Abstract
Level of physical activity is linked to improved glucose homeostasis. We determined whether exercise alters the expression and/or activity of proteins involved in insulin-signal transduction in skeletal muscle. Wistar rats swam 6 h per day for 1 or 5 days. Epitrochlearis muscles were excised 16 h after the last exercise bout, and were incubated with or without insulin (120 nM). Insulin-stimulated glucose transport increased 30% and 50% after 1 and 5 days of exercise, respectively. Glycogen content increased 2- and 4-fold after 1 and 5 days of exercise, with no change in glycogen synthase expression. Protein expression of the glucose transporter GLUT4 and the insulin receptor increased 2-fold after 1 day, with no further change after 5 days of exercise. Insulin-stimulated receptor tyrosine phosphorylation increased 2-fold after 5 days of exercise. Insulin-stimulated tyrosine phosphorylation of insulin-receptor substrate (IRS) 1 and associated phosphatidylinositol (PI) 3-kinase activity increased 2.5- and 3.5-fold after 1 and 5 days of exercise, despite reduced (50%) IRS-1 protein content after 5 days of exercise. After 1 day of exercise, IRS-2 protein expression increased 2.6-fold and basal and insulin-stimulated IRS-2 associated PI 3-kinase activity increased 2.8-fold and 9-fold, respectively. In contrast to IRS-1, IRS-2 expression and associated PI 3-kinase activity normalized to sedentary levels after 5 days of exercise. Insulin-stimulated Akt phosphorylation increased 5-fold after 5 days of exercise. In conclusion, increased insulin-stimulated glucose transport after exercise is not limited to increased GLUT4 expression. Exercise leads to increased expression and function of several proteins involved in insulin-signal transduction. Furthermore, the differential response of IRS-1 and IRS-2 to exercise suggests that these molecules have specialized, rather than redundant, roles in insulin signaling in skeletal muscle.
Impaired insulin action on whole body glucose uptake is a hallmark feature of type 2 diabetes mellitus. Increased physical exercise has been linked to improved glucose homeostasis and enhanced insulin sensitivity. Immediately after an acute bout of exercise in humans (1, 2) and rodents (3–5), insulin sensitivity is enhanced. However, exercise training is also associated with enhanced glucose tolerance and insulin action in healthy (6, 7) or insulin-resistant (8–10) humans and obese insulin-resistant rodents (11). These improvements cannot be fully attributed to the effects of the last bout of exercise (9, 12). Importantly, improvements in glucose tolerance can be observed in people with mild type 2 diabetes mellitus within 1 week of moderate exercise training (9). The molecular mechanism for enhanced glucose uptake with exercise training may be partly related to increased expression and activity of key proteins known to regulate glucose metabolism in skeletal muscle (13).
In skeletal muscle, the acute effect of insulin to increase glucose transport is mediated by translocation of the glucose transporter isoform 4 (GLUT4) from an intracellular pool to the plasma membrane (14, 15). The intracellular signaling pathway by which insulin mediates glucose transport involves signal transduction through the insulin receptor (IR), whereby tyrosine phosphorylation of the IR β-subunit leads to phosphorylation of adapter proteins, including members of the insulin-receptor substrate (IRS) family (16). Evidence is emerging that the different IRS molecules may have a specialized role in mediating metabolic and mitogenic effects of insulin (17, 18). To date, four different IRS molecules have been cloned (19). IRS proteins act as docking proteins for downstream signaling molecules containing Src homology 2 domains, including the 85-kDa regulatory subunit of phosphatidylinositol (PI) 3-kinase (19). PI 3-kinase has been implicated as a key signaling transducer in insulin-mediated GLUT4 translocation and glucose transport (16, 19). Defects in insulin signal transduction through the IRS-1/PI 3-kinase pathway are associated with reduced GLUT4 translocation and glucose transport activity in skeletal muscle from type 2 diabetic patients (20, 21).
An acute bout of exercise elicits an insulin-independent increase in glucose transport through translocation of GLUT4 to the cell surface (15, 22). Several hours thereafter (≈3 h), a persistent increase in muscle insulin sensitivity of glucose transport occurs because of increased GLUT4 translocation rather than increased signal transduction at the level of the IR and IRS-1 (23). Thus, the immediate effects of acute exercise on glucose homeostasis occur primarily at the level of GLUT4 traffic rather than insulin signal transduction at the level of PI 3-kinase (15). However, the metabolic adaptations that occur in skeletal muscle through exercise training may involve changes in expression of proteins involved in glucose transport and metabolism. An early and rapid response to acute exercise is an increase (2-fold) in GLUT4 protein expression 16 h after one prolonged (6-h) swim bout (24). Nevertheless, adaptive responses to prior exercise may not be limited to changes in GLUT4 expression, as increased expression and/or function of proteins involved in insulin signal transduction may occur (25).
The aim of the present study was to determine whether changes in expression and/or activity of key proteins in the insulin signal transduction pathway to glucose transport occur in skeletal muscle 16 h after 1 or 5 days of exercise. A greater understanding of the molecular mechanisms by which exercise leads to enhanced glucose metabolism in skeletal muscle may lead to the identification of new targets for the treatment of insulin resistance in type 2 diabetes mellitus.
Materials and Methods
Materials.
