Abstract
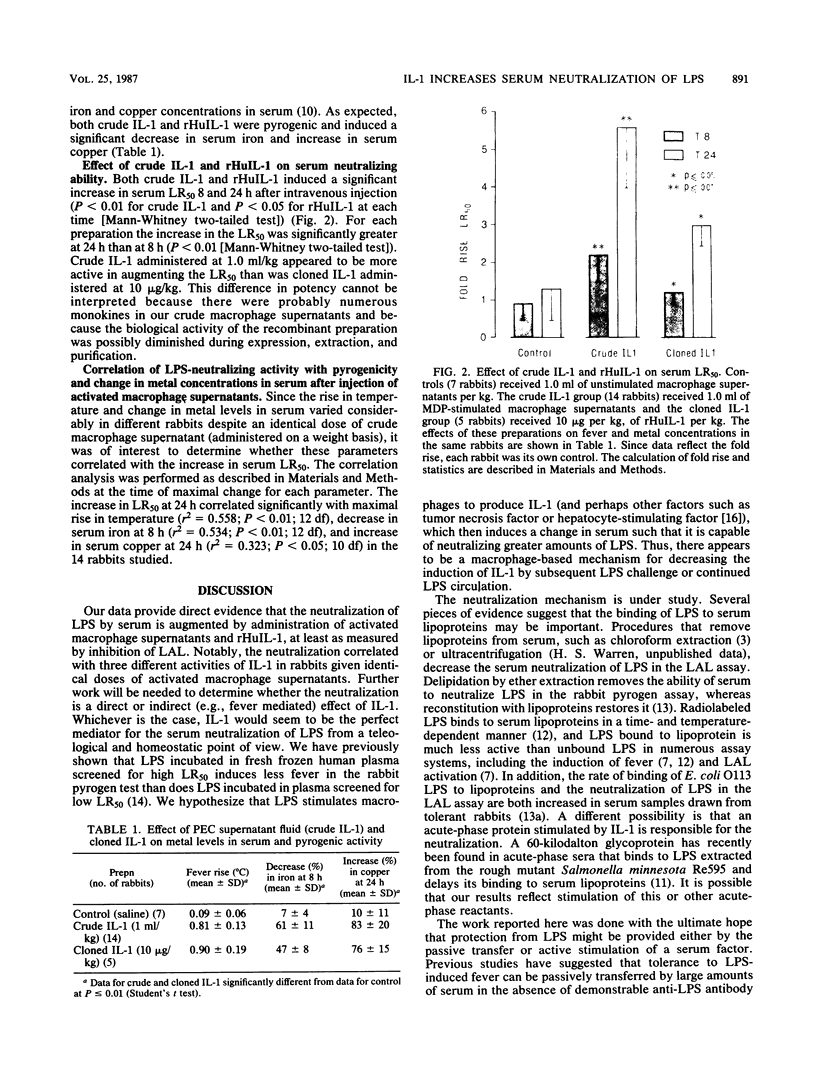
We have previously described an assay to quantify the serum neutralization of bacterial lipopolysaccharide which is based on a spectrophotometric Limulus amoebocyte lysate test (T.J. Novitsky, P.F. Roslansky, G.R. Siber, and H.S. Warren, J. Clin. Microbiol. 21:211-216, 1985). Studies since have shown that serum samples drawn from patients with leukemia and fever, gram-negative or gram-positive bacterial infections, or shock caused by gram-negative bacteria neutralize approximately 10-fold more lipopolysaccharide than do samples from normal controls. These findings suggested that the increased neutralization might reflect an acute-phase response and raised the question of whether it might be under the control of interleukin-1. To answer this question, we studied the neutralization of lipopolysaccharide in serum samples drawn from rabbits before and after the administration of crude interleukin-1, prepared from activated macrophage supernatants, and recombinant human interleukin-1. Crude interleukin-1 induced a 5.7-fold increase in serum neutralization 24 h after intravenous injection, and cloned interleukin-1 induced a 3.0-fold increase (P less than or equal to 0.01 and 0.05, respectively). In individual rabbits given identical doses of crude interleukin-1 on a weight basis, the serum-neutralizing ability correlated significantly with three activities of interleukin-1: rise in temperature (r2 = 0.558; P less than or equal to 0.01), decrease in serum iron (r2 = 0.534; P less than or equal to 0.01), and increase in serum copper (r2 = 0.323; P less than or equal to 0.05). We conclude that the increase in neutralization of bacterial lipopolysaccharide by serum samples drawn from patients with inflammatory states is mediated, at least in part, by interleukin-1, presumably through the induction of acute-phase serum proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dinarello C. A., Cannon J. G., Mier J. W., Bernheim H. A., LoPreste G., Lynn D. L., Love R. N., Webb A. C., Auron P. E., Reuben R. C. