Abstract
Gastric cancer is a highly virulent neoplasm with high morbidity and mortality. Although the benefit of radiation therapy (RT) combined with chemotherapy in gastric cancer has been established, challenges remain in providing accurate and safe radiation delivery. Improved understanding of patterns of gastric cancer relapse and tumor spread, and of organ motion in the abdomen, has allowed for implementation of more conformal radiation techniques. At a minimum, successful implementation of conformal RT requires a detailed understanding of gastric anatomy and radiobiologic principles, an individualized assessment of organ motion, precise patient immobilization techniques, and adequate physics and dosimetry expertise. To aid the practicing clinician, the Gastric Surgical Adjuvant Radiotherapy Consensus Report and National Comprehensive Cancer Network have recently formulated detailed recommendations on simulation, treatment planning, target volumes, and dose limits for select critical normal structures. The practicing clinician is urged to draw upon the multitude of resources now available to ensure that optimal RT for gastric cancer is delivered safely and accurately.
Gastric cancer is the fourth most common malignancy worldwide.1 In the United States, where it is less common than in other parts of the world, there were an estimated 21,500 new cases and 10,880 deaths from the disease in 2008.2 Surgery is the mainstay of treatment for medically operable, surgically resectable patients. However, following primary resection alone, 60% of patients with invasion through the muscularis propria or with positive lymph nodes have local relapse. For 20%, local relapse in the tumor bed, regional nodes, stump, or anastomosis represents the only site of failure.3 Gastric cancer portends a poor prognosis overall, with an estimated 5-year survival rate of 20%.1
ATTEMPTS TO IMPROVE SURGICAL OUTCOMES: POSTOPERATIVE RADIATION THERAPY
The impetus to evaluate chemoradiotherapy (CRT) in the postoperative setting came from the demonstration of efficacy of CRT for unresectable or sub-totally resected gastric cancer. In an early study, a group from the Mayo Clinic randomized 48 patients with unresectable disease to concurrent CRT with 5-fluorouracil (5-FU) and radiation therapy (RT) at 35 to 40 Gy vs. placebo and noted improved median survival of 13 months vs. 6 months in the CRT group, with 12% of CRT patients surviving 5 years.4 The Gastrointestinal Tumor Study Group (GITSG) randomized 90 patients with positive nodes and gross or microscopic residual disease to postoperative chemotherapy alone with 5-FU and methyl-CCNU vs. postoperative CRT with 50 Gy split course RT and bolus 5-FU.5 With a minimum follow-up of 5 years, a statistically significant disease-free survival (DFS) benefit was noted in the CRT arm (16% vs. 7%, P < .05).
More recently, the Intergroup 0116 trial (INT 0116) firmly established the role of RT in the postoperative setting for gastric cancer. In this trial, 558 patients with stage IB-IV M0 adenocarcinoma of the stomach or gastroesophageal junction were randomized to adjuvant CRT vs. observation following complete resection.6 Adjuvant therapy included a 5-day course of daily 5-FU and leucovorin followed by 45 Gy RT delivered in 25 fractions with modified doses of 5-FU and leucovorin on the first 4 and last 3 days of RT. One month after completion of RT, two additional cycles of 5-FU and leucovorin were delivered. Overall, 68% of patients had stage T3 or T4 disease and 85% had node-positive disease. At 3 years, statistically significant benefits in DFS (48% vs. 31%, P < .01) and overall survival (50% vs. 41%, P < .03) were noted among patients receiving adjuvant CRT. With a median follow-up of 5 years, median survival was 36 months in the CRT arm vs. 27 months in the surgery-only arm. Of note, only 10% of patients underwent the recommended D2 dissection and 54% underwent D0 resection. Given that no differences were noted in rates of distant metastatic disease, the overall survival benefit has been attributed to improvements in locoregional control, suggesting that chemotherapy is exerting its maximum effect as a radiosensitizer.