3-O-Methyl[3H]glucose and [14C]mannitol were obtained from American Radiolabeled Chemicals. Human insulin (Actrapid) was from Novo Nordisk (Copenhagen). The GLUT4 polyclonal antibody was from Biogenesis (Poole, U.K.). The glycogen synthase antibody was from Oluf Pedersen (Steno Memorial Hospital, Gentofte, Denmark). The IR monoclonal antibody CT3 was from Ken Siddle (Cambridge University, Cambridge, U.K.), and IRS-1 polyclonal antibody was from Ton Maassen (Leiden University, Leiden, the Netherlands). IRS-1 monoclonal antibody and anti-phosphotyrosine antibodies were from Transduction Laboratories (Lexington, KY). IRS-2 polyclonal antibody was from Upstate Biotechnology (Lake Placid, NY). Phospho-Akt polyclonal antibodies were from New England Biolabs. These antibodies were produced by immunizing rabbits with a keyhole limpet hemocyanin (KLH)-coupled synthetic phospho-peptide (phospho-specific Akt) or synthetic peptide (total Akt) corresponding to residues 466–479 of mouse Akt. Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse IgG were from Bio-Rad. Reagents for enhanced chemiluminescence (ECL) were from Amersham. All other reagents were analytical grade (Sigma).
Animals.
Female Wistar rats (120–130 g) were purchased from B & K Universal (Sollentuna, Sweden) and housed in the animal facility at the Karolinska Hospital for 1 week before use. Rats were maintained on a 12-h light-dark cycle and were provided free access to water and standard rodent chow; they were randomly assigned to one of three groups: 1-day-exercised, 5-day-exercised, or sedentary control. The Animal Ethical Committee of the Karolinska Institute approved all protocols.
Exercise Protocol.
Rats were acclimated to swimming for 10 min per day for 2 days. The swimming protocol was a modification of a previously published procedure (24). Rats swam in groups of six, in plastic barrels 45 cm in diameter, filled to a depth of ≈50 cm. Water temperature was maintained was 34–35°C. Animals performed two 3-h exercise bouts, separated by one 45-min rest period. After the last exercise bout, animals were fed ad libitum. Approximately 16 h after the last exercise bout, rats were anesthetized with intraperitoneal injection of sodium pentobarbital (5 mg/100 g body weight) and epitrochlearis muscles were removed.
Muscle Incubations and Glucose Transport.
Media were prepared from a pregassed (95% O2/5% CO2) Krebs Henseleit buffer (KHB) containing 5 mM Hepes and 0.1% BSA (RIA grade). Epitrochlearis muscles were incubated (20 min) in a shaking water bath (30°C) in 2 ml of KHB, supplemented with 20 mM mannitol, with or without 120 nM insulin. Muscles were transferred to KHB containing 8 mM 3-O-methyl[3H]glucose (438 μCi/mmol; 1 μCi = 37 kBq) and 12 mM [14C]mannitol (42 μCi/mmol) and incubated (10 min) with or without insulin. Glucose transport activity was assessed as described (26) and expressed as micromoles per milliliter of intracellular water per hour.
Glycogen Content and Glycogen Synthase (GS) Activity.
Glycogen content was measured fluorometrically in HCl extracts of epitrochlearis muscle as described by Lowry and Passonneau (27). Results are expressed as millimoles of glucose per kilogram of wet weight. GS activity was determined by measuring the incorporation of [14C]glucose from uridine 5′-diphosphate-glucose into glycogen as described (28).
Insulin Signaling Assays.
Muscles were incubated (30 min) without insulin as described above in 2 ml of KHB containing 5 mM glucose and 15 mM mannitol. Thereafter, muscles were incubated in identical media with or without 120 nM insulin. Muscles were incubated with insulin for 30 min for GS and Akt phosphorylation measurements or for 4 min for all other protocols. Muscles were frozen in liquid nitrogen and stored at −80°C for subsequent analysis.
Western Blot Analysis and Immunoprecipitations.
Muscles were homogenized in 0.6 ml of ice-cold lysis buffer containing 135 mM NaCl, 1 mM MgCl2, 2.7 mM KCl, 20 mM Tris⋅HCl (pH 8.0), 0.5 mM Na3VO4, 10 mM NaF, 1% Triton X-100, 10% (vol/vol) glycerol, 0.2 mM phenylmethylsulfonyl fluoride, and 10 μg/ml each of aprotinin, leupeptin, and pepstatin. Insoluble material was removed by centrifugation (12,000 × g for 10 min at 4°C). Protein was determined with a bicinchoninic acid (BCA) protein assay (Pierce). Lysates were resuspended in Laemmli sample buffer. Protein expression was analyzed in lysates of basal (non-insulin-stimulated) muscle. Proteins were separated by SDS/PAGE, transferred to poly(vinylidene difluoride) (PVDF) membranes (Millipore), blocked with 7.5% nonfat milk, washed with TBST (10 mM Tris⋅HCl, pH 7.5/100 mM NaCl/0.02% Tween 20) and incubated with primary antibodies overnight at 4°C. Membranes were washed with TBST and incubated with appropriate secondary antibody. Proteins were visualized by ECL and quantified by densitometry.
Aliquots of supernatant (750 μg) were immunoprecipitated overnight at 4°C with appropriate antibodies as indicated in figures. Immunoprecipitates were collected on protein A-Sepharose beads (Pharmacia Biotech) and washed three times in lysis buffer, twice in 0.1 M Tris, pH 8.0/0.5 M LiCl, once in 10 mM Tris, pH 7.6/0.15 M NaCl/1 mM EDTA, and once in 20 mM Hepes/5 mM MgCl2/1 mM DTT. Pellets were resuspended in Laemmli sample buffer and subjected to immunoblot analysis.
