Abstract
The incidence of gastric cancer in Turkey, which is between the higher incidence in the Eastern world and lower incidence in the West, is 9.6 cases/100,000 population in men and 5.7/100,000 in women. Mean age at diagnosis is 56 years. Gastric cancer is the second leading cause of cancer deaths in men and the third in women. The relatively high rate of gastric cancer in Turkey appears to be mainly due to dietary factors, and there are etiologic and epidemiologic differences among regions of the country. Although type of surgical resection may vary from center to center, D2 dissection is increasingly preferred in experienced clinics, with an associated mortality of about 3.3%. Adjuvant chemoradiotherapy is widely used. Few chemotherapy regimens are used in advanced disease. Gastric cancer remains an important public health problem in Turkey. With the adoption of healthier dietary practices and screening programs in endemic areas, the mortality from gastric cancer is expected to decrease.
The incidence and mortality of gastric cancer, which was once the most common and deadliest cancer world wide, is decreasing due to a drop in the rate of distal gastric cancer in the Western world. In the United States, for example, gastric cancer was the most common malignancy at the beginning of the 20th century, whereas it currently ranks 13th in men and 9th in women. The global incidence of gastric cancer varies widely by region. High-risk areas include East Asia (China, Japan), Eastern Europe, and parts of Central and South America. Incidence rates are relatively lower in Southern Asia, North and East Africa, North America, Australia, and New Zealand.1
Turkey belongs geographically to both Asia Minor and southeastern Europe, spanning the Anatolian peninsula on the northern Mediterranean Sea in a corner between Europe, Asia, and Africa. Located at a crossroad between Eastern and Western traditions, Turkey has a very rich historic and cultural background. It also has a relatively high rate of gastric cancer. The purpose of this article is to review the available data on the burden of gastric cancer in Turkey, current treatment standards, and efforts directed at reducing the threat of this serious health problem.
INCIDENCE OF GASTRIC CANCER IN TURKEY
Gastric cancer is the second most common cancer, after lung cancer, worldwide, with an estimated 870,000 new cases and 650,000 deaths each year.2 In Turkey, the incidence of gastric cancer is between the higher incidence in the Eastern world and the lower incidence in the West.2,3 It is the second most common cancer in men, following lung cancer, accounting for 8.72% of all tumors in men, with an incidence of 9.6 cases/100,000 population.2,4 Among women, gastric cancer is the third most common cancer, following breast and colorectal cancer, accounting for 6.89% of all cancers, with an incidence of 5.7 cases/100,000 population.2,4
Gastric cancer is the second-leading cause of cancer death in men, after lung cancer, with a crude death rate of 5.84/100,000; and it is the third-leading cause in women, after lung and breast cancers, with a crude rate of 3.7/100,000. The age-standardized incidence rate of gastric cancer for 1998 to 2002 was 12.2 vs. 6.4 per 100,000 for men vs. women, consistent with the male:female incidence ratio of 2:1 observed elsewhere in the world (Table 1).2 The mean age of patients diagnosed with gastric cancer in Turkey is 56 years.
Table 1.
Comparison of gastric cancer incidence in Turkey and the United States
| Gastric cancer incidence | United States | Turkey |
|---|---|---|
| Crude rate | ||
| Male | 8.7/100,000 | 9.6/100,000 |
| Female | 4.7/100,000 | 5.7/100,000 |
|
Age-adjusted rate | ||
| Male | 9.7/100,000 | 12.2/100,000 |
| Female | 4.1/100,000 | 6.4/100,000 |
EPIDEMIOLOGY AND RISK FACTORS
Gastric cancer in Turkey appears to be associated with dietary habits. A case-control study showed that gastric cancer patients in Turkey consumed less fresh fruit, yellow-green vegetables, and meats, while consuming more salted food, condiments, and salt, compared with the control group.5 There was no difference between the two groups with regard to the consumption of starches, fried foods, cereals, milk, dairy products, or tea. Dark black tea, produced in the Black Sea region of the country, is the most common beverage in the country and is consumed in large amounts by both men and women. Although black tea is rich in antioxidant content, consumption of tea was not found to be protective. In Turkey, tea is preferred as a hot drink, which may be associated with thermal trauma, and could be a counterbalancing factor for any potential protective effect against gastric cancer. Alcohol consumption was not associated with increased risk of gastric cancer. Stomach cancer patients brushed their teeth less frequently and had poorer dental health compared with the control group. Refrigeration of food was less common in the gastric cancer group. Many of these risk factors are likely associated with low socioeconomic status.