The Surveillance, Epidemiology, and End Results (SEER) database has been used to evaluate the adoption and efficacy of adjuvant therapy since INT 0116 provided the phase III data justifying adjuvant CRT. For example, Coburn et al used the SEER database to identify patients with gastric cancer diagnosed within the 4 years preceding or following publication of INT 0116 results to determine rates of adoption of adjuvant RT and factors associated with its use.7 This analysis found that use of adjuvant RT doubled in the years following publication of INT 0116. Factors associated with the use of adjuvant RT on multivariate analysis included age, SEER region, marital status, assessed lymph nodes, tumor depth, and nodal status.
Two analyses using the SEER database have specifically examined survival outcomes associated with increased adoption of adjuvant RT in the years following publication of INT 0116. Kozak et al noted significantly improved 3-year overall survival among patients treated after INT-0116.8 The survival advantage was noted among all patients with gastric cancer and among a subset who would have been potentially eligible for inclusion in INT 0116. Similarly, Coburn et al used the SEER database to identify all patients who had undergone resection of nonmetastatic gastric cancer between 2000 and 2003 and compared survival among patients who did vs. did not receive adjuvant RT.9 Significant improvements in median overall survival were noted among patients with stage III and stage IV M0 disease who received adjuvant RT (31 and 20 months, respectively) compared with those who did not receive adjuvant RT (24 and 15 months, respectively).
PREOPERATIVE RADIATION THERAPY
Preoperative CRT offers several theoretic advantages. Intact vasculature unperturbed by surgical manipulation may allow for better chemotherapy penetrability and greater radiosensitivity of aerated cells. Preoperative radiation delivery also allows for improved target identification and, thus, potentially smaller radiation fields, which could minimize treatment toxicity. Preoperative RT may also allow for tumor downstaging and reduced probability of residual microscopic disease at surgery. Finally, delivery of RT prior to surgery helps to ensure that patients receive all components of their multimodality treatment.
Preoperative RT has been evaluated in a number of randomized trials. Zhang et al randomized 370 patients with carcinoma of the gastric cardia to 40 Gy preoperative RT followed by surgery vs. surgery alone.10 At 5 and 10 years, statistically significant improvements were noted in overall survival among patients receiving preoperative RT; at 10 years, overall survival was 20% in the preoperative group vs. 13% in the surgery-alone group (P = .009). In 2002, Skoropad et al reported outcomes among 102 patients with resectable gastric cancer randomized to 20 Gy preoperative RT delivered in 5 fractions vs. surgical resection alone.11 A trend toward improved survival was noted in the RT arm (10-year survival of 32% vs. 18%, P = .555).
In addition to the randomized trials, several single-arm prospective trials or retrospective analyses have suggested efficacy of preoperative CRT.12–18 Ajani et al conducted a prospective multi-institutional trial of preoperative CRT in patients with operable localized gastric adenocarcinoma to assess rates of R0 resection and pathologic complete response, safety, and survival.12 Preoperative CRT consisted of two 28-day cycles of 5-FU, leucovorin, and cisplatin followed by 45 Gy of radiation with concurrent 5-FU. Among 33 eligible patients, rates of R0 resection and pathologic complete response were 70% and 30%, respectively, and median survival was 33.7 months. Patients who achieved a partial or complete pathologic response were noted to have significantly longer median survival than those patients with less than a partial response (63.9 vs. 12.6 months, P < .03).
These findings provided the impetus for the Radiation Therapy Oncology Group 9904 trial, a phase II trial of preoperative CRT for localized stage IB, II, or III gastric cancer.19 The treatment regimen consisted of two cycles of induction 5-FU, leucovorin, and cisplatin followed by concurrent CRT and surgical resection. Among 43 patients enrolled, the pathologic complete response rate was 26%, 77% underwent R0 resection, and 50% underwent D2 resection. Toxicity was felt to be acceptable. Based on encouraging results in this trial, the authors suggested a randomized comparison of preoperative vs. postoperative CRT. Fiorica et al published a metaanalysis in 2007 that included nine randomized trials, four of surgery alone vs. preoperative radiotherapy and five of surgery alone vs. postoperative CRT.20 Both 3- and 5-year mortality were significantly reduced with preoperative RT, and 5-year mortality was significantly reduced with adjuvant CRT.