PI 3-Kinase Activity.
IRS-1 or IRS-2 immunoprecipitates were washed as described above and resuspended in 40 μl of buffer (20 mM Hepes, pH 7.5/180 mM NaCl). PI 3-kinase activity was assessed as described (29). The band corresponding to PI-3-phosphate was quantified with a phosphorimager (Bio-Rad).
Akt Phosphorylation.
Muscle lysate (40 μg) was subjected to SDS/PAGE, proteins were transferred to poly(vinylidene difluoride) membranes, and immunoblot analysis was performed. Membranes were incubated with phosphospecific antibodies. The phospho-Akt antibody recognizes Akt when phosphorylated at Ser-473 (Akt phosphorylation). Phosphorylated proteins were visualized by ECL and quantified by densitometric scanning.
Statistics.
The paired t test was used to assess differences between two treatments within a group. All other differences were determined by one-way ANOVA. Fischer's least significant difference post hoc analysis was used to identify significant differences. Results are reported as mean ± SEM.
Results
Glucose Transport and Metabolism.
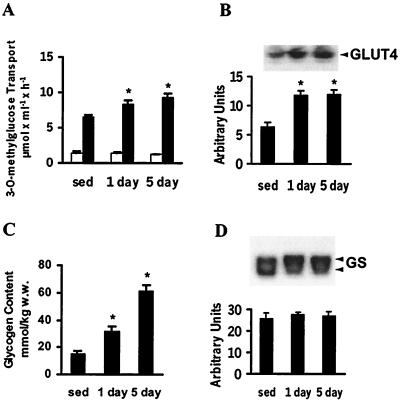
All measurements were performed on epitrochlearis muscle obtained from rats, 16 h after 1 or 5 days of exercise. Sedentary rats were used as controls. Insulin-stimulated (120 nM) glucose transport was ≈30% and 50% higher in epitrochlearis muscle from 1- and 5-day-exercised rats, respectively, compared with sedentary rats (P < 0.05; Fig. 1A). GLUT4 content was increased 2-fold in epitrochlearis muscle 16 h after 1 day of exercise (P < 0.05 vs. sedentary rats; Fig. 1B), with no further increase noted after 5 days of exercise. Muscle glycogen content was increased 2.0- and 4.2-fold 16 h after 1 and 5 days of exercise, respectively (P < 0.05; Fig. 1C). GS protein expression was not altered by exercise (Fig. 1D). However, 16 h after 1 or 5 days of exercise, GS protein displayed a reduced electrophoretic mobility, suggesting that it was phosphorylated and inactive (30, 31). Insulin did not significantly increase GS activity above the basal in muscle from exercised rats (data not shown).
Figure 1.
Insulin-stimulated 3-O-methylglucose transport, GLUT4 protein expression, glycogen concentration, and GS phosphorylation/protein expression in epitrochlearis muscle. (A) Sixteen hours after the last swim bout, epitrochlearis muscles from sedentary (sed) and 1- or 5-day-exercised rats were incubated with (filled bars) or without (open bars) 120 nM insulin, and 3-O-methylglucose transport was assessed. Values are mean ± SEM for 6 or 7 rats. (B) Muscle lysates (25 μg of protein) were subjected to SDS/PAGE and immunoblotted with polyclonal anti-GLUT4 antibody. (Upper) Representative GLUT4 immunoblot. Graph is mean ± SEM for 6 to 8 rats. (C) Glycogen content was assessed in muscle obtained as described for A. Graph is mean ± SEM for glycogen content for 6 or 7 rats. (D) Protein expression of GS in muscle lysates (50 μg) determined by immunoblot analysis with polyclonal anti-GS antibody. (Upper) Representative immunoblot of GS phosphorylation and expression in muscle. Phosphorylation was detected by reduced mobility of GS protein during SDS/PAGE (7.5% gel). Graph is mean ± SEM for GS protein expression in 6 or 7 rats. *, P < 0.05 vs. sedentary rats.
IR Expression and Tyrosine Phosphorylation.
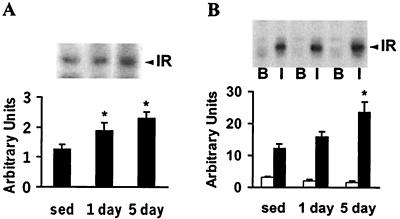
IR β-subunit expression increased ≈50% 16 h after 1 day of exercise (P < 0.05 vs. sedentary rats; Fig. 2A). Although insulin-stimulated IR tyrosine phosphorylation increased 1.3-fold after 1 day of exercise, this increase was not statically significant (P = 0.09; Fig. 2B). After 5 days of exercise, IR protein expression and tyrosine phosphorylation increased 2-fold (P < 0.05 vs. sedentary rats; Fig. 2 A and B, respectively).
Figure 2.
Protein expression and insulin-stimulated phosphorylation of IR β-subunit. Epitrochlearis muscles were obtained from rats described for Fig. 1A and incubated with or without 120 nM insulin (4 min). (A) Muscle lysates (50 μg) were subjected to SDS/PAGE (7.5% gels) and immunoblotted with monoclonal anti-IR antibodies. (Upper) Representative immunoblot of IR protein expression. Graph is mean ± SEM for 8 or 9 rats. (B) Tyrosine phosphorylation of IR. Muscle lysates (750 μg) were immunoprecipitated with polyclonal anti-phosphotyrosine antibody, subjected to SDS/PAGE (7.5% gels), and immunoblotted with a monoclonal anti-phosphotyrosine antibody. (Upper) Representative immunoblot of basal (B; open bar) and insulin-stimulated (I; filled bar) tyrosine phosphorylation of the IR. Graph is mean ± SEM for 5 or 6 rats. *, P < 0.05 vs. sedentary rats (sed).
