Abstract
Background
Several lines of evidence suggest that the lifetime prevalence of alcohol dependence among women has increased in recent decades, but has not risen significantly for men. Early age at onset of drinking (AOD) is strongly correlated with risk for alcohol dependence and there is evidence that mean AOD has also decreased, particularly for women. The present report sought to confirm the trends in AOD and to determine the extent to which they might account for secular trends in alcohol dependence.
Methods
Repeated cross-sectional analyses of data from two large, national epidemiological surveys were conducted to enable estimates of cross-cohort differences while controlling for age-related factors. Regression analyses were used to compute risk for alcohol dependence associated with birth cohort membership, before and after inclusion of AOD as a covariate.
Results
Both men and women born between 1944 and 1963 had earlier ages of onset for drinking than did the earliest birth cohort analyzed (1934–43). However, the net decrease in AOD was twice as large for women (3.2 years) than that for men (1.6 years). After adjusting for AOD, differences in lifetime prevalence between different birth cohorts of women were rendered non-significant, indicating that AOD accounts for a substantial portion of change in the lifetime prevalence of alcohol dependence.
Conclusions
These results suggest that a decrease in AOD accounts for much of the increase in lifetime alcohol dependence among women. AOD is likely to be an indicator of dynamic, and therefore modifiable risk behaviors impacting risk for alcohol dependence.
Keywords: alcohol dependence, prevalence, secular trends, race, women, age at onset
Introduction
Several lines of evidence suggest that secular changes in prevalence and relative sex distribution of alcohol use disorders have occurred in recent decades. Reviewing results of several epidemiological surveys, Greenfield and colleagues noted a decrease in the male-to-female lifetime prevalence ratio for alcohol use disorders between the early 1980s and early 1990s, based on data from the Epidemiological Catchment Area Study (ECA), National Comorbidity Survey (NCS) and National Longitudinal Alcohol Epidemiologic Survey (NLAES) (Greenfield et al., 2003). In a community-based study, Holdcraft and Iacono noted an apparent increase in the lifetime prevalence of DSM-III-R alcohol dependence for younger cohorts, with the rise in prevalence being particularly strong for women (Holdcraft and Iacono, 2002). We recently extended previous findings by simultaneously analyzing lifetime prevalence data for alcohol dependence from the combined National Longitudinal Alcohol Epidemiologic Survey (NLAES, 1991–92) and National Epidemiologic Survey on Alcohol and Related Conditions (NESARC, 2001–02) (Grucza et al, 2008). These analyses suggested that there that there were significant increases in the lifetime prevalence of drinking and alcohol dependence for women born between 1954 and 1983, compared with earlier birth cohorts but no significant secular trends among men
Early onset of alcohol use, operationalized as age at first drink, or age at onset of drinking (AOD), is a robust predictor of subsequent alcohol dependence, substance-related problems, and maladaptive use patterns (Agrawal et al., 2006; DeWit et al., 2000; Grant and Dawson, 1997; Grant et al., 2006; Hingson et al., 2000; Hingson et al., 2006; Schuckit and Russell, 1983). Each one-year decrement in age at onset of drinking is associated with an increase in odds for alcohol dependence as high as 21% (Prescott and Kendler, 1999). However, age at onset of drinking is correlated with a number of other risk factors such as attitudes toward drinking, peer associations, familial liability for drinking and alcohol dependence, and related variables (Hawkins et al., 1997; McGue et al., 2001a; McGue et al., 2001b; Pedersen and Skrondal, 1998). Accordingly, there is debate over whether or not early onset drinking is a direct cause of alcohol dependence or a non-causal correlate, indicative of pre-existing liability to alcohol dependence (DeWit et al., 2000; Grant, 1998; Grant et al., 2006; Prescott and Kendler, 1999; Prescott and Kendler, 2001). Nonetheless, it is clear that age at onset of drinking is, at minimum, a consistent indicator of liability for alcohol dependence. Evidence suggests that onset ages for drinking have become earlier for both men and women, and that these changes have been substantially larger for women (Holdcraft and Iacono, 2002). Thus, with regard to gender differences, secular trends in onset ages for drinking appear to parallel those for alcohol dependence.
The purpose of this report is to evaluate secular trends in ages at onset for drinking and to determine whether they can account for secular changes in alcohol dependence. To accomplish this, we utilize data from the combined NLAES and NESARC, two nationally representative surveys that were conducted ten years apart. As with our previous study, which used these data sets to examine birth cohort differences in the lifetime prevalence of alcohol dependence, the advantage of analyzing both samples simultaneously is that it is possible to study different birth cohorts while controlling for age-related effects, which might include recall effects, differential mortality, and other confounders. These age-related effects could bias estimates of secular trends in alcohol dependence, age at onset of drinking, and the relation between the two. Hence, the first objective of these analyses is to assess the magnitude of differences in mean age at onset of drinking in different birth cohorts sampled at the same age. Our second objective is to determine whether any such changes in age at onset of drinking can account for the previously documented secular changes in the lifetime prevalence of alcohol dependence, in particular, the increase in risk among women born between 1954 and 1983.
Methods
Survey Description
The NLAES (1991–92) and NESARC (2001–02) surveys focused on alcohol and drug use, DSM-IV substance use disorders, associated impairment and comorbid disorders in samples representative of the adult, non-institutionalized, civilian population of the United States. There were many methodological similarities between the two surveys, including the sampling universe and instrumentation used to assess alcohol dependence and other disorders. Blacks were oversampled in both surveys and Hispanics were oversampled in the NESARC. Face-to-face interviews were administered by experienced lay interviewers from the U.S. Census Bureau. Respondents were informed about measures taken to ensure the confidentiality of the information they provided and informed consent was obtained from all subjects. Ethical review and approval of all procedures was conducted by the U.S. Census Bureau and U.S. Office of Management and Budget. The final NESARC sample consisted of 43,093 persons; overall raw response rate was 81%. The final NLAES sample consisted of 42,862 persons with a response rate of 90%. Further details for both surveys, and comparative descriptions of methods are available elsewhere (Compton et al., 2004; Grant, 1997; Grant et al., 2004; Grant et al., 2003).
