Abstract
BACKGROUND:
Due to its high efficacy and technical simplicity, percutaneous endoscopic gastrostomy (PEG) has gained wide-spread use. Local infection, occurring in approximately 2% to 39% of procedures, is the most common complication in the short term. Risk factors for local infection are largely unknown and therefore – apart from calculated antibiotic prophylaxis – preventive strategies have yet to be determined.
OBJECTIVE:
To assess the potential patient- and procedure-related risk factors for peristomal infection following PEG tube placement.
METHODS:
Potential patient-related (eg, age, sex, diseases, body mass index, concomitant antibiotic therapy) and procedure-related (endoscopist experience, institutional factors, findings on endoscopy) risk factors and their coincidence with local infection, defined as a positive peristomal infection three days after PEG tube placement, were evaluated at two institutions. A standardized antibiotic prophylaxis was not performed. The peristomal infection score was also evaluated in 390 patients.
RESULTS:
Using a multivariate binary regression analysis, four risk factors were established as relevant for local infection after PEG: clinical institution (OR 6.69; P=0.0001), size of PEG tubes (15 Fr versus 9 Fr; OR 2.12; P=0.05), experience of the endoscopist (more than 100 investigations versus less than 100 investigations; OR 0.54; P=0.05) and the existence of a malignant underlying disease (OR 2.28; P=0.019).
CONCLUSIONS:
Similar to other endoscopic interventions, local infection as a complication of PEG tube placement depends on the experience of the endoscopist. Institutional factors also play a significant role. Additional risk factors include PEG tube size and underlying diseases. These findings indicate that the local infection after PEG tube placement may be influenced by both endoscopy-associated factors and by the underlying disease status of the patient.
Keywords: Infection score, Percutaneous endoscopic gastrostomy, Risk factors
Abstract
HISTORIQUE :
En raison de sa grande efficacité et de sa simplicité technique, l’utilisation de la gastrostomie endoscopique percutanée (GEP) s’est généralisée. L’infection locale, qui se manifeste dans environ 2 % à 39 % des interventions, en est la complication la plus courante à court terme. Les facteurs de risque d’infection locale sont largement méconnus. C’est pourquoi aucune stratégie préventive n’a encore été déterminée, à part une prophylaxie antibiotique calculée.
OBJECTIF :
Évaluer les facteurs de risque potentiels d’infection péristomiale reliés aux patients et aux interventions après l’installation d’une sonde de GEP.
MÉTHODOLOGIE :
Les facteurs de risque pouvant être reliés au patient (p. ex., âge, sexe, maladies, indice de masse corporelle, antibiothérapie concomitante) et à l’intervention (expérience de l’endoscopiste, facteurs reliés à l’établissement, résultats de l’endoscopie) et leur coïncidence avec l’infection locale, définie par une infection péristomiale positive trois jours après l’installation de la sonde de GEP, ont été évalués dans deux établissements. On n’a pas procédé à une prophylaxie antibiotique standardisée. On a également évalué l’indice d’infection péristomiale chez 390 patients.
RÉSULTATS :
Au moyen d’une analyse de régression binaire multivariée, on a établi que quatre facteurs de risque favorisaient une infection locale après la GEP : l’établissement clinique (RRR 6,69; P=0,0001), la dimension des sondes de GEP (15 Fr par rapport à 9 Fr; RRR 2,12; P=0,05), l’expérience de l’endoscopiste (plus de 100 interventions par rapport à moins de 100 ; RRR 0,54; P=0,05) et l’existence d’une maladie sous-jacente maligne (RRR 2,28; P=0,019).
CONCLUSIONS :
À l’instar des autres interventions endoscopiques, l’infection locale comme complication de l’installation d’une sonde de GEP dépend de l’expérience de l’endoscopiste. Des facteurs reliés à l’établissement peuvent également jouer un rôle important. Les autres facteurs de risque incluent la dimension de la sonde de GEP et les maladies sous-jacentes. Ces observations indiquent que l’infection locale après l’installation d’une sonde de GEP peut dépendre à la fois de facteurs reliés à l’endoscopie et de la maladie sous-jacente du patient.
