Abstract
PURPOSE
To study the nature of the deposits in Avellino corneal dystrophy (ACD) worsening after laser in situ keratomileusis (LASIK), and suggest a mechanism for histopathogenesis.
DESIGN
Interventional case report.
METHODS
A 28-year-old woman previously diagnosed with bilateral ACD underwent bilateral LASIK. The corneal dystrophy progressively worsened bilaterally, one year later. A penetrating keratoplasty was subsequently performed on the right eye at 31 years of age, and in the left eye a year later. The clinical and histopathologic findings of the corneal graft of the right eye were reported in the literature, with positivity to the Masson trichrome stain, negative staining with Congo red, and heterozygosity for the Arg124His mutation by serum DNA studies. Histopathologic studies of the corneal graft of the left eye were conducted at the University of Texas Southwestern Medical Center.
RESULTS
Histopathologic examination of the excised cornea showed the Masson trichrome positive deposits present from underneath the Bowman layer to the LASIK interface, with absence of deposits posterior to the latter. In contrast to the prior report describing findings in the corneal graft of the left eye, the deposits stained lightly with Congo red, but failed to show birefringence under polarized light, or fluorescence with thioflavin T.
CONCLUSION
Accelerated deposits developing after LASIK in ACD eyes seem to harbor pre-amyloid features. The epithelium is likely to be the culprit, in a pathway independent of with human transforming growth hormone beta (TGF-beta), with deposits developing in the anterior stroma and the stromal interface.
Granular corneal dystrophy type II (avellino corneal dystrophy, combined granular corneal dystrophy with amyloid) is an autosomal dominant disorder which usually appears during the first or second decade of life, and is characterized by corneal opacities that resemble rings, disks, stars, or snowflakes.1–5 Originally described in patients and families with ancestry traced to Northern Italy, specifically around the region of Avellino, it has since been reported all around the world.6–8
METHODS
The condition, along with other types of granular corneal dystrophies, lattice corneal dystrophies, and Reis-Buckler dystrophy, results from a specific mutation in the BIGH3 gene on chromosome 5q31, dictating a TGF-beta (TGFB)-induced cell adhesion protein, keratoepithelin (68 Kda), which is mainly expressed by the corneal epithelium and normally present in the corneal stroma, with highest density in the Bowman layer.9,10,18 –20 In the case of Avellino corneal dystrophy, a mutation in codon 124 of BIGH3 gene (histidine replacing arginine) is responsible for all studied cases.9,10