As reviewed above, numerous data now indicate a survival benefit associated with RT for gastric carcinoma. Nevertheless, actual delivery of adjuvant RT poses a significant challenge. Major or minor treatment errors were identified in 35% of RT plans submitted for INT 0116, and 36% of patients were unable to complete the prescribed course of CRT in INT 0116 due to toxicity. In the trial, 54% of patients in the CRT group experienced grade 3 or higher hematologic toxicity and 33% experienced grade 3 or higher gastrointestinal toxicity.6
CHALLENGES IN DELIVERY OF ADJUVANT RADIATION THERAPY
The primary challenges associated with safe and effective delivery of adjuvant RT are accurate target delineation and critical normal structure avoidance.
Improved Target Delineation
Initial efforts in improving RT following publication of INT 0116 were aimed at improving target delineation. The 2002 Gastric Surgical Adjuvant Radiotherapy Consensus Report discussed gastric anatomy and pathways of tumor spread, described patterns of failure, and detailed treatment planning guidelines to aid in successful implementation of adjuvant RT.3 Based on patterns of locoregional recurrence, this report mandated coverage of the gastric tumor bed, the anastomosis or stumps, and the regional lymphatics. The report made several detailed recommendations to aid in successful design and delivery of postoperative RT, including aggressive nutritional support, oral administration of barium at the time of simulation to identify the anastomosis and gastric stump, review of preoperative computed tomography (CT) scans to identify the preoperative location of the tumor and regional lymphatics, and placement of radiopaque clips at the time of surgical resection. Shortly thereafter, Tepper and Gunderson published a report entitled Radiation Treatment Parameters in the Adjuvant Postoperative Therapy of Gastric Cancer.21 This report provided detailed guidelines on appropriate radiation treatment volumes stratified by primary tumor site within the stomach and by tumor stage.
In acknowledgement of the difficulties in designing postoperative RT fields, the current National Comprehensive Cancer Network (NCCN) guidelines provide detailed recommendations, stratified by tumor site within the stomach, on appropriate margins, nodal regions, and areas at risk that should be encompassed in a radiation field for gastric carcinoma.22 It is emphasized that the selection of treatment technique for postoperative gastric cancer should be site-specific and individualized. Individual anatomic variations should dictate the selective use of advanced RT planning.
For radiation delivery in the preoperative or postoperative setting, the pretreatment diagnostic studies including endoscopic ultrasound, upper gastrointestinal tract series, and CT scans should be used to aid in identification of tumor and pertinent nodal groups. In the postoperative setting, clip placement can be used to aid in identification of the gastric tumor bed, anastomosis, and stumps. Treatment of the residual stomach postoperatively should depend on perceived risk of relapse vs. likelihood of normal tissue morbidity.
For primary tumors of the proximal one-third stomach and gastroesophageal junction, a 3- to 5-cm margin of distal esophagus, the medial left hemidiaphragm, and the adjacent pancreatic body should be included. For primaries of the middle and distal one-third stomach, the pancreatic body and pancreatic head, respectively, should also be included. For distal one-third primaries, the proximal duodenum should be covered if the gastroduodenal junction is involved; the first and second part of the duodenum should be included if treatment is preoperative, and a 3- to 5-cm margin of duodenal stump should be included if treatment is postoperative.
The nodal areas at risk can be stratified by primary tumor site within the stomach. For all subsites, the perigastric, suprapancreatic, and celiac nodes are at risk. For proximal gastric primaries, the adjacent paraesophageal lymph nodes are also at risk and their inclusion in the radiation field should be considered. For both middle and distal one-third gastric primaries, the porta hepatis and pancreaticoduodenal nodes are also at risk. For middle one-third primaries, the splenic hilar nodes are at risk as well. Along with the specific nodal areas at risk based on primary tumor site of origin, the relative risk of nodal metastases at any specific location depends on both the primary tumor site of origin and such other factors as width and depth of gastric wall invasion.