Since its introduction by Gauderer et al (1), percutaneous endoscopic gastrostomy (PEG) has rapidly become routine practice and the method of choice for long-term enteral feeding. PEG is now a widely accepted procedure for patients at high risk of malnutrition.
PEG tube placement accounts for the second most common indication for upper gastrointestinal (GI) endoscopy in hospitalized patients in the USA with approximately 260,000 PEG kits produced annually (2). In addition, the quantity and the relevance of PEG tube placement is reflected by a myriad of publications concerning the subject and has continuously increased over the past two decades (2).
Nutritional safety and efficacy, and the technical simplicity of the procedure (3) are the main reasons for the popularity of this enteral nutrition delivery technique. PEG tube placement is a treatment modality with an extraordinarily high success rate of almost 99% and a mortality rate of only 1% (4,5). Minor complications are observed in 2% to 30% of procedures, most commonly consisting of local infections, depending on the studies (5,6). Consequently, the control of local infection was the main subject of several prospective, randomized clinical trials investigating the value of prophylactic antibiotic therapy (7–12). Based on the results of a meta-analysis (13), The European Society of Gastrointestinal Endoscopy and the American Society for Gastrointestinal Endoscopy recommended the use of antibiotic prophylaxis before PEG tube-placement in all patients. However, due to the heterogeneity of the results in the trials mentioned above, these recommendations are not unchallenged (14). Furthermore, the British Society of Gastroenterology and German Society of Digestive and Metabolic Diseases (15) do not recommend the routine use of antibiotic prophylaxis.
The discrepancies in clinical outcomes found in these trials cannot only be due to the methodological differences, including definition of peristomal infection or the antibiotic agent used for prophylaxis. For example, Gossner et al (8) found a peristomal infection rate in the placebo arm of a trial, which was lower than that of several treatment arms of other trials (7,9,10). This is a potential bias with underlying institutional risk factors or other influencing parameters. If this was the case, a risk-adaptive rather than a general antibiotic prophylaxis previous to PEG tube placement would be a desirable approach. This is even more likely the case because routine antibiotic prophylaxis may not only cause allergic reactions but also contribute to the proliferation of methicillin-resistant Staphylococcus strains (16,17), with potential health and economic consequences (18).
The aim of the present study was to identify patient- and procedure-related risk factors for the occurrence of a local wound infection after PEG tube placement.
METHODS
Patient selection
Patients who underwent PEG tube insertion at two institutions of the University in Erlangen-Nuremberg, Germany, during a two-year period were included in the study after giving written consent. The prospective monitoring of the patients was performed by the nutritional support team of the First Department of Medicine.
PEG tube placement according to the ‘pull method’ (1,19), was performed in two separate units of the Friedrich-Alexander University hospital, Erlangen-Nuremberg, Germany: the endoscopic unit of the First Department of Medicine and in the Department of Otolaryngology. Commercially available PEG tubes (Freka PEG Set, Fresenius-Kabi; Germany) were used with tube sizes ranging from 9 to 15 Fr. No standardized antibiotic prophylaxis was applied (14), but was documented in case of prescription for other indications. The insertion procedure was performed using a standard technique. This included an extensive, local, threefold mechanical disinfection of the abdominal wall using sterile compresses and a commercially available alcohol-based (propan-2-ol 63.0 g; ie, 72% vol/vol), coloured disinfection solution (Cutasept G, Bode Chemie, Germany). Differences between departments included the anesthetic management during the investigation; the endoscopic unit of the First Department of Medicine used an analgosedation with pethidin and midazolam, while the Department of Otolaryngology, Head and Neck Surgery, conducted the PEG tube placement using intubation narcosis, although the placement was performed during an upper panendoscopy of the oropharyngeal and esophageal tract. Nutritional supplementation was initiated 4 h after the positioning of the PEG tube. The endoscopists (main examiners) and assistants were categorized according to their experience (ie, trainee: less than 100 endoscopies performed; advanced: more than 100 endoscopies performed).
