Abstract
Introduction
Cu-ATSM, a hypoxia imaging agent, has been shown to be predictive of response to traditional cancer therapies in patients with a wide range of tumors. It is known that the environment of the tumor results in a myriad of physiological consequences, including hypoxia, alterations in metabolism and proliferation. In an effort to better characterize the relationships between Cu-ATSM and other prominent radiopharmaceuticals, this current study was undertaken to compare the regional distribution of 64Cu-ATSM with 18F-FMISO, 18F-FDG and 18F-FLT in 9L tumors.
Methods
Taking advantage of the different half-life of 18F (t½ = 110 min) in comparison to 64Cu (t½ = 12.7 h), we undertook a dual tracer autoradiography study in 9L tumors. Four groups were examined: (a) 18F-FMISO, 2 h p.i. and 64Cu-ATSM 10 min p.i. (b) 18F-FMISO, 2 h p.i. and 64Cu-ATSM 24 h p.i. (c) 18F-FDG, 1 h p.i. and 64Cu-ATSM 10 min p.i., and (d) 18F-FLT, 1 h p.i. and 64Cu-ATSM 10 min p.i‥ Small animal PET imaging was performed in 9L tumor-bearing rats with imaging on concurrent days comparing 64Cu-ATSM with 18F-FMISO and 18F-FLT.
Results
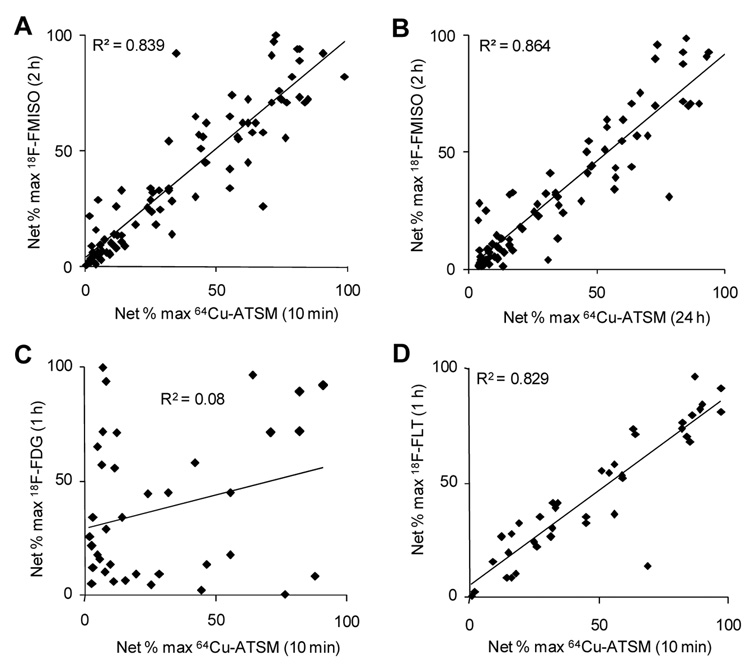
It was shown that the regional distribution of 18F-FMISO and 64Cu-ATSM showed an excellent correlation when the 64Cu-ATSM had been allowed to distribute for either 10 min (R2 = 0.84) or 24 h (R2 = 0.86). The regional comparisons between 64Cu-ATSM (10 min) and 18F-FDG (1 h) resulted in a very poor correlation (R2 = 0.08) between the regional uptake of the two agents. The comparison between 18F-FLT and 64Cu-ATSM showed a strong relationship (R2 = 0.83) between the two tracers. The small-animal PET images for the distribution comparisons between 64Cu-ATSM and 18F-FMISO and 18F-FLT were in agreement with the data generated from the autoradiography studies.
Conclusions
The data show that it is important to remember that a number of different metabolic situations can exist when considering the relationship between regions of high glucose uptake, proliferation and hypoxia.
Keywords: hypoxia, proliferation, metabolism
1. Introduction
The non-invasive imaging of hypoxia is of significant importance, given that the onset of hypoxia in malignant tissues is associated with and influenced by a myriad of complicated physiological processes [1–5]. Copper(II)-diacetyl-bis(N4-methylthiosemicarbazone), or Cu-ATSM, is a hypoxia imaging agent that shows rapid delineation of tumor hypoxia (< 1 hour) in high tumor to background tissue ratios (tumor to blood ratios ≫ 2.0) [6, 7]. In clinical studies Cu-ATSM has been shown to be predictive of response to traditional cancer therapies in patients with rectal, lung, and uterine cervix cancer [8–11]. In these same studies concurrent imaging with [18F]fluoro-2-deoxy-d-glucose (18F-FDG) showed no predictive value.