Diagnostic Measures
Outcome variables in these analyses include lifetime alcohol dependence among lifetime drinkers and age at onset of drinking. Lifetime alcohol dependence in the NLAES and NESARC was assessed with the Alcohol Use Disorder and Associated Disabilities Interview Schedule-DSM-IV version (AUDADIS-IV, Grant et al., 2001) which covers DSM-IV substance use syndromes for past 12-month and life time frames. To minimize the effect of changes to the AUDADIS-IV interview between the NLAES and NESARC, we eliminated items appearing in one survey questionnaire, but not the other (2 NLAES items and 1 NESARC item; see also Grucza et al, 2008). Age at onset of drinking was assessed with the following question “About how old were when you first started drinking, not counting small tastes or sips of alcohol?”
Additional Variables
Race/ethnicity was assessed by self-report. Analyses stratified by race/ethnicity were limited to subjects whose membership could be categorized as White, Black, or Hispanic because of low subsample sizes for groups. Because of the ten-year time-span between surveys, ten years represents the maximum age-category window that will allow subjects with non-overlapping birth years to be compared across surveys at similar ages. To minimize loss of statistical power due to stratification, subjects were categorized into ten-year age groups (18–27, 28–37, 38–47, and 48–57), allowing comparison of adjacent, non-overlapping birth cohorts across surveys. Treating age as a categorical, rather than a continuous variable allows estimation of secular changes without assuming a linear relationship between outcome variables and time (e.g., a monotonic increase in prevalence with birth hear).
Sample Composition
To mitigate the potential effects of differential mortality on cohort composition, analyses were limited to subjects aged 57 and younger at time of survey (eliminating n=12,019 / 11,696 from the NLAES and NESARC, respectively). In order to minimize changes in cohort composition due to in-migration between the NLAES and the NESARC, analyses were limited to U.S. born subjects (eliminating n=3,358 / 6,013). Out-migration can be considered to be statistically negligible for U.S. born adults (population loss of ~0.02% per year Fernandez, 1995; Lauderdale, 2001). Finally, because both age at onset of drinking and alcohol dependence are contingent upon alcohol use, subjects who reported never having consumed 12 drinks in a single year were excluded from the analyses (n=7,500 / 7,231). The sample sizes for the present analyses were 19,985 for the NLAES and 18,153 for the NESARC.
Analytical Design
Because of similarities in sampling universe, definitions of outcome variables, and other methodological characteristics, simultaneous analysis of the NLAES and NESARC constitutes a repeated cross-sectional analysis, which involves sampling one population at two points in time (in contrast with a panel design, which involves one sample assessed at two or more time points; (Firebaugh, 1997). When subjects from the NESARC, conducted in 2000–01, are grouped by age and compared with subjects of the same age range from the NLAES, conducted in 1990–91, the primary difference between the two is the range of birth years, which differs by ten years. The present analyses focus on the subset of subjects aged 18 to 57 at the time of survey, grouped into ten-year birth cohorts. Hence, subjects born between 1944 and 1973 were represented in both surveys, while those born between 1974 and 1983 were represented in the NESARC only, and those born between 1934 and 1943 are represented in the NLAES only. Ten-year birth cohorts from the NESARC were compared with the same-aged cohort in the NLAES, which represents individuals born in the ten-year period immediately prior to the NESARC birth cohort.
Statistical Analyses
Comparisons of ages of onset for drinking (AOD), the first objective of this study, were conducted by computing mean AOD for each ten-year birth cohort in each survey, and comparing results for same-aged groups across surveys. Cross-cohort differences in mean age at onset were assessed using two-sample Z-tests. The second objective of the study, determining whether cross-cohort differences in alcohol dependence can be attributed to changes in AOD, was accomplished using logistic regression analyses of combined NLAES and NESARC data. In the first step of these analyses, each pair of same-aged, temporally adjacent birth cohorts were combined, and odds ratios associated with birth cohort membership were computed (i.e., each NESARC cohort was compared with the immediately preceding NLAES birth cohort). In the second step, AOD was added to the regression model to determine the degree to which any significant risk associated with cohort membership can be explained by AOD as a risk factor.
Based on our reports demonstrating differences in secular trends for a alcohol use disorders by sex and race/ethnicity (Grucza et al, 2008; (Holdcraft and Iacono, 2002; Caetano and Clark, 1998), men and women were examined separately for all analyses. Furthermore, comparisons were first conducted with all racial/ethnic groups combined, and subsequently on groups stratified by race/ethnicity.
All statistical analyses were conducted using the SUDAAN statistical software package (RTI International, 2004). Variance estimation utilized a Taylor linearization method appropriate for the complex design of each survey. Significance of between-survey differences in drinking onset ages were assessed using two-sample Z-tests.
Results
Does recalled AOD change with Age?
It is possible that reporting of AOD is influenced by systematic age-related measurement error, as appears to be the case for lifetime prevalence of alcohol dependence (Grucza et al, 2008). Although our approach of comparing equivalent age groups across the two surveys essentially controls for this, ensuring that the magnitude of any such bias is small can increase confidence in such comparisons. To this end, we compared mean AOD in each birth cohort from the NLAES with the same birth cohort ten years later in the NESARC. To facilitate these comparisons, NESARC subjects were excluded if they reported drinking onset in the ten years prior to the survey. Excluding subjects whose drinking was initiated between the NLAES and the NESARC, eliminates the effect of late-onset drinking on the mean AOD for the NESARC (tentatively assuming accurate recall of AOD). Mean AOD for each cohort of men and women born between 1944 and 1973, the birth year limits for cohorts represented in both surveys, are listed in Table 1. There was no overall mean difference for men between the two surveys, while there was a small (0.1 year) mean difference for women (p=0.08). There were some larger and statistically significant differences in individual cohort comparisons, for example, the 1964–73 birth cohort of women tended to report later ages of onset in the NESARC, with a mean difference of 0.3 years. Even this difference, however, is relatively small (0.3% of the variance). We conclude that there is no evidence for large or systematic age-related bias in retrospective reporting of AOD.
Table 1.
