up scans versus those without; mean(SD) and percent(number). Columns are compared by t-test and Fisher’s exact test where appropriate.
| Eligible for Sleep Subtudy | Participants with Zero Baseline Calcification | |||||
|---|---|---|---|---|---|---|
| Non-participants | Participants | p-value | Rescanned | Not rescanned | p-value | |
| N | 144 | 670 | 495 | 72 | ||
| Self-Reported Sleep (hr) | 6.5(1.4) | 6.5(1.2) | .745 | 6.5(1.2) | 6.5(1.4) | .574 |
| Measured Sleep (hr) | - | 6.1(1.1) | - | 6.1(1.0) | 6.1(1.3) | .925 |
| Baseline Calcification Prevalence | 9.9%(13) | 7.6%(47) | .378 | - | - | - |
| Followup Calcification Incidence | 14.5%(11) | 12.3%(61) | .597 | 12.3%(61) | - | - |
| Race-Sex Groups | .002 | .292 | ||||
| White Male | 30.6%(44) | 26.4%(177) | 25.7%(127) | 38.5%(20) | ||
| Black Male | 26.4%(38) | 16.1%(108) | 15.6%(77) | 23.6%(17) | ||
| White Female | 17.3%(25) | 27.9%(198) | 30.1%(149) | 26.4%(19) | ||
| Black Female | 25.7%(37) | 29.6%(187) | 28.7%(142) | 22.2%(16) | ||
| Age | 39(3.7) | 40(3.6) | .048 | 40(3.5) | 39(3.9) | .042 |
| Current Smokers | 20.8%(30) | 21.0%(141) | .999 | 18.2%(90) | 27.8%(20) | .078 |
| High Density Lipoprotein (mg/dl)* | 50(15) | 51(15) | .690 | 52(15) | 48(12) | .023 |
| Low Density Lipoprotein (mg/dl)* | 115(32) | 113(32) | .458 | 111(30) | 118(34) | .114 |
| Systolic Blood Pressure (mmHg) | 112(13) | 111(14) | .492 | 110(14) | 112(12) | .355 |
| Diagnosed Diabetes | 7.6%(11) | 10.6%(29) | .072 | 4.4%(22) | 5.6%(4) | .841 |
| Framingham Ten-Year Risk% | 1.6(1.8) | 1.8(2.6) | .394 | 1.7(2.3) | 2.1(3.7) | .215 |
1 mmol/l high-density or low-density lipoprotein cholesterol= 39 mg/dl
Figure 2. Coronary calcification incidence by mean sleep duration.
Confidence intervals are 95% binomial intervals; test for trends is logistic regression of incidence on group value (ie, lowest category = 3; highest category = 8). For continuous adjusted analysis see Table 3. Three self-reports were missing.
All statistical analysis was carried out using Stata 9.0 SE (StataCorp, College Station, TX).
Results
Table 1 compares baseline demographic, self-reported sleep and cardiovascular risk characteristics of eligible sleep study participants who did versus did not enroll in the sleep study, and sleep study participants at risk for incident calcification with and without follow-up calcification data. Sleep study participation did not vary by self-perceptions of usual sleep hours (p=0.745). Of the 72 at-risk without follow-up scans, 35 did not return for year-20 follow-up at all, and 37 were not rescanned (scheduling difficulties were often the reason). Measured sleep was very similar for those with and without outcome data (p=0.925).
Table 2 provides descriptive statistics of study participants. Where laboratory values were recorded as integers, we have grouped them as close to tertiles as possible. Figure 2 displays the unadjusted relationship between actigraphic and self reported sleep hours and calcification incidence; the proportion of persons developing calcification decreases monotonically as actigraphic sleep hours increases. Self report sums to a lower number of observations due to non-response in the initial CARDIA questionnaire.
Table 2.
Study sample characteristics among CARDIA participants with sleep data and with baseline and follow-up coronary calcification measurements.
