Abstract
Near-term fetuses of different mammalian species, including humans, exhibit functional sensory and learning capabilities. The neurobiological literature indicates that the unborn organism processes sensory stimuli present in the amniotic fluid, retains this information for considerable amounts of time, and is also capable of associating such stimuli with biologically relevant events. This research has stimulated studies aimed at the analysis of fetal and neonatal learning about ethanol, a topic that constitutes the core of the present review. Ethanol has characteristic sensory (olfactory, taste, and trigeminal) attributes and can exert pharmacologic reinforcing effects. The studies under examination support the hypothesis that low to moderate levels of maternal ethanol intoxication during late pregnancy set the opportunity for fetal learning about ethanol. These levels of prenatal ethanol exposure do not generate evident morphologic or neurobehavioral alterations in the offspring, but they exert a significant impact upon later ethanol-seeking and intake behaviors. Supported by preclinical and clinical findings, this review contributes to strengthening the case for the ability of prenatal ethanol exposure to have effects on the postnatal organism.
Keywords: ethanol, fetal learning, associative conditioning
This review analyzes the impact of low to moderate prenatal levels of exposure to ethanol on later responding to ethanol and stimuli associated with ethanol. These levels of ethanol exposure might be viewed as “safe” but nevertheless have negative effects that might not be noticed for many years.
We previously reviewed the literature, examining possible associations between early ontogenetic experiences with alcohol and subsequent affinity for ethanol ingestion and sensitivity to its reinforcing effects (postabsorptive consequences that increase behaviors aimed at obtaining ethanol or that generate preferences to stimuli signaling such consequences) (1). This review took into account fetal and infantile experiences involving acute or chronic exposure to ethanol and how they exerted significant effects upon subsequent ethanol responsiveness. One of our basic conclusions was that early alcohol exposure can recruit sensory and learning capabilities of the developing organism and this recruitment can result in ethanol-related memories that in turn modulate subsequent patterns of detection and acceptance of ethanol (1). After the publication of this article, we realized that when focusing on prenatal ethanol experiences there were two major problems in understanding how such an immature organism is capable of processing ethanol-related information. The first problem is the teratologic perspective that prevails in studies upon effects of prenatal ethanol exposure: that is, developing organisms are implicitly viewed as relatively passive receptors of a drug that can exert short and long-lasting detrimental effects anatomically and physiologically. These effects can jeopardize sensory and learning capabilities (for reviews see Refs. 2–5). Maybe this image is incomplete. Is it possible that low to moderate levels of exposure to ethanol will recruit functional capabilities of the fetus? If this is true, which consequences might be expected? These questions allude to the second problem of how prenatal ethanol exposure influences later alcohol-seeking or ingestive behaviors. To understand how fetuses might learn about ethanol, we first need to examine ethological and psychobiological studies aimed at identifying the likelihood of prenatal sensory, perceptual, and learning capabilities. In the present article, we first examine the scientific framework associated with fetal functional capabilities in altricial mammals, including humans. This knowledge provides the foundation for analyzing how these capabilities are recruited when the fetus is exposed to low to moderate ethanol levels. We also include recent research concerning neurobiological mechanisms that determine or modulate fetal learning about ethanol and possible interactions between fetal and later ethanol-related experiences. It is our hope that a joint consideration of experimental and clinical issues linked to fetal sensory and learning capabilities will help broaden our conception of how prenatal ethanol exerts effects later in life.
Many studies with animals have shown that prenatal exposure to ethanol increases the offspring’s postnatal responsiveness to ethanol (1). This finding inspired a study aimed at determining whether human newborns exhibit differential responsiveness to ethanol odor as a function of maternal ethanol consumption during pregnancy (6). The hope was to eventually devise a noninvasive tool for diagnosis, at birth, of children at risk of fetal ethanol effects. None of the mothers in this study was diagnosed as an alcoholic, and examination of their intake patterns showed the absence of binge-like drinking episodes or chronic patterns of drinking. The aim was to test the potential diagnostic aid in a relatively normal population rather than in a population in which the expectation of fetal alcohol syndrome was relatively high.
Mothers were subdivided into two groups, infrequent or light drinkers (mothers who drank less than four times a month and whenever they did consume, the overall amount was equal to or less than 8.8 ± 1.7 g of absolute ethanol) versus moderate drinkers (mothers who consumed at least once a week, reaching a level of absolute ethanol ingestion equivalent to 22.1 ± 2.4 g per occasion) (7, 8). None of the babies born to these mothers presented prototypical signs of fetal alcohol syndrome, and the groups did not differ in birth-related morphologic and neurobehavioral parameters. Reactivity of the newborns to ethanol or lemon odor was evaluated through a habituation-dishabituation technique. Habituation refers to a nonassociative learning process characterized by a gradual decrement in responsiveness to a stimulus in a given context. Presentation of a novel stimulus reinstates the level of responding and allows exclusion of processes such as motor fatigue or sensory adaptation that can also be responsible for behavioral decrements as a function of stimulus repetition (9). Babies born to moderate drinkers exhibited, in terms of motor activity, greater responsiveness to ethanol odor than did newborns delivered by infrequent drinkers. There were no behavioral differences in response to lemon odor. The difference between babies of moderate and infrequent drinkers was clear when ethanol was first presented during the habituation phase or when it was employed after habituation to the lemon odor. In a follow-up study Faas et al. (10) reported that the heightened response to ethanol odor in babies born to moderate drinkers was still observable 7 to 10 days after delivery. Facial activity was analyzed by using the Facial Action Coding System developed by Ekman and Friesen (11) to codify the hedonic value of the motor expressions. During habituation, infants born to moderate drinkers exhibited higher positive hedonic reactions (smiling, suckling, and licking) towards ethanol odor than newborns delivered by abstemious or light drinkers. No differences emerged when facial responsiveness elicited by lemon odor were compared or when aversive gestures (e.g., gaping and nose and eye wrinkling) promoted by ethanol or lemon were analyzed (10).
The example in the preceding paragraph illustrates that, despite the absence of physical malformations or changes in the capability to detect and respond to novel olfactory cues resulting from prenatal exposure to low or moderate doses of ethanol, the offspring’s response to ethanol will probably be changed (10). What dose of ethanol intake would be safe during pregnancy? In most countries the governmental recommendation is that no ethanol be consumed during pregnancy, although until recently the recommendation in Great Britain was that one to two drinks on one or two occasions each week is not likely to alter development of the fetus (12). In that context it is notable that two glasses of wine (peak blood ethanol level of 30 mg/100 ml) consumed by a pregnant mother on a single occasion late in gestation has been found to disrupt the fetus’s organization of behavioral states (particularly active sleep), reduce its eye movements, and dramatically suppress its breathing (13). Chronic effects of such a dose are uncertain, although repeated episodes of this or slightly higher amounts of such drinking are known to result, for instance, in correspondingly greater disruption of sleep states (14). Perhaps more important, it is now clear that postnatal consequences of such exposure are quite serious for the risk of ethanol abuse in adolescence (1, 9). In this respect the present review emphasizes a surprising sensitivity to ethanol’s chemosensory features early in development. These chemosensory characteristics allude to the perception of ethanol as a combination of sweet and bitter tastes, retronasal odor volatiles, and oral irritation (15, 16). Also emphasized is the limited exposure to ethanol needed to sensitize the developing animal’s detection of ethanol and disposition to ingest ethanol. An important mechanism in this effect is cognition, illustrated by clear evidence that the fetus engages in learning about ethanol characteristics and its associates. In the following sections of this review, we will analyze fetal sensory and learning capabilities and how these processes can modulate subsequent ethanol intake or seeking patterns.
Basic Sensory and Learning Capabilities of the Fetus
Animal Studies
Definitive experiments to assess the effects of ethanol on brain function require tests of animal models and could not ethically be conducted with humans. The most frequent animals tested for this purpose are rodents, which have a number of scientific advantages. For instance, the rate of brain development in this animal is remarkably rapid. The rat brain is a good bit more immature at birth than is the human’s. In humans, the brain goes through a period of rapid physical growth, beginning in the third trimester and continuing into postnatal life. In rats, the brain growth spurt (part of the third-trimester equivalent) occurs primarily during the first and second postnatal week, yet within the next 2 weeks the rapid growth of the rat’s nervous system yields a brain equivalent in maturation to that of a 10- to 12- year-old human (17, 18). This allows study of the effects of ethanol on the fetal rat brain late in gestation, which will be seen to promote subsequent affinity for ethanol and its abuse. Such a model also allows tests of the effects of ethanol on the postnatal animal when its brain corresponds in some significant ways to that of the human fetus and subsequently during a period corresponding to the human’s early childhood.
Until the beginning of the past decade, the prenatal environment was viewed as affording limited possibilities, at most, for the fetus to acquire information from its surroundings. Neurophysiologic and anatomic development, not mental activity, was seen as the business of gestation. Since then, however, the scientific community has developed techniques and strategies enabling tests of the fetus’s cognitive processing of a variety of stimuli encountered in the womb.
There is now a general agreement that the fetus is exposed to considerable stimulation in utero and that maternal behavior and physiology contribute significantly to fetal experience (19–23). Studies to assess maternal activity in the rat during pregnancy have shown that fetuses are exposed to a dynamic series of different stimuli. When pregnant females ambulate or climb walls, fetuses suffer lineal or angular acceleration. Mechanical pressure is generated when the mother displays behaviors such as self-grooming, and episodes of vibration are caused by hind limb scratching. In other words, maternal activities transmit sensory experiences as acceleration, pressure, and vibrations to fetuses. In general, we could consider the uterine environment as highly stimulating for the offspring, especially during the last stages of gestation (21). The capability to react to cutaneous, vestibular, and thermal sensory cues emerges very early during mammalian ontogeny (24). Neonatal re-exposure to a variety of stimuli experienced during late gestation or during vaginal delivery triggers significant behavioral (general activity) and autonomic (heart rate) changes indicative of orienting responses to familiar stimuli (25).
In rat and mouse fetuses the main olfactory system appears to be the dominant chemosensory system mediating prenatal olfaction (26, 27). The accessory olfactory systems also may undergo stimulation during prenatal life (28, 29). Coppola and Millar (29) suggest that the vomeronasal organ actively samples stimuli during prenatal life in the rat, but other studies suggest that the vomeronasal organ is unlikely to function in the prenatal period in mice or rat fetuses, because the anatomic route for external stimuli to reach the receptor surface is blocked during this stage (27, 30).
Near-term rat fetuses are sensitive to a variety of intraorally administered chemosensory cues in gas or liquid phases. For example, lemon, mint, and cyclohexanone elicit fixed motor action patterns (e.g., facial wiping) that anticipate postnatal behaviors directed by airborne olfactory cues (22, 31). Autonomic changes (e.g., bradycardia) indicative of olfactory processing have also been observed after intraoral stimulation with salient chemical substances (32).
Prenatal chemosensory experiences not only elicit fetal orienting responses but also may modulate several behavioral patterns during postnatal stages. For initial postnatal feeding (suckling), diet preferences as well as maternal-infant interactions seem to be modulated by prenatal chemosensory experiences. During and after labor, the dam licks herself, and this behavior results in the placement of amniotic fluid cues on its ventrum to guide neonates toward nipples. The first suckling response in newborn rats is partially guided by the chemosensory characteristics of the amniotic fluid (32). When this fluid has been contaminated during late gestation with an arbitrary odorant (e.g., lemon), neonates effectively attach to a nipple scented with the prenatally experienced cue (21). In addition, prenatal experiences with specific chemosensory cues can determine long-lasting behavioral effects. Smotherman (33) reported that rats given only one administration of an apple juice solution into the amniotic fluid during gestational day (GD) 20 showed a clear preference for apple juice during adulthood.
Habituation, one of the simplest learning processes, has been observed in many species during the course of late prenatal development. For example, the administration of lemon flavor directly into the oral cavity of a rat fetus increases its general motor activity, considerably reduces its heart rate, and elicits facial wiping behavior. After repeated stimulation with lemon odor, behavioral and autonomic responding stabilizes, reaching basal levels of responsiveness. The possibility that progressive decrements in responding to the lemon odor were due to fatigue rather than habituation was ruled out through the use of novel stimuli that reinstated the original levels of responding (34, 35).
Near-term fetuses are also capable of associating independent stimuli presented in close temporal contiguity. Prenatal associative memories have been generated as early as GD 17 by employing a conditioned taste aversion paradigm. Generally, a salient chemosensory stimulus (conditioned stimulus, CS) administered directly into the amniotic fluid is paired with an intraperitoneal injection of LiCl (lithium chloride) that induces a toxic aversive state (36–38). Rats treated in this way prenatally avoid suckling from maternal nipples odorized with the CS, take longer than controls to approach a dam tainted by the CS, and spend less time in nesting material odorized with this chemosensory cue (37).
Long-term effects of prenatal associative learning have also been evaluated during late adolescence (39). Pregnant rats on either GD 15 and GD 16 or GD 18 and GD 19 had access to a garlic solution that was or was not associated (controls) with the induction of LiCl toxicosis. During adolescence, garlic consumption patterns were evaluated. Adolescents given garlic paired with LiCl prenatally exhibited reliable conditioned taste aversions, an effect that was particularly robust when conditioning took place during GD 18 and GD 19.