IRS Protein Expression and PI 3-Kinase Activity.
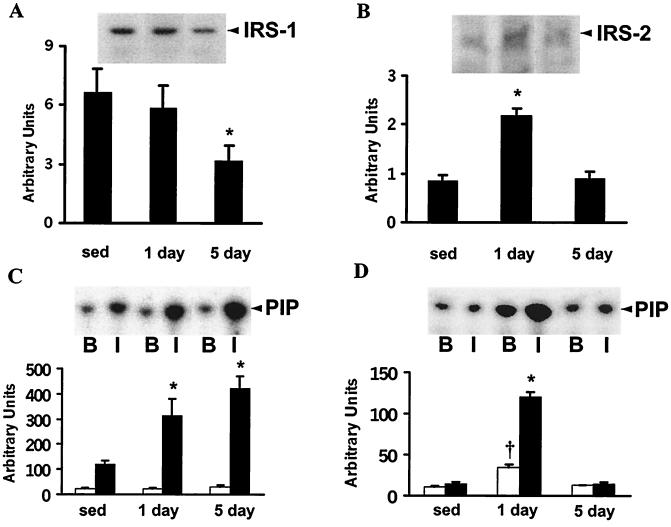
IRS-1 protein expression in epitrochlearis muscle from sedentary and 1-day-exercised rats was similar. Five-day exercise training led to a 2-fold decrease in IRS-1 expression (P < 0.05 vs. sedentary rats; Fig. 3A). IRS-2 is another substrate for the insulin receptor kinase and it shares many of the structural features of IRS-1. However, it is not entirely clear whether these two proteins have specialized or redundant roles in mediating intracellular effects of insulin. In contrast to IRS-1, IRS-2 expression increased 2.6-fold 16 h after 1 day of exercise (P < 0.05; Fig. 3B) and expression was restored to sedentary levels 16 h after 5 days of exercise. Thus, exercise has a divergent effect on IRS-1 and -2 expression.
Figure 3.
IRS protein expression and PI 3-kinase activity. Epitrochlearis muscles were incubated as described in the legend of Fig. 2 and lysates were prepared. (A) Lysates from non-insulin-stimulated muscle (50 μg) were to subjected to SDS/PAGE (7.5% gels) and immunoblotted with a monoclonal anti-IRS-1 antibody. (Upper) Representative immunoblot of IRS-1 expression. Graph is mean ± SEM for 8 or 9 rats. (B) Lysates were subjected to SDS/PAGE as described for A and immunoblotted with a polyclonal anti-IRS-2 antibody. (Upper) Representative immunoblot of IRS-2 expression. Graph is mean ± SEM for 8 or 9 rats. (C) Lysates (500 μg) were immunoprecipitated with polyclonal anti-IRS-1 antibody and PI 3-kinase activity was assessed. Incorporation of 32P into PI-3-phosphate for basal (B; open bar) or insulin-stimulated (I; filled bar) muscle was assessed with a phosphorimager. (Upper) Representative phosphorimage. Graph is mean ± SEM for 7 to 9 rats. (D) Lysates (750 μg) were immunoprecipitated with polyclonal anti-IRS-2 antibody and PI 3-kinase activity was assessed. (Upper) Representative phosphorimage. Graph is mean ± SEM for 3 or 4 rats. For A and B, *, P < 0.05 vs. sedentary rats. For C and D, *, P < 0.05 vs. insulin-stimulated muscle from sedentary rats; †, P < 0.05 vs. non-insulin-stimulated muscle from sedentary rats.
To explore whether the divergent effect of exercise on IRS-1 and -2 protein expression was associated with changes in downstream signaling, PI 3-kinase activity was assessed. Insulin-stimulated IRS-1-associated PI 3-kinase activity (Fig. 3C) was markedly increased 2.6- and 3.5-fold 16 h after 1 and 5 days of exercise (P < 0.05 vs. sedentary rats). The increase in IRS-1-associated PI 3-kinase activity in 5-day-exercised rats was evident despite reduced IRS-1 protein expression. We next assessed IRS-2-associated PI 3-kinase activity (Fig. 3D). Basal activity was increased 2.8-fold 16 h after 1 day of exercise (P < 0.05 vs. sedentary rats), consistent with increased IRS-2 protein expression. Insulin-stimulated IRS-2-associated PI 3-kinase activity was also assessed. In contrast to our findings for IRS-1, insulin was without effect on IRS-2-associated PI 3-kinase activity in muscle from sedentary or 5-day-exercised rats. However, after 1 day of exercise, insulin-stimulated activity increased 3-fold (P < 0.05) vs. basal conditions in 1-day-exercised rats and 9-fold (P < 0.05) compared with insulin-stimulated muscle from sedentary rats.
Insulin-Stimulated Tyrosine Phosphorylation of IRS-1.