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- DuBose D. A., Lemaire M., Basamania K., Rowlands J. Comparison of plasma extraction techniques in preparation of samples for endotoxin testing by the Limulus amoebocyte lysate test. J Clin Microbiol. 1980 Jan;11(1):68–72. doi: 10.1128/jcm.11.1.68-72.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEDMAN H. H. Passive transfer of protection against lethality of homologous heterologous endotoxins. Proc Soc Exp Biol Med. 1959 Nov;102:504–506. doi: 10.3181/00379727-102-25296. [DOI] [PubMed] [Google Scholar]
- Greisman S. E., Young E. J., Carozza F. A., Jr Mechanisms of endotoxin tolerance. V. Specificity of the early and late phases of pyrogenic tolerance. J Immunol. 1969 Dec;103(6):1223–1236. [PubMed] [Google Scholar]
- Greisman S. E., Young E. J., DuBuy B. Mechanisms of endotoxin tolerance. 8. Specificity of serum transfer. J Immunol. 1973 Nov;111(5):1349–1360. [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Lipton J. M., Dietschy J. M. Biological activity, lipoprotein-binding behavior, and in vivo disposition of extracted and native forms of Salmonella typhimurium lipopolysaccharides. J Clin Invest. 1982 Oct;70(4):877–888. doi: 10.1172/JCI110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
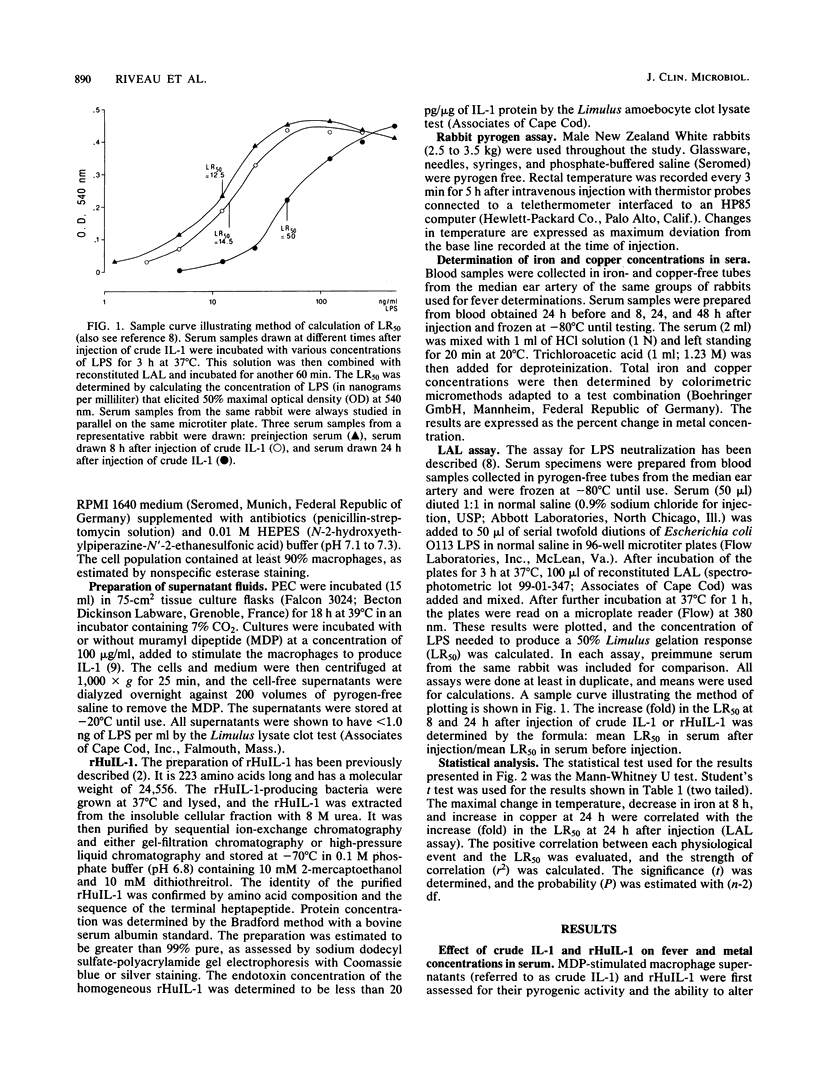
- Novitsky T. J., Roslansky P. F., Siber G. R., Warren H. S. Turbidimetric method for quantifying serum inhibition of Limulus amoebocyte lysate. J Clin Microbiol. 1985 Feb;21(2):211–216. doi: 10.1128/jcm.21.2.211-216.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveau G., Parant M., Chedid L. Changes in rabbit febrile responses to muramyl dipeptide (MDP) after coupling to a synthetic carrier. J Leukoc Biol. 1984 Aug;36(2):111–122. doi: 10.1002/jlb.36.2.111. [DOI] [PubMed] [Google Scholar]