Prenatal conditioned aversions seem to be mediated by glutamate receptors. Mickley et al. (40) evaluated the generation of aversive conditioned responses in fetuses previously given an NMDA receptor antagonist (ketamine). Rat fetuses (GD 19) were given injections of the NMDA receptor antagonist or saline and then exposed to saccharin associated with LiCl toxicity. When re-exposed to saccharin (GD 21), fetuses given ketamine injections failed to exhibit changes in facial responsiveness seen in controls given saline injections. Apparently blockade of glutamatergic receptors interfered with the acquisition and/or expression of fetal conditioned aversion.
The rat pup’s first postnatal suckling response to a maternal nipple is essential for the survival of the neonate. A technique based on the use of a surrogate nipple, applied to the fetus as well as the neonate, has allowed systematic examination of how suckling behavior develops and how early learned experiences modulate subsequent nipple attachment (41). In near-term fetuses (GD 20–GD 21) a single experience comprising access to a surrogate nipple explicitly paired with milk infusions is sufficient to establish conditioned nipple-grasping responses (42, 43). The point is to illustrate the rat fetus’s capability for learning and memory to which we shall return.
Human Studies
As is the case in other altricial species (see the preceding text), the sense of smell in humans is functional long before birth. During the last gestational trimester the human main olfactory system is functional in terms of odor detection and discrimination and also appears to mediate olfactory learning and memory processes (for reviews see Refs. 44, 45). The first suckling response in humans seems to be guided by orosensory cues previously perceived by neonates in utero (46). The suckling behavior of human neonates generally is modified by changes in the maternal diet or by the incorporation of novel sensory cues into milk (47–49).
Human neonates are also capable of recognizing and discriminating chemosensory cues previously encountered in the uterus, implying fetal learning. As originally demonstrated by Schaal et al. (50), newborns detect the amniotic fluid odor and remain attracted to it for at least 2 days after delivery. In a follow-up study these authors demonstrated that 3-day-old newborns preferentially orient to the odor of the biological mother, showing clear familiarization effects of the prenatal experience (31, 51, 52). A few hours after birth, human neonates show orienting head movements, attachment, and suckling behaviors when exposed to the maternal breast scented with amniotic fluid that was collected during labor (45). The scent of the amniotic fluid also exerts calming effects (crying decrements) for babies separated from their biological mother. These effects are significantly higher in magnitude than those recruited by only maternal breast odor, which emphasizes the prenatal olfactory contribution (53). This capacity for human neonates to orient to odors encountered as fetuses was an impetus for the study by Faas et al. (10) mentioned in the introduction of the present paper.
The neonate’s preference for smells experienced in utero decreases during the first days of postnatal life as a function of new experiences with alternative odorants. Babies evaluated 4 or 5 days after birth clearly prefer odors characteristic of the maternal breast over chemosensory stimuli that were present in utero (54). Marlier et al. (55) studied whether human neonates are capable of discriminating intrauterine olfactory cues from odorants typically perceived during breastfeeding. Two-day-old babies are capable of recognizing the scent of the amniotic fluid as well as that of colostrum when these cues are presented independently. They show similar preferences for these odorants in a two-way location test. At 4 days of age, the infants are still capable of discriminating the odors of amniotic fluid and maternal milk, but now they clearly show preferences for the milk. These results suggest that babies go through a period of transition in terms of their preference for biological chemosensory cues, from amniotic fluid to colostrum, and then to maternal milk (55, 56). As mentioned, morphologic and histologic studies in mammals indicate that olfactory subsystems responsible for chemosensory perception start developing during early gestational stages. In the human being, these systems are functional during late gestation, even when they have not yet completed maturity (57). Additional studies suggest that vomeronasal epithelial cells are already functional in 5-month-old fetuses (58).
As is the case in various animal species (e.g., rabbits [59, 60], sheep [50], and rats [33, 61, 62]), prenatal human experiences derived from chemosensory constituents of the maternal diet have a profound effect upon later responsiveness to these constituents. For example, orofacial responsiveness to the smell of anise has been evaluated in human neonates whose mothers had or had not consumed anise-flavored sweets during the last 2 weeks of pregnancy. Babies of mothers who had not consumed anise expressed more negative orofacial responses toward this stimulus than babies prenatally exposed to this cue through their mothers’ ingestion of anise. Mouthing responses to anise were also higher in the babies prenatally exposed to this odor (62). Prenatal chemosensory experience in humans apparently can shape orofacial correlates of hedonic processing in the infant.
In summary, substantial neuroethologic research conducted during the past two decades has shown that the nearterm fetus is sensitive to chemosensory stimuli administered directly into the amnion or that result from maternal ingestion. Prenatal exposure to these stimuli determines postnatal detection of related chemosensory events and helps regulate hedonic responsiveness to them. Studies also indicate that the fetus can associate flavored stimuli with aversive (e.g., lithium) and appetitive (milk) unconditioned stimuli. We now examine how tests of these phenomena inspired and have merged with the analysis of fetal exposure to ethanol.
Learning About Ethanol in the Fetus
Before examining fetal responsiveness to ethanol and the possibility of prenatal associative learning mediated by ethanol’s reinforcing effects, we will briefly provide an overview of how developing rats react to and encode information about ethanol (1, 63). This overview should help clarify the basis for short-term and long-term effects of fetal exposure to ethanol.
Under a variety of experimental circumstances, infantile familiarization with ethanol odor has been observed to promote olfactory preferences for ethanol and heightened ethanol consumption. These effects can be detected soon after ethanol exposure or even during adolescence and adulthood (64–66). Infant rats also process ethanol’s chemosensory attributes during the course of an acute state of intoxication induced by intragastric administrations of ethanol doses that range between 1.5 and 3.0 g/kg (67). Nonmetabolic elimination of ethanol (via urination, respiration, and/or salivation) and hematogenic stimulation of nasal and vomeronasal receptors seem to explain these findings (68–70). Further evidence indicative of sensory processing of ethanol derived from the state of intoxication in infants has been reported by Lopez and Molina (71). After sequential episodes of ethanol intoxication, infants showed heightened predisposition to ingest not only an ethanol-flavored solution but also a quinine-sucrose mixture known to represent a psychophysical equivalent of ethanol’s taste (72–74).
Several associative learning studies have indicated that experience with ethanol during infancy is capable of modifying later profiles of acceptance and reactivity to ethanol. When infantile ethanol intoxication is associated with an intraoral infusion of a sweet reinforcer, pups subsequently exhibit heightened ethanol intake and ethanol odor preferences (75). Opposite effects are encountered after ethanol intoxication paired with aversive nociceptive stimulation (76). Similar conditioned aversions have been reported after ambient ethanol odor stimulation associated with the induction of a toxic state caused by an emetic drug (LiCl or apomorphine). In contrast, if ethanol odor is presented during recovery from apomorphine toxicosis, infants later exhibit heightened ethanol preferences (64, 77).
Further evidence that infants readily detect ethanol and associate its sensory properties with reinforcers is seen in the context of nursing. Infants intraorally stimulated with milk contaminated with ethanol while suckling from an anesthetized dam express conditioned mouthing responses when they are re-exposed to the odor of ethanol (78). Several studies have also indicated that during the first two postnatal weeks ingestion of minimal amounts of ethanol in maternal milk (a concentration equal to 0.22 % [v/v] ethanol), pups became sensitized to detection of this low ethanol concentration (79–81). These doses cause hypothermia in the dam and disruption of maternal behavior, thus allowing an opportunity for the infants to learn an association between ethanol and altered maternal states (82, 83).
Not only are infant rats sensitive to processing ethanol’s sensory properties as conditioned stimuli when paired with either appetitive or aversive reinforcers, but also they are also highly reactive to ethanol’s postabsorptive motivational effects. As with adult animals, high ethanol doses (1.2–3.0 g/kg yielding peak blood ethanol levels equivalent to 70–175 mg/100 ml, respectively) (84) act as aversive unconditioned stimuli in the infant rats, supporting the acquisition of chemosensory as well as tactile-conditioned aversions (65, 85–88). Recently, it was reported that after pairing a surrogate nipple or a novel odor with low ethanol doses yielding 15–20 mg/100 ml blood ethanol levels, newborn rats (3–24 hrs old) increase their appetitive suckling activity in the presence of this nipple or odor; thus, this result indicated positive reinforcement from the ethanol (89–91). There is corresponding evidence that ethanol doses yielding blood ethanol levels ranging from very low (15 mg/100 ml) to moderately high (80 mg/100 ml) are likely to exert negative reinforcing (antianxiety) effects in infant rats (92).
This evidence illustrates the vast potential for learning about ethanol early in infancy, whether as a signal for other appetitive or aversive consequences or as an appetitive or aversive consequence itself, signaled by other events.
Brief Fetal Experiences with Ethanol in the Amniotic Fluid
The notion that detection and retention of ethanol’s sensory properties are not restricted to postnatal life originated in a series of studies performed during the early 1990s. The leading question was whether brief exposure to ethanol, directly administered into the amniotic fluid, would allow later ethanol recognition. The strategy was to avoid fetal intoxication and hence teratologic effects or post-absorptive motivational consequences. The procedure took place during the last GD and included externalization of the uterine horns to allow administration of ethanol or a control odorant (lemon) into the amniotic sacs. Ten minutes after ethanol or lemon odorant were administered into the amniotic sacs, pups were delivered by cesarean section. Peak ethanol levels in the amniotic fluid were equivalent to 100 mg/100 ml, and ethanol was undetectable in fetal blood. Eight days later, pups prenatally exposed to ethanol showed a significantly greater preference for ethanol odor and greater ingestion of ethanol than did controls (93). Additional studies further indicated enhanced autonomic orienting to ethanol odor after the brief fetal experiences with ethanol (94). This experience with ethanol odor also facilitated appetitive learning and inhibited aversive learning later in life when this odor was associated with pleasant (sucrose) or unpleasant (peripheral nociception) unconditioned stimuli, respectively (95).
These experiments leave little doubt that the near-term fetus processes ethanol’s chemosensory characteristics and that this experience can predispose the animal to react positively to these characteristics. Were these effects solely determined by mere exposure to ethanol’s chemosensory properties? Apparently, this was not the case. Recall that ethanol contamination of the amniotic fluid occurred just a few minutes before cesarean delivery and, in some conditions, stroking procedures (i.e., tactile stimulation of the newborn that resembles maternal behaviors leading to behavioral activation of the newborn and hence optimization of survival rates) (24, 96). Stroking procedures act as reinforcers capable of supporting associative learning to novel odorants in the newborn rat, as assessed through neural and behavioral levels of expression (97). Under this perspective it is possible that the fetus not only processed ethanol odor but also associated it with birth-related stimuli biologically relevant to survival. This hypothesis was supported by demonstration of the following: (i) the temporal delay between fetal exposure to ethanol and the combination of cesarean delivery and stroking were negatively correlated with postnatal motor orienting to ethanol odor (98), (ii) postnatal nonreinforced exposure to ethanol odor markedly weakened subsequent reactivity to the ethanol odor previously presented in close temporal contiguity with the birth process (a phenomenon that resembles extinction of associative learning) (99), and (iii) postnatal pairing of ethanol with behaviorally activating stimulation can promote retrieval of or further strengthen fetal memories of the prenatal ethanol exposure (100). These results suggest that the fetus engaged in associative learning involving ethanol and birth-related stimuli.
Fetal Learning About Ethanol Derived from Maternal Intoxication with the Drug
During pregnancy, ethanol ingested by or administered to dams is rapidly distributed throughout amniotic fluid and fetal blood. Pregnant rats intragastrically administered 1.0 or 2.0 g/kg ethanol once each day near the end of gestation, GD 17–GD 20, deliver viable offspring that are indistinguishable morphologically from neonates born to those of control dams. More specifically, no teratogenic effects are observed in terms of litter size, birth body weight, size and weight of brain hemispheres, cerebellum and olfactory bulbs, weight of the placenta, and length of the umbilical cord. Pharmacokinetic analysis indicated that peak amniotic fluid and maternal and fetal blood levels are very similar within each prenatal ethanol dose (1.0 g/kg: about 40 mg/ 100 ml; 2.0 g/kg: about 120 mg/100 ml) (101).
In humans, blood ethanol levels similar to that encountered with the 1.0 g/kg ethanol dose in rat dams are observed after consumption of two to four standard drinks. This correspondence is very rough and subject to error induced by species-related differences in ethanol metabolism. The ethanol content encountered by the rat fetuses in the prenatal milieu recruits processing of ethanol’s chemosensory characteristics (95). Behavioral studies have confirmed this by demonstrating that neonates born to ethanol-treated dams rapidly detect the presence of ethanol in an air stream. This effect is highly specific because it does not generalize to other volatile substances (e.g., lemon odor) (101).