| Category | N | Sleep hours Mean(SD) | Baseline Calcification Prevalence %(N) | Five Year Calcification Incidence %(N) |
|---|---|---|---|---|
| All Subjects | 535 | 6.1(1.0) | 7.5(40) | 12.3(61) |
| Sex/Race | ||||
| White Male | 144 | 6.1(.88) | 11.8(17) | 20.5(26) |
| Black Male | 85 | 5.2(1.1) | 9.4(8) | 23.4(18) |
| White Female | 157 | 6.7(.84) | 5.1(8) | 4.7(7) |
| Black Female | 149 | 5.9(.92) | 4.7(7) | 7.0(10) |
| Age (years) | ||||
| <38 | 121 | 6.0(1.1) | 3.3(4) | 8.5(10) |
| 38–41 | 193 | 6.1(1.0) | 7.3(14) | 10.1(18) |
| 42+ | 219 | 6.0(1.0) | 9.6(21) | 16.7(33) |
| Smoking Status | ||||
| Never | 337 | 6.1(1.0) | 5.0(17) | 10.9(35) |
| Past | 93 | 6.3(.93) | 8.6(8) | 9.4(8) |
| Current | 105 | 5.8(1.2) | 14.3(5) | 20.0(18) |
| High Density Lipoprotein Tertile (mg/dl)* | ||||
| (14,43) | 177 | 5.8(1.0) | 10.2(18) | 25.2(40) |
| (43,56) | 179 | 6.1(1.0) | 7.3(13) | 7.2(12) |
| (57,114) | 173 | 6.3(1.0) | 4.6(8) | 5.5(9) |
| Low Density Lipoprotein Tertile (mg/dl)* | ||||
| (39,98) | 174 | 6.1(1.1) | 5.2(9) | 9.7(16) |
| (98,124) | 171 | 6.1(.96) | 5.3(9) | 7.4(12) |
| (125,209) | 175 | 6.1(1.0) | 11.4(20) | 18.7(29) |
| Systolic Blood Pressure Tertile (mmHg) | ||||
| (83,103) | 170 | 6.3(.94) | 4.1(7) | 6.7.(11) |
| (104,114) | 181 | 6.1(1.0) | 6.6(12) | 13.0(22) |
| (115,181) | 184 | 5.9(1.1) | 11.4(21) | 17.2(28) |
| Diagnosed Diabetes | ||||
| No | 508 | 6.1(1.0) | 7.5(38) | 12.1(57) |
| Yes | 23 | 6.0(1.1) | 4.3(1) | 13.6(3) |
| Framingham Ten-Year Risk% | ||||
| <=1% | 402 | 6.1(1.0) | 4.7(19) | 9.1(35) |
| 2–4% | 97 | 6.0(1.0) | 14.4(14) | 20.5(17) |
| >=5% | 36 | 6.0(.96) | 19.4(7) | 31.0(9) |
| Apnea Risk | ||||
| Low | 465 | 6.1(1.0) | 6.9(32) | 12.0(52) |
| High | 70 | 5.8(1.0) | 11.4(8) | 14.5(9) |
Table 3 presents the associations of sleep metrics and calcification from logistic regression. Unadjusted logistic regression yields a significant reduction in the odds of incident calcification with increasing measured sleep duration; the unadjusted odds ratio is 0.57 (95% CI 0.44–0.73) per hour. Adjusting for age, sex, race, education, smoking and apnea risk, longer measured sleep duration was associated with reduced calcification incidence: an odds ratio of 0.67 (95% CI 0.49–0.91) per hour. Additionally adjusting for key cardiovascular risk factors had little effect on the odds ratio for measured sleep. None of the alternate metrics was significantly associated with incident calcification (Table 3).
Table 3.
Logistic regression results of incident coronary calcification on separate models with alternate sleep measurements.
| Covariate | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Actigraph-measured Sleep (per hour)* | 0.57 | (0.44,.73) | <.001 |
| Actigraph-measured Sleep (per hour)** | 0.67 | (0.49, 0.91) | 0.01 |
| Actigraph-measured Sleep (per hour)*** | 0.66 | (0.48, 0.92) | 0.01 |
| Self-reported Sleep (per hour)** | 0.87 | (0.67, 1.13) | 0.30 |
| Fragmentation Index (per SD=7.7 points)** | 1.07 | (0.80, 1.42) | 0.66 |
| PSQI Score (per SD=2.9 points)** | 1.21 | (0.88, 1.65) | 0.24 |
| Epworth Score (per SD=4.0 points)** | 1.26 | (0.96, 1.66) | 0.10 |
Unadjusted (n=495)
Models are adjusted for race, sex, age, smoking, education, and apnea risk (n=494).
Model additionally adjusted for BMI, HDL, LDL, BP, diabetes (n=478).
Stratifying by sex suggested a stronger measured sleep duration effect for women (N=291; sleep OR=0.48, 95% CI 0.27–0.85) than for men (N=203; sleep OR=0.76, 95% CI.52–1.10), however the interaction term in a combined model was not significant (p=.122). In stratified regression models there was no suggestion that effects vary by race: OR=0.61, 95% CI 0.39–0.96 for whites (N=275) and OR=0.64, 95% CI 0.41–1.02 for blacks (N=219). In combined regression, the interaction term of race and sleep was not significant (p=.916). Stratifying by apnea risk, there was a suggestion of a stronger effect for those at high risk (OR=0.38, 95% CI 0.13–1.10) compared to low risk (OR=0.72, 95% CI 0.52–1.01), but the interaction was not significant (p=.507).