- Riveau G., Parant M., Damais C., Parant F., Chedid L. Dissociation between muramyl dipeptide-induced fever and changes in plasma metal levels. Am J Physiol. 1986 Apr;250(4 Pt 1):C572–C577. doi: 10.1152/ajpcell.1986.250.4.C572. [DOI] [PubMed] [Google Scholar]
- Tobias P. S., Ulevitch R. J. Control of lipopolysaccharide-high density lipoprotein binding by acute phase protein(s). J Immunol. 1983 Oct;131(4):1913–1916. [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R. The modification of biophysical and endotoxic properties of bacterial lipopolysaccharides by serum. J Clin Invest. 1978 Dec;62(6):1313–1324. doi: 10.1172/JCI109252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF S. M., MULHOLLAND J. H., WARD S. B., RUBENSTEIN M., MOTT P. D. EFFECT OF 6-MERCAPTOPURINE ON ENDOTOXIN TOLERANCE. J Clin Invest. 1965 Aug;44:1402–1409. doi: 10.1172/JCI105245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Knights C. V., Siber G. R. Neutralization and lipoprotein binding of lipopolysaccharides in tolerant rabbit serum. J Infect Dis. 1986 Nov;154(5):784–791. doi: 10.1093/infdis/154.5.784. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Novitsky T. J., Ketchum P. A., Roslansky P. F., Kania S., Siber G. R. Neutralization of bacterial lipopolysaccharides by human plasma. J Clin Microbiol. 1985 Oct;22(4):590–595. doi: 10.1128/jcm.22.4.590-595.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Novitsky T. J., Martin P., Roslansky P. F., Siber G. R. Endotoxin neutralizing capacity of sera from different patient populations assessed by the Limulus lysate test. Prog Clin Biol Res. 1987;231:341–348. [PubMed] [Google Scholar]
- Woloski B. M., Fuller G. M. Identification and partial characterization of hepatocyte-stimulating factor from leukemia cell lines: comparison with interleukin 1. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1443–1447. doi: 10.1073/pnas.82.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]