It is obvious that the macroenvironment of the tumor results in a wide range of physiological consequences, including hypoxia, alterations in metabolism and proliferation. An understanding of the relationships among these complicated interactions is of importance to both the basic scientist and the clinician. The relationship between hypoxia, proliferation and metabolism has partly been explored in clinical studies. A comparison between 18F-fluoromisonidazole (18F-FMISO) and 18F-FDG uptake in humans demonstrated that some hypoxic tumors can have modest glucose metabolism, whereas some highly metabolic tumors are not hypoxic, showing discordance in tracer uptake that was tumor type specific [12]. 18F-FDG uptake has been shown to correlate with tumor proliferative rates in lymphomas [13] and NSCLC [14]. However, Buck et al showed that 18F-fluorothymidine (18F-FLT) correlated significantly better with the proliferative activity of lung tumors than did 18F-FDG [15].
In an effort to better characterize the relationships between Cu-ATSM and other validated radiopharmaceuticals, this study was undertaken to compare the regional distribution of 64Cu-ATSM with 18F-FDG (metabolism) and 18F-FLT (proliferation). A comparison was also performed with 18F-FMISO, given that the retention mechanisms for these two hypoxia tracers are different. By exploitation of the different half lives of 18F (t½ = 110 min) and 64Cu (t½ = 12.7 h), we undertook a dual tracer autoradiography study in a simple model of cancer, the 9L gliosarcoma model, in order to better understand the regional distribution of each tracer in comparison to each other.
2. Material and Methods
2.1. Materials
Unless otherwise stated, all chemicals were purchased from Aldrich Chemical Company, Inc. (Milwaukee, WI). All solutions were prepared using distilled, deionized water (Milli-Q®; >18 MΩ resistivity). High specific activity 64Cu was produced on a CS-15 biomedical cyclotron at Washington University School of Medicine via published methods. 64Cu-ATSM was synthesized by methods previously described [16], and 18F-FMISO was synthesized according to procedures by McCarthy, Dence and Welch [17]. 18F-FLT was produced by a modified version of Machulla et al [18]. All radiopharmaceuticals were used in >98 % radiochemical purity, as determined by radio-TLC and radio-HPLC.
2.2. Animal Models
All animal experiments were conducted in compliance with the Guidelines for the Care and Use of Research Animals established by Washington University’s Animal Studies Committee. The 9L cells were a generous gift from the Brain Tumor Research Center, University of California (San Francisco). All studies were performed on 150–165 g female Fisher 344 rats (Charles River Laboratories, Wilmington MA). The Fischer rats were implanted subcutaneously with 1 × 107 9L gliosarcoma cells in the right flank and were used at day 14 of tumor growth. On the day of study the tumors were approximately 1.5 – 2 g, and on postmortem examination had varying degrees of necrosis. In all cases, rats were anesthetized with 1–2% isoflurane (with room air) for administration of radiopharmaceuticals.
2.3. Dual Tracer Ex vivo Autoradiography
All electronic autoradiography was performed on an InstantImager Electronic Autoradiography System from Packard Instrument Co. (Meriden, CT). Four groups of 9L-bearing animals (n = 5 each group) were examined, in order to compare the regional distributions of the fluorinated tracers 18F-FMISO, 18F-FLT and 18F-FDG. The four groups were (a) 18F-FMISO, 2 h post injection and 64Cu-ATSM 10 min post injection (b) 18F-FMISO, 2 h post injection and 64Cu-ATSM 24 h post injection (c) 18F-FDG, 1 h post injection and 64Cu-ATSM 10 min post injection, and (d) 18F-FLT, 1 h post injection and 64Cu-ATSM 10 min post injection. All rats received an i.v. injection of 1.0 – 1.2 mCi of the required 18F tracer, followed by 64Cu-ATSM. The administration of 50 – 60 µCi of 64Cu-ATSM occurred after 110 min for (a) and 50 min after for (c) and (d). For (b), each rat (n = 5) received an i.v. injection of 240 µCi of 64Cu-ATSM, followed 22 h later by 18F-FMISO. After euthanasia, the tumors were excised and frozen whole in Miles, Inc., Tissue-Tek Embedding Medium (Elkhart, IN). Slices (1 mm thick) were mounted and placed in the InstantImager® in order to visualize the distribution of radioactivity. The slices were not washed at any time and were not treated with any preservative. Autoradiography of the slices initially visualized the 18F localization (1 or 2 h distribution) and was repeated 24 h later, in order to visualize the 64Cu-ATSM (10 min or 24 h distribution). Standard radioisotopic mixtures were also made and imaged in order to ensure that >95% of the initial image was generated by the 18F and that only 64Cu activity remained in the 24-h images.
