Abstract
In France, to practice as a pharmacist, one needs a “diplome d'état de Docteur en Pharmacie” This degree is awarded after 6 or 9 years of pharmacy studies, depending on the option chosen by the student. The degree is offered only at universities and is recognized in France as well as throughout the European Union.
Each university in France is divided into faculties called Unité de Formation et de Recherche (UFR). There are 24 faculties of pharmacy or UFRs de pharmacie. A national committee develops a pharmacy education program at the national level and each faculty adapts this program according to its specific features and means (eg, faculty, buildings). The number of students accepted in the second year is determined each year by a Government decree (numerus clausus).
Successive placements, totalling 62 weeks, progressively familiarize the student with professional practice, and enable him/her to acquire the required competencies, such as drug monitoring and educating and counselling patients. Challenges facing community pharmacies in the next 10 years are patient education, home health care, and orthopaedics; in hospital pharmacies, empowering pharmacists to supervise and validate all prescriptions; and finally, research in pharmacy practice.
Keywords: international pharmacy education, France
INTRODUCTION
In France, one needs a “diplome d'état de Docteur en Pharmacie” to practice as a pharmacist. This degree is awarded after 6 or 9 years of pharmacy studies, depending on the option chosen by the student. The degree is offered only at universities and is recognized in France as well as throughout the European Union.
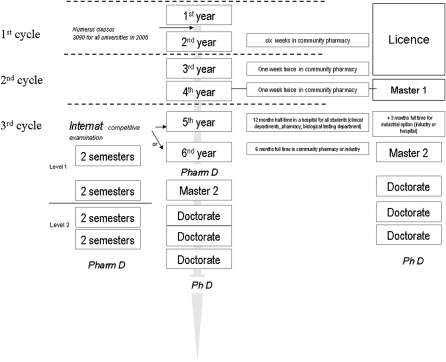
Before 1980, any student could register in pharmacy studies. However, since 1980, access to the second year of the program (and thus to the profession of pharmacy) has been conditional on success in a competitive examination at the end of the first year. Since the Laustriat reform in 1984, pharmacy education in France has consisted of either 6 or 9 years of training.1 Students in the fifth year complete a 1-year internship at a university hospital, the “university hospital year” (UHY).2 At the end of this year, students can choose to do an additional year of internship and then go into practice in community or industrial pharmacy or apply for an Internat.3 This is a 4-year paid internship and training concluded by a special degree, the Diplome d'Etudes Spécialisées (DES). In both the 6- and 9-year degree programs, students must defend a thesis for their final degree, the diplome d'état de Docteur en Pharmacie or doctor of pharmacy (PharmD) degree (Figure 1).
Figure 1.
Pharmacy education in France: le Diplôme de Docteur en Pharmacie (PharmD) awarded after 6 years of studies or 9 years at the end of the Internat.
Number of Students
The number of pharmacy students varies across universities and from year to year within each program.4 For instance, at the University Paris-Descartes, although 1000 students register for the first year, only 266 positions are available for the second year. Although the number of first-year students varies across pharmacy faculties in France, the proportion of students accessing the second year is similar (one third to one quarter of those in the first year). Students are admitted to the second year according to their rank in a competitive examination, until the quota is reached. European students are accepted within the quota. Non-European students and/or internationally educated pharmacists are accepted in excess of the quota, within a limit of 8% of the total quota. However, non-European pharmacists are not permitted to practice in community pharmacy in France or need special authorization to do so. Similarly, the quota of Internat positions available each year is fixed and entrance is decided by a competitive examination. Both quotas are fixed each year by a decree from the Ministry of Higher Education and Research and the Ministry of Health, Youth and Sports.5,6 These Ministries set the number of students that will be accepted each year. Since 1980 when the quota system was introduced, there have been 3 major changes: the number of second-year admissions, initially set at 2800, was reduced to 2500 in 1983 and then to 2250 in 1985 (19% fewer students than in 1980). This quota was increased in 2002, when it was decided to increase it progressively to 3090 by 2008.5 The quota is modified according to the population pyramid, the observed loss of qualified pharmacists, and the needs expressed by professionals. The gender balance of the profession appears to have stabilized at 2 qualified female students for every qualified male student.