Within-Cohort Comparisons of AOD by Sex
|
Drinking |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age
|
N
|
Mean AOD (SE)
|
Comparison: |
|||||||
| Birth Cohort | NLAES | NESARC | NLAES | NESARC | NLAES | NESARC | Difference (SE)a | P | ||
| Men | ||||||||||
| 1964–73 | 18–27 | 28–37 | 2,297 | 1,921 | 16.7 | 0.06 | 16.9 | 0.07 | +0.1 (0.09) | 0.11 |
| 1954–63 | 28–37 | 38–47 | 3,162 | 2,691 | 17.4 | 0.06 | 17.4 | 0.07 | +0.0 (0.09) | 0.79 |
| 1944–53 | 38–47 | 48–57 | 2,606 | 2,109 | 18.5 | 0.08 | 18.3 | 0.09 | −0.2 (0.12) | 0.07 |
| Total | 18–47 | 48–57 | 8,065 | 6,721 | 17.6 | 0.04 | 17.6 | 0.04 | 0.0 (0.06) | 0.79 |
| Women | ||||||||||
| 1964–73 | 18–27 | 28–37 | 2,380 | 2,072 | 17.1 | 0.05 | 17.4 | 0.07 | +0.3 (0.09) | <0.001 |
| 1954–63 | 28–37 | 38–47 | 3,655 | 2,510 | 18.2 | 0.06 | 18.4 | 0.08 | +0.2 (0.10) | 0.05 |
| 1944–53 | 38–47 | 48–57 | 2,596 | 1,891 | 20.3 | 0.11 | 20.2 | 0.11 | −0.1 (0.16) | 0.43 |
| Total | 18–47 | 48–57 | 8,631 | 6,473 | 18.5 | 0.05 | 18.6 | 0.05 | +0.1 (0.07) | 0.08 |
Difference in mean AOD between NESARC and NLAES for the birth cohort listed in the first column.
Mean values for Age at Onset of Drinking (AOD)
To assess cross-cohort differences in mean age at onset of drinking, same-aged ten-year birth cohorts from the NLAES and NESARC were compared; for example, NESARC subjects who were 48–57 years old at interview were born between 1944 and 1953, while NLAES subjects who were 48–57 years old were born between 1934 and 1943. By comparing cohorts that are categorically matched for age, any observed differences in AOD are related to differences between birth-cohorts, rather than age-related changes within a cohort, such as the influence of differences in length of the period of risk (due to age), recall effects, differential mortality, etc. Birth cohorts were also stratified by sex and race/ethnicity to examine whether any cohort differences would differ by these variables.
Results are shown in Table 2 for men and in Table 3 for women. For men, the 1944–53 birth cohort had significantly earlier ages of onset for drinking (change of −0.7 years, p<0.001) than did their counterparts born between 1934–43. A further reduction in AOD was seen for the 1954–63 birth cohort compared with the previous group (−0.9 years, p<0.001); the next birth cohort (1964–73) did not differ significantly from their predecessor, and a slight increase in AOD was seen for the most recent cohort (1974–83) when compared to their immediate predecessor. To summarize, the mean AOD for men was reduced by 1.6 years between the birth years 1944 and 1963, leveled off for the 1964–73 cohort and rose by 0.5 years for the 1974–83 cohort. Qualitatively, these patterns were consistent across racial/ethnic groups (Table 2), but smaller subsample sizes for Blacks and Hispanics preclude definitive statements.
Table 2.
Cross-Cohort Comparisons of AOD -- Men
|
Drinking |
||||||||
|---|---|---|---|---|---|---|---|---|
| Birth Cohort
|
N
|
Mean AOD (SE)
|
Comparison: |
|||||
| Age | NLAES | NESARC | NLAES | NESARC | NLAES | NESARC | Difference (SE)a | P |
| All Men | ||||||||
| 18–27 | 1964–73 | 1974–83 | 2,314 | 1,829 | 16.8 (0.1) | 17.2 (0.1) | +0.5 (0.08) | <0.001 |
| 28–37 | 1954–63 | 1964–73 | 3,206 | 2,281 | 17.4 (0.1) | 17.6 (0.1) | +0.1 (0.09) | 0.13 |
| 38–47 | 1944–53 | 1954–63 | 2,644 | 2,757 | 18.5 (0.1) | 17.6 (0.1) | −0.9 (0.09) | <0.001 |
| 48–57 | 1934–43 | 1944–53 | 1,635 | 2,165 | 19.1 (0.1) | 18.4 (0.1) | −0.7 (0.12) | <0.001 |
| White | ||||||||
| 18–27 | 1964–73 | 1974–83 | 1,902 | 1,127 | 16.7 (0.1) | 17.2 (0.1) | +0.5 (0.10) | <0.001 |
| 28–37 | 1954–63 | 1964–73 | 2,682 | 1,501 | 17.4 (0.1) | 17.4 (0.1) | 0.0 (0.10) | 0.76 |
| 38–47 | 1944–53 | 1954–63 | 2,225 | 1,951 | 18.4 (0.1) | 17.6 (0.1) | −0.9 (0.10) | <0.001 |
| 48–57 | 1934–43 | 1944–53 | 1,377 | 1,530 | 19.1 (0.1) | 18.4 (0.1) | −0.6 (0.13) | <0.001 |
| Black | ||||||||
| 18–27 | 1964–73 | 1974–83 | 246 | 281 | 17.7 (0.2) | 17.8 (0.2) | +0.1 (0.24) | 0.67 |
| 28–37 | 1954–63 | 1964–73 | 306 | 391 | 18.4 (0.2) | 18.9 (0.2) | +0.5 (0.29) | 0.11 |
| 38–47 | 1944–53 | 1954–63 | 283 | 455 | 19.2 (0.3) | 18.2 (0.2) | −1.0 (0.31) | 0.005 |
| 48–57 | 1934–43 | 1944–53 | 184 | 391 | 20.3 (0.6) | 18.6 (0.3) | −1.7 (0.39) | 0.01 |
| Hispanic | ||||||||
| 18–27 | 1964–73 | 1974–83 | 126 | 381 | 16.6 (0.2) | 17.1 (0.2) | +0.4 (0.27) | 0.16 |
| 28–37 | 1954–63 | 1964–73 | 148 | 356 | 17.0 (0.3) | 17.6 (0.3) | +0.5 (0.35) | 0.22 |
| 38–47 | 1944–53 | 1954–63 | 93 | 320 | 18.4 (0.4) | 17.5 (0.3) | −0.8 (0.42) | 0.09 |
| 48–57 | 1934–43 | 1944–53 | 52 | 213 | 18.6 (0.5) | 17.8 (0.4) | −0.8 (0.59) | 0.22 |
Difference in mean AOD between NESARC and NLAES cohorts corresponding to the ages listed in the first column.
