Confirmation of patency of the portal vein by either ultrasound or angiography is a routine part of the evaluation of patients being considered for hepatic transplantation (1). Complete thrombosis of the portal vein usually has been viewed as precluding successful orthotopic hepatic replacement (1). In addition, some pediatric patients present with extremely small (less than 4 to 5 millimeters in diameter) portal veins which, although patent, have proved to be thick walled and sclerotic. Our recent experience has shown that, in both of these situations, successful and complete revascularization of hepatic allografts is quite feasible.
Two patients, ages 47 and 21 years, presented with complete thrombosis of the portal vein. In four pediatric patients ranging in age from one to five years, the portal vein proved to be patent but measured less than 4 millimeters in diameter (Fig. 1). Furthermore, when the vessels were transected, their luminal caliber was found to be further reduced by sclerosis. Another instance involved a seven year old boy who had a portal vein thrombosis with recanalization.
Fig. 1.
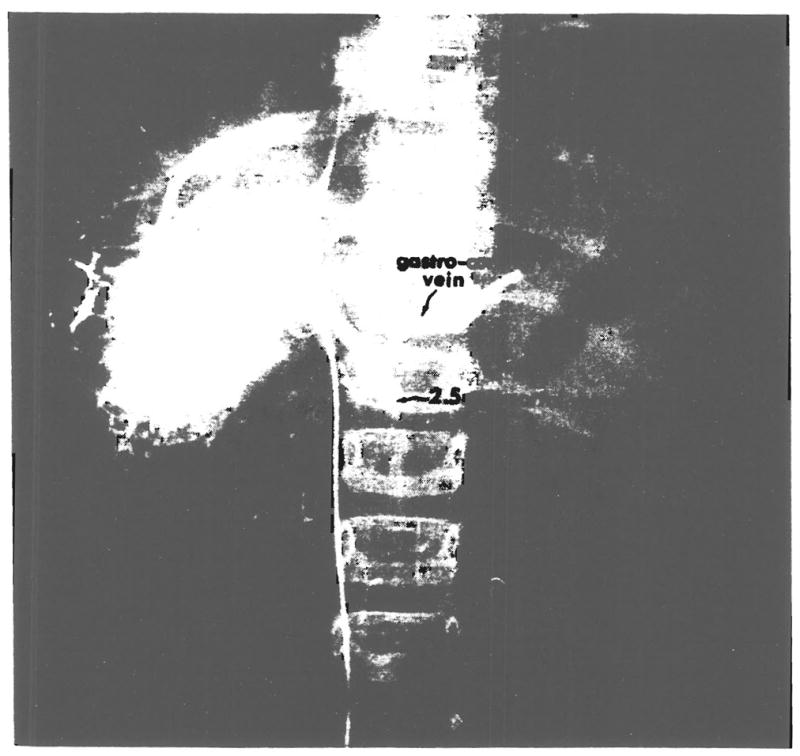
Wedged hepatic venogram showing patent, 2.5 millimeter portal vein.
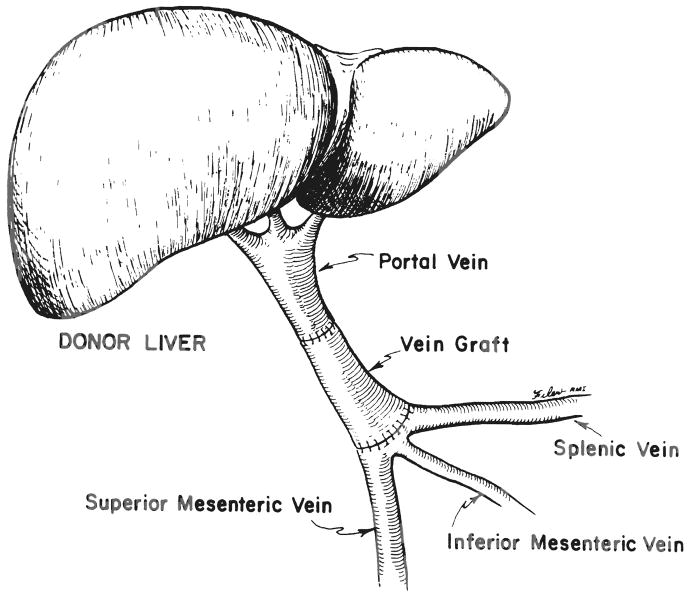
In all seven of these instances, restoration of adequate portal venous flow to the allograft was achieved by dissecting out the area of confluence between the splenic and superior mesenteric veins in order to obtain a patent vessel of adequate caliber. More importantly, in four of these instances, vessel grafts were required in order to span the distance from the more proximal recipient vessel to the hepatic allograft portal vein (Fig. 2). On the other hand, in three instances involving pediatric patients, the portal vein on the hepatic allograft was of adequate length to obviate the use of a vein graft.