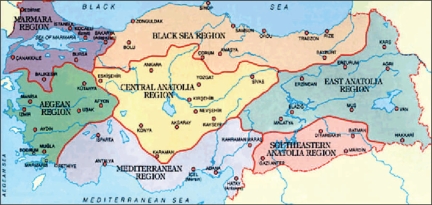
Turkey is a large country of 780,000 square km (approximately 302,535 square miles) with a population of 70.5 million, and etiologic and epidemiologic differences exist among geographical regions with respect to gastric cancer risk (Figure 1). Gastric cancer is seen much more often in the central, northeastern, and eastern regions of the country, where the incidence is almost twice that of colorectal cancer.6,7 Many studies have explored the underlying cause of these differences, with the main risk factors appearing to be dietary. In these regions, food is preserved and cooked in more traditional ways that include salting and pickling; and charcoal, wood, and dried cow dung are still used as fuel for cooking.
Figure 1.
Modern Turkey is bordered by eight countries and geographically divided into seven regions—the Mediterranean, Black Sea, Marmara, Aegean, Central Anatolia, Eastern Anatolia, and Southeastern Anatolia. Numerous etiologic and epidemiologic differences exist among the various geographical regions with respect to gastric cancer risk.
The use of dried cow dung as fuel is especially common in the Van region of eastern Turkey, where gastroesophageal cancers are endemic. Analyses of benzo(a)pyrene and 1,2-benzanthracene levels in cooked foods in this region showed high levels of these carcinogens in bread and meat, indicating a carcinogenic role of traditional foods baked or cooked using animal manure or fuel oil.8 Levels of nitrates and nitrites in all traditional foods and nitrite levels in drinking water were also significantly elevated in the Van region, suggesting that a nitrate- and nitrite-rich traditional diet might play a role in endemic upper gastrointestinal cancers.9
Increased Helicobacter pylori infection also appears to be an important factor in increased risk of gastric cancer in Turkey.10,11 Although studies showed no difference between cases of gastric cancer in western and eastern Turkey in terms of gender, age, anemia, location of the tumor, and frequency of atrophic gastritis, data from 4,065 cases collected from 16 centers between 1990 and 2000 showed that H. pylori was detected significantly more frequently in distal tumors in the east vs. the west (65.7% vs. 38.7%, P = .02).10,11
Other regional variations include differences in seeking health services, as reflected in a greater delay from the beginning of symptoms to diagnosis in the western region when compared to the eastern part of Turkey (6.98 months in the west vs. 4.24 months in the east, P = .0001). However, the rate of tumor resectability was higher in western patients than in eastern patients (63.4% vs. 31.6%, P = .0001). Empirical ulcer treatment was more commonly employed in the east vs. west (52.4% vs. 78.2%, P = .0001). Smoking rates did not differ between groups (48.6% in the east vs. 49.2% in the west), but alcohol consumption was more common in the west (15.5% vs. 6.9%, P = .043). Although the incidence of proximal tumors was higher in the western group, the difference was not statistically significant (26.9% vs. 20.9%, P = .201). Signet ring cell carcinoma was significantly more common in the west (19.0% vs. 12.6%, P = .003). There was not a significant difference in the rate of atrophic gastritis between the two groups (13.9% in the east vs. 21.7% in the west, P = .070). However, H. pylori infection (75% vs. 40.5%, P = .003) and intestinal metaplasia (68.4% vs. 18.1%, P = .0001) were more common in the eastern group.10
In Turkey, tumors of the gastric cardia account for less than 15% of gastric cancers. The intestinal metaplasia rate is 24.9% and atrophic gastritis occurs in approximately 20% of gastric cancer cases. Constituting only 2.6% to 3.6% of all gastric cancers, early gastric cancer is rare in Turkey in comparison to other parts of the world, including Japan (rates of 46% to 50%), Britain (18%), and Korea (16.4%).12
TREATMENT
Surgery
Although type of surgical resection may vary from center to center, and from surgeon to surgeon, D2 dissection is increasingly preferred in experienced surgery clinics; the mortality associated with such resection is approximately 3.3%, decreasing to 1.2% with increasing expertise.13 The rate of morbidity with D2 dissection increases with total gastrectomy (24.4%) and when splenectomy is performed (48.5%), whereas the rate is lower with D2 dissection in distal gastrectomy (9.2%). Following D2 dissection, 75% of recurrences are distant metastases to peritoneum, bone, liver, and lung, whereas 5% are local recurrences and 20% are locoregional recurrences. The 5-year survival rates are 78% in stage I, 42% in stage II, and 35% in stage III disease.