Table 3.
Cross-Cohort Comparisons of AOD -- Women
| Birth Cohort
|
N
|
Mean AOD (SE)
|
Comparison: |
|||||
|---|---|---|---|---|---|---|---|---|
| Age | NLAES | NESARC | NLAES | NESARC | NLAES | NESARC | Difference (SE)a | P |
| All Women | ||||||||
| 18–27 | 1964–73 | 1974–83 | 2,405 | 1,940 | 17.1 (0.1) | 17.6 (0.1) | +0.5 (0.09) | <0.001 |
| 28–37 | 1954–63 | 1964–73 | 3,697 | 2,611 | 18.2 (0.1) | 18.3 (0.1) | +0.1 (0.11) | 0.31 |
| 38–47 | 1944–53 | 1954–63 | 2,639 | 2,614 | 20.3 (0.1) | 18.7 (0.1) | −1.6 (0.13) | <0.001 |
| 48–57 | 1934–43 | 1944–53 | 1,445 | 1,956 | 22.1 (0.2) | 20.6 (0.2) | −1.6 (0.19) | <0.001 |
| White | ||||||||
| 18–27 | 1964–73 | 1974–83 | 1,919 | 1,196 | 17.0 (0.1) | 17.6 (0.1) | +0.6 (0.10) | <0.001 |
| 28–37 | 1954–63 | 1964–73 | 2,978 | 1,670 | 17.9 (0.1) | 18.0 (0.1) | +0.1 (0.11) | 0.52 |
| 38–47 | 1944–53 | 1954–63 | 2,226 | 1,768 | 20.3 (0.1) | 18.5 (0.1) | −1.8 (0.13) | <0.001 |
| 48–57 | 1934–43 | 1944–53 | 1,195 | 1,374 | 22.1 (0.2) | 20.4 (0.2) | −1.6 (0.21) | <0.001 |
| Black | ||||||||
| 18–27 | 1964–73 | 1974–83 | 267 | 337 | 18.1 (0.2) | 18.2 (0.2) | +0.2 (0.25) | 0.55 |
| 28–37 | 1954–63 | 1964–73 | 499 | 508 | 19.7 (0.2) | 19.6 (0.2) | 0.0 (0.32) | 0.95 |
| 38–47 | 1944–53 | 1954–63 | 292 | 494 | 21.0 (0.4) | 20.2 (0.4) | −0.8 (0.43) | 0.17 |
| 48–57 | 1934–43 | 1944–53 | 185 | 397 | 22.9 (0.7) | 21.0 (0.3) | −1.9 (0.53 | 0.01 |
| Hispanic | ||||||||
| 18–27 | 1964–73 | 1974–83 | 149 | 368 | 17.6 (0.2) | 17.2 (0.3) | −0.4 (0.37) | 0.24 |
| 28–37 | 1954–63 | 1964–73 | 142 | 382 | 18.8 (0.3) | 18.9 (0.3) | +0.1 (0.33) | 0.78 |
| 38–47 | 1944–53 | 1954–63 | 77 | 319 | 21.2 (0.7) | 19.5 (0.4) | −1.7 (0.49) | 0.02 |
| 48–57 | 1934–43 | 1944–53 | 43 | 157 | 22.3 (1.1) | 22.2 (0.7) | −0.1 (0.96) | 0.91 |
Difference in mean AOD between NESARC and NLAES cohorts corresponding to the ages listed in the first column.
Trends in AOD for women followed a similar pattern, but, importantly, the reduction in AOD seen for the 1944–53 and 1954–63 birth cohorts, each compared with their immediate predecessors, was larger for women than for men (−1.6 years in each case, p<0.001, vs. −0.8 and −0.9 years for the corresponding male cohorts). Hence, there was a 3.2 year reduction in mean AOD between birth years 1944 and 1963, followed by a leveling off for the 1964–73 birth cohort, and a small increase (0.5 years) for the 1974–83 cohort. The pattern was clearest among White women, but small subsample sizes for minority groups again preclude definitive statements.
The Role of AOD in Secular Changes in Alcohol Dependence
We sought to determine whether cohort differences in risk for alcohol dependence among drinkers could be partially attributable to the changes in AOD documented in Tables 1 and 2. In order to address this, each ten-year NESARC cohort was combined with the immediately preceding ten-year birth cohort from the NLAES. In the first step of each analysis, the odds ratio associating risk for alcohol dependence and birth cohort membership was computed. In the second step, AOD was added to the model. Table 4 lists odds ratios for cross-cohort comparisons of men, before and after inclusion of AOD. As documented previously, there were no significant differences in lifetime risk for alcohol dependence between different cohorts of men (Grucza et al, 2008). We show here that adjusting for AOD has little effect on the odds ratios; for all comparisons, odds ratios associated with cohort membership remain near 1.0 for men. One noteworthy trend, however, is that for the 1954–63 birth cohort of men, relative to the 1944–53 birth cohort (38–47 year old, all race/ethnicity combined), there is a drop in odds ratio from 1.10 (95% CI: 0.94, 1.28) to 0.94 (95% CI: 0.80, 1.09) after including AOD in the model. Though neither odds ratio is significant alone, the point estimate prior to adjustment for AOD falls outside of the 95% confidence interval of the adjusted value. This suggests a near-significant mediation of cohort-related risk by AOD.
Table 4.