To generate a comparison between the focal uptake of 64Cu-ATSM and the 18F-agents, a ‘template’ was generated from the 18F image by use of the equipment software to yield the net % max uptake in each defined region. Briefly, the template generated by the software consists of an x–y grid that contains regions of interest (ROI’s) that have been determined by threshold levels defined automatically. The system uses three user-specified parameters to locate and create regions of interest based on separation, sensitivity and background. As a control in this study, regions of interest were also defined manually. The manual operation was performed where every individual tumor slice was first analyzed for the absolute number of counts. The ROIs based on separation were defined as percentages of the overall number of counts within the tumor ROI that showed the highest concentration of activity with fixed levels of sensitivity and background for individual slices. For both the manual and automatic analysis of the autoradiographs, identical templates and data were obtained.
The template generated for each 18F image was overlaid on the complementary 64Cu-ATSM image (i.e., the same tumor slice after 18F decay) in order to generate the 64Cu distribution data. As a control, a template was also generated on the 64Cu image and then overlaid on the complementary 18F image to generate the 18F distribution data. The data presented (net % max) are defined as [a/b ×100 %], where a = number of counts/mm2 in a defined ROI within a tumor slice and b = number of counts/mm2 in the ROI with the highest number of counts from the same tumor slice. These analyses were performed on 10 tumor slices for each study group. Each tumor slice had at least 10 ROIs defined within its boundaries.
2.4. Small-Animal PET Comparisons
All small-animal PET imaging was undertaken on the microPET-Focus 220 [19] (Concorde MicroSystems Inc., Knoxville, TN) and were coregistered with CT images from a MicroCAT II System (ImTek Inc., Knoxville, TN). Isoflurane (1–2%) was used as an inhaled anesthetic to induce and maintain anesthesia during imaging. Drugs were administered via the tail vein. Two separate studies were performed in 9L bearing rats (n = 4 per group). (A) On Day 1, 150 µCi of 64Cu-ATSM were injected into the animals and imaged 10 min post-injection. The animals were allowed to waken and on Day 2 (28 hours after 64Cu-ATSM) were injected with 1.5 mCi 18F-FMISO and imaged at 2 h post-injection; (B) On Day 1, 150 µCi of 64Cu-ATSM were injected into the animals and imaged 10 min post-injection. The animals were allowed to waken and on Day 2 (28 hours after 64Cu-ATSM) were injected with 1.5 mCi 18F-FLT and imaged at 1 h post-injection. All PET scans consisted of a single static 5-min collection. The image registration between CT and PET images was accomplished by using a landmark registration technique, AMIRA image display software (AMIRA, TGS Inc, San Diego, CA). The registration method proceeds by rigid transformation of the microCT images from landmarks provided by fiducial markers directly attached to the animal bed.
3. Results
3.1. Dual Tracer Ex vivo Autoradiography
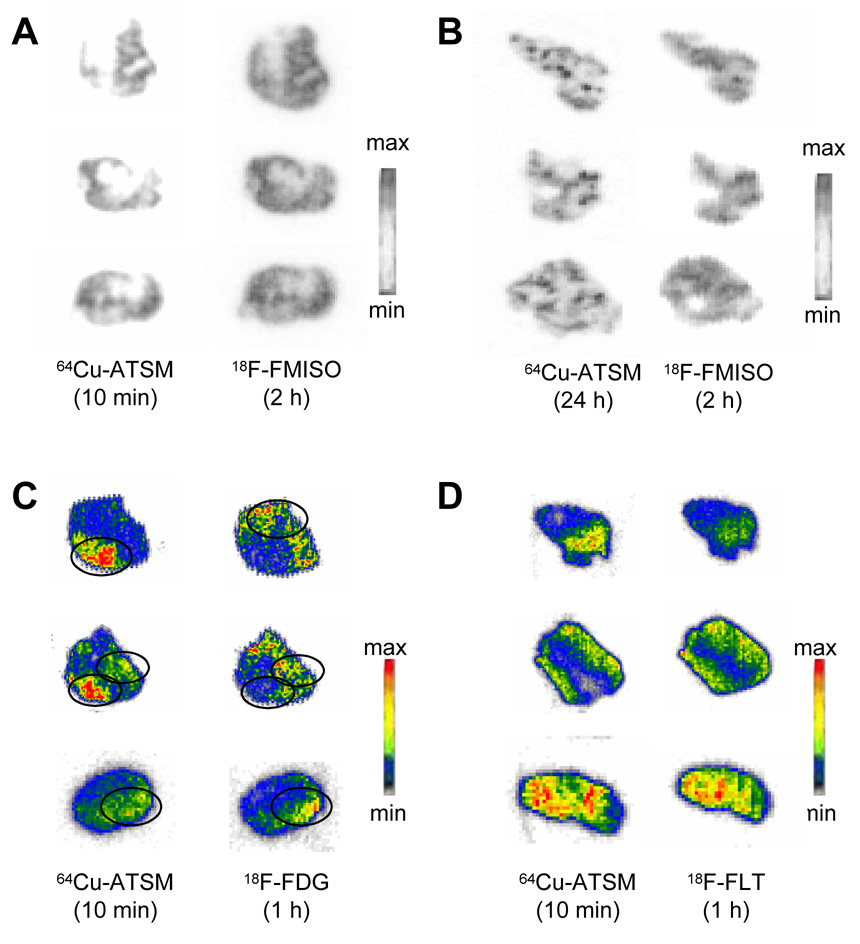
Shown in Figure 1A and 1B are representative sample slices from the autoradiographic studies comparing distribution of 18F-FMISO (2 h) and 64Cu-ATSM (10 min or 24 h). Figures 2A and 2B demonstrate the correlation of the regional uptake of 64Cu at both time points with the 2-h distribution of 18F-FMISO. It is evident, both through visual analysis and quantitative comparison, that the regional distributions of 18F-FMISO and 64Cu-ATSM show an excellent correlation when the 64Cu-ATSM has been allowed to distribute for either 10 min (R2 = 0.84) or 24 h (R2 = 0.86). It is also evident from Figure 1B that Cu-ATSM at 24-h results in a more intense uptake within small discreet regions of the tumor volume, more so than is seen with the corresponding 18F-FMISO image.