In a subsequent study, neonates prenatally exposed to ethanol during GD 17–GD 20 were evaluated in terms of motor responses elicited by the amniotic fluid of the dam, ethanol, or a combination of both stimuli presented in a gas phase (102). The results were surprising: ethanol-exposed neonates reacted to the odor of ethanol in the same way control neonates did to amniotic fluid, the natural scent that predominates for controls during gestation.
Two-week-old infant rats born to dams treated with 0.0, 1.0, or 2.0 g/kg ethanol during late gestation were tested for consumption of five solutions that varied in their similarity to the flavor of ethanol: sucrose, highly appetitive to infant rats and sharing the sweet taste of ethanol; quinine, which shares the bitter aversive flavor of ethanol; a mixed solution of sucrose and quinine, known to be psychophysically equivalent to the flavor of ethanol (72, 103); ethanol itself; and water. Consumption of sucrose, quinine, or water was not affected by prenatal treatment, yet prenatal ethanol exposure resulted in heightened consumption of the ethanol solution and its sensory equivalent (sucrose-quinine) (102). Recent studies have confirmed these results, and the use of taste reactivity tests indicate that the palatability of ethanol is enhanced as a function of prenatal ethanol experience and that exposure to ethanol during late gestation subsequently increases ethanol consumption during adolescence (104, 105). This latter effect was markedly attenuated when naloxone, a nonselective opioid antagonist, was administered to the pregnant female in conjunction with ethanol or when postnatal re-exposure to ethanol was also accompanied by naloxone administration (106). The opioid system modulates responsiveness to ethanol’s reinforcing effects as well as ethanol’s palatability (106–111). The opioid system appears functional during late gestation and has been demonstrated to regulate hedonic contents of fetal and infantile associative memories (42, 112–114).
Are these changes in subsequent response to ethanol determined solely by prenatal exposure to ethanol’s sensory attributes? This question deserves several considerations. From a pharmacokinetic approach, ethanol not only accumulates in the amniotic fluid after maternal administration but also is distributed in fetal and maternal circulatory systems. From a psychobiological perspective, the levels of ethanol in the fetus reach or excede, according to maternal dosage, those known to render reinforcing effects capable of supporting associative learning in infant and adult organisms (1, 63, 90, 91, 114, 115). Integrating both perspectives, maternal ethanol treatment could result in reinforcing consequences as well as sensory processing of ethanol that might become associated by the fetus; however, how can we analyze the hypothesis that ethanol intoxication supports fetal associative learning while the possible impact of pre-exposure to ethanol’s sensory effects is adequately controlled for?
The experimental strategy chosen was to employ a nonethylic chemosensory cue as the CS that, when administered to the dam, would allow the fetus to process its sensory attributes and become associated with ethanol’s central consequences through arrangement of a close contingency between the occurrences of the former and the latter. Cineole (the main component of eucalyptus oil) was chosen as the CS. This substance is nontoxic at low concentrations, has salient aromatic characteristics, and has a low molecular weight to allow rapid distribution in the prenatal milieu (116, 117). Also important, ethanol and cineole have similar pharmacokinetic profiles in terms of distribution and elimination rates in maternal blood and in the prenatal environment (118). Knowing the temporal pharmacokinetic profiles of cineole in the amniotic fluid and of ethanol in the fetus set the basis for controlling temporal contiguity between these elements (cineole as the CS and maternal-fetal intoxication as the unconditioned stimulus).
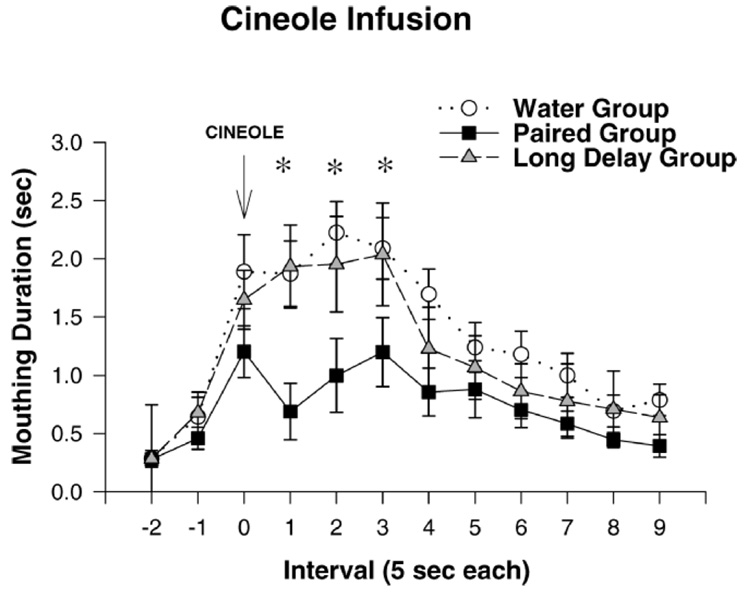
During GD 17–GD 20 pregnant rats were administered cineole in close temporal proximity with maternal ethanol administration (2.0 g/kg) (paired group). A second group of dams received cineole and ethanol administrations separated by a 4-hr time interval to minimize the contingency between these events (long-delay control). Hence, the sole difference between paired and long-delay fetuses was length of the time separating cineole and ethanol intoxication. Rat fetuses and infants condition poorly or not at all with long-delay intervals. This provides a conservative control condition for assessing associative learning (119–121). A third group of dams only received water. The experimental strategy proved to be successful: cineole-ethanol associative memories were expressed in a chemosensory test during the third postnatal week of life. Although prenatal treatment did not affect mouthing to infused milk, it clearly affected mouthing in response to cineole (Fig. 1). Infants prenatally exposed to paired presentations of cineole and ethanol intoxication mouthed significantly less to infused cineole than did controls (118). In other words, these results support the notion of infantile expression of conditioned responses elicited by an olfactory stimulus that was prenatally associated with maternal-fetal ethanol intoxication.
Figure 1.
Mouthing duration (sec) generated by cineole intraoral infusions as a function of prenatal treatment (water group, paired group, and long-delay group) and interval of the test (−2, −1, 0, 1, 2, 3, 4, 5, 6, 7, 8, and 9 mins). Intraoral stimulation took place during interval 0. Vertical lines represent the standard errors of the means (adapted from Ref. 118). The asterisk denotes significant differences between the paired group and the remaining prenatal treatments.
The effects of prenatal olfactory learning supported by prenatal ethanol exposure have also been analyzed in terms of the newborn’s first suckling response. Prenatal associative learning procedures again included presentation of cineole (CS) paired or unpaired with the onset of acute ethanol intoxication (US). How the presence or absence of the prenatal CS modulated the structure of the first suckling response was addressed through tests with a surrogate nipple that delivered milk as a consequence of neonatal attachment (122–124). Neonates prenatally exposed to cineole associated with ethanol intoxication exhibited heightened responsiveness to the nutritive surrogate nipple when in the presence of the odor used as a CS during gestation (125). These results are depicted in Figure 2. Similar effects were obtained when the newborn in the paired condition was briefly re-exposed to cineole odor before testing, but not if newborns were exposed to a novel odorant before or during exposure to the nipple (Fig. 3A).
Figure 2.
Time of attachment (seconds) to a nutritive surrogate nipple as a function of prenatal treatment (water group, paired group, and long-delay group) and the odor presented at testing (cineole or no odor). The test had a total duration of 10 mins. Vertical lines represent the standard errors of the means (adapted from Ref. 125). The asterisk denotes significant differences between the paired group stimulated with cineole and the remaining treatment conditions.
Figure 3.
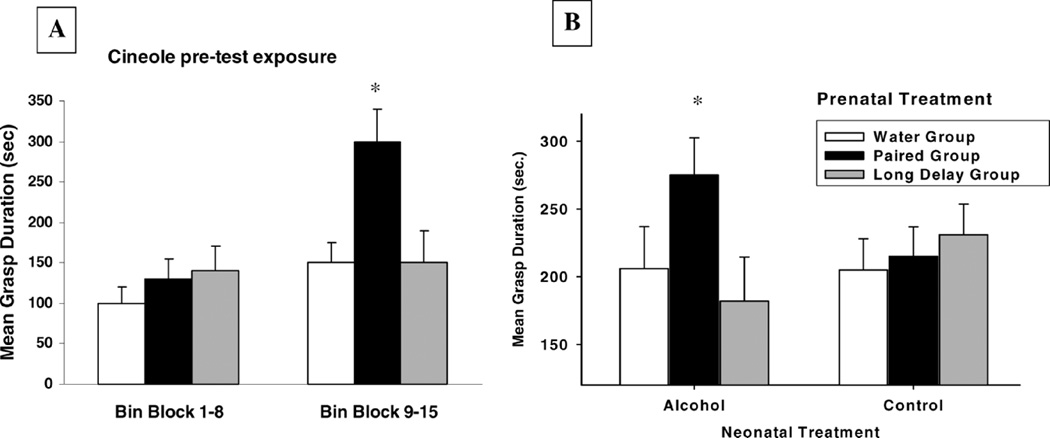
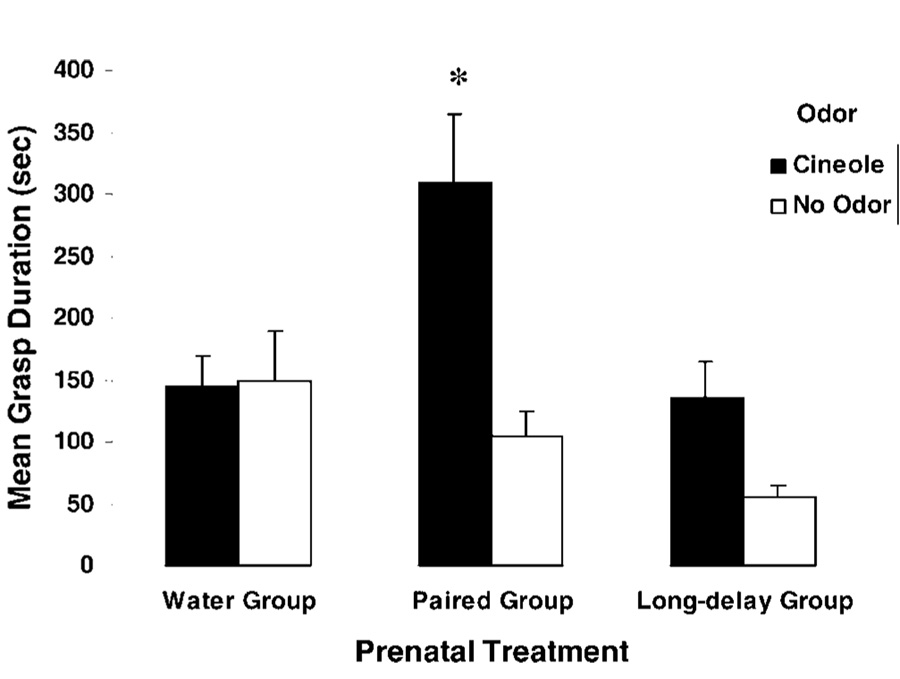
(A) Mean grasp duration (seconds) elicited by a nutritive surrogate nipple as a function of prenatal treatment (water group, paired group, and long-delay group) when newborns were exposed to cineole odor before the test situation. Mean grasp durations correspond to the first and second half of the test (1–8 and 9–15 mins, respectively) (adapted from Ref. 125). (B) Mean grasp duration (seconds) to a nutritive surrogate nipple as a function of prenatal treatment (water group, paired group, and long-delay group). Neonates were pretreated with intragastric administrations of ethanol or water before being tested. Vertical lines represent the standard errors of the means. Asterisks denote significant differences between the paired group and the remaining prenatal treatments (A) and the significant difference existing between paired animals treated with ethanol before the test and the remaining control conditions (B).
Additional evidence supports the hypothesis that prenatal ethanol exposure can act as a US capable of supporting associative learning. An acquired memory may be reactivated when the organism is re-exposed to one or more elements involved in the acquisition of the original learning experience; among others are redundant contextual cues, contingent presentations of the CS and US, or even the US alone (126–133). This reactivation effect also has been observed after cineole-ethanol pairings during late prenatal life. Newborns prenatally treated with cineole in association with ethanol exhibited heightened responding to a cineole-scented surrogate nipple containing milk. This effect was particularly strong when, before the test, neonates were intoxicated with an ethanol dose (0.75 g/kg) that yielded blood ethanol levels similar to those attained during prenatal treatment, as if this US reactivated the memory of its prenatal association with cineole (Fig. 3B).
A recent correlational study that focused on maternal sensitivity to ethanol and neonatal responsiveness to ethanol’s sensory cues adds to the growing body of evidence suggesting that fetal exposure to ethanol comprises perception of its sensory attributes in conjunction with physiologic effects inherent to the state of intoxication. One of the multiple physiologic effects of ethanol intoxication is disruption of thermoregulatory homeostasis (134). In adult rats, ethanol-induced hypothermia contributes to the drug’s ability to establish conditioned aversions (132, 133). Infants are also sensitive to ethanol’s disruption of thermoregulation (87). Neonates exhibit marked behavioral changes in response to specific thermal challenges and exhibit conditioning when thermal cues are employed as reinforcers (25, 26, 136–139).