Fig. 2.
Vessel graft for portal vein reconstruction.
The vessel grafts were obtained as part of the routine liver procurement procedure as has been our practice for a number of years (2). To this end, following removal of the abdominal organs, the terminal abdominal aorta and inferior vena cava in continuity with the entire complex of iliac vessels are excised, placed in tissue culture medium and transported in ice along with the liver. The usefulness of arterial grafts has been described previously (3).
In the first patient, an iliac vein was used, and in the second and fourth patients, the distal end of the inferior vena cava was a better size match. In the third patient, a donor pulmonary artery was used as a graft because both the inferior vena cava and iliac vein grafts were unsatisfactory.
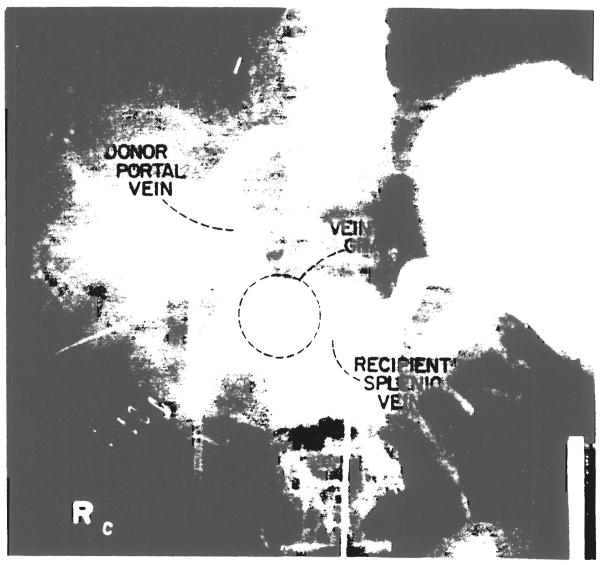
All patients have made uneventful recoveries except for the third patient who died of acute rejection on the tenth postoperative day but was found at autopsy to have a patent portal vein graft. The six surviving patients have now been observed from one to 24 months. Repeat angiograms in the second patient studied, fours weeks after transplantation, showed a patent portal vein (Fig. 3).
Fig. 3.
Angiograms of the second patient showing patent vessel graft.
In the course of the recipient operation for hepatic replacement, early recognition of a thrombosed or otherwise inadequate portal vein, combined with an aggressive approach at obtaining control of the more proximal area of confluence of the splenic and superior mesenteric veins, has resulted in a successful transplantation for several of the patients, one of whom died at a later date because of acute rejection. The dissection required, behind the neck of the pancreas and under the duodenum, has not resulted in any injury to these organs or in additonal hemorrhage.
Acknowledgments
Supported by research grams from the Veterans Administration; by project gram No. AM-29961 from the National Institutes of Health, and by gram No. RR-00084 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health.
References
- 1.Van Thiel DH, Schade RR, Starzl TE, et al. Liver transplantation in adults. Hepatology. 1982;5:637–640. doi: 10.1002/hep.1840020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Halgrimson CG, Koep LJ, et al. Vascular homografts from cadaveric organ donors. Surg Gynecol Obstet. 1979;149:737. [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw BW, Jr, Iwatsuki S, Starzl TE. Alternative methods of hepatic graft arterialization. Surg Gynecol Obstet. 1984;159:490–493. [PMC free article] [PubMed] [Google Scholar]