Fig. 1.
Ex vivo electronic autoradiographs of the regional in vivo uptake of (A, B) 18F-FMISO, (C) 18F-FDG, (D) 18F-FLT with 64Cu(ATSM) in 9L gliosarcomas. The images shown are representative of the typical dual-tracer autoradiographs obtained from all of the slices of the 9L tumors from rats. Shown in each panel are three representative slices chosen at random from a total of 120 slices from 5 tumors. Shown are slices with following 18F tracer collection (right) and the 64Cu-ATSM distribution (left) in the same tumor slices. At the time of imaging, the 18F images are generated 99% by 18F. Following 24 h decay the images are generated from 95+% 64Cu.
Fig. 2.
A direct measurement of the regional uptake of the 18F-agents and 64Cu-ATSM in 9L tumors. The correlation is based on the direct measurement of the net % maximum uptake (cpm) of the 18F agent and 64Cu-ATSM by use of software supplied with the Packard InstantImager. A significant correlation is seen between the regional uptake following 18F-FMISO (2 h distribution) and 64Cu-ATSM at (A) 10 min distribution and (B) 24 h distribution. No correlation is seen between the regional uptake following (C) 18F-FDG (1 h) and 64Cu-ATSM (10 min), but a significant correlation is observed between the distribution of (D) 18F-FLT (1 h) and 64Cu-ATSM (10 min).
Figure 1C shows the regional comparisons between 64Cu-ATSM (10 min) and 18F-FDG (1 h). Visually, it appears that there are regions within the tumor with uptake similar for both tracers but also regions in which there is a complete discrepancy of uptake, where uptake of Cu-ATSM is not mirrored by uptake of 18F-FDG. The quantitative analysis of these images (Figure 2C) confirms this, as demonstrated by a poor correlation (R2 = 0.08) between the regional uptake of the two agents. The uptake of 18F-FLT and 64Cu-ATSM appear visually identical (Figure 1D), which is confirmed by quantitative analysis (R2 = 0.83) (Figure 2D).
3.2. Small-Animal PET Comparisons
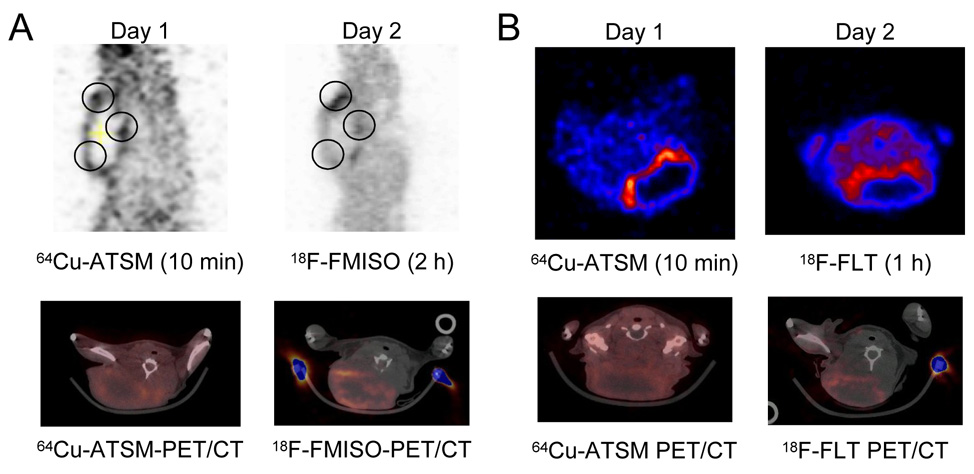
The small-animal PET images for the distribution comparisons between 64Cu-ATSM and 18F-FMISO and 18F-FLT are given in Figure 3A and 3B, respectively. As with the autoradiography studies, similarities in regional uptake are noted between the two tracers even after 10 min of Cu-ATSM distribution. The images comparing 64Cu-ATSM and 18F-FLT are also in agreement with the data generated from the autoradiography studies. The intense uptake of tracers is on the tumor edge (the tumor had a histologically confirmed necrotic center).