Taking into account these observations coupled with the fact that fetuses detect and retain ethanol’s sensory cues after maternal administration, we asked whether ethanol’s thermoregulatory disruptions correlated with subsequent neonatal responsiveness to ethanol’s odor. Hypothermia was the prevailing effect in ethanol-treated dams. Some hypothermia was also observed in water-treated dams, which serendipitously provided an advantageous control condition. Significant correlations were obtained between the degree of ethanol-induced prenatal thermal changes and the duration of neonatal activity triggered by ethanol odor. With greater ethanol-induced hypothermia, there was greater neonate activity in response to the ethanol odor (140). The correlation was highly specific: no comparable results occurred for the relationship between ethanol-induced hypothermia and the newborn’s response to a novel odorant. Water-treated dams yielded no comparable correlation.
This correlational study strongly suggested that physiologic changes in the prenatal milieu, derived from the state of ethanol intoxication, act as important components in modulating fetal ethanol learning processes (118, 125, 141, 142). Although these results cannot address a causal relation between hypothermia as a US and fetal learning about ethanol’s chemosensory cues, these results emphasize that analysis of early memories involving experiences with ethanol requires vigilant consideration of contingencies existing between ethanol’s sensory properties and a variety of ethanol’s physiologic consequences. Similar vigilance is required for analysis when young animals or humans detect ethanol’s chemosensory properties while intoxicated or when interacting with biological counterparts (e.g., the mother or age-related counterparts) that exhibit behavioral and/or physiologic disruptions caused by ethanol (73, 79, 80, 143–147).
That the consequences of prenatal ethanol experiences are dependent not only on ethanol’s sensory properties but also on its diffuse consequences of its unconditioned effects has recently received further experimental support. Prenatal exposure to ethanol has been found to alter the neonatal rat’s susceptibility to reinforcement from low doses of ethanol. Pregnant females were given ethanol or water during late gestation or were untreated. After cesarean delivery, newborns were exposed to a surrogate nipple providing water, paired or unpaired with intraperitoneal injections of 0.0, 0.25, 0.50, or 0.75 g/kg ethanol. After conditioning, ethanol reinforcement effects were evaluated by the pup’s response to an empty surrogate nipple. It was clear that ethanol reinforcement was more effective in animals prenatally exposed to the drug: the range of doses capable of exerting reinforcing effects was more broad for these newborns, and the reinforcing effects themselves were stronger than those found in rats from prenatal control treatments (115) (Fig. 4). This study may represent the first empirical evidence for sensitization to ethanol’s positive reinforcing capabilities as a function of late gestational exposure to ethanol and can be added to other examples of sensitization of the fetus to ethanol’s effects (148). Such sensitization may help us understand why animals prenatally exposed to ethanol consistently show heightened proclivity to accept ethanol for ingestion or to exhibit specific responsiveness to ethanol odor.
Figure 4.
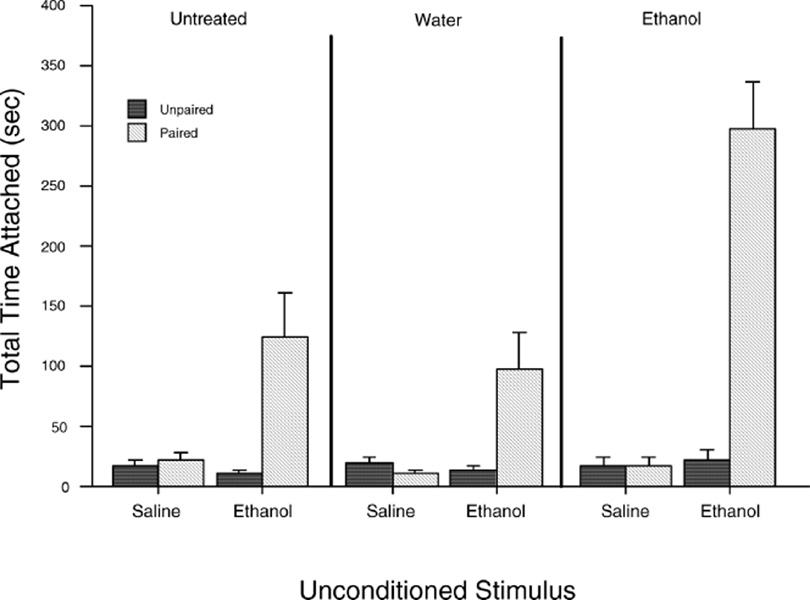
Time of attachment to a surrogate nipple as a function of prenatal treatments and neonatal conditioning procedures. The data corresponding to ethanol as an unconditioned stimulus employed during postnatal life are representative of collapsed values of 0.25, 0.50, and 0.75 g/kg ethanol. Vertical lines represent the standard errors of the means (adapted from Ref. 115).
When do fetuses become capable of associative learning mediated by ethanol’s reinforcing consequences? This question remains unanswered. Yet, there is experimental evidence indicating that chemosensory, associative learning, and memory capabilities progressively develop during GD 16 through GD 19 in the rat (vaginal delivery occurs on GD 21–GD 22) (149). Recent studies confirm that this learning capability is markedly developed in the neonate and that ethanol reinforcement is dependent on central nervous system effects. Neonates delivered by cesarean section express conditioned olfactory preferences when exposed to an odorant (lemon) that had been previously paired with an intracisternal administration of ethanol (i.e., direct administration of the drug into the cerebrospinal fluid). Ethanol doses that were effective in terms of mediating appetitive learning ranged between 25 and 200 mg/100 ml. Central administration of the drug virtually eliminated the possibility that ethanol’s chemosensory or caloric attributes were involved in ethanol’s reinforcing capabilities (150).
As is the case in adult rats (151–155), the endogenous opioid system modulates ethanol’s positive reinforcing effects in neonatal rats (156). Specifically, intracisternal injections of selective antagonists of mu or kappa receptors (d-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 [CTOP] or nor-binaltorphimine, respectively) reduce or eliminate ethanol’s reinforcing effects in animals born by cesarean delivery. These results and those indicating that naloxone suppresses ethanol’s appetitive effects during late gestation (106) seem to support the hypothesis that positive hedonic components of fetal memories about ethanol are mediated by the activation of the endogenouss opioid system. This hypothesis does not rule out alternative neurochemical mechanisms that could be responsible for the reinforcing effects of low to moderate ethanol doses during late gestation. For example, the dopaminergic mesolimbic system, which plays a critical role in the rewarding properties of ethanol and other drugs of abuse (153, 157), is significantly affected by prenatal exposure to moderate ethanol doses. In rhesus monkeys, voluntary ethanol consumption (0.6 g/kg ethanol daily) during early gestation or throughout pregnancy results in a reduction or blunting of the offspring’s dopaminergic function. On the contrary, moderate ethanol intake in midgestation to late gestation induces heightened dopaminergic function as operationalized through the ratio existing between striatal dopamine (D2 receptors) binding and dopamine synthesis (158). These findings serve to illustrate the distinct possibility that even low to moderate ethanol exposure can affect brain reward mechanisms (e.g., dopaminergic and opiate systems) that can determine the hedonic valence of ethanol’s effects during fetal exposure or later in ontogeny.
Beyond the analysis of possible neurobiological mechanisms that may determine the reinforcing effects of low to moderate ethanol doses during late gestation, there is also a need to examine whether this exposure can affect early learning and memory processes. As indicated by Schneider et al. (159), there are very few preclinical studies aimed at determining the effects of moderate ethanol exposure during different gestational stages despite the fact that this pattern of consumption is common during human pregnancy (160). Rats exposed to low ethanol doses (blood ethanol concentration ≤30 mg/100 ml) throughout gestation show impairments in the acquisition of a spatial learning task accompanied by long lasting alterations in hippocampal glutamate-dependent synaptic neurotransmission (161). From an electrophysiologic perspective, relatively low ethanol levels (60 mg/100 ml) are sufficient to strengthen glutamatergic synaptic neurotransmission in the newborn rat, an effect that is partially mediated by disruptions in neurosteroid (pregnenolone sulfate) production (162). In other words, there is now evidence that during a stage in development characterized by a brain growth spurt, similar to the one observed during the third gestational trimester in humans (17, 163), ethanol leads to a premature hippocampal synaptic stabilization. A recent study has also indicated that during the period of rapid synaptogenesis in the mouse, a single ethanol dose (0.63 g/kg) yielding relatively low peak blood ethanol levels (57 mg/100 ml) is sufficient to trigger a significant neuroapoptosis response (164). The specific impact of these neurobiological disruptions upon sensory and learning processes occurring under the state of intoxication during these early stages in development remains to be elucidated. Detrimental effects are likely to be expected, but they appear not to be sufficient to impede fetal detection and learning about ethanol’s sensory and postabsorptive attributes.
Does exposure to low or moderate ethanol doses during late gestation also affect subsequent sensitivity to activating or sedative effects of the drug that may in turn regulate ethanol intake patterns? Only a few studies have analyzed the possibility of differential psychomotor profiles elicited by ethanol intoxication as a function of late prenatal ethanol exposure. For example, Chotro and Spear (148) reported that rat fetuses (GD 20) are more sensitive to the sedative behavioral effects of ethanol (2.0 g/kg) when previously exposed to 1.0 or 2.0 g/kg ethanol during GD 17–GD 19. This psychopharmacologic outcome appears to be transient because one day after birth rats no longer exhibit sensitization to ethanol’s sedative effects (141). Recently, this phenomenon has been examined later in infancy (postnatal days 12 and 13). Infant rats exposed during late gestation to water or ethanol (2.0 g/kg) were intragastrically administered with either 0.5 or 2.5 g/kg ethanol. The highest ethanol dose induced biphasic motor effects (hyperactivity followed by hypoactivity) when infants were tested in a novel environment. These effects were similar across prenatal treatments (165).
Interactions Between Prenatal and Postnatal Experiences with Ethanol
Earlier we referred to two studies (95, 99) supporting the notion that fetal experiences with ethanol are likely to interact with postnatal learning about ethanol, perhaps the first evidence of this kind. In both studies ethanol was directly administered into the amniotic fluid just before birth. This brief experience was sufficient to modulate postnatal conditioning to ethanol odor. More specifically, the brief prenatal exposure to ethanol facilitated appetitive conditioning to ethanol odor and inhibited aversive conditioning to ethanol odor during the pups’ second postnatal week (95). The second study focused on the likelihood that ethanol odor processing in utero can be associated with behaviorally activating tactile stimulation occurring soon after birth. The latter US mimics a portion of the maternal stimulation routinely experienced by newborn rats and also can promote olfactory preference learning in newborn rats (97, 99, 166–168). When paired with tactile activation in the second study (99), prenatal exposure to ethanol odor facilitated subsequent neonatal learning comprising temporally contiguous presentations of ethanol odor and tactile stimulation.
Later studies endorsed the hypothesis that sequential fetal and infantile experiences with ethanol may result in the developing organism becoming still more responsive to ethanol’s sensory properties. For example, infantile exposure to ethanol’s chemosensory properties directly or through interaction with an intoxicated sibling facilitated subsequent expression of cardiac and behavioral responsiveness to ethanol odor, but only if infants had also been exposed to ethanol as fetuses during the last 4 GDs (169). Two recent studies have extended these findings and aid in the explanation of this pattern of results.
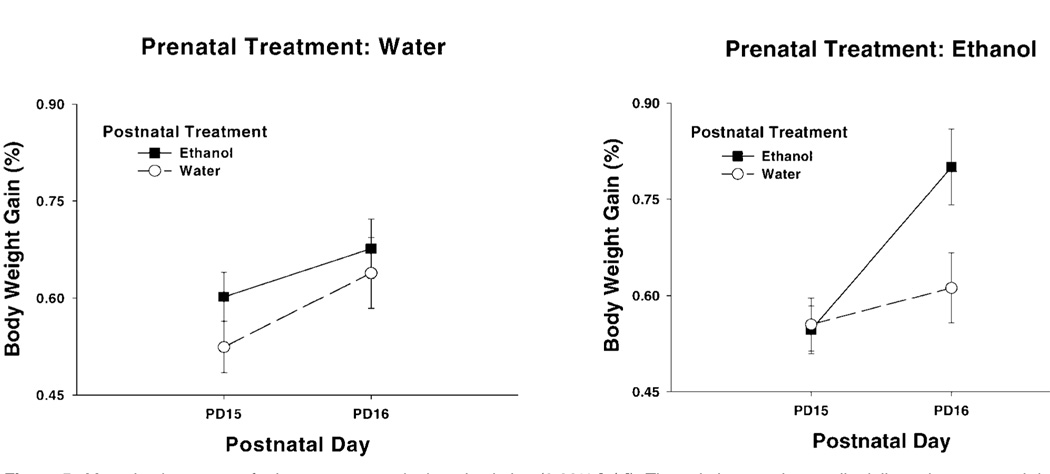
In the first study, detection of low concentrations of ethanol in water (0.22% [v/v] ethanol solution) was altered by sequential prenatal and postnatal ethanol experiences derived from maternal ethanol intoxication in both cases. Near-term pregnant females were given water or an ethanol dose (2.0 g/kg) known to promote fetal sensory processing. Half of each of these groups was then exposed to ethanol during the nursing period, and half was not. Infant rats were tested for their intake of a 0.22% (v/v) ethanol solution, a concentration similar to that encountered in the amniotic fluid and in breast milk when dams were intoxicated while pregnant or while breastfeeding. Beginning the day after the first intake test, infant rats exposed to ethanol both prenatally and postnatally consumed significantly more ethanol than animals from alternative maternal treatments (84) (Fig. 5). Apparently, information acquired during prenatal and postnatal exposure to ethanol facilitates subsequent responsiveness to very small concentrations of ethanol, even if it is presented in a different vehicle from that in which it was previously experienced (water rather than amniotic fluid or milk). It is unlikely that these effects were determined by differential behavioral sensitivity to ethanol’s psychomotor effects. As originally stated by Pueta et al. (84), the maximum amount of absolute ethanol that pups could consume in these tests was equivalent to 0.03 g/kg. Hence, it appears that both prenatal and nursing experiences with ethanol sensitized the rats’ perception of ethanol’s chemosensory attributes, with greatest sensitization occurring from a combination of these early experiences (84, 148, 170).