The Effect of AOD on Cross-Cohort Differences in Lifetime Prevalence of Alcohol Dependence Among Lifetime Drinkers: Men
|
Birth Years |
N |
Prevalence (%) |
Odds Ratios |
|||||
|---|---|---|---|---|---|---|---|---|
| Age | NLAES | NESARC | NLAES | NESARC | NLAES | NESARC | OR (95% CI)‡ | OR Adjusted for AOD (95% CI)‡ |
| All | ||||||||
| 18–27 | 1964–73 | 1974–83 | 2,314 | 1,829 | 32.7 | 33.1 | 1.02 (0.87, 1.20) | 1.14 (0.97, 1.35) |
| 28–37 | 1954–63 | 1964–73 | 3,206 | 2,281 | 27.6 | 27.0 | 0.97 (0.84, 1.12) | 0.98 (0.84, 1.13) |
| 38–47 | 1944–53 | 1954–63 | 2,644 | 2,757 | 24.4 | 26.1 | 1.10 (0.94, 1.28) | 0.94 (0.80, 1.09) |
| 48–57 | 1934–43 | 1944–53 | 1,635 | 2,165 | 18.0 | 20.5 | 1.17 (0.96, 1.43) | 1.08 (0.88, 1.32) |
| White | ||||||||
| 18–27 | 1964–73 | 1974–83 | 1,902 | 1,127 | 33.8 | 35.3 | 1.07 (0.89, 1.29) | 1.22 (1.01, 1.48)* |
| 28–37 | 1954–63 | 1964–73 | 2,682 | 1,501 | 28.5 | 28.1 | 0.98 (0.83, 1.16) | 0.98 (0.82, 1.16) |
| 38–47 | 1944–53 | 1954–63 | 2,225 | 1,951 | 24.2 | 26.1 | 1.10 (0.93, 1.30) | 0.94 (0.79, 1.11) |
| 48–57 | 1934–43 | 1944–53 | 1,377 | 1,530 | 17.4 | 20.0 | 1.19 (0.95, 1.48) | 1.12 (0.89, 1.40) |
| Black | ||||||||
| 18–27 | 1964–73 | 1974–83 | 246 | 281 | 22.1 | 19.9 | 0.88 (0.52, 1.49) | 0.89 (0.51, 1.54) |
| 28–37 | 1954–63 | 1964–73 | 306 | 391 | 21.8 | 18.8 | 0.83 (0.55, 1.27) | 0.89 (0.58, 1.37) |
| 38–47 | 1944–53 | 1954–63 | 283 | 455 | 21.2 | 23.3 | 1.13 (0.75, 1.69) | 1.00 (0.66, 1.52) |
| 48–57 | 1934–43 | 1944–53 | 184 | 391 | 24.5 | 19.2 | 0.73 (0.44, 1.23) | 0.64 (0.38, 1.08) |
| Hispanic | ||||||||
| 18–27 | 1964–73 | 1974–83 | 126 | 381 | 36.5 | 31.4 | 0.80 (0.48, 1.34) | 0.83 (0.49, 1.42) |
| 28–37 | 1954–63 | 1964–73 | 148 | 356 | 25.6 | 26.8 | 1.06 (0.61, 1.84) | 1.12 (0.63, 1.99) |
| 38–47 | 1944–53 | 1954–63 | 93 | 320 | 30.3 | 31.4 | 1.05 (0.55, 2.00) | 0.91 (0.47, 1.73) |
| 48–57 | 1934–43 | 1944–53 | 52 | 213 | 21.2 | 21.9 | 1.04 (0.43, 2.51) | 0.75 (0.30, 1.84) |
Notes:
Odds Ratio for NESARC relative to NLAES; corresponds to OR for birth years included in NESARC age group (third column), relative to the preceding birth cohort, which is represented in the NLAES (second column).
p<0.05
The odds ratios for cross-cohort comparisons of women are shown in Table 5. As previously demonstrated (Grucza et al, 2008), women experienced an increase in risk for lifetime alcohol dependence beginning with the 1954–1963 birth cohort compared to their immediate predecessors born between 1944 and 1953 (third row of Table 5). There was no further increase in risk for later birth cohorts, but neither was their a return to earlier levels of risk; i.e., the 1964–83 birth cohorts were at equally high risk as the 1954–63 birth cohort, who were at 1.53-fold higher odds than the previous (1944–53) cohort. The analyses stratified by race/ethnicity demonstrate that the increase in risk was significant for White and Hispanic women, but not Black women. In the second step of the analysis, in which AOD was incorporated into the model, the odds ratio for the 1954–63 vs. 1944–53 birth cohort comparisons were rendered non-significant. In other words, the key finding with regard to secular trends, i.e., the elevated prevalence for the 1954–63 cohort relative to their predecessors, relative to their immediate predecessors, is largely explainable by changes in AOD. In the analyses unstratified by race/ethnicity, the odds ratio was reduced from 1.53 prior to inclusion of AOD, to 1.15 after including AOD. This corresponds to a reduction in the logistic regression coefficient (log OR) of more than 70%. These are the same birth cohorts for which the smaller, marginally significant mediation effect was seen in men (i.e., the 1954–63 birth cohort relative to the 1944–53 cohort). In the analyses stratified by race/ethnicity, the results for White women paralleled those for the whole sample, while a significant birth-cohort effect remained for Hispanic women after incorporation of AOD into the model.
Table 5.