CRITICAL NORMAL STRUCTURE AVOIDANCE
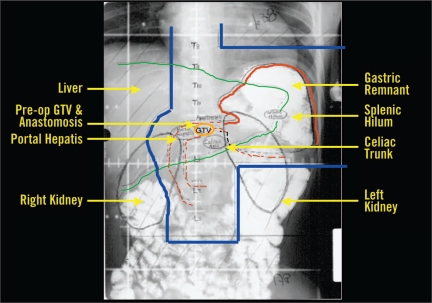
An additional challenge in implementing adjuvant RT for gastric cancer is the close proximity of several dose-limiting critical structures, including kidneys, liver, heart, lung, and spinal cord. Sixty percent of the liver should receive less than 30 Gy, at least two thirds of one kidney should receive no more than 20 Gy, the spinal cord dose should not exceed 45 Gy, and one third of the heart should receive less than 50 Gy. Although specific dose-volume histogram parameters are controversial for the lung, the lung volume and doses should be kept to a minimum. Regarding optimal beam arrangement to encompass the target areas at risk, the INT 0116 trial protocol recommended an AP-PA (anteroposterior-posteroanterior) field arrangement (Figure 1). As reviewed below, numerous recent efforts have concentrated on implementation of more complex field arrangements in an attempt to improve the limitation of dose to critical normal structures beyond what can be achieved using the AP-PA technique.
Figure 1.
Postoperative anterior-posterior radiation field following complete resection of distal gastric cancer.
Abbreviations: pre-op = preoperative; GTV = gross tumor volume.
3-DIMENSIONAL CONFORMAL RADIATION THERAPY
An early retrospective review of 63 patients treated with postoperative RT with or without chemotherapy at the Mayo Clinic between 1980 and 1996 suggested improved toxicity outcomes associated with use of four or more radiation fields.23 In this series, 22% of patients treated with AP-PA techniques developed grade 4 or 5 complications vs. 4% of patients treated with 4 or more fields. Soyfer et al at the Institute of Radiotherapy in Tel Aviv, Israel, implemented a non-coplanar 3-dimensional conformal RT (3D CRT) technique that used four fields, including right and left laterals, an anterior craniocaudal oblique field, and an anterior caudal-cranial oblique field.24 A total of 19 patients each underwent planning using three techniques: non-coplanar 3D CRT, AP-PA, and four-field box. The 3D CRT technique resulted in equivalent clinical target volume coverage with significantly decreased dose to the kidneys and spinal cord.
Leong et al at the Peter MacCallum Cancer Centre in Melborne, Australia, developed a split-field mono-isocentric conformal technique using six radiation fields.25 This technique divides the planning target volume (PTV) into two abutting sections, the upper half including the tumor bed, anastomosis, and splenic hilar nodes and the lower half including the subpyloric, pancreaticoduodenal, and paraaortic nodes. The upper half is treated with an anterior field, a posterior field, and a left lateral field that is angled as necessary to avoid the spinal cord. The lower half is treated with a right lateral, left lateral, and anterior field that are angled to minimize kidney dose. A total of 15 patients were each planned using the split-field conformal technique and a standard AP-PA arrangement. Dose-volume histogram comparisons revealed improved PTV coverage and lower RT doses to the kidneys and spinal cord using the split-field conformal technique.
To aid in target localization, CT simulation with IV and/or oral contrast and 3D treatment planning should be used. Use of an immobilization device is also recommended for set-up reproducibility. Regarding optimal field arrangement, AP-PA fields can be weighted anteriorly to decrease spinal cord dose. A four-field technique is feasible when the target stomach is sufficiently anterior to allow for a 1.5- to 2-cm margin while sparing the spinal cord on the lateral fields. The unconventional multifield arrangements may allow for improved target coverage and critical normal structure avoidance.
It should be noted that without careful target definition, the target volumes at risk that would have been encompassed in APPA or multifield techniques could be excluded when using oblique or noncoplanar beams. Variations in stomach filling and respiratory motion should be taken into consideration when designing optimal field arrangements. Proper determination of treatment volumes should be a multidisciplinary effort including surgical, medical, and radiation oncology input, as well as input from gastroenterologists, radiologists, and pathologists.