Figure 5.
Mean intake scores of a low concentrated ethanol solution (0.22% [v/v]). The solution was intraorally delivered at postnatal days 15 and 16. Pups at this age have the capability to ingest or reject intraorally delivered liquids. Intake scores are operationalized through the percentage of increases in the pups’ body weights. These scores were calculated as follows: 100×[(postinfusion weight – preinfusion weight) ÷ preinfusion weight]. Groups are defined as a function of prenatal and postnatal experiences with ethanol (water-water, water-ethanol [EtOH], EtOH-water or EtOH-EtOH). Vertical lines represent the standard errors of the means (adapted from Ref. 83).
The second study was meant to analyze the persistence of prenatal ethanol effects into adolescence in terms of social interaction with an ethanol-intoxicated partner and preference for ethanol odor (171). Dams were treated during late gestation with ethanol or with water. During adolescence, rats were tested in terms of social interactions with an ethanol-intoxicated or a sober partner. Infant and periadolescent rats show preferences for ethanol odor after they had interacted with intoxicated counterparts, a phenomenon probably regulated by sensory exposure to ethanol derived from the partner’s nonmetabolic elimination of the drug (143–145). Prenatal ethanol experience heightened social investigation of intoxicated partners but had no effect on social behavior with a sober partner. Fetal experience with ethanol was also observed to increase the adolescent’s preference for ethanol odor (171).
It seems clear that the combination of fetal and infantile experience with ethanol results in heightened sensitivity to ethanol’s chemosensory attributes. Is there any evidence that this combination also has a special impact on ethanol’s reinforcing consequences? No significant effects have been observed in tests of conditioned aversion to high doses of ethanol during later infancy (84, 141). At present, we know of no tests of the interaction between gestational and infantile exposure to ethanol on subsequent sensitivity to positive or negative (antianxiety-like) reinforcing effects of ethanol.
Final Considerations: Relationships Between Preclinical and Human Studies of Effects of Moderate Prenatal Exposure
The link between prenatal ethanol exposure and subsequent use and abuse of ethanol has been the focus of a significant number of epidemiologic and animal studies, most of which focused on the impact of high levels of exposure to ethanol (1, 172). There is considerable agreement that ethanol exposure during pregnancy results in either a predisposition to consume (102, 104, 105) or changes upon the pattern of reactivity to ethanol (6, 10, 93–95, 100–102, 159, 161) later in life. Recent epidemiologic studies have systematically indicated that even when controlling for variables known to affect ethanol use and abuse (e.g., genetic predisposition as assessed through family history of alcoholism, gender, co-use of other psychotropic agents throughout gestation, and different environmental factors), prenatal ethanol exposure strongly predicts later ethanol drinking patterns and ethanol-related problems (173–177). The reliable association between fetal ethanol exposure and juvenile alcohol problems has lead Baer and colleagues (174) to conclude that “studies of alcoholism etiology and family history need to include consideration of even modest levels of fetal alcohol exposure.’’ The mechanisms that may account for the above mentioned association are diverse and complex. Direct effects of ethanol upon neurochemical systems known to regulate ethanol-seeking or intake behaviors (e.g., dopamine [178, 179] and GABA [180, 181]) as well as teratologic consequences leading toward hyperactivity, altered emotional states, sleep disorders, or mental retardation cannot be discarded in the structure or modulation of ethanol use and abuse (182–184).
On the other hand, the focus of the present review is related with studies of effects of low to moderate ethanol exposure during ontogenetic stages characterized by the emergence of functional sensory and learning systems. Infant rats as well as human babies are shown in this review to express behaviors indicative of prenatal memories about ethanol. In a variety of experimental circumstances, preclinical research has shown that these ethanol experiences result in the following: (i) detection and retention of sensory information about ethanol, (ii) processing of ethanol’s postabsorptive effects that have an impact upon maternal-fetal physiology, (iii) associative learning mediated by ethanol’s effects, (iv) increased sensitivity to reinforcing motivational components of the state of intoxication, and (v) apparent establishment of a behavioral foundation that modulates the impact of subsequent ethanol-seeking and intake patterns.
These findings, the well-known teratogenic effects of ethanol, and the negative correlation between onset of ethanol experience and severity of subsequent drug-related problems support primary preventive health policies that are reluctant to accept as “safe” any amount of ethanol during pregnancy. In this respect, the literature reviewed in the present work adds to a growing body of evidence indicating negative health outcomes derived from even modest maternal ethanol intake and should help to emphasize the existing recommendation of abstinence during pregnancy (185–187).
Acknowledgments
This research, a collaborative project between the Research Foundation of SUNY Binghamton and Instituto de Investigacion Medica Mercedes y Martin Ferreyra, was supported by grants R01 AA1960-06, R01 AA013098, and R01 AA015992 from the National Institute on Alcohol Abuse and Alcoholism (N.E.S.); grant PICT 5-14024 from the Agencia Nacional de Promocion Cientifica y Tecnologica (J.C.M.); and fellowships of Secretaria de Ciencia y Tecnica, Universidad Nacional de Cordoba, Argentina (M.P.) and Consejo Nacional de Investigaciones Cientificas y Tecnicas, Argentina (P.A.).
We thank Catriona A. Kirkwood, Olga B. Haymal, and Teri Tanenhaus for their technical support.
References
- 1.Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- 2.Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 2005;230:389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- 3.Roebuck TM, Mattson SN, Riley EP. Prenatal exposure to alcohol: effects on brain structure and neuropsychological functioning. In: Hannigan JH, Spear LP, Spear NE, Goodlett CR, editors. Alcohol and Alcoholism: Effects on Brain and Development. Hillsdale, NJ: Lawrence Erlbaum Associates; 1999. pp. 1–16. [Google Scholar]
- 4.Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 5.West JR. Recent findings on the mechanisms by which alcohol damages the developing nervous system. Alcohol Alcohol. 1994 Suppl 2:395–399. [PubMed] [Google Scholar]
- 6.Faas AE, Spontón ED, Moya PR, Molina JC. Differential responsiveness to alcohol odor in human neonates: effects of maternal consumption during gestation. Alcohol. 2000;22:7–17. doi: 10.1016/s0741-8329(00)00103-8. [DOI] [PubMed] [Google Scholar]
- 7.Dawson DA, Grant BF, Chow PS. Gender differences in alcohol intake. In: Hunt WA, Zahkari S, editors. Stress Gender and Alcohol-Seeking Behavior. Bethesda, MD: National Institute of Alcohol Abuse and Alcoholism Research; 1995. pp. 3–121. [Google Scholar]
- 8.Scher MS, Richardson GA, Robles N, Geva D, Goldschmidt L, Dahl RE, Sclabassi RJ, Day NL. Effects of prenatal substance: altered maturation of visual evoked potentials. Pediatr Neurol. 1998;18:236–243. doi: 10.1016/s0887-8994(97)00217-8. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- 10.Faas AE. Aplicaciones del aprendizaje no asociativo en la evaluación neonatal. PhD thesis. Argentina: Universidad Nacional de Cordoba; 2001. Estudios funcionales del sistema nervioso central del recién nacido. [Google Scholar]
- 11.Ekman P, Friesen W. Facial Action Coding System: A technique for measurement of facial movement. Palo Alto, CA: Consulting Psychologist Press; 1978. [Google Scholar]
- 12.Hankin JR. Fetal alcohol syndrome prevention research. Alcohol Res Health. 2002;26:58–65. [PMC free article] [PubMed] [Google Scholar]
- 13.Dillner L, Josefson D, Karcher H, Sheldon T, Dorozynski A, Zinn C. Alcohol—pushing the limits. BMJ. 1996;312:7–9. doi: 10.1136/bmj.312.7022.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scher M, Richardson GA, Coble PA, Day NL, Stoffer DS. The effects of prenatal alcohol and marijuana exposure: disturbances in neonatal sleep cycling and arousal. Pediatr Res. 1988;24:101–105. doi: 10.1203/00006450-198807000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Chotro MG, Arias C. Exposure to low and moderate exposure of alcohol on late gestation modifies infantile response to and preference for alcohol in rats. Ann Ist Super Sanita. 2006;42:22–30. [PubMed] [Google Scholar]
- 16.Bachmanov AA, Kiefer SW, Molina JC, Tordoff M, Duffy VB, Bartoshuk L, Mennella JA. Chemosensory factors influencing alcohol perception, preferences and consumption. Alcohol Clin Exp Res. 2003;26:220–231. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 19.Lanier SA, Hayes JE, Duffy VB. Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Physiol Behav. 2005;17:821–831. doi: 10.1016/j.physbeh.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Fifer WP, Moon CM. The effects of fetal experience with sound. In: Lecaunet JP, Fifer WP, Krasnegor N, Smotherman WP, editors. Fetal Development: A Psychobiological Perspective. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. pp. 351–368. [Google Scholar]
- 21.Pedersen PE, Blass EM. Prenatal and postnatal determinants of the 1st suckling episode in albino rats. Dev Psychobiol. 1982;15:349–355. doi: 10.1002/dev.420150407. [DOI] [PubMed] [Google Scholar]
- 22.Ronca AE, Lamkin CA, Alberts JF. Maternal contributions to sensory experience in the fetal and newborn rat (Rattus norvigecus) J Comp Psychol. 1993;107:61–74. doi: 10.1037/0735-7036.107.1.61. [DOI] [PubMed] [Google Scholar]
- 23.Smotherman WP, Robinson SR. Behavior of rat fetuses following chemical or tactile stimulation. Behav Neurosci. 1988;102:24–34. doi: 10.1037//0735-7044.102.1.24. [DOI] [PubMed] [Google Scholar]
- 24.Smotherman WP, Robinson SR. The amniotic sac as scaffolding: prenatal ontogeny of an action pattern. Dev Psychobiol. 1991;24:463–485. doi: 10.1002/dev.420240703. [DOI] [PubMed] [Google Scholar]
- 25.Alberts JF. Sensory-perceptual development in the Norway rat: a view toward comparative studies. In: Kail R, Spear NE, editors. Comparative Perspectives on Memory Development. Hillsdale, NJ: Lawrence Erlbaum Associates; 1984. pp. 65–102. [Google Scholar]
- 26.Ronca AE, Alberts JF. Sensory stimuli associated with gestation and parturition evoke cardiac and behavioral responses in the fetal rat. Dev Psychobiol. 1994;55:270–282. [Google Scholar]
- 27.Coppola DM, O’Connel RJ. Perinatal olfaction in the mouse: developmental morphology of the vomeronasal channel in preliminary 2-deoxyglucose studies in utero. Program of the 11th Meeting of the Association of Chemoreception Sciences (Achems XI) Sarasota; Florida. 1989. [Google Scholar]
- 28.Coppola DM. The role of the main and accessory olfactory systems in prenatal olfaction. In: Marchlewska-Koj A, Lepri JJ, Müller-Schwarze D, editors. Chemical Signals in Vertebrates. Vol. 9. New York: Plenum Press; 2001. pp. 189–196. [Google Scholar]