The Effect of AOD on Cross-Cohort Differences in Lifetime Prevalence of Alcohol Dependence Among Lifetime Drinkers: Women
|
Birth Years |
N |
Prevalence (%) |
Odds Ratios |
|||||
|---|---|---|---|---|---|---|---|---|
| Age | NLAES | NESARC | NLAES | NESARC | NLAES | NESARC | OR (95% CI)‡ | OR Adjusted for AOD (95% CI)‡ |
| All | ||||||||
| 18–27 | 1964–73 | 1974–83 | 2,405 | 1,940 | 22.4 | 21.3 | 0.94 (0.79, 1.12) | 1.06 (0.89, 1.26) |
| 28–37 | 1954–63 | 1964–73 | 3,697 | 2,611 | 18.2 | 18.8 | 1.04 (0.89, 1.22) | 1.04 (0.88, 1.22) |
| 38–47 | 1944–53 | 1954–63 | 2,639 | 2,614 | 11.1 | 16.0 | 1.53 (1.26, 1.87)*** | 1.15 (0.93, 1.43) |
| 48–57 | 1934–43 | 1944–53 | 1,445 | 1,956 | 9.3 | 11.2 | 1.24 (0.94, 1.63) | 1.18 (0.90, 1.56) |
| White | ||||||||
| 18–27 | 1964–73 | 1974–83 | 1,919 | 1,196 | 23.4 | 22.0 | 0.92 (0.76, 1.12) | 1.08 (0.88, 1.32) |
| 28–37 | 1954–63 | 1964–73 | 2,978 | 1,670 | 18.4 | 19.3 | 1.06 (0.89, 1.26) | 1.05 (0.88, 1.26) |
| 38–47 | 1944–53 | 1954–63 | 2,226 | 1,768 | 10.9 | 15.8 | 1.52 (1.22, 1.90)*** | 1.11 (0.87, 1.42) |
| 48–57 | 1934–43 | 1944–53 | 1,195 | 1,374 | 8.9 | 11.2 | 1.29 (0.96, 1.75) | 1.22 (0.90, 1.65) |
| Black | ||||||||
| 18–27 | 1964–73 | 1974–83 | 267 | 337 | 11.5 | 13.8 | 1.23 (0.67, 2.24) | 1.30 (0.69, 2.43) |
| 28–37 | 1954–63 | 1964–73 | 499 | 508 | 15.6 | 12.5 | 0.77 (0.49, 1.23) | 0.76 (0.48, 1.21) |
| 38–47 | 1944–53 | 1954–63 | 292 | 494 | 12.2 | 13.4 | 1.11 (0.65, 1.91) | 0.99 (0.57, 1.72) |
| 48–57 | 1934–43 | 1944–53 | 185 | 397 | 9.2 | 10.2 | 1.13 (0.56, 2.27) | 1.11 (0.54, 2.28) |
| Hispanic | ||||||||
| 18–27 | 1964–73 | 1974–83 | 149 | 368 | 15.1 | 24.6 | 1.83 (0.99, 3.40) | 1.62 (0.87, 3.02) |
| 28–37 | 1954–63 | 1964–73 | 142 | 382 | 18.3 | 21.7 | 1.24 (0.69, 2.22) | 1.21 (0.66, 2.23) |
| 38–47 | 1944–53 | 1954–63 | 77 | 319 | 7.6 | 21.5 | 3.31 (1.35, 8.12)*** | 2.64 (1.07, 6.52)* |
| 48–57 | 1934–43 | 1944–53 | 43 | 157 | 9.5 | 15.7 | 1.78 (0.59, 5.32) | 1.81 (0.58, 5.64) |
Notes:
Odds Ratio for NESARC relative to NLAES; corresponds to OR for birth years included in NESARC age group (third column), relative to the preceding birth cohort, which is represented in the NLAES (second column).
p<0.05
p<0.01
p<0.001
Summary and Graphical Analysis
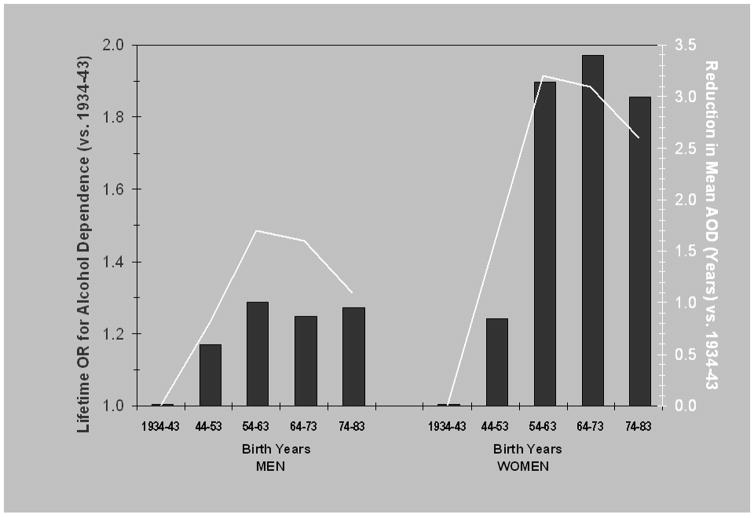
Figure 1 illustrates the cumulative secular trends in risk for alcohol dependence, and their relation to changes in AOD. Cumulative odds ratios for each birth cohort (unadjusted), relative to the earliest (1934–43) birth cohort, are plotted separately for men and women. Whereas the odds ratios listed in Tables 3 and 4 compare each birth cohort to the immediately preceding cohort, the cumulative odds ratios involve the product of those listed in the tables, such that all are computed relative to the 1934–43 birth cohort. The cumulative reduction in mean AOD, relative to the 1934–43 birth cohort is also plotted. A close correspondence between change in AOD and risk for alcohol dependence is seen for both men and women across birth cohorts. Although secular trends in alcohol dependence among men did not meet statistical significance, it is noteworthy that the qualitative patterns for both lifetime alcohol dependence and AOD are similar to that observed for women, but with smaller magnitudes of change.
Figure 1.
Summary of changes in prevalence and mean age at onset of drinking across birth cohorts. Bars represent odds ratios for alcohol dependence among drinkers, referenced to the 1934–43 birth cohort. Lines represent net change in mean AOD, relative to the 1934–43 birth cohort.
Discussion
Previous analyses of the combined NLAES/NESARC samples exploited the repeated cross-sectional design of these two surveys, which were conducted ten years apart, and demonstrated increased prevalence of lifetime alcohol dependence among White and Hispanic women born between 1954 and 1983, relative to those born before 1954, as well as lower rates of abstinence for women born in recent decades (Grucza et al, 2008). These differences held even after accounting age-related effects, which might include differences in recall and other factors, by comparing different birth cohorts interviewed at the same age. The key finding of the present study is that much of the increase in risk for alcohol dependence among women may be attributable to earlier onset ages for drinking during recent decades. Specifically, the primary difference discovered in between-cohort comparisons, namely, the lifetime prevalence of alcohol dependence in the 1954–63 birth cohort of women compared with that for 1944–53 cohort, was rendered statistically non-significant once AOD was accounted for, with a corresponding 71% reduction in effect size. A suggestive mediation effect was observed among men when these birth cohorts were compared, though secular trends in lifetime prevalence of alcohol dependence among men were not statistically significant. Subsequent (1964–1983) cohorts were at equivalent levels of risk as the 1954–63 cohort, both before and after accounting for AOD (Table 5). Hence, much of the increased risk for alcohol dependence among women, which began with the 1954–63 birth cohort, is accounted for by earlier AOD. This pattern was most pronounced for White women; secular trends for Hispanic women were only slightly reduced by controlling for AOD and no significant secular trends were observed for Black women before or after accounting for AOD.