Although the IRS-1 expression in epitrochlearis muscle was not altered by 1 day of exercise training, a 1.9-fold increase in insulin-stimulated tyrosine phosphorylation of IRS-1 was observed (10.6 ± 2.9 vs. 20.9 ± 3.5 arbitrary units for sedentary vs. 1-day-exercised rats; P = 0.08). Thus, even after 1 day of exercise, there was a strong tendency for increased signal transduction at the level of IRS-1. Despite a 50% reduction in IRS-1 protein expression 16 h after 5 days of training, insulin-stimulated tyrosine phosphorylation was increased 3-fold (10.6 ± 2.9 vs. 30.6 ± 4.8 arbitrary units for 5-day-exercised vs. sedentary rats; P < 0.05).
Akt Expression and Phosphorylation.
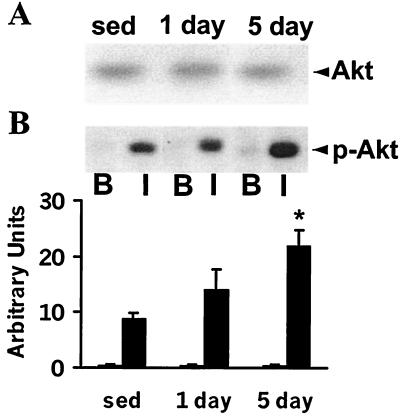
Exercise training did not alter Akt protein expression in epitrochlearis muscle (data not shown, representative immunoblot shown Fig. 4A). Furthermore, 1 day of exercise did not alter insulin-stimulated Akt phosphorylation (Fig. 4B) (P = 0.07 vs. sedentary rats). However, 5 days of exercise led to a 3-fold increase in insulin-stimulated Akt phosphorylation (P < 0.05 vs. insulin-stimulated muscle from sedentary rats).
Figure 4.
Akt protein expression and phosphorylation. Muscles were incubated with or without 120 nM insulin (30 min). Muscle lysates (40 μg) were subjected to SDS/PAGE (10% gels) and immunoblotted with a polyclonal anti-Akt antibody (A: measuring expression) or a phospho-specific polyclonal anti-p-Akt antibody (B: measuring phosphorylation). A representative immunoblot of basal (B) and insulin-stimulated (I) Akt phosphorylation is shown. Graph is mean ± SEM for basal (open bar) and insulin-stimulated (filled bar) conditions for 5 or 6 rats. *, P < 0.05 vs. sedentary rats.
Discussion
Exercise training has multiple effects on glucose metabolism and gene expression. Nevertheless, little is known of the molecular mechanism by which exercise training leads to increased insulin responsiveness for glucose transport in skeletal muscle. Here we provide evidence for a molecular mechanism to account for increased insulin action in skeletal muscle after exercise. Rats were subjected to swim exercise for 1 or 5 days, and 16 h after the last exercise bout, epitrochlearis muscle was studied. Exercise was associated with a marked increase in expression and phosphorylation/activity of several proteins involved in insulin signal transduction in skeletal muscle (Table 1). Although 1 day of exercise led to an increase in IR protein expression, insulin-stimulated tyrosine phosphorylation of the IR was not significantly enhanced. However, 16 h after 5 days of exercise, IR expression and insulin-stimulated tyrosine phosphorylation were increased 2-fold. Nevertheless, exercise-induced changes at the level of the IR are unlikely to be the sole explanation for the increased metabolic response to insulin, because insulin-stimulated glucose transport activity and GLUT4 protein expression were dramatically increased already after 1 day of exercise.
Table 1.
Changes in phosphorylation/activity (function) and expression in muscle after exercise
| Protein | Function
|
Expression
|
||
|---|---|---|---|---|
| 1 day | 5 days | 1 day | 5 days | |
| Glucose transport/GLUT4 | ▴ | ▴ | ▴ | ▴ |
| | | | | | | | | |
| GS | ◂-▸ | ◂-▸ | ◂-▸ | ◂-▸ |
| Glycogen content | ▴ | ▴▴ | ||
| | | | | | |||
| IR | ▴ | ▴ | ◂-▸ | ▴ |
| | | | | | | ||
| IRS-1 | ▴ | ▴▴ | ◂-▸ | | |
| | | | | | ▾ | ||
| PI 3-kinase activity | ▴ | ▴▴ | ||
| | | | | | |||
| IRS-2 | ▴ | ◂-▸ | ||
| | | ||||
| PI 3-kinase activity | ▴ | ◂-▸ | ||
| | | ||||
| Akt | ◂-▸ | ▴ | ◂-▸ | ◂-▸ |
| | | ||||
In contrast to our findings for the IR, changes in IRS-1 protein expression were not observed 16 h after 1 day of exercise. However, insulin-stimulated tyrosine phosphorylation of IRS-1 tended to be increased after 1 day of exercise. Surprisingly, IRS-1 protein expression was reduced 16 h after 5 days of exercise, despite a profound increase in insulin-stimulated IRS-1 tyrosine phosphorylation. Thus, repeated exercise is associated with either increased degradation or decreased synthesis of IRS-1. Although hyperinsulinemia leads to IRS-1 degradation (32), it is unlikely to account for the exercise-induced reduction in IRS-1 protein content, as exercise is known to lower insulin levels (33). Rather, reduced IRS-1 expression may occur as a negative-feedback mechanism in response to increased IR expression and phosphorylation.