There are 24 faculties of pharmacy in France. The number of second-year admissions varies across faculties, for example, there are 67 for Limoges, and 266 for each of the 2 Parisian faculties, Paris Descartes and Paris Sud. Each faculty offers 3 programs of study: one that prepares students for community pharmacy practice, another for jobs in the pharmaceutical industry, and a third for taking the Internat, the national competitive examination. It is possible for students to change from one program to another under certain conditions. In the entire country, only 12% of students successfully complete the Internat and are permitted to specialize. For the Internat, medical biology accounts for 40% of the positions offered and hospital pharmacy accounts for about 33%, although this proportion is increasing with the current demand.7
Criteria for Admission to Pharmacy School
In France, high school is concluded by a terminal examination, the Baccalauréat. Passing the Baccalauréat entitles the student to enter any program in any French university. In other words, there is no selection after the Baccalauréat for the first year of university education. Anyone who gets a passing grade may therefore register as an undergraduate in the faculty of pharmacy, regardless of the type of Baccalauréat and their marks. Students have the right of repeating a year once without approval from the faculty.
To limit failures at the end of the first year, Paris Descartes University communicates to applicants what their chance of success is before they register for pharmacy school. The competitive examination at the end of the first year is based on questions requiring simple answers, multiple-choice questions, and open- or short-response questions, or essays. Questions are based on different topics. For example, at University Paris Descartes the topics include: human anatomy and embryology, chemistry, mathematics, genetics, botany, physics-biophysics, jurisprudence, knowledge and delivery of medications, biochemistry and molecular biology, cellular biology, and animal biology.8 This examination is independently organized by each faculty of pharmacy, the questions being set by the professors or lecturers teaching at that faculty. The passing criteria for the fist year are different from the following years: students pass the competitive examination when their rank is above the quota determined each year, and fail when their rank is below it. In the University Paris Descartes, students are encouraged not to repeat the first year if their rank at the end of the first year is below 600. The number of positions available for candidates wishing to change a course of study depends on the number of first applicants, the number of students repeating a year, and the space available in the facilities.
Promotion to the next year is contingent on students' performance on end-of-semester or end-of-year examinations: students must obtain a mean score of 10/20 to continue on to the next year. An examining committee of professors decides on scores that are less than 10/20. Oral examinations may be organized but are rare, particularly in faculties with large numbers of students. In addition, tutorials are of great importance during studies. They are compulsory and graded.
During the fifth year, which is spent in a university hospital (UHY internship), the students must pass a “major diseases” examination, including an oral examination based on the analysis of a clinical case. Similarly, the internship at the end of the sixth year option is validated by a role-play in which the student must dispense a prescription; give the jury a scientific commentary of the prescription; and provide counselling and information to the patient.
The Internat is similar for the pharmacy and medical biology (clinical pathology) options and has 5 sections: (1) mathematical sciences, physics, and chemistry; (2) health sciences; (3) sciences for the public health and environmental health; and (4) clinical pharmacy, pathology, and biology applied to clinical pharmacy for bacterial and viral infections, haematology, and other infections; and (5) therapeutics. (Details regarding the program can be found at: http://www.cnci.univ-paris5.fr/pharmacie/ Programme.3) There are 3 written tests including exercises, analyses of clinical cases, and multiple-choice questions based on the 5 sections of the program. Applicants have 2 opportunities to sit for the examination (once for Northern France and once for Southern France).
Curriculum in French Pharmacy Programs
Candidates wishing to obtain the degree of Docteur en Pharmacie must pass the learning assessments associated with all academic courses and clinical/practice placements and must defend a thesis9.