In the present study, patients with a mean (± SD) age of 60±13 years (range 18 to 69 years) were included. For each patient, written consent from the patient or from the legal guardian of the patient was obtained. Exclusion criteria for PEG placement in the present study included ascites, peritonitis, peritoneal carcinosis, coagulation disorders (prothrombin time of less than 50%; thrombocytes less than 50×109/L) and patients missing written consent.
Determination of local infection
The patients from both hospital units were supported and evaluated by the same nurses of the nutritional team of the First Department of Medicine. A standardized doctor’s visit ensured homogeneous evaluation of the local wound infection. PEG patients were visited before PEG tube placement as well as on a fixed schedule three days after PEG tube placement. Additional visits were performed if clinically indicated. At each visit, the sterile dressing was removed, the outer fixation was loosened and the PEG tube was carefully pushed 2 cm to 4 cm into the stoma. The puncture site was carefully inspected and cleaned with sterile dressings, and, if necessary, followed by gently pulling the tube back until a slight resistance was felt. Then, a sterile dressing with slit compresses under the fixation plate was applied again with a dressing set (Fresenius Kabi, Germany).
To evaluate local infection, the peristomal infection was assessed three days after PEG tube placement. The peristomal infection comprises the observed extension of local inflammatory signs such as erythema, induration and exudate including the quality of secretion. Peristomal infections were scored and were defined to be positive if three or more of the following criteria were observed: erythema (score of 0 to 3; with a circumference of 0 cm = 0, less than 0.5 cm = 1, 0.5 cm to 1.0 cm = 2, and greater than 1.0 cm = 3); induration (score of 0 to 3; with a circumference of 0 cm = 0, less than 0.5 cm = 1, 0.5 cm to 1.0 cm = 2, and greater than 1.0 cm = 3); and exudate (score of 0 to 3; no compresses needed = 0 or the input of 1, 2 or 3 compresses = 1, 2 and 3, respectively) and/or purulent secretion.
Assessment of potential risk factors for local inflammation
The following factors were evaluated as possible risk factors for local wound infection: age, sex, underlying disease entity (malignant versus nonmalignant), body mass index (BMI), antibiotic therapy, endoscopist experience (ie, main examiner, assistant), institution, presence of other diseases (eg, cardiovascular disease and diabetes mellitus), endoscopic findings (erythema or atrophy of the mucosa, esophagitis) and size of the PEG tube (9 Fr versus 15 Fr).
Statistical methods
All data were documented and electronically stored using an Access 1997 database (Microsoft, USA). Subsequently, data were retrieved, then analyzed using SSPS 12.0 (SPSS Inc, USA) for further statistical evaluation. For the evaluation of risk factors, a multivariate regression analysis was performed, estimating the occurrence of wound infection after PEG tube-placement depending on patient characteristics and PEG tube placement procedure. Statistical significance was set at P≤0.05.
RESULTS
Characterization of patient- and procedure-related parameters
A total of 390 patients were included in the present study (300 men and 90 women). The mean age of the patients was 60±13 years. The mean BMI was 23±4 kg/m2. All PEG tubes were inserted endoscopically according to the the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines. PEG tubes of two different sizes were used for insertion: 9 Fr in 250 patients and 15 Fr in 140 patients (Table 1). Indications for PEG tube insertion are listed in Table 2.
TABLE 1.
Patient and procedure characteristics
| Age, years (mean ± SD) | 60±13 |
| Sex, n (men/women) | 300/90 |
| Body mass index (mean ± SD), kg/m2 | 23±4 |
| PEG tube size (9 Fr/15 Fr) | 250/140 |
| Endoscopist (main examiner/assistant) | 319/119 |
PEG Percutaneous endoscopic gastrostomy
TABLE 2.