Despite reduced IRS-1 protein expression, insulin-stimulated tyrosine phosphorylation of IRS-1 and IRS-1-associated PI 3-kinase activity was markedly increased 16 h after 5 days of exercise. Improved insulin responsiveness on glucose transport may be related to enhanced signal transduction at the level of the IRS proteins and PI 3-kinase. These findings are clinically relevant, because insulin-stimulated tyrosine phosphorylation of IRS-1 and PI 3-kinase activity is reduced in skeletal muscle from type 2 diabetic patients (20). Thus, exercise training may be one therapeutic strategy to restore impaired insulin signal transduction in skeletal muscle from type 2 diabetic patients.
Perhaps the most striking and unexpected finding was the dramatic increase in IRS-2 expression in epitrochlearis muscle 16 h after 1 day of exercise. The increase in IRS-2 protein expression was associated with functional changes, as noted by increased insulin-stimulated IRS-2-associated PI-3 kinase activity. Increased IRS-2 expression in human pancreatic cancer cells is linked to excessive growth stimulation of the malignancy (34, 35). Thus, the increase in IRS-2 protein expression in skeletal muscle 16 h after 1 day of exercise may occur in response to enhanced mitogenic signaling, as mitogen-activated protein kinase phosphorylation is increased during a single exercise bout (36, 37). IRS-2 mediates insulin-stimulated glucose transport in a manner similar to that of IRS-1 (38, 39). The increase in IRS-2 associated PI 3-kinase activity may contribute to insulin-stimulated glucose transport after 1-day exercise training. However the exact contribution of IRS-2 to insulin-stimulated glucose transport activity has yet to be determined.
The serine/threonine kinase Akt (PKB/Rac), a downstream target of PI 3-kinase, has been implicated to play a role in growth factor signaling to protein synthesis and glucose transport (40–43), although the requirement for Akt in the activation of glucose transport has been challenged (44, 45). Akt may also play a role in the development of the myogenic program (46), raising the possibility that Akt may interact with transcription factors. Increased insulin-stimulated Akt phosphorylation in skeletal muscle 16 h after 1 or 5 days of exercise is likely to result from increased signal transduction through PI 3-kinase. Repeated swim exercise for 5 days led to a 2.3-fold increase in insulin-stimulated Akt phosphorylation. Although Akt does not appear to be directly involved in exercise-mediated glucose transport (37), enhanced insulin signal transduction at the level of Akt 16 h after exercise may contribute to increased synthesis of muscle proteins and/or glucose transport.
Exercise-induced metabolic adaptations in skeletal muscle are not limited to increased GLUT4 protein expression. However, effects of exercise on GLUT4 expression cannot be underestimated. Ren and co-workers (24) reported that 1 or 2 days of swim exercise was associated with an ≈2-fold overexpression of GLUT4 and a proportional increase in insulin-stimulated glucose transport in epitrochlearis muscle from overnight-fasted animals in which muscle glycogen concentration was not increased. Our findings in fed animals are consistent with those of Host and co-workers (47): maximal insulin-stimulated glucose transport activity was not dramatically increased in epitrochlearis muscle from fed rats studied 16 h after 2 days of swimming. In our study, exercise led to a dramatic increase in glycogen content in epitrochlearis muscle, with no change in glycogen synthase expression. Immunoblot analysis revealed reduced electrophoretic mobility of glycogen synthase protein in muscle 16 h after 1 or 5 days of exercise, providing evidence for glycogen synthase phosphorylation and inactivation (30, 31). Although 5 days of exercise led to a greater increase in insulin-stimulated glucose transport activity compared with 1 day, the magnitude of this increase was less than observed in fasted rats (24). Our data are consistent with the observation that glycogen supercompensation after carbohydrate feeding in exercise-trained rats is associated with reduced insulin-stimulated glucose transport activity (48, 49). GLUT4 vesicles have been suggested to be associated with glycogen particles in a saturable manner (50). Thus, under fasted conditions, when glycogen levels are low, there may be a larger pool free of GLUT4 available for recruitment to the cell surface.
GLUT4 overexpression in skeletal muscle prevents impaired whole-body glucose homeostasis associated with various states of insulin resistance (51, 52). Physical training increases skeletal muscle GLUT4 expression and improves glucose intolerance and whole-body insulin action (7, 10, 13, 52). However, exercise-induced increases in GLUT4 protein and insulin-stimulated glucose transport activity are accompanied by dramatic changes in expression and function of several proteins in the insulin signal transduction cascade. Our results provide evidence that increased insulin-stimulated glucose transport after exercise training is not limited to increased GLUT4 protein expression. Collectively, these proteins undergo a dynamic regulation in response to prior exercise. Here we provide evidence that IRS-1 and IRS-2 undergo differential regulation in skeletal muscle in response to exercise. Furthermore, our findings suggest that IRS-1 and IRS-2 have specialized, rather than redundant, roles in mediating insulin signal transduction in skeletal muscle. The nature of these specialized roles remains to be determined.
Acknowledgments
This study was supported by grants from the Novo-Nordisk Foundation, the Swedish Diabetes Association, Thurings Foundation, Tore Nilson's Foundation, Marcus and Amalia Wallenbergs Foundation, Lars Hiertas Memorial Foundation, Foundation for Strategic Research, and the Swedish Medical Research Council.