Fig. 3.
Representative PET images from 18F-FMISO or 18F-FLT and 64Cu-ATSM imaging in the same animals. On Day 1, 150 µCi 64Cu-ATSM was injected into the animals and imaged on the microPET-FOCUS 10 min post-injection; then on Day 1, the same animals were injected with 1.5 mCi of the respective 18F-FLT compound and reimaged at the required time point. (A) Sagittal PET images comparing the distribution of 10 min 64Cu-ATSM distribution with 2 h 18F-FMISO in the same tumor slice. Black circles denote similar regions of uptake, with a clearer delineation of regional uptake in the 64Cu-ATSM image. Below are representative transaxial PET/CT slices through the same point in the tumor. Histology confirmed a necrotic center to the tumor. (B) Transaxial PET images that compare the distribution of 10 min 64Cu-ATSM distribution with 1 h 18F-FLT in the same tumor slice. Below are representative transaxial PET/CT slices through the same point in the tumor. Histology confirmed a necrotic center to the tumor.
4. Discussion
4.1. Regional Uptake of 64Cu-ATSM and 18F-FMISO Show a Strong Correlation
One of the most popular agents for the PET imaging of hypoxia has been 18F-FMISO [12, 20, 21]. Although Cu-ATSM has been validated for use in vitro and in vivo in multiple tumor models [6] and it is a very effective PET agent for clinically delineating many hypoxic human malignancies [8–11], there has been a concern that its ability to delineate hypoxia may be tumor-dependent. In 2005, it was shown in vitro that the hypoxia-selectivity of 64Cu-ATSM was found to be cell line dependent. It was demonstrated that there was variation in the 64Cu cellular accumulation, with uptake in normoxic cells being anywhere from two to nine times lower than that in hypoxic cells, depending upon the cell line [22]. This study was followed by an intricate in vivo comparison of 64Cu-ATSM and 18F-FMISO in tumor-bearing rats with direct pO2 tumor measurements, autoradiography and fluorescent microscopy [23]. In rats bearing the R3327-AT rat prostate tumor, there was a poor correlation between the intra-tumoral distribution of 18F-FMISO and 64Cu-ATSM except at later times (16–20 hr post injection). However, in the same study 18F-FMISO and 64Cu-ATSM images were also acquired in nude rats bearing xenografts derived from the human squamous cell carcinoma cell line, FaDu. For the FaDu tumor model, the early and late 64Cu-ATSM PET images were similar and were in general concordance with the 18F-FMISO scans. Following these discrepancies, a recent publication showed that the ability of Cu-ATSM to delineate hypoxia was suspect primarily in prostate tumors in a manner that that was directly related to the expression of fatty acid synthase [24]. It was, therefore, important to continue to validate the use of 64Cu-ATSM against 18F-FMISO in this 9L gliosarcoma model. A previous study compared the autoradiographic distributions of 64Cu-ATSM with a well-established hypoxia marker drug (EF5) in 9L tumors; there was a close correlation between 64Cu- ATSM uptake and hypoxia in 9L tumors [25].
In the current study a comparison was made between the distribution of 18F-FMISO (2 h) and early and late distribution time points for 64Cu-ATSM (10 min and 24 h). Our data show that the distribution of 64Cu-ATSM at both 10 min and 24 h correlates highly with the 2-h distribution of 18F-FMISO. The similarity of the image quality of the radionuclides has been demonstrated (Figure 1A and 1B) and it is, therefore, interesting that the 64Cu-ATSM images show a more focal (localized) distribution of activity. Observed differences in distribution may be attributed to differences in retention mechanisms for the agents and the poor target:background ratios associated with the nitromidazoles. The distribution of 64Cu-ATSM at 24 h does have a greater correlation with 18F-FMISO than the 10 min distribution; however, these differences are not significant and confirm that examination of 64Cu-ATSM at earlier time points is suitable for the delineation of hypoxia within tumors.
4.2. Regional Uptake of 64Cu-ATSM and 18F-FDG Do Not Correlate in 9L Tumors
18F-FDG is utilized widely in many aspects of PET diagnostic medicine, including cancer diagnosis and diseases of the brain and heart. The mechanism of 18F-FDG trapping follows the well-documented glucose biochemical pathway. The direct measurement of glucose metabolism with 18F-FDG also yields valuable information about tumor localization and quantitation. A previous dual-tracer study with 64Cu-ATSM and 18F-FDG in VX2 tumors (implanted into Japanese white rabbits) showed that the major accumulation of 64Cu-ATSM was observed around the outer rim of the tumor masses, which consisted mainly of active hypoxic cells, and 18F-FDG was distributed more widely with highest levels in the inner regions where pre-necrotic cells were mainly observed [26]. An additional study compared the intratumoral distribution of 64Cu-ATSM and 18F-FDG in four mice-tumor models (LLC1, Meth-A, B16 and colon26) with the immunohistochemical staining of proliferating cells (Ki67), blood vessels (CD34 or von Willebrand factor), and apoptotic cells (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling method) [27]. It was revealed that there are regions within the tumor that contain cells of different phenotypes that can be distinguished by the use of 64Cu-ATSM and 18F-FDG [27].