Underlying indications for percutaneous endoscopic gastrostomy tube insertion (n=390)
| Indication | n |
|---|---|
| Malignant disease | 314 |
| Neurological disease | 21 |
| Heart disease | 33 |
| Other benign disease | 22 |
Positive local inflammation score
Of the 390 evaluated patients who underwent PEG tube placement, 216 (55.4%) presented with erythema of various degrees, 174 (44.7%) with exudate, 15 (3.8%) with peristomal induration and 105 (26.9%) with purulent secretion three days after insertion. The peristomal infection was positive in 131 patients (33.6%). Most frequently, the infection index was positive due to purulent secretion from the insertion wound.
Severe complications
In one patient, severe abscess formation of the abdominal wall was observed, resulting in the removal of the PEG tube. None of the patients died as a consequence of PEG tube placement.
Risk factors for peristomal infection after endoscopic PEG insertion
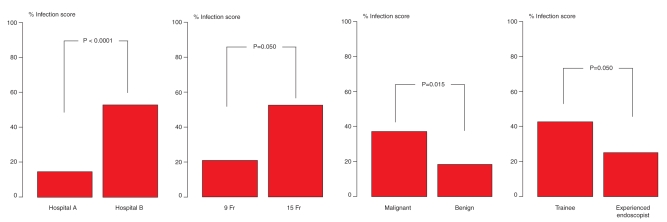
In evaluating the above-mentioned potential risk factors, no association could be established between a positive peristomal infection and age, sex, endoscopic findings, antibiotic therapy or BMI (Table 1). The underlying disease (ie, malignant versus benign disease; P=0.019; Table 3, Figure 1) proved to be associated with a positive infection score. Interestingly, a significant difference in terms of local inflammation was detected for different institutions (P<0.0001). Additionally, the diameter of the PEG tubes used (9 Fr versus 15 Fr; P=0.050) and the experience of the endoscopist (P=0.050) influenced the outcome significantly.
TABLE 3.
Risk factors for peristomal infection after percutaneous endoscopic gastrostomy (PEG) tube placement
| Risk factors | OR | 95% CI | P |
|---|---|---|---|
| Different institution* | 6.69 | 4.072–10.998 | 0.0001 |
| Endoscopist experience: (>100 endoscopies versus <100 endoscopies) | 0.54 | 0.288–1.000 | 0.050 |
| PEG tube diameter (15 Fr versus 9 Fr) | 2.12 | 0.993–4.521 | 0.050 |
| Underlying disease (malignant versus benign) | 2.28 | 1.147–4.529 | 0.019 |
Department of Otolaryngology versus First Department of Medicine
Figure 1).
Occurrence of percutaneous endoscopic gastrostomy (PEG) infection dependent on institution: hospital A (Department of Medicine) versus hospital B (Department of Otolaryngology); size of PEG tube (9 Fr versus 15 Fr); underlying disease (malignant versus benign) and endoscopist experience (trainee: <100 endoscopies performed; advanced: >100 endoscopies performed)
An experienced endoscopist (ie, more than 100 PEG tube placement procedures performed) was associated with a lower infection score (P=0.021) in the multivariate analysis, but no influence was detected with regard to the puncturist (Table 3 and Figure 1).
DISCUSSION
In the present prospective, controlled study, we demonstrated that local infection following PEG tube insertion is significantly associated with different risk factors such as a malignant underlying disease, institutional factors, experience of the endoscopic team and the PEG tube size.
The peristomal infection was assessed on the third day after PEG placement as a hallmark of infection (20). A positive infection score was found in 33.6% of our patients and this result was comparable with previous results (9–11,20–23).
A major problem in interpreting the development of local wound infection after PEG tube placement is the questionable clinical significance of an infection score. Despite the fact that a score may be positive, the infection is mostly of minor severity and generally well-controlled by local therapy. For example, Külling et al (24) showed that in seven prospective studies, only 1.3% of patients without antibiotic prophylaxis required advanced (eg, surgical) therapy for treatment of severe wound infection. This was the case in one of 390 patients (0.3%) in the present series. Therefore, a positive peristomal infection index score is more a surrogate for a potential, rather than an existing, clinical problem. Sample size calculations probably account for the use of these sensitive infection scores, but due to the comparability of results, another approach would be prudent.