Pharmacy studies are divided into 3 cycles:
The first cycle includes the first and second years. During the first cycle, students acquire basic scientific knowledge in biology, physics, mathematics, chemistry, physiology, and public health.
-
The second cycle includes the third and fourth years. Training is based on coordinated teaching, involving several disciplines and self-learning. The courses taught in the third and fourth years constitute a basic pharmaceutical training, allowing all students to acquire:
○Essential fundamental knowledge specific to each discipline, building upon the knowledge acquired in the first and second years. This provides students with a solid scientific background, enabling them to become specialists in drug treatment and clinical biology.
○Biomedical and therapeutic knowledge from coordinated interdisciplinary teaching courses, preparing them for overall management of the patient in the framework of the various functions of the pharmacist (community pharmacist, industry, hospital pharmacy, biology, research).
A balance is achieved between these 2 modes of learning by assigning equal numbers of hours to each during the third and fourth years (Table 1).
Table 1.
Coordinated Teaching Topics
The coordinated teaching program takes into account the need to achieve several objectives. Students are expected to have assimilated by the end of the second cycle:
Knowledge of drugs, from their design to their therapeutic use;
Knowledge of the principal diseases for which the main classes of therapeutic drugs are used;
Knowledge of the organization of the health system and public health issues: prevention (particularly iatrogenic conditions), monitoring, information and education of patients, etc.
The coordinated teaching program should also enable the students to acquire communication skills and to develop a capacity for analytic and critical thinking by making the student aware of the demands of the profession of pharmacy in terms of patient care. With this aim in mind, each coordinated teaching topic is illustrated by clinical applications presented, in particular, by lecturers in clinical pharmacy and clinical biology.
The second cycle also includes a common core course (CCC) and optional courses: students must pass 2 optional units to go on to the fifth year, one of which must be a professional pre-orientation unit. The CCC includes all the mandatory courses of the first 3 years and a training course, Preparation for Hospital Positions, taught in the fourth year. A placement (studentship) in a pharmacy, designed to illustrate coordinated teaching, is organized in the third and fourth years.9
The third cycle includes the fifth and sixth years. As seen above, during the fifth year all students are completing their UHY during which they receive academic instruction and hospital training. A complete academic year consists of 60 European Credits Transfer System (ECTS) credits. During the fifth and sixth years, the students must pass the examinations for 4 teaching units (2 in the fifth and 2 in the sixth), one hospital training course, and a professional placement (pharmacy or industry), and must defend their thesis.
Hospital training consists of a 12-month part-time placement in a hospital. Students taking the industrial option must also successfully complete a 3-month placement during the fifth year. This placement should focus on a university hospital project in research or with industrial applications completed at an industrial site or in an approved hospital, university, or research laboratory facility. Some students complete 4 teaching units in the fifth year and are able to take their Master course during the sixth year. Twenty percent of the students choose this path, particularly those interested in the industrial option.9
Specialization
The candidates selected through the Internat examination (during the fifth or sixth year) can choose to follow 1 of 4 different specialized degrees (the Diplôme d'Études Spécialisées or DES).10
DES in Hospital and Organizations Pharmacy.
The DES in hospital and organizations pharmacy leads to pharmacy careers in hospitals or public health. Holders of this DES are entitled to sit for the competitive examination for hospital practitioners to become hospital pharmacists (this status is identical for doctors, pharmacists, and dentists within hospitals, meaning they receive identical rank and wage but have different duties). They can also sit for other competitive examinations for administrative positions (eg, Inspection of Pharmacies). The skills acquired by students taking this course also qualify them for positions as pharmacists in private clinics.
Hospital pharmacists have diverse functions, depending on administrative approval from regional authorities: the production of drugs and sterile medical devices (cytotoxic chemicals, parenteral nutrition, preparations for oral, local or parenteral routes); control (uniformity of mass and of the content of hospital preparations); purchase and management of same; responsibility for radiopharmacy; sterilization of instruments; and identification of every batch of drugs derived from plasma and clinical trials. Pharmacists also have to check and dispense prescriptions. These clinical pharmacy activities are made in the clinical wards or within the pharmacy department. Hospital pharmacists are involved in multidisciplinary meetings with radiologists, oncologists, and haematologists where decisions about patients are discussed. All hospital activities must adhere to quality assurance criteria based on the institution's quality assurance system.