This current study demonstrates that 9L tumors contain regions of high metabolism that are not very hypoxic and vice versa. It is reasonable and possible for regions of high 18F-FDG uptake to also show both high and low 64Cu-ATSM uptake. Tumor cells in close proximity to the vasculature would presumably have high 18F-FDG uptake, whereas cells remote from the vascular bed could be hypoxic. It is, therefore, possible that both conditions could exist within the same tumor. These data are in agreement with the previously reported animal [27] and clinical data [8–10, 12].
4.3. Regional Uptake of 64Cu-ATSM and 18F-FLT Show a Strong Correlation
18F-FLT has been proposed as a PET tracer for cell proliferation [28, 29]. It is retained in proliferating cells after phosphorylation by thymidine kinase (TK1). 18F-FLT is taken up by cells and phosphorylated by TK1, which leads to intracellular trapping within the cell. The retention of FLT within the cell provides a measure of cellular TK activity, an enzyme that is closely tied to cellular proliferation. It is apparent that the relationships between proliferation, hypoxia and metabolism are complicated. Studies that compare proliferation with hypoxia markers have led to a wide range of results. In an effort to characterize the distribution of hypoxia and proliferation in human squamous cell carcinoma of the cervix via an immunohistochemical approach, it was shown in 1997 that there was adirect relationship between hypoxia measured by pimonidazole binding and proliferation markers [30]. In a clinical imaging study in 2006, the relationship between 18F-FMISO and tumor markers of hypoxia, proliferation, and angiogenesis showed a weakly positive correlation between both 18F -FMISO (R2 = 0.64) and 18F-FDG uptake (R2 = 0.75) and the proliferative marker Ki67 in peripheral tumor, with no correlation seen in the central tumor [31].
The results in this current study would suggest, at least in the 9L tumor model, that regions of hypoxia as delineated by 64Cu-ATSM also demonstrate increased levels of proliferation as shown by the kinetics of 18F-FLT. This is in contrast to the observation by Tanaka et al that abundant positive nuclear staining of Ki67 in tumor cells was observed in the regions of high 18F-FDG uptake, and was hardly observed in regions of 64Cu-ATSM accumulation [27], and, similar to microvessel density, the number of Ki67 positive cells increased with 18F-FDG uptake but decreased with 64Cu-ATSM localization. The reasons for this discrepancy on the macro-level can be related to the fact that hypoxia might develop in different tumors through mechanisms unrelated to oxygen supply. Freyer et al., have demonstrated in spheroids that the oxygen consumption rate may increase as much as 5-fold in cells undergoing active proliferation [32, 33]. It has also been shown that angiogenesis occurs in tumors of a very small size (100 cells) [34], and it is reasonable to assume that high oxygen consumption rates in tumors could stimulate angiogenesis by the lowering of pO2. Moreover, in well-developed tumors, regions with high proliferative rates may create a locoregional hypoxia that is not directly related to the degree of vascularity, perfusion, or oxygen supply.
5. Conclusions
This present study highlights that a number of different situations can exist when considering the complex relationships between regions of high metabolism, proliferation and hypoxia. The 9L tumor exhibits many of the physiological attributes that could be apparent in the clinical situation: tumors with regions of varied metabolism, proliferation and hypoxia which may, or may not, be related to each other but are not discernible due to the resolution restrictions of the imaging modality. Specifically, the 9L tumors contain regions of high metabolism (high FDG uptake) that are not very hypoxic (low Cu-ATSM retention) and vice versa. Also seen are regions that demonstrate increased uptake of both tracers and vice versa. In regard to proliferation, regional hypoxia within the 9L tumor strongly correlates to increased levels of proliferation as shown by the kinetics of 18F-FLT. It is apparent that Cu-ATSM is a clinically relevant PET agent that has enormous value in the imaging of oncological hypoxia [6–11] and it has clearly been shown with Cu-ATSM that the 9L tumor has regions of hypoxia within its margins. These regions, at the resolution of the autoradiography instrumentation, are also highly proliferative but have varied levels of glucose metabolism.
Acknowledgements
This work was supported by the United States Department of Energy (DE-FG02-87ER60212). The production of copper radionuclides at Washington University is supported by a grant from the National Cancer Institute (1 R24 CA86307). We wish to thank Debbie Sultan, Todd Perkins and Thomas Voller for production of 64Cu, John Engelbach, and Lynne Jones for technical assistance, and Bill Margenau for cyclotron support. We also thank Dr. Joseph B. Dence for his editing of this manuscript.