In our analysis, the application of antibiotics had no influence on the occurrence of a peristomal infection. Due to rather low infection rates in the department of medicine – as documented by a positive infection score in 18% of patients – antibiotic prophylaxis is not routinely given in our department. Therefore, application of antibitotics was only peformed if required for other reasons. Due to the low number of patients who required antibiotic therapy, a positive (ie, prophylactic) effect could not be confirmed, as shown by multiple randomized studies, reflecting a statistical problem rather than arguing against the studies mentioned above.
Dormann et al (25) found that patients with malignancies are at greater risk for peristomal infections than those with nonmalignant diseases. This finding is strongly supported by data in the present study that indicated a highly significant association of peristomal infection with malignancy as the underlying disease.
In the present study, experience with the technical procedure was associated with lower infection rates after PEG tube placement. Several endoscopy trials demonstrated that experience in terms of numbers and frequency of endoscopic interventions determines the outcome of such endoscopic procedures as endoscopic retrograde cholangiopancreatography (26), sphincterotomy (27) or application of hemoclips (28). It was surprising that we found such an influence in PEG tube placement, a procedure that is considered to be as technically easy. Therefore, the importance of expertise and experience in PEG tube placement may also have been underestimated as risk factors for the development of infection to date. What, in particular, makes the difference between an ‘experienced’ and a ‘trainee’ team? We can only speculate; however, a rapid identification of the optimal puncture site would subsequently lead to shorter investigation times, smaller incisions and perhaps less traction on the PEG tube (29), and these factors may contribute to a reduced local infection rate.
In our study, two different diameters of PEG tubes (9 Fr and 15 Fr) were used. According to the ESPEN guidelines, it is advisible to use 15 Fr PEG tubes because PEG tubes with smaller diameters are associated with higher rates of clogging. The result of our study revealed significantly more peristomal infections after placing 15 Fr PEG tubes than after the placement of 9 Fr tubes. These differences in diameter account for a 1.7-fold greater circumference of the tube (9.4 mm versus 15.7 mm), respectively) and consequently lead to a correspondingly greater surface area in the tube. In a previous study (30), the material of the PEG tube (silicone versus polyurethane) was shown to produce significantly different complication rates. In analogy, the extension of the surface area may also be responsible for such a finding. This, however, should prompt a prospective randomized study comparing two different sizes of PEG tubes in relation to peristomal infection.
The finding of an institutional risk factor for peristomal infection is subject to the suspicion that bias contributed to the different results in previous randomized trials for antibiotic prophylaxis. In our study, a positive infection score was seen in 50% of the patients in the department of otolaryngology compared with 18% in the department of medicine. Interestingly, this almost exactly reflects the range of positive scores in the studies mentioned above (9–11,20–23). We do not know what particular factors are responsible for this finding, but in the department of otolaryngology, almost all PEG tubes were placed during intubation narcosis for panendoscopy of the upper oropharyangeal esophageal tract. Other, hitherto unknown factors may also be responsible for the observed difference, but are awaiting clarification in further prospective studies.
SUMMARY
We were able to identify four risk factors for peristomal infection after PEG placement in the present prospective study: malignancy as underlying disease; experience of the PEG tube placement team; the diameter of the PEG tube; and most importantly, institutional factors. These findings could lead to further prospective trials substantiating our results and may add PEG tube insertion to a list of endoscopy procedures that require experience with the technique to avoid postprocedural infections.