Abbreviations
- GLUT4
insulin-responsive glucose transporter isoform 4
- IR
insulin receptor
- IRS
insulin-receptor substrate
- PI
phosphatidylinositol
- GS
glycogen synthase
References
- 1.Zierath J R. Acta Physiol Scand. 1995;155,(Suppl. 626):1–96. [PubMed] [Google Scholar]
- 2.Devlin J T, Hirshman M, Horton E D, Horton E S. Diabetes. 1987;36:434–439. doi: 10.2337/diab.36.4.434. [DOI] [PubMed] [Google Scholar]
- 3.Wallberg-Henriksson H. Acta Physiol Scand. 1987;131,(Suppl. 564):1–80. [PubMed] [Google Scholar]
- 4.Richter E A, Garetto L P, Goodman M N, Ruderman N B. J Clin Invest. 1982;69:785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallberg-Henriksson H, Constable S H, Young D A, Holloszy J O. J Appl Physiol. 1988;65:909–913. doi: 10.1152/jappl.1988.65.2.909. [DOI] [PubMed] [Google Scholar]
- 6.Seals D R, Hagberg J M, Allen W K, Hurley B F, Dalsky G P, Ehsani A A, Holloszy J O. J Appl Physiol. 1984;56:1521–1525. doi: 10.1152/jappl.1984.56.6.1521. [DOI] [PubMed] [Google Scholar]
- 7.Dela F, Mikines K J, Von Linstow M, Secher N H, Galbo H. Am J Physiol. 1992;263:E1134–E1143. doi: 10.1152/ajpendo.2006.263.6.E1134. [DOI] [PubMed] [Google Scholar]
- 8.Trovati M, Carta Q, Cavalot F, Vitali S, Banaudi C, Lucchina S G, Fiocchi F, Emanuelli G, Lenti G. Diabetes Care. 1984;7:416–420. doi: 10.2337/diacare.7.5.416. [DOI] [PubMed] [Google Scholar]
- 9.Rodgers M A, Yamamoto C, King D S, Hagberg J M, Ehsani A A, Holloszy J O. Diabetes Care. 1988;11:613–618. doi: 10.2337/diacare.11.8.613. [DOI] [PubMed] [Google Scholar]
- 10.Hughes V A, Fiatarone M A, Fielding R A, Kahn B B, Ferrara C M, Shepherd P R, Fisher E C, Wolfe R R, Elahi D, Evans W J. Am J Physiol. 1993;264:E855–E862. doi: 10.1152/ajpendo.1993.264.6.E855. [DOI] [PubMed] [Google Scholar]
- 11.Cortez M Y, Torgan C E, Brozinick J T, Ivy J L. Am J Physiol. 1991;261:E613–E619. doi: 10.1152/ajpendo.1991.261.5.E613. [DOI] [PubMed] [Google Scholar]
- 12.Mikines K J, Sonne B, Tronier B, Galbo H. J Appl Physiol. 1989;66:704–711. doi: 10.1152/jappl.1989.66.2.704. [DOI] [PubMed] [Google Scholar]
- 13.Hjeltnes N, Galuska D, Björnholm M, Aksnes A-K, Lannem A, Zierath J R, Wallberg-Henriksson H. FASEB J. 1998;12:1701–1712. doi: 10.1096/fasebj.12.15.1701. [DOI] [PubMed] [Google Scholar]
- 14.Hirshman M F, Goodyear L J, Wardzala L J, Horton E D, Horton E S. J Biol Chem. 1990;265:987–991. [PubMed] [Google Scholar]
- 15.Lund S, Holman G D, Schmitz O, Pedersen O. Proc Natl Acad Sci USA. 1995;92:5817–5821. doi: 10.1073/pnas.92.13.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn C R. Diabetes. 1994;43:1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- 17.Araki E, Lipes M A, Patti M E, Bruning J C, Haag B L, III, Johnson R S, Kahn C R. Nature (London) 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 18.Whithers D J, Gutierrez J S, Towery H, Burks D J, Ren J-M, Previs S, Zhang Y, Bernal D, Pons S, Shulman G I, et al. Nature (London) 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 19.White M F. Mol Cell Biochem. 1998;182:3–11. [PubMed] [Google Scholar]
- 20.Björnholm M, Kawano Y, Lehtihet M, Zierath J R. Diabetes. 1997;46:524–527. doi: 10.2337/diab.46.3.524. [DOI] [PubMed] [Google Scholar]
- 21.Zierath J R, He L, Gumá A, Odegaard-Wahlström E, Klip A, Wallberg-Henriksson H. Diabetologia. 1996;39:1180–1189. doi: 10.1007/BF02658504. [DOI] [PubMed] [Google Scholar]
- 22.Douen A G, Ramlal T, Rastogi S A, Bilan P J, Cartee G D, Vranic M, Holloszy J O, Klip A. J Biol Chem. 1990;265:13427–13430. [PubMed] [Google Scholar]
- 23.Hansen P A, Nolte L A, Chen M M, Holloszy J O. J Appl Physiol. 1998;85:1218–1222. doi: 10.1152/jappl.1998.85.4.1218. [DOI] [PubMed] [Google Scholar]
- 24.Ren J-M, Semenkovich C F, Gulve E A, Gao J, Holloszy J O. J Biol Chem. 1994;269:14396–14401. [PubMed] [Google Scholar]
- 25.Kim Y, Inoue T, Nakajima R, Nakae K, Tamura T, Tokuyama K, Suzuki M. Biochem Biophys Res Commun. 1995;210:766–773. doi: 10.1006/bbrc.1995.1725. [DOI] [PubMed] [Google Scholar]
- 26.Wallberg-Henriksson H, Zetan N, Henriksson J. J Biol Chem. 1987;262:7665–7671. [PubMed] [Google Scholar]