DES in Industrial and Biomedical Pharmacy.
The DES in industrial and biomedical pharmacy leads to careers in the pharmaceutical industry (control of processes for the manufacture of medical products, etc).
DES in Specialised Pharmacy.
The DES in specialized pharmacy provides cutting-edge training in particular topics (genetics, biotechnology, virology, pharmacogenomics, public health, and pharmacoeconomics). In principle, it leads to careers in teaching and research in public sector laboratories or in the research and development sector of the pharmaceutical industry.
DES in Medical Biology.
The DES in medical biology provides training common to medical biologists. This training is mandatory for those wishing to carry out biological testing in analytical laboratories, whether these laboratories are private medical testing companies, or belong to hospitals or specialist facilities (eg, agro-food industry, environmental analyses, water testing). In France, biologists have a monopoly for analyses carried out on ambulatory patients. These analyses must be carried out in a medical biology laboratory under the responsibility of its directors or deputy directors. Biologists may only work in a single medical biology laboratory in which they must hold 75% of the capital. France is one of the few countries in which pharmacists play a major role in medical biology activities: 85% of the directors or deputy directors of private laboratories have pharmacy degrees; the other 15% are medical biologists. The DES in medical biology has been structured as 2 levels since 2003. For the first level (4 semesters), the intern must validate placements in haematology, biochemistry, and bacteriology, together with a placement in parasitology or immunology. At the end of the first level, a teaching commission assesses the intern's planned career path. The intern must select 1 of 2 options for level 2: a DES in specialist medical biology or a DES in polyvalent medical biology. Placements are organized during this second level according to the option and professional path chosen.
It is envisioned to combine the DES in hospital and organization pharmacy and the DES in industrial and biomedical pharmacy into a single entity, a DES in pharmacy, with 2 professional orientations: hospital pharmacy practice and research (PH-PR), and industrial and biomedical pharmacy (PIBM). This new DES in pharmacy degree program will have 2 levels. The first 4 semesters will comprise level 1. During the first year, 2 placements must be validated in 2 of the 4 accredited domains of hospital pharmacy:
clinical pharmacy: filling and dispensing prescriptions, counselling
medical equipment: sterilization
health economics
compounding and controlling drugs
During the internship, validation of the PH-PR option requires validation of at least 6 semesters in the 4 domains of hospital pharmacy. The PIBM option requires validation of a placement in industry during level 2.
It is also envisioned to transform the DES in specialized pharmacy into a DES in innovation and research applied to drugs and health (IRAMS). This degree will contribute to hospital skills training and to the development of knowledge in certain innovative disciplines, such as pharmacotoxicology, pharmacokinetics, public health and environment, pharmaco-epidemiology, gene and cell therapy, hospital hygiene, biotechnology and nanosciences, diagnosis in vitro, nutrition, and new therapies (bio-organs, biomaterials, etc).
Specialization through the Internat is not essential for most of the careers exercised by pharmacists because only medical biology requires the corresponding DES. There is currently a demand to make the DES hospital pharmacy practice and research (PH-PR) an essential requirement for careers in this field. During the Internat, students work in a hospital, industry, or administration and are paid during the 4 years.
Clinical Training and Practical Experiences
The teaching objectives of the various pharmacy placements planned during the curriculum depend on the type of placement. They should enable the student to progressively become familiarized with and competent in professional practice, including drug monitoring and patient counselling.
The 6-week placement before the start of the third year aims to provide all pharmacy students, regardless of their subsequent professional activity, with insight into the role of dispensing pharmacists and their role in public health. At the end of the training, students have to be competent regarding dose schedules, plants, and chemical compounds.