- 29.Coppola DM, Millar LC. Stimulus access to the accessory olfactory system in the prenatal and perinatal rat. Neuroscience. 1994;60:463–468. doi: 10.1016/0306-4522(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 30.Coppola DM, Budde J, Millar L. The vomeronasal duct has a protracted postnatal development in the mouse. J Morphol. 2005;218:59–64. doi: 10.1002/jmor.1052180105. [DOI] [PubMed] [Google Scholar]
- 31.Smotherman WP, Robinson SR. Rat fetuses respond to chemical stimuli in gas phase. Physiol Behav. 1990;97:863–868. doi: 10.1016/0031-9384(90)90010-2. [DOI] [PubMed] [Google Scholar]
- 32.Smotherman WP, Robinson SR, Ronca AE, Alberts JF, Hepper P. Heart rate responses to the rat fetus and neonate to a chemosensory stimulus. Physiol Behav. 1991;50:47–52. doi: 10.1016/0031-9384(91)90496-b. [DOI] [PubMed] [Google Scholar]
- 33.Smotherman WP. In utero chemosensory experience alters taste preferences and corticosterone responsiveness. Behav Neural Biol. 1982;36:61–68. doi: 10.1016/s0163-1047(82)90245-x. [DOI] [PubMed] [Google Scholar]
- 34.Smotherman WP, Robinson SR. Habituation in the rat fetus. Q J Exp Psychol B. 1992;44:215–230. doi: 10.1080/02724999208250613. [DOI] [PubMed] [Google Scholar]
- 35.Smotherman WP, Robinson SR. Habituation to chemosensory stimuli in the rat fetus: effects of endogenous kappa opioid activity. Behav Neurosci. 1993;107:611–617. doi: 10.1037//0735-7044.107.4.611. [DOI] [PubMed] [Google Scholar]
- 36.Smotherman WP. Odor aversion learning by the rat fetus. Physiol Behav. 1982;29:769–771. doi: 10.1016/0031-9384(82)90322-5. [DOI] [PubMed] [Google Scholar]
- 37.Smotherman WP, Robinson SR. The rat fetus in its environment: behavioral adjustments to novel, familiar, aversive and conditioned stimuli presented in utero. Behav Neurosci. 1985;99:521–530. doi: 10.1037//0735-7044.99.3.521. [DOI] [PubMed] [Google Scholar]
- 38.Stikord G, Kimble DP, Smotherman WP. In utero taste/odor aversion conditioning in the rat. Physiol Behav. 1982;28:5–7. doi: 10.1016/0031-9384(82)90093-2. [DOI] [PubMed] [Google Scholar]
- 39.Gruest N, Richer P, Hars B. Emergence of long-term memory for conditioned aversion in the rat fetus. Dev Psychobiol. 2004;44:189–198. doi: 10.1002/dev.20004. [DOI] [PubMed] [Google Scholar]
- 40.Mickley GA, Remmers-Roeber DR, Crouse C, Pelusso R. Ketamine blocks a taste-mediated conditioned motor response in perinatal rat. Pharmacol Biochem Behav. 2000;66:547–552. doi: 10.1016/s0091-3057(00)00250-1. [DOI] [PubMed] [Google Scholar]
- 41.Robinson SR, Hoeltzel TCM, Cooke KM, Umphress SM, Smother-man WP, Murrish DE. Oral capture and grasping of an artificial nipple by rat fetuses. Dev Psychobiol. 1992;26:375–387. doi: 10.1002/dev.420250802. [DOI] [PubMed] [Google Scholar]
- 42.Petrov ES, Varlinskaya EI, Smotherman WP. Classical conditioning of responses to an artificial nipple in the rat fetus: mu and kappa opioid systems. Dev Psychobiol. 2000;37:59–72. doi: 10.1002/1098-2302(200009)37:2<59::aid-dev1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Arnold HM, Robinson SR, Spear NE, Smotherman WP. Conditioned opioid activity in the rat fetus. Behav Neurosci. 1993;107:963–969. doi: 10.1037//0735-7044.107.6.963. [DOI] [PubMed] [Google Scholar]
- 44.Schaal B, Coureaud G, Marlier L, Soussignan R. Fetal olfactory cognition preadapts neonatal behavior in mammals. In: Marchlewska-Koj A, Lepri JL, Muller-Schwarze, editors. Chemical Signals in Vertebrates 9. New York: Kluwer Academic/Plenum Publishers; 2001. pp. 197–204. [Google Scholar]
- 45.Schaal B, Hummel T, Soussignan R. Olfaction in the fetal and premature infant: functional status and clinical implications. Clin Perinatol. 2004;31:261–285. doi: 10.1016/j.clp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Varendi H, Porter RH, Weimberg J. Attractiveness for amniotic fluid odor: evidence of prenatal learning? Acta Paediatr. 1996;85:1223–1227. doi: 10.1111/j.1651-2227.1996.tb18233.x. [DOI] [PubMed] [Google Scholar]
- 47.Mennella JA, Beauchamp GK. The transfer of alcohol to human milk: effects on flavor and the infant’s behavior. N Engl J Med. 1991;325:981–985. doi: 10.1056/NEJM199110033251401. [DOI] [PubMed] [Google Scholar]
- 48.Mennella JA, Beauchamp GK. Early flavor experiences: when do they start? Nutr Today. 1994;29:25–31. [Google Scholar]
- 49.Mennella JA. Mother’s milk: a medium for early flavor experiences. J Hum Lact. 1995;11:39–45. doi: 10.1177/089033449501100122. [DOI] [PubMed] [Google Scholar]
- 50.Schaal B, Marlier L, Soussignan R. Responsiveness to the odour of amniotic fluid in the human neonate. Biol Neonate. 1995;67:397–406. doi: 10.1159/000244192. [DOI] [PubMed] [Google Scholar]
- 51.Schaal B, Marlier L, Soussignan R. Olfactory function in the human fetus: evidence from selective neonatal responsiveness to the odor of amniotic fluid. Behav Neurosci. 1998;112:1438–1449. doi: 10.1037//0735-7044.112.6.1438. [DOI] [PubMed] [Google Scholar]
- 52.Schaal B, Soussignan R, Marlier L. Olfactory cognition at the start of life: the perinatal shaping of selective odor responsiveness. In: Rouby C, Schaal B, Dubois D, Gervais RB, Holley A, editors. Olfaction, Taste, and Cognition. New York: Cambridge University Press; 2002. pp. 421–440. [Google Scholar]
- 53.Varendi H, Christensson K, Porter RH, Wimberg J. Soothing effect of amniotic fluid smell in newborn infants. Early Hum Dev. 1998;51:47–55. doi: 10.1016/s0378-3782(97)00082-0. [DOI] [PubMed] [Google Scholar]
- 54.Varendi H, Porter RH, Winberg J. Natural odour preferences of newborn infants change over time. Acta Paediatr. 1997;86:985–990. doi: 10.1111/j.1651-2227.1997.tb15184.x. [DOI] [PubMed] [Google Scholar]
- 55.Marlier L, Schaal B, Soussignan R. Neonatal responsiveness to the odor of amniotic and lacteal fluids: a test of perinatal chemosensory continuity. Child Dev. 1998;69:611–623. [PubMed] [Google Scholar]
- 56.Marlier L, Schaal B. Human newborns prefer human milk: conspecific milk odor is attractive without postnatal experience. Child Dev. 2005;76:155–168. doi: 10.1111/j.1467-8624.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 57.Schaal B, Orgeur P, Rognon C. Odor sensing in the human fetus: anatomical, functional and chemoecological bases. In: Lecaunet JP, Fifer WP, Krasnegor N, Smotherman WP, editors. Fetal Development: A Psychobiological Perspective. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. pp. 205–238. [Google Scholar]
- 58.Takami S, Yukimatsu M, Matsumura G, Nishiyama F. Vomeronasal epithelial cells of human fetuses contain immunoreactivity for G proteins, Goα and Giα2. Chem Senses. 2001;26:517–522. doi: 10.1093/chemse/26.5.517. [DOI] [PubMed] [Google Scholar]
- 59.Bilkó A, Altbäcker V, Hudson R. Transmission of food preference in the rabbit: the means of information transfer. Physiol Behav. 1994;56:907–912. doi: 10.1016/0031-9384(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 60.Semke E, Distel H, Hudson R. Specific enhancement of olfactory receptor sensitivity associated with fetal learning of food odors in the rabbit. Naturwissenschaften. 1995;82:148–149. doi: 10.1007/BF01177279. [DOI] [PubMed] [Google Scholar]
- 61.Hepper PG. Adaptative fetal learning: prenatal exposure to garlic affects postnatal preferences. Anim Behav. 1988;36:935–936. [Google Scholar]
- 62.Schaal B, Marlier L, Soussignan R. Human foetuses learn odours from their pregnant mother’s diet. Chem Senses. 2000;25:729–737. doi: 10.1093/chemse/25.6.729. [DOI] [PubMed] [Google Scholar]
- 63.Molina JC, Domínguez HD, López MF, Pepino MY, Faas AE. The role of fetal and infantile experience with alcohol in later recognition and acceptance patterns of the drug. In: Hanningan JH, Spear NE, Spear LP, Goodlett CR, editors. Alcohol and Alcoholism: Brain and Development. Hillsdale, NJ: Lawrence Erlbaum Associates; 1999. pp. 199–228. [Google Scholar]
- 64.Bannoura MD, Kraebel KS, Spear LP, Spear NE. Effects of preweanling ethanol odor exposure on ethanol preference. Alcohol. 1998;15:213–217. doi: 10.1016/s0741-8329(97)00122-5. [DOI] [PubMed] [Google Scholar]
- 65.Molina JC, Serwatka J, Spear NE. Changes in alcohol intake resulting from prior experience with alcohol odor in young rats. Pharmacol Biochem Behav. 1984;21:387–391. doi: 10.1016/s0091-3057(84)80100-8. [DOI] [PubMed] [Google Scholar]
- 66.Molina JC, Serwatka J, Spear NE. Alcohol drinking patterns of young adult rats as a function of infantile aversive experiences with alcohol odor. Behav Neural Biol. 1986;46:257–271. doi: 10.1016/s0163-1047(86)90191-3. [DOI] [PubMed] [Google Scholar]
- 67.Molina JC, Chotro MG, Spear NE. Early (preweanling) recognition of ethanol’s orosensory cues resulting from acute ethanol intoxication. Behav Neural Biol. 1989;51:307–325. doi: 10.1016/s0163-1047(89)90961-8. [DOI] [PubMed] [Google Scholar]
- 68.Bachmanov AA, Kiefer SW, Molina JC, Tordoff MG, Duffy VB, Bartoshuk LM, Mennella JA. Chemosensory factors influencing alcohol perception, preferences, and consumption. Alcohol Clin Exp Res. 2003;27:220–231. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirai S, Yashiki T, Matsuzawa T, Mima H. Absorption of drugs from the nasal mucosa of rat. Int J Pharm. 1981;7:317–325. [Google Scholar]
- 70.Maruniak JA, Silver WL, Moulton DG. Olfactory receptors to blood-borne odorants. Brain Res. 1983;265:312–345. doi: 10.1016/0006-8993(83)90348-7. [DOI] [PubMed] [Google Scholar]
- 71.Lopez MF, Molina JC. Chronic alcohol administration in the rat pup: effects upon later consumption of alcohol and other palatable solutions. Addict Biol. 1999;4:169–180. doi: 10.1080/13556219971678. [DOI] [PubMed] [Google Scholar]
- 72.Di Lorenzo P, Kiefer S, Rice A, García J. Neural and behavioral responsivity to ethyl alcohol as a tastant. Brain Res Bull. 1986;3:55–61. doi: 10.1016/0741-8329(86)90071-6. [DOI] [PubMed] [Google Scholar]
- 73.Kiefer SW, Lawrence GJ. The sweet-bitter taste of alcohol: aversion generalization to various sweet-quinine mixtures in the rat. Chem Senses. 1988;13:633–641. [Google Scholar]
- 74.Kiefer SW, Mahadevan RS. The taste of alcohol for rats as revealed by aversion generalization tests. Chem Senses. 1993;18:509–522. [Google Scholar]
- 75.Molina JC, Chotro MG. Acute alcohol intoxication paired with appetitive reinforcement: effects upon ethanol intake in infant rats. Behav Neural Biol. 1989;51:326–345. doi: 10.1016/s0163-1047(89)90974-6. [DOI] [PubMed] [Google Scholar]
- 76.Molina JC, Chotro MG. Acute alcohol intoxication paired with aversive reinforcement: ethanol odor as a conditioned reinforcer in rat pups. Behav Neural Biol. 1989;52:1–19. doi: 10.1016/s0163-1047(89)90122-2. [DOI] [PubMed] [Google Scholar]
- 77.Molina JC, Serwatka J, Spear LP, Spear NE. Differential ethanol olfactory experiences affect ethanol ingestion in preweanlings but not in older rats. Behav Neural Biol. 1985;44:90–100. doi: 10.1016/s0163-1047(85)91226-9. [DOI] [PubMed] [Google Scholar]
- 78.Hunt PS, Kraebel KS, Rabine H, Spear LP, Spear NE. Enhanced ethanol intake in preweanling rats following exposure to ethanol in a nursing context. Dev Psychobiol. 1993;26:133–153. doi: 10.1002/dev.420260302. [DOI] [PubMed] [Google Scholar]
- 79.Molina JC, Pepino MY, Johnson J, Spear NE. The infant rat learns about alcohol through interaction with an intoxicated mother. Alcohol Clin Exp Res. 2000;24:428–437. [PubMed] [Google Scholar]
- 80.Pepino MY, Kraebel KS, López MF, Spear NE, Molina JC. Behavioral detection of low concentrations of ethanol in the preweanling rat. Alcohol. 1998;15:337–353. doi: 10.1016/s0741-8329(97)00154-7. [DOI] [PubMed] [Google Scholar]