In all of the models that singly included the potential mediators listed in the Methods section (individual results not shown), none of the potential mediators substantially changed the sleep coefficient (although many had main effects on calcification incidence), or caused the p-value for the coefficient for sleep to fall below 0.05; adjusted odds ratios for measured sleep ranged from 0.64 to 0.68, and the largest p-value for sleep hours was 0.020. No interaction terms between sleep and other covariates were found to be significant.
The modeled effect of one additional hour of sleep on the odds of incident calcification was equal to the modeled effect of a 16.5 mmHg decrease in systolic blood pressure.
Results for a sensitivity analysis with a higher cut-point for positive calcification (>10 Agatston units) were similar (adjusted OR =0.63, 95% CI 0.44–0.90). Use of relative risk regression (Poisson regression) did not substantially change the result: RR for gain of 1 hour = 0.75, p=0.011, 95% CI 0.60–0.93.
Discussion
We have found a robust and novel association between objectively measured sleep duration and five-year incidence of coronary artery calcification. One hour more sleep decreased the estimated odds of calcification by 33%. Figure 2 shows that the (unadjusted) dose-response relationship held up across the range of measured sleep; the lack of significant heterogeneity by race or sex strengthened this finding. Controlling for potential confounders and mediators did not greatly attenuate the relationship, as seen by multiply-adjusted odds-ratios ranging only between 0.64 and 0.68 and significant adjusted p-values. While this is surprising, there exists a large amount of unexplained variance in cardiac outcomes and hence potential for previously unidentified risk factors. The magnitude of the observed effect was similar to sizable differences in established coronary risk factors, e.g. one additional hour sleep reduced risk similarly to a reduction of 16.5 mmHg systolic blood pressure.
Our study has several limitations. First, too few participants had calcification at baseline for us to examine the rate of further calcification among them. Second, our first wave of sleep measures was taken more than halfway through the period between baseline and follow-up. While calcification may have occurred before sleep measurements, there is no obvious reverse causation mechanism. Finally, actigraphy is unable to measure potentially important dimensions of sleep such as sleep stages, which may underlie the apparent association between duration and calcification. Sleep quality is multidimensional, and there is no perfect metric for measuring it; the apnea-hypopnea index from polysomnography is probably the closest to a “gold standard.” Actigraph-measured fragmentation has not been widely used in research, although one recent study did find a significant correlation between it and obesity in the elderly [33]. How actigraphic fragmentation relates to other measures of sleep quality remains unclear.
However, actigraphy provides several advantages compared to self-reported sleep. Previous data indicate that self reported sleep is only weakly correlated to total sleep time from polysomnography (r = 0.16) [12] but that actigraphy is highly correlated to polysomnography total sleep time (r > 0.90) [19,20]. Actigraphy measured sleep has been shown to be relatively stable year-to-year in this cohort [34], indicating that our measure likely represents sleep duration throughout the study. Actigraphy avoids potential biases in self-reported sleep duration caused by the perception of fatigue in states of poor health [14].
While some participants at risk do not have follow-up data (Table 1), we do not see evidence that omitting them is likely to affect our conclusions. There were a few significant differences between those with follow-up scans and those without, but we adjust for these factors in regression analysis. Importantly, the mean measured and self-reported sleep levels were very similar between those with and without follow-up data.
Because of the well-established association between apnea and cardiac outcomes [35,36,37], our lack of a clinical apnea diagnosis is the study’s main limitation. We used the Berlin Questionnaire to identify high-risk individuals. For apnea to bias our results away from the null hypothesis, apnea must be more prevalent among individuals with short sleep duration, and the Berlin Questionnaire must be so inaccurate as to leave significant residual confounding. Different studies have reported both longer and shorter sleep durations for apnea patients [38–42]. The Berlin Questionnaire has been found to have high sensitivity (0.86) and moderate specificity (0.77) [23], meaning our high-risk group should include almost all of the persons with apnea and a moderate number without. If we stratify by apnea risk, however, the sleep effect among those with low apnea risk is quite similar to the effect in the whole sample (0.72, 95% CI 0.2, 1.01), suggesting that residual apnea confounding is not likely to be responsible for our positive results. The small effect of apnea risk on incidence in Table 2 is likely a result both of the inclusion of persons without apnea in the high-risk group and also the large effect on baseline prevalence; that is, persons with apnea were not in our “at-risk” cohort because they had already developed calcification before baseline.