Students are placed twice for 1-week periods in a community pharmacy in the third year and also in the fourth year to encounter real situations and apply the knowledge they have gained during the coordinated teaching regarding major diseases, therapeutic strategy, classes of therapeutic drugs, drug optimization, patient follow-up, patient education, and compliance. They provide students with opportunities to talk with patients (assessing the patient's knowledge of the disease and follow–up care needed and the patient's expectations, and above all, learning to listen to the patient).
The fifth-year placement consists of working 12 months part-time in a hospital, including a compulsory period of at least 6 months in clinical departments and, preferably, 3 months in a biological testing department and 3 months in a hospital pharmacy.
The professional placement of 6 months in a pharmacy in the sixth year of study is limited to students choosing a third cycle of teaching leading to a career as a pharmacist in a community pharmacy. At the end of this placement, the student must be fully competent concerning dose schedules; plants, and mushrooms, and knowledge of special preparations; recognition of the symptoms of common diseases and ability to give therapeutic and preventive advice regarding these diseases; and coordination of home health care. They must also be able to assess and comment on prescriptions drugs or medical devices, be able to ensure treatment follow up from the patient's medical records, and know how to develop and write a pharmaceutical opinion. They are expected to have learned the essential elements of the daily work of a community pharmacist, including management and administrative aspects, and must know how to integrate into the community pharmacy team (work with other pharmacists and pharmacy technicians).
Objectives
At the end of the sixth year, students destined for careers as pharmacists in a community pharmacy will:
Have extensive knowledge concerning drugs and medical devices and be able to evaluate objectively scientific documents and files relating to new drugs supplied by the appropriate authorities;
Be able to validate a drug treatment strategy prescribed by a doctor, check the dose, establish dose schedules, adapt treatments in the light of physiopathological contraindications and side effects, detect and analyze drug interactions;
Know the clinical value of the principal biological analyses;
Be able to communicate to patients all essential information related to the treatment given and lifestyle recommendations to be followed;
Be able to develop and to write a pharmaceutical opinion and to communicate with the prescriber;
Be able to write a pharmacovigilance notification;
Be able to contribute to the rationalization of health expenditure by informing and educating patients, encouraging better drug use (compliance, self-medication, product selection);
Be able to give high-quality pharmaceutical advice;
Be able to take part in hygiene and public health actions, particularly towards disease prevention;
Be able to act as part of a health care network, and to collaborate actively with other healthcare professionals;
Be able to exercise the profession of pharmacy on a daily basis, and to provide services while continually applying quality assurance principles;
Understand that a community pharmacy is essentially a small- or medium-sized business.
At the end of the fifth year (UHY), students destined for hospital-based pharmacy careers are expected to be able:
To listen to patients and interpret medical records;
To establish professional relations with members of the entire health-care team.
To integrate their future role as interns into the overall management of the patient. This requires knowledge of the path followed by a drug from its prescription to its administration.
To have the ability to validate a prescription by checking drug history, indications, contraindications, doses, dose schedules, drug interactions and adverse effects.
To have the ability to analyze the prescription of biological tests, to take part in the validation and interpretation of the results of analyses, and to participate in the biological evaluations of the main diseases encountered.
To use, understand, and evaluate different sources of information concerning drugs and biology, making it possible for the student to write and to present patients' biological and treatment records.
To participate in the various tasks required of hospital pharmacists.
To understand and apply the concepts of quality assurance, accreditation and certification.
To participate in hygiene and public health actions.
To speak at least one foreign language.
Highlights of Pharmacy Educational Programs
With a single degree, the students may decide to follow one of several career paths they discover during their studies. In the course of their studies, they are taught about drugs—from their manufacture to their administration to the patient—and trained in their use.
The students benefit from several placements during their studies. Every student must undergo at least 6 months of full-time training in a university hospital. The last years of training are accomplished through the resolution of clinical case studies in both the pharmacy and Internat options. This is also available to students choosing the industrial option on a less-developed basis. During the Internat option (4 years), students are paid about €1500 per month.