- 81.Pepino MY, Spear NE, Molina JC. Nursing experiences with an alcohol intoxicated dam counteracts appetitive conditioned responses towards alcohol. Alcohol Clin Exp Res. 2001;25:18–24. [PubMed] [Google Scholar]
- 82.Pepino MY, Abate P, Spear NE, Molina JC. Heightened ethanol intake in infant and adolescent rats following nursing experiences with an ethanol-intoxicated dam. Alcohol Clin Exp Res. 2004;28:895–905. doi: 10.1097/01.alc.0000128223.95184.c9. [DOI] [PubMed] [Google Scholar]
- 83.Pueta M, Abate P, Spear NE, Molina JC. Interactions between pre-and early postnatal alcohol-related memories: impact upon alcohol acceptance patterns. Int J Comp Psicol. 2005;18:207–224. [Google Scholar]
- 84.Pautassi RM, Sanders S, Miller S, Spear N, Molina JC. Early ethanol’s anxiolytic effects assessed through an unconditional stimulus revaluation procedure. Alcohol Clin Exp Res. 2006;30:448–459. doi: 10.1111/j.1530-0277.2006.00049.x. [DOI] [PubMed] [Google Scholar]
- 85.Molina JC, Bannoura MD, Chotro MG, McKinzie DL, Arnold HM, Spear NE. Alcohol-mediated tactile conditioned aversions in infant rats: devaluation of conditioning through alcohol-sucrose association. Neurobiol Learn Mem. 1996;66:121–132. doi: 10.1006/nlme.1996.0053. [DOI] [PubMed] [Google Scholar]
- 86.Hunt PS, Molina JC, Spear LP, Spear NE. Ethanol-mediated taste aversions and state-dependency in preweanling (16 day old) rats. Behav Neural Biol. 1990;54:300–322. doi: 10.1016/0163-1047(90)90650-u. [DOI] [PubMed] [Google Scholar]
- 87.Hunt PS, Spear LP, Spear NE. An ontogenetic comparison of ethanol-mediated taste aversion learning and ethanol-induced hypothermia in preweanling rats. Behav Neurosci. 1991;105:971–983. doi: 10.1037//0735-7044.105.6.971. [DOI] [PubMed] [Google Scholar]
- 88.Pautassi RM, Godoy JC, Spear NE, Molina JC. Ethanol’s responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol Clin Exp Res. 2002;26:644–654. [PubMed] [Google Scholar]
- 89.Cheslock SJ, Varlinskaya EI, Petrov ES, Silveri MM, Spear LP, Spear NE. Ethanol as a reinforcer in the newborn’s first suckling experience. Alcohol Clin Exp Res. 2001;25:391–402. [PubMed] [Google Scholar]
- 90.Petrov ES, Varlinskaya EI, Smotherman WP. Self-administration of ethanol and saccharin in newborn rats: effects on suckling plasticity. Behav Neurosci. 2001;115:1318–1331. [PubMed] [Google Scholar]
- 91.Petrov ES, Varlinskaya EI, Spear NE. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcohol Clin Exp Res. 2003;27:1583–1591. doi: 10.1097/01.ALC.0000089960.62640.58. [DOI] [PubMed] [Google Scholar]
- 92.Pautassi RM, Melloni C, Ponce LF, Molina JC. Acute ethanol counteracts the acquisition of aversive olfactory learning in infant rats. Alcohol. 2005;36:99–105. doi: 10.1016/j.alcohol.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 93.Chotro MG, Molina JC. Acute ethanol contamination of the amniotic fluid during gestational day 21: postnatal changes in ethanol responsiveness in rats. Dev Psychobiol. 1990;23:535–547. doi: 10.1002/dev.420230608. [DOI] [PubMed] [Google Scholar]
- 94.Chotro MG, Molina JC. Bradycardic responses elicited by alcohol odor in rat neonates: influence of in utero experience with alcohol. Psychopharmacology (Berl) 1992;106:491–496. doi: 10.1007/BF02244820. [DOI] [PubMed] [Google Scholar]
- 95.Chotro MG, Cordoba NE, Molina JC. Acute prenatal experience with alcohol in the amniotic fluid: interactions with aversive and appetitive alcohol orosensory learning in the rat pup. Dev Psychobiol. 1991;24:431–451. doi: 10.1002/dev.420240605. [DOI] [PubMed] [Google Scholar]
- 96.Ronca AE, Alberts JF. Perinatal stimulation and adaptation of the neonate. Acta Paediatr Suppl. 1996;416:8–15. doi: 10.1111/j.1651-2227.1996.tb14271.x. [DOI] [PubMed] [Google Scholar]
- 97.Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: early olfactory learning. Behav Neural Biol. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- 98.Molina JC, Chotro MG. Association between chemosensory stimuli and cesarean delivery in rat fetuses: neonatal presentation of similar stimuli increases motor activity. Behav Neural Biol. 1991;55:42–60. doi: 10.1016/0163-1047(91)80126-y. [DOI] [PubMed] [Google Scholar]
- 99.Domínguez HD, Chotro MG, Molina JC. Alcohol in the amniotic fluid prior to cesarean delivery: effects of subsequent exposure to alcohol odor upon alcohol responsiveness. Behav Neural Biol. 1993;60:129–138. doi: 10.1016/0163-1047(93)90229-b. [DOI] [PubMed] [Google Scholar]
- 100.Domínguez HD, López MF, Molina JC. Interactions between perinatal and neonatal associative learning defined by contiguous olfactory tactile stimulation. Neurobiol Learn Mem. 1999;71:272–288. doi: 10.1006/nlme.1998.3882. [DOI] [PubMed] [Google Scholar]
- 101.Domínguez HD, López MF, Chotro MG, Molina JC. Perinatal responsiveness to alcohol’s chemosensory cues as a function of prenatal alcohol administration during gestational days 17–20 in the rat. Neurobiol Learn Mem. 1996;65:103–112. doi: 10.1006/nlme.1996.0012. [DOI] [PubMed] [Google Scholar]
- 102.Domínguez HD, López MF, Molina JC. Neonatal responsiveness to alcohol odor and infant alcohol intake as a function of alcohol experience during late gestation. Alcohol. 1998;16:109–117. doi: 10.1016/s0741-8329(97)00169-9. [DOI] [PubMed] [Google Scholar]
- 103.Kiefer SW. Alcohol palatability and taste reactivity. Neurosci Biobehav Rev. 1995;19:133–141. doi: 10.1016/0149-7634(94)00027-x. [DOI] [PubMed] [Google Scholar]
- 104.Arias C, Chotro MG. Increased preference for ethanol in the infant rat after prenatal ethanol exposure, expressed on intake and taste reactivity tests. Alcohol Clin Exp Res. 2005;29:337–346. doi: 10.1097/01.alc.0000156115.35817.21. [DOI] [PubMed] [Google Scholar]
- 105.Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: a conditioned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- 106.Arias C, Chotro MG. Increased palatability of ethanol after prenatal ethanol exposure is mediated by the opioid system. Pharmacol Biochem Behav. 2005;83:434–442. doi: 10.1016/j.pbb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 107.Acquas E, Meloni M, Di Chiara G. Blockade of delta-opioid receptors in the nucleus accumbens prevents ethanol-induced stimulation of dopamine release. Eur J Pharmacol. 1993;230:239–241. doi: 10.1016/0014-2999(93)90809-v. [DOI] [PubMed] [Google Scholar]
- 108.Critcher EC, Lin CI, Patel J, Myers RD. Attenuation of alcohol drinking in tetrahydroisoquinoline-treated rats by morphine and naltrexone. Pharmacol Biochem Behav. 1983;18:225–229. doi: 10.1016/0091-3057(83)90367-2. [DOI] [PubMed] [Google Scholar]
- 109.Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HWD, Koob GF, Gold LH. mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- 110.Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O’Brien CP. A comparison of the effects of the opioid antagonists’ naltrexone, naltrindole and β-funaltrexamine on ethanol consumption in the rat. Alcohol. 1998;15:281–289. doi: 10.1016/s0741-8329(97)00131-6. [DOI] [PubMed] [Google Scholar]
- 111.Hill KG, Kiefer SW. Naltrexone treatment increases the aversiveness of alcohol in outbreed rat. Alcohol Clin Exp Res. 1997;19:279–284. [PubMed] [Google Scholar]
- 112.Roth TL, Sullivan RM. Consolidation and expression of a shock-induced odor preference in rat pups is facilitated by opioids. Physiol Behav. 2003;78:135–142. doi: 10.1016/s0031-9384(02)00961-7. [DOI] [PubMed] [Google Scholar]
- 113.Varlinskaya EI, Petrov ES, Smotherman WP. Classical conditioning in the fetal rat: reinforcing properties of dynorphin A (1–13) Behav Neurosci. 1996;110:154–167. doi: 10.1037//0735-7044.110.1.154. [DOI] [PubMed] [Google Scholar]
- 114.Spear NE, Molina JC. Animal studies may reveal the consequences of early exposure to alcohol for later patterns of use and abuse in humans. In: Carroll ME, Overmier JB, editors. Animal Research for Human Health: Advancing Human Welfare Through Behavioral Science. Washington, DC: American Psychological Association; 2001. pp. 85–99. [Google Scholar]
- 115.Nizhnikov ME, Molina JC, Varlinskaya EI, Spear NE. Prenatal ethanol increases postnatal ethanol reinforcement. Alcohol Clin Exp Res. 2006;30:34–45. doi: 10.1111/j.1530-0277.2006.00009.x. [DOI] [PubMed] [Google Scholar]
- 116.Keller K, Hansel R, Chandler RF. Adverse Effects of Herbal Drugs. Berlin: Springer Verlag; 1992. [Google Scholar]
- 117.Reynolds JEF, editor. The Extra Pharmacopoeias. London: The Pharmaceutical Press; 1989. Martindale; p. 1063. [Google Scholar]
- 118.Abate P, Pepino MY, Domínguez HD, Spear NE, Molina JC. Fetal associative learning mediated through maternal alcohol administration. Alcohol Clin Exp Res. 2000;24:39–47. [PubMed] [Google Scholar]
- 119.Spear NE, Rudy J. Tests of learning and memory in the developing rat. In: Shair HN, Barr GA, Hofer MA, editors. Developmental Psychobiology: Current Methodological and Conceptual Issues. New York: Oxford University Press; 1991. pp. 84–113. [Google Scholar]
- 120.Miller JS, Molina JC, Spear NE. Ontogenetic differences in the expression of odor aversion learning in 4 and 8 day old rats. Dev Psychobiol. 1990;23:319–330. doi: 10.1002/dev.420230404. [DOI] [PubMed] [Google Scholar]
- 121.Rudy J, Cheatle M. Ontogeny of associative learning: acquisition of odor aversions by neonatal rats. In: Spear NE, Campbell B, editors. Ontogeny of Learning and Memory. New Jersey: Lawrence Erlbaum Associates; 1979. pp. 157–188. [Google Scholar]
- 122.Petrov ES, Varlisnkaya EI, Smotherman WP. The newborn rat ingests fluids through a surrogate nipple: a new technique for the study of early suckling behavior. Physiol Behav. 1997;62:693–696. doi: 10.1016/s0031-9384(97)00310-7. [DOI] [PubMed] [Google Scholar]
- 123.Smotherman WP, Goffman D, Petrov ES, Varlinkaya EI. Oral grasping of a surrogate nipple by the newborn rat. Dev Psychobiol. 1997;31:3–17. doi: 10.1002/(sici)1098-2302(199707)31:1<3::aid-dev2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 124.Varlinskaya EI, Petrov ES, Cheslock SJ, Spear NE. A new model of ethanol self-administration in newborn rats: gender effects on ethanol ingestion through a surrogate nipple. Alcohol Clin Exp Res. 1999;23:1368–1376. [PubMed] [Google Scholar]
- 125.Abate P, Varlinskaya EI, Cheslock SJ, Spear NE, Molina JC. Neonatal activation of alcohol-related prenatal memories: impact upon the first suckling response. Alcohol Clin Exp Res. 2002;26:1512–1522. doi: 10.1097/01.ALC.0000034668.93601.8F. [DOI] [PubMed] [Google Scholar]
- 126.Deweer B, Sara SJ, Hars B. Contextual cues and memory retrieval in rats: alleviation of forgetting by a pretest exposure to background stimuli. Animal Learn Behav. 1980;8:255–272. [Google Scholar]
- 127.Miller JS, Jagielo JA, Spear NE. Differential effectiveness of various prior-cuing treatments in the reactivation and maintenance of memory. J Exp Psychol Anim Behav Process. 1991;17:249–258. doi: 10.1037//0097-7403.17.3.249. [DOI] [PubMed] [Google Scholar]
- 128.Miller RR, Kasprow WJ, Schachtman TR. Retrieval variability sources and consequences. Am J Psychol. 1986;99:145–218. [PubMed] [Google Scholar]
- 129.Rescorla RA, Heth DC. Reinstatment of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Process. 1975;1:88–95. [PubMed] [Google Scholar]
- 130.Spear NE, Riccio DC. Memory: Phenomena and Principles. Boston: Allyn & Bacon; 1994. pp. 53–83. [Google Scholar]
- 131.Rodríguez WA, Horne CA, Padilla JL. Effects of glucose and fructose on recently reactivated and recently acquired memories. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:1285–1317. doi: 10.1016/s0278-5846(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 132.Spear NE. Retrieval of memories in animals. Psychol Rev. 1973;80:163–194. [Google Scholar]