INTENSITY-MODULATED RADIATION THERAPY
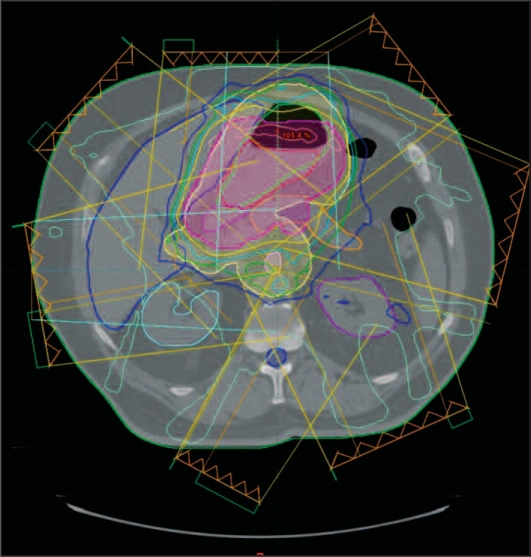
Several recent reports have examined intensity modulated radiation therapy (IMRT) for the delivery of postoperative radiation (Figure 2). In order to assess the potential advantages of IMRT for the delivery of adjuvant radiation, Ringash et al at Princess Margaret Hospital in Toronto, Ontario planned 20 patients with both a conformal five-field technique and a seven- to nine-field coplanar sliding window IMRT technique.26 Blinded gastrointestinal radiation oncologists were provided with dose-volume histograms and organ dose summaries for both plans and asked to select which of the two plans provided optimal PTV coverage and critical organ sparing. For 19 of the 20 cases, a preferred plan was identified; IMRT was the preferred plan in 17 of the 19 cases based on improved PTV coverage and/or improved sparing of the spinal cord, kidneys, liver, and/or heart.
Figure 2.
IMRT reduces the radiation dose to both kidneys. Figure shows isodose distributions of an IMRT plan: the gross tumor volume (red), planning tumor volume of 45 Gy (yellow), and planning tumor volume of 50.4 Gy (violet) are shown on the contours. The purple line represents the 80% isodose.
Wieland et al performed a dosimetric plan comparison of AP-PA, four-field box, step and shoot IMRT, and Peacock tomotherapy among 15 patients who were treated postoperatively for gastric cancer.27 The IMRT plans, compared to conventional 3D planning, reduced dose to the kidney with highest exposure by 50%.
Although most series of IMRT have been limited to dosimetric plan comparisons, one small series described outcomes among 7 patients treated with IMRT.28 The IMRT plans provided excellent target coverage and significantly reduced liver and kidney doses when compared with AP-PA and three-field plans. No patient experienced greater than grade 2 acute gastrointestinal toxicity.
A number of limitations of IMRT were identified in the aforementioned series. For example, the investigators at Princess Margaret Hospital stated that IMRT was associated with reduced dose homogeneity, emphasizing that this could be a concern if, for example, hot spots occurred in the small bowel.26 These investigators also caution that there is a need for detailed information regarding organ motion in the upper abdomen and that implementation of breath hold or gating techniques may be necessary prior to adoption of IMRT in routine clinical practice.
ACCOUNTING FOR ORGAN MOTION
Accounting for tumor motion is a longstanding problem in the practice of radiation oncology. Respiratory motion degrades anatomic position reproducibility during imaging, distorts the shapes of the tumor target, displaces the organs, and causes errors during radiation delivery. Accurate real-time localization of the target with 4- dimensional computed tomography (4DCT) technology may reduce the volume of the normal tissue irradiated and therefore allow a higher conformal radiation dose to be delivered to the target without increasing normal tissue complications.
Significant mobility of abdominal organs secondary to respiratory motion has been demonstrated. Investigators at the University of Pittsburgh obtained 4DCT scans in 13 patients to quantify abdominal organ motion.29 Average superior to inferior organ displacements were 1.3 cm for the liver, spleen, and right kidney and 1.1 cm for the left kidney. Investigators at Princess Margaret Hospital obtained abdominal CT scans in states of free breathing, inhaling, and exhaling for 17 patients undergoing postoperative RT for gastric cancer.10 They subsequently identified several volumes of interest, including those for right and left kidney, liver, stomach, pancreas, celiac axis, and porta hepatis, and then quantified organ motion observed in the respiratory gated scans. Organ motion was found to be significant, with mean 17.5 mm craniocaudal displacement, 5.9 mm AP displacement, and 2.7 mm right to left displacement. Interfraction motion was also significant. The authors recommended that organ motion be incorporated into the PTV for conformal or IMRT planning.