Innovations in Instruction and Assessment
The most important innovations from the last 10 years are
Coordinated teaching;
Problem-based learning;
e-learning: use of interactive voting systems and self-training by viewing recorded cases;
Patient education on major pathologies such as diabetes,11 asthma, cardiac insufficiency, and obesity, to improve therapeutic outcomes (this should be part of the training of all health professionals).
Quality assurance for good pharmacy practice;
Independent professional training;
Student exchanges, international collaborations; and
Programs and teachers assessments.
Accreditation of Pharmacy Education Programs
A national teaching committee, la Commission Pédagogique Nationale des Etudes Pharmaceutiques (CPNEP), has developed an accreditation program at the national level. Each university and faculty of pharmacy adapts this program according to its skills, specific features, and means (teachers, buildings, resources). The proposed modifications are studied by a committee within the faculty of pharmacy, and have to be discussed and endorsed by the University Board of Governors (Conseil d'Administration) before being applied.
In 2006, the Government created an agency, l'Agence d'Evaluation de la Recherche et de l'Enseignement Supérieur (AERES), to evaluate and accredit the doctorate schools, including pharmacy.
PHARMACY PRACTICE
Some regions and towns attract more pharmacists than others. Changes in the number of students admitted to pharmacy programs and special offers from regions to attract health professionals should help satisfy the demand for pharmacists.
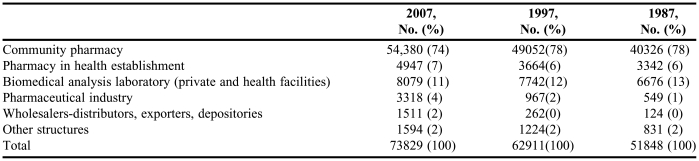
The national degree of Docteur en Pharmacie opens up possibilities for various careers and professions. At the start of 2007, 74% of the professionals listed by the College of Pharmacists (Ordre National des Pharmaciens) — 54,000 pharmacists — practiced in a community pharmacy, and more than half owned the pharmacy in which they worked. Pharmacies remain the principal place of work for pharmacists. Ten percent of pharmacists practice their profession in hospitals and other health and social facilities; this percentage corresponds to 7500 professionals and is almost 1 percentage point higher than it was in 1997 (5,864: 9.3 %) and higher than in 1987 (5,119: 9.9%). In 2007, two thirds of these pharmacists worked in hospital pharmacies (4,947) and one third worked as biologists (2,515).12 The number of pharmacists working in facilities other than pharmacies has increased substantially, and continues to do so; it has doubled since 1987 to almost 20,000 (Table 2).
Table 2.
Employment Distribution of Pharmacists in France in 1987, 1997, and 2007
Pharmacists must register with the College of Pharmacists (Ordre National des Pharmaciens) if they use their pharmacy degree professionally. However, pharmacists are also recruited for their skills to other sectors, in particular in cosmetics, marketing, and the agro-food industry, and medical journalism, in which they do not necessarily make use of their degrees. In such cases, they are not registered with the College and there is less information about how such careers develop. Given that individuals with pharmacy degrees account for about a third of the 20,000 executives working in the pharmaceutical industry, we estimate that about 3000 degree holders are not registered with the College and should be added in 2007.12
Due to the specific training received and skills acquired during pharmacy studies in France, particularly during internships, pharmacists can also opt to work in regulatory agencies, such as the Health Products Safety Agencies (AFSSAPS: Agence Française de Sécurité Sanitaire des Produits de Santé and HAS: Haute Autorité de Santé) or the National Doping Laboratory. The recruitment of pharmacists to other sectors and the high number of female pharmacists (64.7% of newly qualified pharmacists in 2006) have led to the loss of pharmacists from the profession, justifying the increase in the quota of students accepted into pharmacy school.10
France regulates the number of community pharmacies. The 22,561 pharmacies in France cover the entire country, with a community pharmacy for every 2500 or 3000 inhabitants. France has a mean population density of 98 inhabitants per square kilometer.10
Practice in Pharmacy
Pharmacists are responsible for checking prescriptions, but they are not allowed to alter the dosage or stop a treatment without consulting the prescribing doctor, except in emergency situations or if the patient's life is in danger. The pharmacist must provide counselling regarding medications and can dispense emergency contraception to minors, this being covered by the health insurance system as a “prescription” by the pharmacist. Pharmacists' role as educators is major for tobacco cessation. Since 1999, pharmacists have been partners of the health insurance system in efforts to increase the use of generic drugs: unless otherwise specified by the doctor, the pharmacist has the right to replace the original drug with a generic. The profession has committed itself to attaining a substitution rate of 70%.