- 133.Spear NE. The Processing of Memories: Forgetting and Retention. Hillsdale, NJ: Lawrence Erlbaum Associates; 1978. [Google Scholar]
- 134.Myers RD. Alcohol’s effect on body temperature: hypothermia, hyperthermia, or poikilothermia? Brain Res Bull. 1981;7:209–220. doi: 10.1016/0361-9230(81)90085-x. [DOI] [PubMed] [Google Scholar]
- 135.Cunningham CL, Hawks DM, Niehus JS. Role of hypothermia in ethanol-induced conditioned taste aversion. Psychopharmacology. 1988;95:318–322. doi: 10.1007/BF00181940. [DOI] [PubMed] [Google Scholar]
- 136.Johanson IB. Thermotaxis in neonatal rat pups. Physiol Behav. 1979;23:871–884. doi: 10.1016/0031-9384(79)90193-8. [DOI] [PubMed] [Google Scholar]
- 137.Ogilvie DM, Stinson RH. The effect of age on temperature selection by laboratory mice (Mus musculus) Can J Zool. 1966;44:511–517. doi: 10.1139/z66-055. [DOI] [PubMed] [Google Scholar]
- 138.Hoffman CM, Flory GS, Alberts JR. Neonatal thermotaxis improves reversal of a thermally reinforced operant response. Dev Psychobiol. 1999;34:87–99. doi: 10.1002/(sici)1098-2302(199903)34:2<87::aid-dev2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 139.Kraemer PJ, Hoffman H, Randall CK, Spear NE. Devaluation of Pavlovian conditioning in the 10-day-old rat. Anim Learn Behav. 1992;20:219–222. [Google Scholar]
- 140.Abate P, Pepino MY, Spear NE, Molina JC. Fetal associative learning with ethanol: correlations between maternal hypothermia and neonatal responsiveness to chemosensory cues of the drug. Alcohol Clin Exp Res. 2004;28:805–815. doi: 10.1097/01.alc.0000125354.15808.24. [DOI] [PubMed] [Google Scholar]
- 141.Abate P, Spear NE, Molina JC. Fetal and infantile alcohol-mediated associative learning in the rat. Alcohol Clin Exp Res. 2001;25:989–998. [PubMed] [Google Scholar]
- 142.Abate P, Haymal BO, Spear NE, Molina JC. Alcohol-mediated associative learning in utero: neonatal reactivation through re-exposure to the pharmacological effects of the drug. (Abstract) Alcohol Clin Exp Res. 2002;26 Suppl May:151A. [Google Scholar]
- 143.Fernandez-Vidal JM, Molina JC. Socially mediated alcohol preferences in adolescent rats following interactions with an intoxicated peer. Pharmacol Biochem Behav. 2004;79:229–341. doi: 10.1016/j.pbb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 144.Hunt PS, Lant GM, Carroll CA. Enhanced intake of ethanol in preweanling rats following interactions with intoxicated siblings. Dev Psychobiol. 2000;37:90–99. [PubMed] [Google Scholar]
- 145.Hunt PS, Holloway JL, Scordalakes EM. Social interactions with an intoxicated sibling can result in increased intake of ethanol by periadolescent rats. Dev Psychobiol. 2001;38:101–109. doi: 10.1002/1098-2302(200103)38:2<101::aid-dev1002>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 146.Mennella JA, Garcia PL. Children’s hedonic response to the smell of alcohol: effects of parental drinking habits. Alcohol Clin Exp Res. 2000;8:1167–1171. [PubMed] [Google Scholar]
- 147.Noll RB, Zucker RA, Greenberg GS. Identification of alcohol by smell among preschoolers: evidence for early socialization about drugs occurring in the home. Child Dev. 1990;61:1520–1527. [PubMed] [Google Scholar]
- 148.Chotro MG, Spear NE. Repeated exposure to moderate doses of alcohol in the rat fetus: evidence of sensitization to toxic and chemosensory aspects of alcohol. Alcohol Clin Exp Res. 1997;21:360–367. [PubMed] [Google Scholar]
- 149.Gruest N, Richer P, Hars B. Emergence of long-term memory for conditioned aversion in the rat fetus. Dev Psychobiol. 2004;44:189–198. doi: 10.1002/dev.20004. [DOI] [PubMed] [Google Scholar]
- 150.Niznikov ME, Varlinskaya EI, Spear NE. Reinforcing effects of central ethanol injections in newborn rat pups. Alcohol Clin Exp Res. 2006;30:2089–2098. doi: 10.1111/j.1530-0277.2006.00253.x. [DOI] [PubMed] [Google Scholar]
- 151.Froehlich JC. Interactions between alcohol and the endogenous opioid system. In: Zakhari S, editor. Alcohol and the Endocrine System. Bethesda, MD: U.S. Department of Health and Human Services, National Institute on Alcohol Abuse and Alcoholism Research Monograph No. 23; pp. 31–35. [Google Scholar]
- 152.Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 153.Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- 154.Li TK, Spanagel R, Colombo G, McBride WJ, Porrino LJ, Suzuki T, Rodd-Henricks ZA. Alcohol reinforcement and voluntary ethanol consumption. Alcohol Clin Exp Res. 2001;25 Suppl 5:117S–126S. doi: 10.1097/00000374-200105051-00021. [DOI] [PubMed] [Google Scholar]
- 155.McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- 156.Nizhnikov ME, Varlinskaya EI, Petrov ES, Spear NE. Reinforcing properties of ethanol in neonatal rats: involvement of the opioid system. Behav Neurosci. 2006;120:267–228. doi: 10.1037/0735-7044.120.2.267. [DOI] [PubMed] [Google Scholar]
- 157.Hudson R. Olfactory imprinting. Curr Opin Neurobiol. 1993;3:548–552. doi: 10.1016/0959-4388(93)90054-3. [DOI] [PubMed] [Google Scholar]
- 158.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 159.Schneider ML, Moore CF, Barnhart TE, Larson JA, DeJesus OT, Mukheriee J, Nickles RJ, Converse AK, Roberts AD, Kraemer GW. Moderate-level prenatal alcohol exposure alters striatal dopamine system function in rhesus monkeys. Alcohol Clin Exp Res. 2005;29:1685–1697. doi: 10.1097/01.alc.0000179409.80370.25. [DOI] [PubMed] [Google Scholar]
- 160.Ebrahim SH, Luman ET, Floyd RL, Murphy CC, Bennett EM, Boyle CA. Alcohol consumption by pregnant women in the United States during 1988–1995. Obstet Gynecol. 1998;92:187–192. doi: 10.1016/s0029-7844(98)00205-1. [DOI] [PubMed] [Google Scholar]
- 161.Savage DD, Becher M, de la Torre AJ, Sutherland RJ. Dose-dependent effects of prenatal ethanol exposure on synaptic plasticity and learning in mature offspring. Alcohol Clin Exp Res. 2002;26:1752–1758. doi: 10.1097/01.ALC.0000038265.52107.20. [DOI] [PubMed] [Google Scholar]
- 162.Mameli M, Valenzuela CF. Alcohol increases efficacy of immature synapses in a neurosteroid-dependent manner. Eur J Neurosci. 2006;23:835–839. doi: 10.1111/j.1460-9568.2006.04597.x. [DOI] [PubMed] [Google Scholar]
- 163.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 Suppl 3:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Young C, Olney JW. Neuroapoptosis in the infant mouse brain triggered by a transient small increase in blood alcohol concentration. Neurobiol Dis. 2006;22:548–554. doi: 10.1016/j.nbd.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 165.Arias C, Mlewski C, Pautassi R, Spear NE, Molina JC. Infantile behavioral sensitivity to ethanol as a function of late prenatal exposure to the drug (abstract) Alcohol Clin Exp Res. 2007;31:182A. [Google Scholar]
- 166.Hudson R. Olfactory imprinting. Curr Opin Neurobiol. 1993;3:548–552. doi: 10.1016/0959-4388(93)90054-3. [DOI] [PubMed] [Google Scholar]
- 167.Leon M. Neuroethology of olfactory preference. J Neurobiol. 1992;23:1557–1573. doi: 10.1002/neu.480231012. [DOI] [PubMed] [Google Scholar]
- 168.McLean JH, Harley CW. Olfactory learning in the rat pup: a model that may permit visualization of a mammalian memory trace. Neuroreport. 2004;15:1691–1697. doi: 10.1097/01.wnr.0000134988.51310.c3. [DOI] [PubMed] [Google Scholar]
- 169.Chotro MG, Kraebel KS, McKinzie DL, Molina JC, Spear NE. Prenatal and postnatal ethanol exposure influences preweanling rat’s behavioral and autonomic responding to ethanol odor. Alcohol. 1996;13:377–385. doi: 10.1016/0741-8329(96)00027-4. [DOI] [PubMed] [Google Scholar]
- 170.Pepino MY, López MF, Spear NE, Molina JC. Infant rats respond differentially to alcohol after nursing from an alcohol intoxicated dam. Alcohol. 1999;18:189–201. doi: 10.1016/s0741-8329(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 171.Fernández-Vidal JM, Melloni C, Spear NE, Molina JC. Interactions between prenatal alcohol exposure and socially transmitted alcohol preferences in juvenile rats. Alcohol Clin Exp Res. 2003;27 Suppl June:130A. [Google Scholar]
- 172.Malanga CJ, Kosofsky BE. Does drug abuse beget drug abuse? Behavioral analysis of addiction liability in animal models of prenatal drug exposure. Dev Brain Res. 2003;147:47–57. doi: 10.1016/j.devbrainres.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 173.Alati R, Al Mamum A, Williams GM, O’Callagan M, Njman JA, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood. Arch Gen Psychiatry. 2006;63:1009–1016. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- 174.Baer JS, Sampson PD, Barr HM, Conner PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol. 1998;59:533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- 175.Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60:377–386. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- 176.Griesler PC, Kandel DB. The impact of maternal drinking during and after pregnancy on the drinking of adolescent offspring. J Stud Alcohol. 1998;59:292–304. doi: 10.15288/jsa.1998.59.292. [DOI] [PubMed] [Google Scholar]
- 177.Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol and drug dependence. Alcohol Clin Exp Res. 1998;22:914–920. [PubMed] [Google Scholar]
- 178.Roberts AD, Moore CF, DeJesus OT, Barnhart TE, Larson JA, Mukherjee J, Nickles RJ, Schueller MJ, Shelton SE, Schneider ML. Prenatal stress, moderate fetal alcohol, and dopamine system function in rhesus monkeys. Neurotoxicol Teratol. 2004;26:169–178. doi: 10.1016/j.ntt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 179.Schen RY, Hannigan JH, Kapatos G. Prenatal ethanol reduces the activity of adult midbrain dopamine neurons. Alcohol Clin Exp Res. 1999;23:1801–1807. [PubMed] [Google Scholar]
- 180.Costa ET, Savage DD, Valenzuela CF. A review of the effects of prenatal or early postnatal ethanol exposure on brain lingand-gated ion channels. Alcohol Clin Exp Res. 2000;24:706–715. [PubMed] [Google Scholar]
- 181.Olney JW. Fetal alcohol syndrome at the cellular level. Addiction Biol. 2004;9:137–149. doi: 10.1080/13556210410001717006. [DOI] [PubMed] [Google Scholar]
- 182.Johnson JL, Leff M. Children of substance abusers: overview of research findings. Pediatrics. 1999;103:1085–1099. [PubMed] [Google Scholar]
- 183.Streissguth A, Barr H, Kogan J, Bookstein F. Primary and secondary disabilities in fetal alcohol syndrome. In: Streissguth A, Kanter J, editors. The Challenge of Fetal Alcohol Syndrome. Overcoming Secondary Disabilities. Seattle, WA: University of Washington Press; 1997. pp. 25–39. [Google Scholar]
- 184.Wong MM, Brower KJ, Fitzgeral HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol Clin Exp Res. 2004;28:578–587. doi: 10.1097/01.alc.0000121651.75952.39. [DOI] [PubMed] [Google Scholar]
- 185.Day NL, Richardson GA. An analysis of the effects of prenatal alcohol exposure on growth: a teratological model. Am J Med Genet C Semin Med Genet. 2004;127:28–34. doi: 10.1002/ajmg.c.30013. [DOI] [PubMed] [Google Scholar]
- 186.Mulder EJ, Morssink LP, van der Schee T, Visser GH. Acute maternal alcohol consumption disrupts behavioral state organization in the near-term fetus. Pediatr Res. 1998;44:774–779. doi: 10.1203/00006450-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 187.National Association of State Alcohol and Drug Abuse Directors, Inc. Alcohol Research on Prenatal Alcohol Exposure, Prevention, and Implication for State AOD Systems. State Issue Brief No. 2 prepared for the National Institute on Alcohol Abuse and Alcoholism. 2005 August