REFERENCES
- 1.Gauderer MW, Ponsky JL, Izant RJ., Jr Gastrostomy without laparotomy: A percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872–5. doi: 10.1016/s0022-3468(80)80296-x. [DOI] [PubMed] [Google Scholar]
- 2.Gauderer MW. Percutaneous endoscopic gastrostomy and the evolution of contemporary long-term enteral access. Clin Nutr. 2002;21:103–10. doi: 10.1054/clnu.2001.0533. [DOI] [PubMed] [Google Scholar]
- 3.Gauderer MW. Percutaneous endoscopic gastrostomy – 20 years later: A historical perspective. J Pediatr Surg. 2001;36:217–9. doi: 10.1053/jpsu.2001.20058. [DOI] [PubMed] [Google Scholar]
- 4.Gossner L, Ludwig J, Hahn EG, Ell C. The risks of percutaneous endoscopic gastrostomy. Dtsch Med Wochenschr. 1995;120:1768–72. doi: 10.1055/s-2008-1055540. [DOI] [PubMed] [Google Scholar]
- 5.Larson DE, Burton DD, Schroeder KW, DiMagno EP. Percutaneous endoscopic gastrostomy: Indications, success, complications, and mortality in 314 consecutive patients. Gastroenterology. 1987;9:48–52. [PubMed] [Google Scholar]
- 6.Loser C, Wolters S, Folsch UR. Enteral long-term nutrition via percutaneous endoscopic gastrostomy (PEG) in 210 patients: A four-year prospective study. Dig Dis Sci. 1998;43:2549–57. doi: 10.1023/a:1026615106348. [DOI] [PubMed] [Google Scholar]
- 7.Dormann AJ, Wigginghaus B, Risius H, et al. A single dose of ceftriaxone administered 30 minutes before percutaneous endoscopic gastrostomy significantly reduces local and systemic infective complications. Am J Gastroenterol. 1999;94:3220–4. doi: 10.1111/j.1572-0241.1999.01523.x. [DOI] [PubMed] [Google Scholar]
- 8.Gossner L, Keymling J, Hahn EG, Ell C. Antibiotic prophylaxis in percutaneous endoscopic gastrostomy (PEG): A prospective randomized clinical trial. Endoscopy. 1999;31:119–24. doi: 10.1055/s-1999-13658. [DOI] [PubMed] [Google Scholar]
- 9.Preclik G, Grune S, Leser HG, et al. Prospective, randomised, double blind trial of prophylaxis with single dose of co-amoxiclav before percutaneous endoscopic gastrostomy. BMJ. 1999;319:881–4. doi: 10.1136/bmj.319.7214.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonas SK, Neimark S, Panwalker AP. Effect of antibiotic prophylaxis in percutaneous endoscopic gastrostomy. Am J Gastroenterol. 1985;80:438–41. [PubMed] [Google Scholar]
- 11.Jain NK, Larson DE, Schroeder KW, et al. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy. A prospective, randomized, double-blind clinical trial. Ann Intern Med. 1987;107:824–8. doi: 10.7326/0003-4819-107-6-824. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Kim JJ, Jang JK, et al. Increased risk of peristomal wound infection after percutaneous endoscopic gastrostomy in patients with diabetes mellitus. Dig Liver Dis. 2002;34:857–61. doi: 10.1016/s1590-8658(02)80256-0. [DOI] [PubMed] [Google Scholar]
- 13.Sharma VK, Howden CW. Meta-analysis of randomized, controlled trials of antibiotic prophylaxis before percutaneous endoscopic gastrostomy. Am J Gastroenterol. 2000;95:3133–6. doi: 10.1111/j.1572-0241.2000.03283.x. [DOI] [PubMed] [Google Scholar]
- 14.Loser C, Keymling M. [Antibiotic prophylaxis before percutaneous endoscopic gastrostomy (PEG catheter)] Z Gastroenterol. 2000;38:271–3. [PubMed] [Google Scholar]
- 15.Loser C, Folsch UR. [Guidelines for treatment with percutaneous endoscopic gastrostomy. German Society of Digestive and Metabolic Diseases] Z Gastroenterol. 1996;34:404–8. [PubMed] [Google Scholar]