Educational Challenges
The establishment of the European Bachelors-Masters-Doctorate (BMD or LMD in French) system is being considered in faculties of medicine, pharmacy, and dentistry.13 The BMD-Health would consist of 4 options: medicine, pharmacy, dentistry, and midwifery. The first year (B1) of the 3-year bachelor's degree would be common to all 4 options, with modules selected by students according to the competitive examination they plan to take. At the end of the first year, the students would be allowed to sit for several competitive examinations. The students would then start to specialize in a particular option during the second year (B2), before specializing fully during the third year (B3). Students would have the opportunity to transfer to another option at the end of B2. The 2 years of the master's degree (M1, M2, or years 4 and 5) would largely involve practical work and placements. The doctorate would then last 3 to 5 years after the fifth year, depending on the specialty selected. The creation of a BMD-Health would make it possible for students to transfer onto other BMD courses if they so desired and would establish equivalence in terms of training with other countries in the European Union.
Future of Pharmacy Practice
Several changes are now occurring in pharmacy practice, especially in pharmacies, and will impact the education program in France:
Because of hospital-community networks, many drugs are now available for outpatients in pharmacies. For example, drugs for treatment of human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS, eg, Combivir, Epivir, Sustiva, Crixivan) or chemotherapy (eg, Xeloda, Navelbine) or anaemia (eg, Aranesp). These drugs have now left the reserves of the hospitals, and require close follow-up care of patients.
There is an increased emphasis on patient education. Networks are now being established for patients with chronic diseases such as diabetes, asthma, or HIV and AIDS to consult with pharmacies and pay for pharmaceutical advice.
Some drugs will be available without a prescription in pharmacies for self-medication.14
Pharmacies will be allowed to subcontract preparations to other community pharmacies or a hospital pharmacy.
Because of sport, travels, and odd jobs, orthopaedics is becoming important in community pharmacy, and pharmacists will have to adapt.
Home health care is increasing in France because of the aging of the population, and given the distribution of pharmacies throughout France, pharmacists are poised to play an important role in this domain.
In hospitals, the pharmaceutical validation of prescriptions will have to be improved. At the present time, not all prescriptions are checked and supervised by a pharmacist. Clinical pharmacy has to be developed for patient security and optimization of patient care.
Research activities in pharmacy are performed by integrated hospital teams based in university hospital centers and an increase is expected
Generally speaking, there is a shortage of physicians in France, and the idea of transferring medical competencies to other health care practitioners is being discussed.
The national teaching committee of pharmacy is currently revising the study program and validating career references (dispensing, industry, hospital pharmacy, and biology). At the end of the validation procedure, adjustment references for pharmacy studies will be proposed.
SUMMARY
In France, the number of pharmacists is controlled by means of a quota. France trains its pharmacists for a minimum of 6 years, which is long by international standards. This is in part due to the importance given to professional placements, including the university hospital year following the 1984 reform. Specialization in hospital pharmacy requires internship training. There is also a specialization in medical biology (France is one of the few countries to provide this) and a specialization geared toward careers in the pharmaceutical industry. However, the main specialization remains community pharmacy. France has a particular organization of pharmacies, which are subject to public service constraints, have a monopoly for dispensing drugs, and must do so within the framework of laws and regulations. These features and efforts to ensure an adequate distribution of pharmacies throughout France contribute to the particularly high density, with 1 pharmacy for every 3000 inhabitants.