This work was supported by a grant from the U. S. Department of Energy (Grant DE FG02-87ER60512). The production of copper radionuclides at Washington University is supported by a grant from the National Cancer Institute (Grant 1 R24 CA86307).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KSC, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, Hill RP, Koch CJ, Krishna MC, Krohn KA, Lewis JS, Mason RP, Melillo G, Padhani AR, Powis G, Rajendran JG, Reba R, Robinson SP, Semenza GL, Swartz HM, Vaupel P, Yang D, Croft B, Hoffman J, Liu G, Stone H, Sullivan D. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 2.Brown JM. The hypoxic cell: A target for selective cancer therapy-Eighteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1999;59:5863–5870. [PubMed] [Google Scholar]
- 3.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature (Lond) 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 4.Höckel M, Schlenger K, Aral B, Mitze M, Schäffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 5.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature (Lond) 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 6.Vavere AL, Lewis JS. Cu-ATSM: A radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans. 2007;43:4893–4902. doi: 10.1039/b705989b. [DOI] [PubMed] [Google Scholar]
- 7.Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med. 1997;38:1155–1160. [PubMed] [Google Scholar]
- 8.Dehdashti F, Grigsby PW, Lewis JS, Laforest R, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM. J Nucl Med. 2008;49:201–205. doi: 10.2967/jnumed.107.048520. [DOI] [PubMed] [Google Scholar]
- 9.Dehdashti F, Grigsby PW, Mintun MA, Lewis JS, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM: relationship to therapeutic response - a preliminary report. Int J Radiat Biol Phys. 2003;55:1233–1238. doi: 10.1016/s0360-3016(02)04477-2. [DOI] [PubMed] [Google Scholar]
- 10.Dehdashti F, Mintun MA, Lewis JS, Bradley J, Govindan R, Laforest R, Welch MJ, Siegel BA. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur J Nucl Med Mol Imaging. 2003;30:844–850. doi: 10.1007/s00259-003-1130-4. [DOI] [PubMed] [Google Scholar]
- 11.Dietz DW, Dehdashti FD, Grigsby PW, Malyapa RS, Myerson RJ, Picus J, Ritter J, Lewis JS, Welch MJ, Siegel BA. Tumor hypoxia detected by Positron Emission Tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing neoadjuvant chemoradiotherapy for rectal carcinoma: a pilot study. Dis Colon Rec. 2008 doi: 10.1007/s10350-008-9420-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajendran JG, Mankoff DA, O’Sullivan F, Peterson LM, Schwartz DL, Conrad EU, Spence AM, Muzi M, Farwell DG, Krohn KA. Hypoxia and glucose metabolism in malignant tumors: Evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004;10:2245–2252. doi: 10.1158/1078-0432.ccr-0688-3. [DOI] [PubMed] [Google Scholar]
- 13.Okada J, Yoshikawa K, Itami M, Imaseki K, Uno K, Itami J, Kuyama J, Mikata A, Arimizu N. Positron emission tomography using fluorine-18-fluorodeoxyglucose in malignant lymphoma: a comparison with proliferative activity. J Nucl Med. 1992;33:325–329. [PubMed] [Google Scholar]
- 14.Vesselle H, Schmidt RA, Pugsley JM, Li M, Kohlmyer SG, Vallières E, E WD. Lung cancer proliferation correlates with [F-18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000;6:3837–3844. [PubMed] [Google Scholar]
- 15.Buck AK, Halter G, Schirrmeister H, Kotzerke J, Wurziger I, Glatting G, Mattfeldt T, Neumaier B, Reske SN, Hetzel M. Imaging proliferation in lung tumors with PET: 18F-FLT Versus 18F-FDG. J Nucl Med. 2003;44:1426–1431. [PubMed] [Google Scholar]
- 16.Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med. 1997;38:1155–1160. [PubMed] [Google Scholar]
- 17.McCarthy TJ, Dence CS, Welch MJ. Application of microwave heating to the synthesis of [18F]fluoromisonidazole. Appl Radiat Isot. 1993;44:1129–1132. doi: 10.1016/0969-8043(93)90118-t. [DOI] [PubMed] [Google Scholar]
- 18.Machulla HJ, Blocher A, Kuntzsch M, Piert M, Wei R, Grierson JR. Simplified labeling approach for synthesizing 3'-deoxy-3'-[18F]fluorothymidine ([18F]FLT) J Radioanal Nucl Chem. 2000;243:843–846. [Google Scholar]