Although the mortality and morbidity from surgical treatment of gastric cancer can be as high as 26.4% and 17.3%, respectively, 14 data from a large cohort of gastric cancer patients receiving D1 and D2 dissection showed mortality of 3.1% and 4.3%, respectively, and morbidity of 6.2% and 27.9%, respectively.14,15 In spite of the significantly increased morbidity (P < .05), D2 dissection was associated with increases in 5-year disease-free survival, from19.0% to 49% (P < .05), and 5-year overall survival, from 36% to 54.0% (P < .05).
In another study reporting an analysis of a large cohort of patients from a single center median, overall survival was 52 months in stage I, 42.4 months in stage II, 33.9 months in stage III, and 9.8 months in stage IV disease.16 These figures indicate that the prognosis of gastric cancer patients in Turkey is comparable to that of patients in Western countries.
The clinicopathologic characteristics of gastric cancer cases undergoing resection were analyzed in a large Turkish Oncology Group study. The mean age of the patients was 57 years (range 19–85 years). Tumors were located in the antrum in 44.5% of cases and in the corpus in 41.3%, whereas cardia tumors accounted for only 7.5% of cases. Disease was stage I in 8.4% of patients, stage II in 14.5%, stage III in 56.7%, and stage IV in 14.5%. Fiveyear survival of the resected patients by stage was 91%, 65%, 33%, and 18%, respectively. Recurrences were mostly locoregional, with recurrence as only distant metastasis being detected in 19.3% of cases.17
Adjuvant Therapy
These relatively favorable results in select patients are not attributable to succesful surgery alone, but also to use of adjuvant therapy. Adjuvant therapy is widely accepted and used in Turkey, with the most common regimen being the Intergroup chemoradiotherapy protocol.18 However, after the reporting of benefits from the MAGIC19 and FNLCC ACCORD07-FFCD 970320 trials, the Turkish Oncology Group initiated a multicenter phase II trial evaluating neoadjuvant therapy in locally advanced gastric cancer. The neoadjuvant regimen consists of two to three cycles of docetaxel, 5-fluorouracil (5-FU), and cisplatin without radiotherapy. Neoadjuvant chemotherapy may be instituted more often, if the study indicates favorable outcomes.
In advanced-stage gastric cancer, several chemotherapy regimens are available. Unfavorable results have been seen with FAM (5-FU/doxorubicin/mitomycin-C), ELF (etoposide/leucovorin/5-FU), and EPE (etoposide/cisplatin/epirubicin) regimens, which have thus fallen out of use.21 Cisplatin plus 120-hour continuous infusion 5-FU (CF), modified epirubicin plus CF (ECF), and EC-UFT (tegafur-uracil) regimens have been most often used. However, since reporting of the TAX-325 study, the docetaxel plus CF (DCF) regimen has now been preferred more commonly.22
Although toxicity such as neutropenic fever and nausea/vomiting can be a problem in patients receiving DCF, neutropenic fever is rare with prophylactic granulocyte colony-stimulating factor and antibiotic use similar to that employed in TAX-324.22,23 Emesis can be controlled successfully with a combination of aprepitant, 5-HT3 antagonists, and steroid premedication. In select Turkish patients, median survival may be as high as 19 months with this regimen.24 Irinotecan-based regimens are usually preferred as a second-line treatment.25
CONCLUSION
As part of the worldwide gastric cancer registry study REGATE, Turkish oncologists are collecting prospective data on patterns of presentation and treatment of gastric cancer. In addition to providing us with current prospective data, our participation in this study will allow us to compare aspects of disease and treatment with current data from other regions of the world, and to monitor changes in presentation and treatment over time.26
Although oncologists are well aware of the gastric cancer problem in Turkey, and experienced in the management of this cancer, gastric cancer remains an important public health problem. Since gastric cancer is decreasing in overall incidence in the Western world, we expect that adoption of more healthy dietary behavior and screening programs in endemic regions will reduce the incidence of, and mortality from, gastric cancer in the foreseeable future.