Acknowledgments
Jean-Luc Audhoui and Sophie Dilag from “Ordre National des Pharmaciens” for statistics about pharmacists
REFERENCES
- 1.Réponse aux questions des étudiants de 5ème A.H.U. http://anepc.org/guides/etudiant/ANEPC-GuideEtudiants_1.pdf. Accessed November 22, 2008.
- 2.Calop J, Brion F. Guides de 5 AHU. Association Nationale des Enseignants de Pharmacie Clinique. Guide du formateur. Available at: http://anepc.org/guides/formateurs.aspx. Accessed November 22, 2008.
- 3.Centre National des Concours d'Internat. Available at http://www.cnci.univ-paris5.fr. Accessed October 31, 2008
- 4.Le Portail Etudiant, formation générale Available at http://www.etudiant.gouv.fr/pid20446/formation-generale.html. Accessed November 22, 2008.
- 5.Nombre des étudiants de première année du premier cycle des études pharmaceutiques autorisés à poursuivre leurs études en pharmacie à la suite des épreuves terminales de l'année universitaire 2007-2008. JORF 26 janvier 2008 art 36. Available at: http://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000017991441&dateTexte. Accessed November 22, 2008.
- 6.Nombre de places offertes au titre de l'année universitaire 2008-2009 pour l'admission en première année du deuxième cycle des études médicales, odontologiques ou pharmaceutiques aux candidats n'ayant pas effectué le premier cycle correspondant. JORF 26 janvier 2008 art 35. http://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000017991438&dateTexte. Accessed November 22, 2008.
- 7.Ordre national des pharmaciens. Pharmacien d'officine: un métier au cœur du système de soin. 2003, Available at: http://www.ordre.pharmacien.fr/fr/pdf/service03.pdf. Accessed November 22, 2008.
- 8.FCB de Pharmacie, 1er année, Faculté des sciences pharmaceutiques et biologiques Université Paris Descartes http://www.pharmacie.univ-paris5.fr/spip.php?article2021. Accessed November 22, 2008.
- 9.Régime des études en vue du diplôme d'état de docteur en pharmacie. Available at: http://ispb.univ-lyon1.fr/ispb_info/index_info.asp ISPB-INFO N°171, 24 octobre 2003 http://ispb.univ-lyon1.fr/ispb_info/ispbinfo_dossierpdf/ispbspecial171.PDF.
- 10.Collet M. Pojection du nombre de pharmaciens en France à l'horizon 2030. DREES. 2005 http://www.sante.gouv.fr/drees/serieetudes/pdf/serieetud54.pdf. Accessed Novembre 22, 2008.
- 11.XXI Journées de l'ordre des pharmaciens. Les Nouvelles Pharmaceutiques 2008, 370: 1–3. http://www.ordre.pharmacien.fr/Actualites/frame_news.asp?actu_id=804. Accessed Novembre 22, 2008.
- 12.Ordre national des pharmaciens. Démographie pharmaceutique française: étude prospective sur 20 ans 2002 http://www.ordre.pharmacien.fr/fr/pdf/etude_prospective.pdf. Accessed Novembre 22, 2008.
- 13.Ambroise-Thomas P, Aurengo A, Loisance D, Queneau P. Organisation des études de médecine, pharmacie, odontologie et maïeutique dans le cadre du système européen LMD 2006. Available at: http://www.univ-paris-diderot.fr/2006/02-acadmedecineLMD.pdf. Accessed October 31, 2008.
- 14.Situation de l'automédication en France et perspectives d'évolution. Marché, comportements, positions des acteurs.2006 http://www.sante.gouv.fr/htm/actu/automedication/rapport.pdf. Accessed November 22, 2008.