- 16.Nunley D, Berk SL. Percutaneous endoscopic gastrostomy as an unrecognized source of methicillin-resistant Staphylococcus aureus colonization. Am J Gastroenterol. 1992;87:58–61. [PubMed] [Google Scholar]
- 17.Mohammed I, Jones BJ. Antibiotic prophylaxis after percutaneous endoscopic gastrostomy insertion. Widespread routine use of prophylactic antibiotics might predispose to increased risk of resistant organisms. BMJ. 2000;320:870–1. [PubMed] [Google Scholar]
- 18.Ogundipe OA, Kar-Purkayastha S. An audit of antibiotics usage and their effect on MRSA infection or colonisation following percutaneous endoscopic gastrostomy in a district general hospital. Int J Clin Pract. 2004;58:632–4. doi: 10.1111/j.1368-5031.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 19.Keymling M. First results with percutaneous endoscopic gastrostomy. Adv Exp Med Biol. 1987;209:227–9. doi: 10.1007/978-1-4684-5302-7_34. [DOI] [PubMed] [Google Scholar]
- 20.Dormann AJ, Wigginghaus B, Risius H, et al. Antibiotic prophylaxis in percutaneous endoscopic gastrostomy (PEG) – results from a prospective randomized multicentre trial. Z Gastroenterol. 2000;38:229–34. doi: 10.1055/s-2000-14862. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad I, Mouncher A, Abdoolah A, et al. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy – a prospective, randomised, double-blind trial. Aliment Pharmacol Ther. 2003;18:209–15. doi: 10.1046/j.1365-2036.2003.01684.x. [DOI] [PubMed] [Google Scholar]
- 22.Sturgis TM, Yancy W, Cole JC, Proctor DD, Minhas BS, Marcuard SP. Antibiotic prophylaxis in percutaneous endoscopic gastrostomy. Am J Gastroenterol. 1996;91:2301–4. [PubMed] [Google Scholar]
- 23.Saadeddin A, Freshwater DA, Fisher NC, Jones BJ. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy for non-malignant conditions: A double-blind prospective randomized controlled trial. Aliment Pharmacol Ther. 2005;22:565–70. doi: 10.1111/j.1365-2036.2005.02578.x. [DOI] [PubMed] [Google Scholar]
- 24.Kulling D, Sonnenberg A, Fried M, Bauerfeind P. Cost analysis of antibiotic prophylaxis for PEG. Gastrointest Endosc. 2000;51:152–6. doi: 10.1016/s0016-5107(00)70410-x. [DOI] [PubMed] [Google Scholar]
- 25.Dormann AJ, Huchzermeyer H, Lippert H. The relevance of systemic complications and the different outcomes of subgroups after percutaneous endoscopic gastrostomy (PEG) Am J Gastroenterol. 2001;96:1951–2. doi: 10.1111/j.1572-0241.2001.03915.x. [DOI] [PubMed] [Google Scholar]
- 26.Freeman ML. Adverse outcomes of ERCP. Gastrointest Endosc. 2002;56(6 Suppl):S273–82. doi: 10.1067/mge.2002.129028. [DOI] [PubMed] [Google Scholar]
- 27.Rabenstein T, Schneider HT, Nicklas M, et al. Impact of skill and experience of the endoscopist on the outcome of endoscopic sphincterotomy techniques. Gastrointest Endosc. 1999;50:628–36. doi: 10.1016/s0016-5107(99)80010-8. [DOI] [PubMed] [Google Scholar]
- 28.Maiss J, Dumser C, Oeztuerk Y, et al. Hemodynamic efficacy of two endoscopic clip devices in the treatment of bleeding vessels – An experimental testing using the compactEASIE model. Endoscopy. 2006;38:575–80. doi: 10.1055/s-2006-925000. [DOI] [PubMed] [Google Scholar]
- 29.Chung RS, Schertzer M. Pathogenesis of complications of percutaneous endoscopic gastrostomy. A lesson in surgical principles. Am Surg. 1990;56:134–7. [PubMed] [Google Scholar]
- 30.Van Den Hazel SJ, Mulder CJ, Den Hartog G, Thies JE, Westhof W. A randomized trial of polyurethane and silicone percutaneous endoscopic gastrostomy catheters. Aliment Pharmacol Ther. 2000;14:1273–7. doi: 10.1046/j.1365-2036.2000.00850.x. [DOI] [PubMed] [Google Scholar]