Calcification as an endpoint also has strengths and weaknesses. Calcification tends to increase over time [32] and is a potent risk factor for coronary events [2,3]. By observing persons in early middle-age we have reduced the possibility that unmeasured health problems confound the association [14]. However, early calcification is not a clinical outcome and coronary events may not necessarily follow.
We have not been able to find previous literature directly relating sleep and calcification. However, we note that previous studies have established a relationship between self-reported sleep duration and related outcomes, such as hypertension [8,43] and coronary events [44]. Sleep apnea has been linked to calcification in a clinical population [35] as well as to heart disease in population-based cohorts [36,37]. Contrary to others [43,44], we find no evidence of a U-shaped relationship. However, such a relationship might be impossible to find in this study population because so few had more than eight hours measured sleep. Also, our sleep measurement avoided the potential problem that self-reports of long sleep are confounded by health factors [14]. Finally, the long sleep-cardiac outcome association could be a feature that emerges at older ages.
We highlight three possible mechanisms to explain this association. First, the determinants of sleep duration are poorly understood, although socioeconomic correlations [10] exist. There may be unknown common factors predicting both sleep and calcification. Second, we may have been unable to adequately assess mediating mechanisms. Our inflammatory marker data are incomplete; fibrinogen and interleukin-6 were available only at follow-up. Cortisol profiles, which have been correlated to both calcification [45] and sleep [46], were not investigated. More frequent measurements may be needed to capture the activity of hypothesized mediators. For example, transient decreases in glucose tolerance following evenings of short sleep [47] might not be detected at either examination. Third, unmeasured diurnal variation of calcification pathways may be at work. For example, blood pressure declines during sleep [48] and significantly predicts [4] calcification incidence.
Future studies will be needed for crucial extensions to these result. First, these results need confirmation in other cohorts. Second, does sleep moderate the rate at which calcification accumulates? Third, will objective sleep tie to coronary disease event outcomes over the long term? While calcification predicts such outcomes, it is difficult to know how and if the predictors of calcification themselves will determine outcomes, or if their impact will be purely mediated by their effect on calcification. Finally, if this association is born out, interventional studies will be needed to guide clinical advice.
Acknowledgments
Research for this study was supported by grant AG 11412 from the National Institute on Aging and the Medical Scientist National Research Service Award T 32 GM07281. CARDIA is supported by US Public Health Service contracts NO1-HC-48047, NO1-HC-48048, NO1-HC-48049, NO1-HC-48050, and NO1-HC-95095 from the National Heart, Lung, and Blood Institute. No funding organization or sponsor aside from the National Heart, Lung, and Blood Institute played a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Diane Lauderdale had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosures: None of the authors have financial interests or affiliations related to the topic of this manuscript aside from those listed on the title page.
Works Cited
- 1.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–62. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 2.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 3.Greenland P, Bonow RO, Brundage RH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk Factors for the Progression of Coronary Artery Calcification in Asymptomatic Subjects: Results From the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 5.Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, Williams OD, Bild DE, Detrano R. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007 May 22;49(20):2013–20. doi: 10.1016/j.jacc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Yan LL, Liu K, Daviglus ML, et al. Education, 15-year risk factor progression, and coronary artery calcium in young adulthood and early middle age: the Coronary Artery Risk Development in Young Adults study. JAMA. 2006;295:1793–1800. doi: 10.1001/jama.295.15.1793. [DOI] [PubMed] [Google Scholar]
- 7.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007 Jun;11(3):163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analysis of the First National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 9.Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci. 2007 May;64(10):1174–86. doi: 10.1007/s00018-007-6529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauderdale D, Knutson K, Yan L, et al. Objectively measured sleep characteristics among early middle-aged adults: The CARDIA Study. Am J Epidemiology. 2006;164(1):5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 11.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield S. Inadequate sleep as a risk factor for obesity: analysis of the NHANES I. Sleep. 2005;28:1265–1272. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 12.Silva GE, Goodwin JL, Sherrill DL, Arnold JL, Bootzin RR, Smith T, Walsleben JA, Baldwin CM, Quan SF. Relationship between reported and measured sleep times: the sleep heart health study (SHHS) J Clin Sleep Med. 2007 Oct 15;3(6):622–30. [PMC free article] [PubMed] [Google Scholar]