Reductions in age at onset of drinking are apparent for both men and women born after 1943, however, the reductions for women were larger than that for men. There has been some return to older ages of onset of drinking for the most recent birth cohorts, but there has been an overall net reduction in AOD, particularly among women (Table 3). The observation that these changes correspond to changes in risk for alcohol dependence among women underscores the need to better understand the sources of changes in AOD and their potential impact on correlated behaviors and comorbid pathologies.
These secular trends in age at onset of drinking may be attributable, in part, to changes in minimum legal drinking age (MLDA) laws. Between 1970 and 1975, the MLDA was lowered in 29 states in the U.S. The 1984 federal minimum drinking age law reversed the earlier statutory changes, by prompting all states to set the minimum age for sales or public consumption of alcohol to 21, a change that was complete by 1989 (O’Malley and Wagenaar, 1991; Wechsler and Sands, 1980). Reduction of the MLDA in many states in the 1970s, would have impacted those born in the 1950s; hence, part of the 1944–53 and all of the 1954–63 birth cohort were exposed to the more liberal laws, and both had lower mean AODs than the 1934–43 birth cohort, for both men and women, though the change in AOD was more dramatic for women. The more conservative laws had their initial impact on those born in 1966 and afterwards and would have been complete for the 1974–83 cohort, who began to reach the age of 18 six years after the transition to a uniform MLDA of 21 was complete. Notably, this cohort had a later onset of drinking than the middle birth cohorts for both men and women.
The fact that a substantially larger decrease in AOD was observed for women, compared with men, raises the possibility that women are more responsive to legal deterrence for alcohol-related behavior than men. While this cannot be ruled out, we found no evidence of this in the literature. For example, male and female high school students were equally responsive to MLDA changes in the 1980’s with regard to frequency of drinking (O'Malley and Wagenaar, 1991). Likewise, studies of DUI behavior suggest that men and women are about equally responsive to legal deterrence (DeJong and Hingson, 1998). On the other hand, DUI behavior for women is more likely to be influenced by social and moral norms (Marelich et al., 2000; Shepherd, 2001). If this is also the case for youthful drinking, it may be that the decreases in AOD for men (1944–53 and 1954–63 cohorts) resulted primarily from changes in MLDA laws, whereas the somewhat larger decreases for women of these cohorts resulted from additive effects of legal changes and changing social norms with regard to women’s drinking. The subsequent increases in AOD for both men and women, which are similar in magnitude, may be primarily attributable to the reversal of the more liberal MLDA laws. Still another interpretation is that earlier cohorts of men already had relatively early ages of onset of drinking, relative to women, and practical limits on the ability to obtain alcohol limited the degree to which the average AOD for men could drop. With this explanation, the near-convergence in AOD for recent cohorts of both men and women necessarily implies a larger change in AOD for women because the earlier cohorts had fairly high AOD.
Regardless of the origins of secular changes in AOD, it is clear that they are associated with secular changes in alcohol dependence, such that a substantial portion of the elevated risk for alcohol dependence among women born after 1953, can be statistically attributed to earlier onset of drinking. Whether changes in AOD are causally linked to alcohol dependence is obviously a more complex question. Drinking among adolescents is correlated with a number of legal (Wagenaar and Toomey, 2002), cultural (Caetano, 1987; Gfroerer and Tan, 2003), familial (Jacob and Johnson, 1997), and individual-specific variables (McGue et al., 2001b), any number of which may contribute to the association between AOD and alcohol dependence. Indeed, some researchers have argued that AOD is a non-causal indicator of pre-existing, heritable liability for alcohol dependence (McGue et al., 2001a; Prescott and Kendler, 1999). However, the correlated changes in AOD and alcohol dependence that have taken place in the U.S. over a relatively short period of time cannot be attributed to changes in the genetic makeup of the population. Therefore, even if AOD is non-causal, it is likely to be closely related to a dynamic, and therefore modifiable risk factor that influences alcohol dependence. Even if there is no direct, causal relation between AOD and alcohol dependence, interventions aimed at early onset drinking might also impact the actual causal factors as well.
Over 240 studies have examined the impact of raising or lowering the minimum legal drinking age on drinking behavior, alcohol-related automobile accidents, and other outcomes (Reviewed by Wagenaar and Toomey, 2002). There is strong support that later MLDA is associated with reduced drinking, even beyond age 21, and with lower rates of alcohol-related accidents (O’Malley and Wagenaar, 1991; Wagenaar and Toomey, 2002). These “natural experiment” studies suggest that changes in youthful drinking behavior induced by exogenous means alter rates of subsequent alcohol-related problems. Though such studies have not yet addressed alcohol dependence, it is clear that reducing or delaying youthful drinking has lasting impact on a variety of alcohol-related outcomes. It would be surprising if changes in the prevalence of alcohol dependence did not accompany changes in the numerous other alcohol-related outcomes that result from changes in youthful drinking behavior.
Conclusion
A strong, consistent association of age at onset of drinking with alcohol dependence and other problems has been previously demonstrated by numerous investigators (Agrawal et al., 2006; DeWit et al., 2000; Grant and Dawson, 1997; Hingson et al., 2000; Hingson et al., 2006; Schuckit and Russell, 1983). We extend such findings here by demonstrating a correspondence between changes in both variables over time, and showing that changes in the outcome (alcohol dependence) can be statistically attributed to changes in a salient risk factor (AOD). Specifically, we demonstrate that the significantly increased lifetime prevalence of alcohol dependence among women born in recent decades may be attributable to earlier age at onset of drinking. Once AOD is taken into account, no statistically significant differences between birth cohorts were observed. The biological plausibility of a causal connection between these variables has been established in animal models. Alcohol consumption among adolescent rats has been shown to result in increased alcohol self-administration later in adulthood, and to result in persistent neurobiological changes in brain regions associated with the rewarding effects of addictive substances (Reviewed by McBride et al., 2005). Hence, the present study adds to the body of evidence, drawn from both basic research and epidemiology, that interventions effecting AOD are likely to also have an impact on alcohol dependence.
Acknowledgments
Analysis and manuscript preparation were supported by NIH-K01DA16618 (RAG), NIH AA12640, DA14363, AA11998 (KKB); NIH-U10AA08401, K02-DA021237 (LJB). NLAES and NESARC data were obtained from CSR, Incorporated and the NIAAA, (http://niaaa.census.gov), respectively. The author’s have no financial interest in this work.