- 27.Lowry O H, Passonneau J V. A Flexible System of Enzymatic Analysis. New York: Academic; 1972. [Google Scholar]
- 28.Thomas J A, Schlender K K, Larner J. Anal Biochem. 1968;25:486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- 29.Krook A, Whitehead J P, Dobson S P, Griffiths M R, Ouwents M, Baker C, Hayward A C, Sen S K, Maassen J A, Siddle K, et al. J Biol Chem. 1997;272:30208–30214. doi: 10.1074/jbc.272.48.30208. [DOI] [PubMed] [Google Scholar]
- 30.DePaoli-Roach A A, Ahmad Z, Camici M, Lawrence J C, Roach P J. J Biol Chem. 1983;258:10702–10709. [PubMed] [Google Scholar]
- 31.Kaslow H R, Lesikar D D. FEBS Lett. 1984;172:294–298. doi: 10.1016/0014-5793(84)81144-8. [DOI] [PubMed] [Google Scholar]
- 32.Rice K M, Turnbow M A, Garner C W. Biochem Biophys Res Commun. 1993;190:961–967. doi: 10.1006/bbrc.1993.1143. [DOI] [PubMed] [Google Scholar]
- 33.Wallberg-Henriksson H, Zierath J R. Sports Med. 1998;25:25–35. doi: 10.2165/00007256-199825010-00003. [DOI] [PubMed] [Google Scholar]
- 34.Schuppin G T, Pons S, Hügl S, Aiello L P, King G L, White M, Rhodes C J. Diabetes. 1998;47:1074–1085. doi: 10.2337/diabetes.47.7.1074. [DOI] [PubMed] [Google Scholar]
- 35.Kornmann M, Maruyama H, Bergmann U, Tangvoranuntakul P, Beger H G, White M F, Korc M. Cancer Res. 1998;58:4250–4254. [PubMed] [Google Scholar]
- 36.Aronson D, Violan M A, Dufresne S D, Zangen D, Fielding R A, Goodyear L J. J Clin Invest. 1997;99:1251–1257. doi: 10.1172/JCI119282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Widegren U, Jiang X J, Krook A, Chibalin A V, Björnholm M, Tally M, Roth R A, Henriksson J, Wallberg-Henriksson H, Zierath J R. FASEB J. 1998;12:1379–1389. doi: 10.1096/fasebj.12.13.1379. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L, Chen H, Lin C H, Cong L-N, McGibbon M A, Sciacchitano S, Lesniak M, Quon M J, Taylor S I. J Biol Chem. 1997;272:29829–29833. doi: 10.1074/jbc.272.47.29829. [DOI] [PubMed] [Google Scholar]
- 39.Miele C, Caruso M, Calleja V, Auricchio R, Oriente F, Formisano R, Condorelli G, Cafieri A, Sawka-Verhelle D, Van Obberghen E, Beguinot F. J Biol Chem. 1999;274:3094–3102. doi: 10.1074/jbc.274.5.3094. [DOI] [PubMed] [Google Scholar]
- 40.Kohn A D, Kovacina K S, Roth R A. EMBO J. 1995;14:4288–4295. doi: 10.1002/j.1460-2075.1995.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R J, Reese C B, Cohen P. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 42.Kohn A D, Summers S A, Birnbaum M J, Roth R A. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 43.Tanti J-F, Grillo S, Grémeaux T, Coffer P J, Van Obberghen E, Le Marchand-Brustel Y. Endocrinology. 1997;138:2005–2010. doi: 10.1210/endo.138.5.5136. [DOI] [PubMed] [Google Scholar]
- 44.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, Kasuga M. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imanaka T, Hayashi H, Kishi K, Wang L, Ishii K, Hazeki O, Katada T, Ebina Y. J Biol Chem. 1998;273:25347–25355. doi: 10.1074/jbc.273.39.25347. [DOI] [PubMed] [Google Scholar]
- 46.Calera M R, Pilch P F. Biochem Biophys Res Commun. 1998;251:835–841. doi: 10.1006/bbrc.1998.9566. [DOI] [PubMed] [Google Scholar]
- 47.Host H H, Hansen P A, Nolte L A, Chen M M, Holloszy J O. J Appl Physiol. 1998;85:133–138. doi: 10.1152/jappl.1998.85.1.133. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds T H, 4th, Brozinick J T, Jr, Rogers M A, Cushman S W. Am J Physiol. 1997;272:E320–E325. doi: 10.1152/ajpendo.1997.272.2.E320. [DOI] [PubMed] [Google Scholar]
- 49.Coderre L, Kandror K V, Vallega G, Pilch P F. J Biol Chem. 1995;270:27584–27588. doi: 10.1074/jbc.270.46.27584. [DOI] [PubMed] [Google Scholar]
- 50.Leturque A, Loizeau M, Vaulont S, Salminen M, Girard J. Diabetes. 1996;45:23–27. doi: 10.2337/diab.45.1.23. [DOI] [PubMed] [Google Scholar]
- 51.Gibbs E M, Stock J L, McCoid S C, Stukenbrok H A, Pessin J E, Stevenson R W, Milici A J, McNeish J D. J Clin Invest. 1995;95:1512–1518. doi: 10.1172/JCI117823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houmard J A, Shinebarger M H, Dolan P L, Leggett-Frazier N, Bruner R K, McCammon M R, Israel R G, Dohm G L. Am J Physiol. 1993;264:E896–E901. doi: 10.1152/ajpendo.1993.264.6.E896. [DOI] [PubMed] [Google Scholar]