- 19.Tai Y-C, Ruangma A, Rowland D, Siegel S, Newport DF, Chow PL, Laforest R. Performance evaluation of the microPET Focus: A third-generation microPET scanner dedicated to animal imaging. J Nucl Med. 2005;46:455–463. [PubMed] [Google Scholar]
- 20.Nunn A, Linder K, Strauss HW. Nitroimidazoles and imaging hypoxia. Eur J Nucl Med. 1995;22:265–280. doi: 10.1007/BF01081524. [DOI] [PubMed] [Google Scholar]
- 21.Rajendran JG, Wilson DC, Conrad EU, Peterson LM, Bruckner JD, Rasey JS, Chin LK, Hofstrand PD, Grierson JR, Eary JF, Krohn KA. [18F]FMISO and [18F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging. 2003;30:695–704. doi: 10.1007/s00259-002-1096-7. [DOI] [PubMed] [Google Scholar]
- 22.Burgman P, O'Donoghue JA, Lewis JS, Welch MJ, Humm JL, Ling CC. Cell line dependent differences in uptake and retention of the hypoxia selective nuclear imaging agent Cu-diacetyl-bis(N4-methylthiosemicarbazone) (Cu-ATSM) Nucl Med Biol. 2005;32:623–630. doi: 10.1016/j.nucmedbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 23.O'Donoghue JA, Zanzonico P, Pugachev A, Wen B, Smith-Jones P, Cai S, Burnazi E, Finn RD, Burgman P, Ruan S, Lewis JS, Welch MJ, Ling CC, Humm JL. Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, pO2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models. Int J Radiat Onc Biol Phys. 2005;61:1493–1502. doi: 10.1016/j.ijrobp.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 24.Vavere AL, Lewis JS. Examining the relationship between Cu-ATSM hypoxia selectivity and fatty acid synthase expression in human prostate cancer cell lines. 2008. Nucl Med Biol. 2008;35:273–279. doi: 10.1016/j.nucmedbio.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan H, Schroeder T, Bowsher JE, Hedlund LW, Wong T, Dewhirst MW. Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) J Nucl Med. 2006;47:989–998. [PubMed] [Google Scholar]
- 26.Obata A, Yoshimoto M, Kasamatsu S, Naiki H, Takamatsu S, Kashikura K, Furukawa T, Lewis JS, Welch MJ, Saji H, Yonekura Y, Fujibayashi Y. Intratumoral distribution of 64Cu-ATSM: a comparison study with FDG. Nucl Med Biol. 2003;30:529–534. doi: 10.1016/s0969-8051(03)00047-7. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka T, Furukawa T, Fujieda S, Kasamatsu S, Yonekura Y, Fujibayashi Y. Double-tracer autoradiography with Cu-ATSM/FDG and immunohistochemical interpretation in four different mouse implanted tumor models. Nucl Med Biol. 2006;33:743–750. doi: 10.1016/j.nucmedbio.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, Obradovich JE, Muzik O, Mangner TJ. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nature Med. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 29.Shields AF, Grierson JR, Stayanoff JC, Lawhorn-Crews JM, Obradovich J, Muzik O, Mangner T. [F-18] FLT can be used to image cell proliferation in vivo. J Nucl Med. 1998;39:228p–228p. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy AS, Raleigh JA, Perez GM, Calkins DP, Thrall DE, Novotny DB, Varia MA. Proliferation and hypoxia in human squamous cell carcinoma of the cervix: first report of combined immunohistochemical assays. Int J Radiat Onc Biol Phys. 1997;37:897–905. doi: 10.1016/s0360-3016(96)00539-1. [DOI] [PubMed] [Google Scholar]
- 31.Cherk MH, Foo SS, Poon AMT, Knight SR, Murone C, Papenfuss AT, Sachinidis JI, Saunder THC, O'Keefe GJ, Scott AM. Lack of correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in non-small cell lung cancer assessed by 18F-fluoromisonidazole and 18F-FDG PET. J Nucl Med. 2006;47:1921–1926. [PubMed] [Google Scholar]
- 32.Freyer JP. Rates of oxygen consumption for proliferating and quiescent cells isolated from multicellular tumor spheroids. Adv Exp Med Biol. 1994;345:335–342. doi: 10.1007/978-1-4615-2468-7_44. [DOI] [PubMed] [Google Scholar]
- 33.Freyer JP, Sutherland RM. A reduction in the in situ rates of oxygen and glucose consumption of cells in EMT6/Ro spheroids during growth. J Cell Physiol. 1985;124:516–524. doi: 10.1002/jcp.1041240323. [DOI] [PubMed] [Google Scholar]
- 34.Li C-Y, Shan S, Huang Q, Braun RD, Lanzen J, Hu K, Lin P, Dewhirst MW. Initial stages of tumor cell-induced angiogenesis: Evaluation via skin window chambers in rodent models. J Natl Cancer Institute. 2000;92:143–147. doi: 10.1093/jnci/92.2.143. [DOI] [PubMed] [Google Scholar]