DISCUSSION
Both the annual incidence of and mortality from gastric cancer exceed one million cases worldwide.1 With the benefit of radiation clearly established, the challenge to optimizing RT comes in ensuring accurate and safe delivery. Improved understanding of patterns of gastric cancer relapse and tumor spread and of organ motion in the upper abdomen have allowed for implementation of more conformal radiation techniques, including IMRT. At a minimum, successful implementation of conformal radiation delivery requires a detailed understanding of gastric anatomy and radiobiologic principles, an individualized assessment of organ motion, precise patient immobilization techniques, and adequate physics and dosimetry expertise.
To aid the practicing clinician, the Gastric Surgical Adjuvant Radiotherapy Consensus Report and the NCCN have recently incorporated detailed recommendations on simulation, treatment planning, target volumes, and dose limits for select critical normal structures. Clinicians are urged to draw upon these and other resources now available to ensure that adjuvant radiation for gastric cancer is delivered effectively, accurately, and safely.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Seigel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Smalley SR, Gunderson L, Tepper J, et al. Gastric surgical adjuvant radiotherapy consensus report: rationale and treatment implementation. Int J Radiat Oncol Biol Phys. 2002;52:283–293. doi: 10.1016/s0360-3016(01)02646-3. [DOI] [PubMed] [Google Scholar]
- 4.Moertel CG, Childs DS, Jr, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2:865–867. doi: 10.1016/s0140-6736(69)92326-5. [DOI] [PubMed] [Google Scholar]
- 5.Gastrointestinal Tumor Study Group A comparison of combination chemotherapy and combined modality therapy for locally advanced gastric carcinoma. Cancer. 1982;49:1771–1777. doi: 10.1002/1097-0142(19820501)49:9<1771::aid-cncr2820490907>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 7.Coburn NG, Guller U, Baxter NN, et al. Adjuvant therapy for resected gastric cancer—rapid, yet incomplete adoption following results of Intergroup 0116 trial. Int J Radiat Oncol Biol Phys. 2008;70:1073–1080. doi: 10.1016/j.ijrobp.2007.07.2378. [DOI] [PubMed] [Google Scholar]
- 8.Kozak KR, Moody JS. The survival impact of the Intergroup 0116 trial on patients with gastric cancer. Int J Radiat Oncol Biol Phys. 2008;72:517–521. doi: 10.1016/j.ijrobp.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Coburn NG, Govindarajan A, Law CH, et al. Stage-specific effect of adjuvant therapy following gastric cancer resection: a population-based analysis of 4,041 patients. Ann Surg Oncol. 2008;15:500–507. doi: 10.1245/s10434-007-9640-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZX, Gu XZ, Yin WB, et al. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)—report on 370 patients. Int J Radiat Oncol Biol Phys. 1998;42:929–934. doi: 10.1016/s0360-3016(98)00280-6. [DOI] [PubMed] [Google Scholar]
- 11.Skoropad V, Berdov B, Zagrebin V. Concentrated preoperative radiotherapy for resectable gastric cancer: 20-years follow-up of a randomized trial. J Surg Oncol. 2002;80:72–77. doi: 10.1002/jso.10102. [DOI] [PubMed] [Google Scholar]
- 12.Ajani JA, Mansfield PF, Janjan N, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004;22:2774–2780. doi: 10.1200/JCO.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Ajani JA, Mansfield PF, Crane CH, et al. Paclitaxel- based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237–1244. doi: 10.1200/JCO.2005.01.305. [DOI] [PubMed] [Google Scholar]