Support: NIH-K01DA16618 (RAG), AA12640, DA14363, AA11998 (KKB), U10AA08401, HG-U01-004422, K02DA021237 (LJB).
References
- Agrawal A, Grant JD, Waldron M, Duncan AE, Scherrer JF, Lynskey MT, Madden PA, Bucholz KK, Heath AC. Risk for initiation of substance use as a function of age of onset of cigarette, alcohol and cannabis use: findings in a Midwestern female twin cohort. Prev Med. 2006;43(2):125–8. doi: 10.1016/j.ypmed.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Caetano R. Acculturation and attitudes toward appropriate drinking among U.S. Hispanics. Alcohol Alcohol. 1987;22(4):427–33. [PubMed] [Google Scholar]
- Caetano R, Clark CL. Trends in alcohol-related problems among Whites, Blacks, and Hispanics: 1984–1995. Alcohol Clin Exp Res. 1998;22(2):534–8. [PubMed] [Google Scholar]
- Chaloupka FJ, Grossman M, Saffer H. The effects of price on alcohol consumption and alcohol-related problems. Alcohol Res Health. 2002;26(1):22–34. [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA. 2004;291(17):2114–21. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- DeJong W, Hingson R. Strategies to reduce driving under the influence of alcohol. Annu Rev Public Health. 1998;19:359–78. doi: 10.1146/annurev.publhealth.19.1.359. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157(5):745–50. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Fernandez E. Estimation of the annual emigration of US born persons by using foreign censuses annd selected administrative data: Circa 1980. US Bureau of the Census; Washington, DC: 1995. [Google Scholar]
- Firebaugh G. Analyzing Repeated Surveys. Sage; Thousand Oaks, CA: 1997. [Google Scholar]
- Gfroerer JC, Tan LL. Substance use among foreign-born youths in the United States: does the length of residence matter? Am J Public Health. 2003;93(11):1892–5. doi: 10.2105/ajph.93.11.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol. 1997;58(5):464–73. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health Res World. 1998;22(2):144–7. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–10. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Hasin DS. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-DSM-IV Version. National Institute on Alcohol Abuse and Alcoholism; Bethesda, Md: 2001. [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74(3):223–34. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Moore TC, Shepard J, Kaplan K. Source and Accuracy Statement: Wave 1 National Epidemiologic Survey on Alchol and Related Conditions (NESARC) National Institute on Alcohol Abuse and Alcoholism; Bethesda, Md: 2003. [Google Scholar]
- Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, True WR, Bucholz KK. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychol Med. 2006;36(1):109–18. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Manwani SG, Nargiso JE. Epidemiology of substance use disorders in women. Obstet Gynecol Clin North Am. 2003;30(3):413–46. doi: 10.1016/s0889-8545(03)00072-x. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Bucholz KK, Rice JP, Bierut LJ. Secular Trends in the Lifetime Prevalence of Alcohol Dependence in the United States: A Re-evaluation. Alcohol Clin Exp Res. 2008;32(5) doi: 10.1111/j.1530-0277.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J Stud Alcohol. 1997;58(3):280–90. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Jamanka A, Howland J. Age of drinking onset and unintentional injury involvement after drinking. Jama. 2000;284(12):1527–33. doi: 10.1001/jama.284.12.1527. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160(7):739–46. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cohort effects on gender differences in alcohol dependence. Addiction. 2002;97(8):1025–36. doi: 10.1046/j.1360-0443.2002.00142.x. [DOI] [PubMed] [Google Scholar]
- Jacob T, Johnson S. Parenting influences on the development of alcohol abuse and dependence. Alcohol Health Res World. 1997;21(3):204–9. [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS. Education and survival: birth cohort, period, and age effects. Demography. 2001;38(4):551–561. doi: 10.1353/dem.2001.0035. [DOI] [PubMed] [Google Scholar]
- Marelich WD, Berger DE, McKenna RB. Gender differences in the control of alcohol-impaired driving in California. J Stud Alcohol. 2000;61(3):396–401. doi: 10.15288/jsa.2000.61.396. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences. Studies with animal models. Recent Dev Alcohol. 2005;17:123–42. doi: 10.1007/0-306-48626-1_6. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res. 2001a;25(8):1166–73. [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001b;25(8):1156–65. [PubMed] [Google Scholar]
- O’Malley PM, Wagenaar AC. Effects of minimum drinking age laws on alcohol use, related behaviors and traffic crash involvement among American youth: 1976–1987. J Stud Alcohol. 1991;52(5):478–91. doi: 10.15288/jsa.1991.52.478. [DOI] [PubMed] [Google Scholar]
- Pedersen W, Skrondal A. Alcohol consumption debut: predictors and consequences. J Stud Alcohol. 1998;59(1):32–42. doi: 10.15288/jsa.1998.59.32. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: a noncausal association. Alcohol Clin Exp Res. 1999;23(1):101–7. [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Early age at first alcoholic drink. Am J Psychiatry. 2001;158(9):1530. doi: 10.1176/appi.ajp.158.9.1530-a. [DOI] [PubMed] [Google Scholar]
- RTI International. SUDAAN Language Manual, Release 9.0. Research Triangle Institute; Research Triangle Park, NC: 2004. [Google Scholar]
- Schuckit MA, Russell JW. Clinical importance of age at first drink in a group of young men. Am J Psychiatry. 1983;140(9):1221–3. doi: 10.1176/ajp.140.9.1221. [DOI] [PubMed] [Google Scholar]
- Shepherd JP. Criminal deterrence as a public health strategy. Lancet. 2001;358(9294):1717–22. doi: 10.1016/S0140-6736(01)06716-2. [DOI] [PubMed] [Google Scholar]
- Wagenaar AC, Toomey TL. Effects of minimum drinking age laws: review and analyses of the literature from 1960 to 2000. J Stud Alcohol Suppl. 2002;(14):206–25. doi: 10.15288/jsas.2002.s14.206. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Sands E. Minimum-age laws and youthful drinking: An introduction. In: Wechsler H, editor. Minimum Drinking Age Laws. Lexington Books; Lexington, MA: 1980. pp. 1–10. [Google Scholar]