Footnotes
Disclosures of Potential Conflicts of Interest
Dr. Yalcin indicated no potential conflicts of interest.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2002: cancer incidence. Mortality and prevalence worldwide. IARC cancer base no. 5, version 2.0. Lyon (France): IARC Press; 2004. [Google Scholar]
- 3.Mackay J, Jemal A, Lee NC, et al. The Cancer AtlasAmerican Cancer Society,2006
- 4.Karaoğuz H, İçli H. Cancer problem in Türkiye. J Ankara Med School. 1993;15:547–558. [Google Scholar]
- 5.Demirer T, Icli F, Uzunalimoglu Ö, et al. Diet and stomach cancer incidence: a case-control study in Turkey. Cancer. 1990;65:2344–2348. doi: 10.1002/1097-0142(19900515)65:10<2344::aid-cncr2820651030>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Firat D, Celik I. Cancer statistics in Turkey and in the world. 1993–1995. Turkish Association for Cancer Research and Control; Ankara. 1998. [Google Scholar]
- 7.Tuncer I, Topcu N, Ugras S, et al. Van gölü havzasında gastrointestinal kanserlerin dağılımı; 1002 olgunun analizi. T Klin J Gastroenterohepatol. 2003;14:161–166. [Google Scholar]
- 8.Türkdoğan KM, Dağoglu G, Akman N, et al. Dietary benzo(a)pyrene and 1,2-benzanthracene levels in an endemic upper gastrointestinal (oesophageal and gastric) cancer region of Turkey. J Nutr Environ Med. 2003;13:103–108. [Google Scholar]
- 9.Türkdoğan KM, Testereci H, Akman N, et al. Dietary nitrate and nitrite levels in an endemic upper gastrointestinal (esophageal and gastric) cancer region of Turkey. Turk J Gastroenterol. 2003;14:50–53. [PubMed] [Google Scholar]
- 10.Yalcin B, Zengin N, Aydın F, et al. The clinical and pathological features of patients with gastric cancer in Turkey: A Turkish Oncology Group study. Turk J Cancer. 2006;36:108–115. [Google Scholar]
- 11.Bor S, Vardar R, Ormeci N, et al. Prevalence patterns of gastric cancers in Turkey: model of a developing country with high occurrence of Helicobacter pylori. J Gastroenterol Hepatol. 2007;22:2242–2245. doi: 10.1111/j.1440-1746.2006.04737.x. [DOI] [PubMed] [Google Scholar]
- 12.Buğra D.Erken evre mide kanserine yaklaşım. Cerrahi ilkeler ve Türkiye’de durumMide tümörleri sempzyumu, İstanbul, 17–18 December 2004. Available at: http://www.medonk.com/ppt/mts/Dursun_Bugra.ppt#256
- 13.Demirci S. Unpublished data, partly presented at the National Cancer Congress; Antalya, Turkey. 2003. [Google Scholar]
- 14.Nazli O, Derici H, Tansug T, et al. Survival analysis after surgical treatment of gastric cancer: review of 121 cases. Hepatogastroenterol. 2007;54:625–629. [PubMed] [Google Scholar]
- 15.Yildirim E, Celen O, Berberoglu U. The Turkish experience with curative gastrectomies for gastric carcinoma: is D-2 dissection worthwhile? J Am Coll Surg. 2001;192:25–37. doi: 10.1016/s1072-7515(00)00779-1. [DOI] [PubMed] [Google Scholar]
- 16.Demir G, Buyukunal E, Kizilkilic E, et al. Gastric cancer in Turkey: a single center experience of 683 cases. J Clin Oncol. 2004;22(suppl 14) (abstr 4254) [Google Scholar]
- 17.Demir G, Ünsal D, Zengin N, et al. Opere 840 mide kanserli hastada prognostik faktörler. TOG Gastrointestinal Tümörler Grubu Çalışması, Medical Oncology Congress; Antalya. 2008. (abstr SB15) [Google Scholar]
- 18.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 20.Boige V, Pignon J, Saint-Aubert B, et al. Final results of a randomized trial comparing preoperative 5-fluorouracil/cisplatin to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J Clin Oncol. 2007;25 (abstr 4510) [Google Scholar]
- 21.Icli F, Celik I, Aykan F, et al. A randomized phase III trial of etoposide, epirubicin, and cisplatin versus 5-fluorouracil, epirubicin, and cisplatin in the treatment of patients with advanced gastric carcinoma. Turkish Oncology Group. Cancer. 1998;83:2475–2480. [PubMed] [Google Scholar]
- 22.Ajani JA, Moiseyenko VM, Tjulandin S, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3205–3209. doi: 10.1200/JCO.2006.10.4968. [DOI] [PubMed] [Google Scholar]
- 23.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 24.Kılıçkap S, Ateş Ö, Aksoy S, et al. Metastatik mide kanserinde taksoter-sisplatin-fluorourasil’in etkinlik analizi: diğer tedavi rejimlerine göre sağkalım avantajı sağlar mı? II. Tıbbi Onkoloji Kongresi, Antalya, 2008, (abstr 0 SB13)
- 25.Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–1457. doi: 10.1093/annonc/mdn166. [DOI] [PubMed] [Google Scholar]
- 26.Yalcin S, Roth A, Zalcberg JR, et al. Registry of gastric cancer treatment evaluation (REGATE): baseline characteristics of 10,299 patients from 22 countries, 2009 Gastrointestinal Cancers SymposiumSan Francisco, CAJanuary 15–17, 2009(abstr 43) [Google Scholar]