- 13.Lauderdale DS, Knutson KL, Yan LJ, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008 November;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knutson KL, Turek FW. The U-shaped association between sleep and health: the 2 peaks do not mean the same thing. Sleep. 2006 Jul 1;29(7):878–9. doi: 10.1093/sleep/29.7.878. [DOI] [PubMed] [Google Scholar]
- 15.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 16.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005 Jan;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990 Mar 15;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 18.Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H. The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Perceptual & Motor Skills. 1997;85(1):207–16. doi: 10.2466/pms.1997.85.1.207. [DOI] [PubMed] [Google Scholar]
- 19.Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27:158–65. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- 20.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 22.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 23.Netzer N, Stoohs R, Netzer C, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 24.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999 May;46(5):437–43. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 25.Cook W, Medley D. Proposed hostility and pharisaic-virtue scales for the MMPI. J Appl Psychol. 1954;238:414–8. [Google Scholar]
- 26.Evans PD, Moran P. The Framingham type A scale, vigilant coping, and heart-rate reactivity. J Behav Med. 1987;10:311–21. doi: 10.1007/BF00846544. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exercise. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- 29.Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. 2007 Mar-Apr;13(3–4):178–84. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiner AP, Carty CL, Carlson CS, Wan JY, Rieder MJ, Smith JD, Rice K, Fornage M, Jaquish CE, Williams OD, Tracy RP, Lewis CE, Siscovick DS, Boerwinkle E, Nickerson DA. Association between patterns of nucleotide variation across the three fibrinogen genes and plasma fibrinogen levels: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Thromb Haemost. 2006 Jun;4(6):1279–87. doi: 10.1111/j.1538-7836.2006.01907.x. [DOI] [PubMed] [Google Scholar]
- 31.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 32.Yoon HC, Emerick AM, Hill JA, Gjertson DW, Goldin JG. Calcium begets calcium: progression of coronary artery calcification in symptomatic subjects. Radiology. 2002;224:236–241. doi: 10.1148/radiol.2241011191. [DOI] [PubMed] [Google Scholar]
- 33.van den Berg JF, Knvistingh Neven A, Tulen JH, Hofman A, Witteman JC, Miedema HM, et al. Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. Int J Obes (Lond) 2008;32:1083–1090. doi: 10.1038/ijo.2008.57. [DOI] [PubMed] [Google Scholar]
- 34.Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA study. Sleep. 2007 Jun 1;30(6):793–6. doi: 10.1093/sleep/30.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung HH, Han H, Lee JH. Sleep apnea, coronary artery disease, and antioxidant status in hemodialysis patients. Am J Kidney Dis. 2005 May;45(5):875–82. doi: 10.1053/j.ajkd.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Marin JM, Carrizo SJ, Vicente E, Augusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2000;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 37.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–618. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 38.Hedner J, Pillar G, Pittman SD, Zou D, Grote L, White DP. A novel adaptive wrist actigraphy algorithm for sleep-wake assessment in sleep apnea patients. Sleep. 2004;27:1560–6. doi: 10.1093/sleep/27.8.1560. [DOI] [PubMed] [Google Scholar]
- 39.Hastings PC, Vazir A, O’Driscoll DM, Morrell MJ, Simonds AK. Symptom burden of sleep-disordered breathing in mild-to-moderate congestive heart failure patients. Eur Respir J. 2006;27:748–755. doi: 10.1183/09031936.06.00063005. [DOI] [PubMed] [Google Scholar]
- 40.García-Díaz E, Quintana-Gallego E, Ruiz A, Carmona-Bernal C, Sánchez-Armengol A, Botebol-Benhamou G, Capote F. Respiratory polygraphy with actigraphy in the diagnosis of sleep apnea-hypopnea syndrome. Chest. 2007;131(3):725–32. doi: 10.1378/chest.06-1604. [DOI] [PubMed] [Google Scholar]
- 41.Zou D, Grote L, Peker Y, Lindblad U, Hedner J. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep. 2006 Mar 1;29(3):367–74. doi: 10.1093/sleep/29.3.367. [DOI] [PubMed] [Google Scholar]
- 42.Patel SR, Blackwell T, Redline S, Ancoli-Israel S, Cauley JA, Hillier TA, Lewis CE, Orwoll ES, Stefanick ML, Taylor BC, Yaffe K, Stone KL. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008 Oct 21; doi: 10.1038/ijo.2008.198. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29(8):1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 44.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003 Jan 27;163(2):205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 45.Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006 Sep-Oct;68(5):657–61. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- 46.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997 Oct;20(10):865–70. [PubMed] [Google Scholar]
- 47.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999 Oct 23;354(9188):1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 48.Staessen J, Bulpitt CJ, O’Brien E, Cox J, Fagard R, Stanton A, Thijs L, Van Hulle S, Vyncke G, Amery A. The diurnal blood pressure profile. A population study. Am J Hypertens. 1992 Jun;5(6 Pt 1):386–92. doi: 10.1093/ajh/5.6.386. [DOI] [PubMed] [Google Scholar]