- 14.Allal AS, Zwahlen D, Bründler MA, et al. Neoadjuvant radiochemotherapy for locally advanced gastric cancer: long-term results of a phase I trial. Int J Radiat Oncol Biol Phys. 2005;63:1286–1289. doi: 10.1016/j.ijrobp.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 15.Balandraud P, Moutardier V, Gionannini M, et al. Locally advanced adenocarcinomas of the gastric cardia: results of pre-operative chemoradiotherapy. Gastroenterol Clin Biol. 2004;28:651–657. doi: 10.1016/s0399-8320(04)95043-9. [DOI] [PubMed] [Google Scholar]
- 16.Klautke G, Foitzik T, Ludwig K, et al. Neoadjuvant radiochemotherapy in locally advanced gastric carcinoma. Strahlenther Onkol. 2004;180:695–700. doi: 10.1007/s00066-004-9194-z. [DOI] [PubMed] [Google Scholar]
- 17.Lowy AM, Feig BW, Janjan N, et al. A pilot study of preoperative chemoradiotherapy for resectable gastric cancer. Ann Surg Oncol. 2001;8:519–524. doi: 10.1007/s10434-001-0519-1. [DOI] [PubMed] [Google Scholar]
- 18.Wydmañski J, Suwinski R, Poltorak S, et al. The tolerance and efficacy of preoperative chemoradiotherapy followed by gastrectomy in operable gastric cancer, a phase II study. Radiother Oncol. 2007;82:132–136. doi: 10.1016/j.radonc.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Ajani JA, Winter K, Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953–3958. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
- 20.Fiorica F, Cartei F, Enea M, et al. The impact of radiotherapy on survival in resectable gastric carcinoma: a meta-analysis of literature data. Cancer Treat Rev. 2007;33:729–740. doi: 10.1016/j.ctrv.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Tepper JE, Gunderson LL. Radiation treatment parameters in the adjuvant postoperative therapy of gastric cancer. Semin Radiat Oncol. 2002;12:187–195. doi: 10.1053/srao.2002.30827. [DOI] [PubMed] [Google Scholar]
- 22.NCCN Clinical Practice Guidelines in Oncology. Gastric Cancer V.1.2008. Available at http://www.nccn.org/professionals/physician_gls/PDF/gastric.pdf Accessed 11 June 2008.
- 23.Henning GT, Schild SE, Stafford SL, et al. Results of irradiation or chemoirradiation following resection of gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 2000;46:589–598. doi: 10.1016/s0360-3016(99)00446-0. [DOI] [PubMed] [Google Scholar]
- 24.Soyfer V, Corn BW, Melamud A, et al. Three-dimensional non-coplanar conformal radiotherapy yields better results than traditional beam arrangements for adjuvant treatment of gastric cancer. Int J Radiat Oncol Biol Phys. 2007;69:364–369. doi: 10.1016/j.ijrobp.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 25.Leong T, Willis D, Joon DL, et al. 3D conformal radiotherapy for gastric cancer—results of a comparative planning study. Radiother Oncol. 2005;74:301–306. doi: 10.1016/j.radonc.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Ringash J, Perkins G, Brierley J, et al. IMRT for adjuvant radiation in gastric cancer: a preferred plan? Int J Radiat Oncol Biol Phys. 2005;63:732–738. doi: 10.1016/j.ijrobp.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Wieland P, Dobler B, Mai S, et al. IMRT for postoperative treatment of gastric cancer: covering large target volumes in the upper abdomen: a comparison of a step-and-shoot and an arc therapy approach. Int J Radiat Oncol Biol Phys. 2004;59:1236–1244. doi: 10.1016/j.ijrobp.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 28.Milano MT, Garofalo MC, Chmura SJ, et al. Intensity- modulated radiation therapy in the treatment of gastric cancer: early clinical outcome and dosimetric comparison with conventional techniques. Br J Radiol. 2006;79:497–503. doi: 10.1259/bjr/43441736. [DOI] [PubMed] [Google Scholar]
- 29.Brandner ED, Wu A, Chen H, et al. Abdominal organ motion measured using 4D CT. Int J Radiat Oncol Biol Phys. 2006;65:554–560. doi: 10.1016/j.ijrobp.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 30.Ringash J, Wysocka B, Kassam Z, et al. Abdominal organ motion during conformal radiotherapy for gastric cancer. Int J Radiat Oncol Biol Phys. 2006;66:S271–S272. doi: 10.1016/j.ijrobp.2009.04.046. [DOI] [PubMed] [Google Scholar]