Phototherapeutic keratectomy has been successful in improving the visual acuity of visually significant Avellino corneal dystrophies (ACD). However, recurrences after phototherapeutic keratectomy (PTK) and photorefractive keratectomy (PRK) have been documented.11,12 Laser in situ keratomileusis (LASIK) in patients with mild to moderate dystrophy has been reported to systematically worsen the disease condition, with histopathologic findings recently being described, but the mechanism has not been formulated to date.13–19 A previous article published in the American Journal of Ophthalmology by Aldave and associates reported on a 28-year-old woman with an accelerated granular-lattice corneal dystrophy course after LASIK, with a bilateral penetrating keratoplasty (PK) performed thereafter.16 Histopathologic findings of the corneal graft of the right eye were presented, which included negative Congo red staining, with the conclusion that there is an accelerated TGFB-induced protein response in patients with ACD after excimer laser ablation. We had the chance to examine the patient subsequently at the University of Texas Southwestern Medical Center. The second corneal graft, performed in the left eye, was later sent to us for histopathological evaluation. Although many findings were similar to the ones described by the previous report, based on the first corneal graft of the right eye, there were important additional findings which reconcile the histologic picture with previous findings by Lee and associates, and we believe are vital to report to improve our understanding of the pathophysiology of the disease discussed, the etiology of the deposits seen, and the potential mechanisms of their accelerated formation after LASIK.14
CASE
A 28-year-old woman had been diagnosed since the age of 23 as having bilateral granular corneal dystrophy with bilateral central anterior stromal “fleck-like” crystals. She underwent bilateral LASIK, and in the early follow-up visits, few deposits were noted bilaterally anterior to and mainly in the interface. A year later, an increase in fine white granular deposits were noted in the flap interface in both eyes. Over two years, the interface deposits were noted to be denser in both eyes with decrease in uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA) in both eyes and poor brightness acuity test (BAT) results. Subsequently, the LASIK flap was lifted in the right eye and the interface was scraped and 0.02% mitomycin C (MMC) was applied for two minutes, but the deposits started to increase again along the interface only few months later, and a PK was undertaken in the right eye. The clinical and histopathologic findings were described in details in the report of Aldave and associates, who later performed a BIGH3 gene screening using polymerase chain reaction on a blood sample from the patient confirming heterozygousity for the Arg124His mutation.16
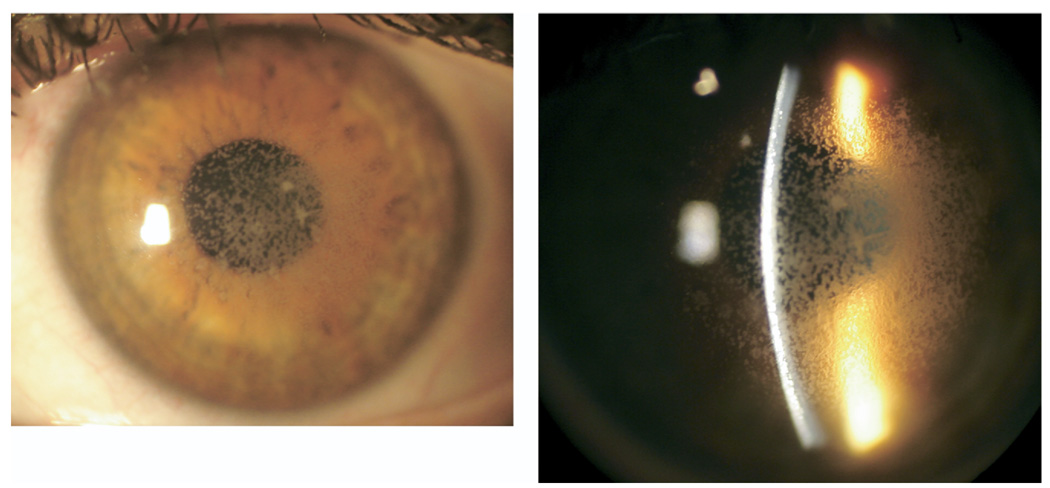
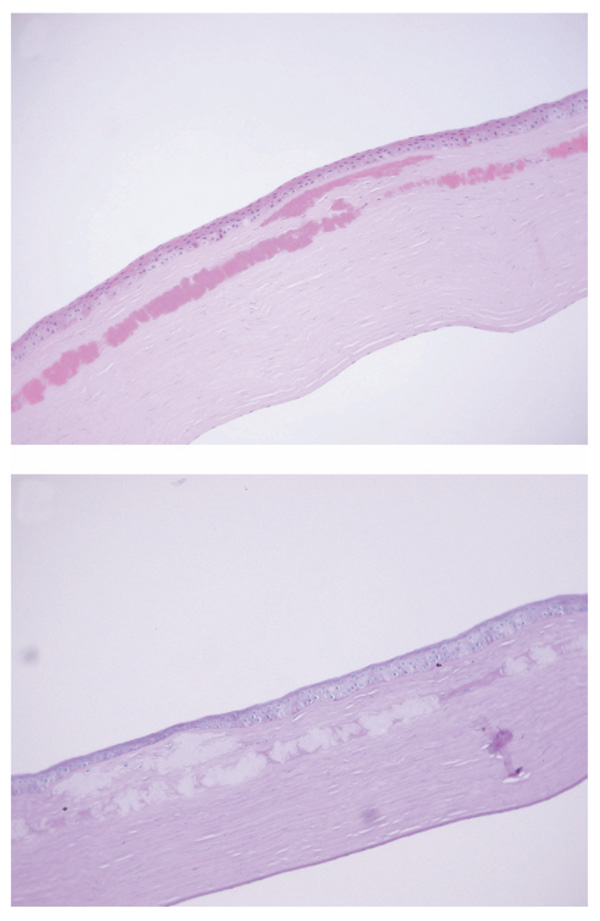
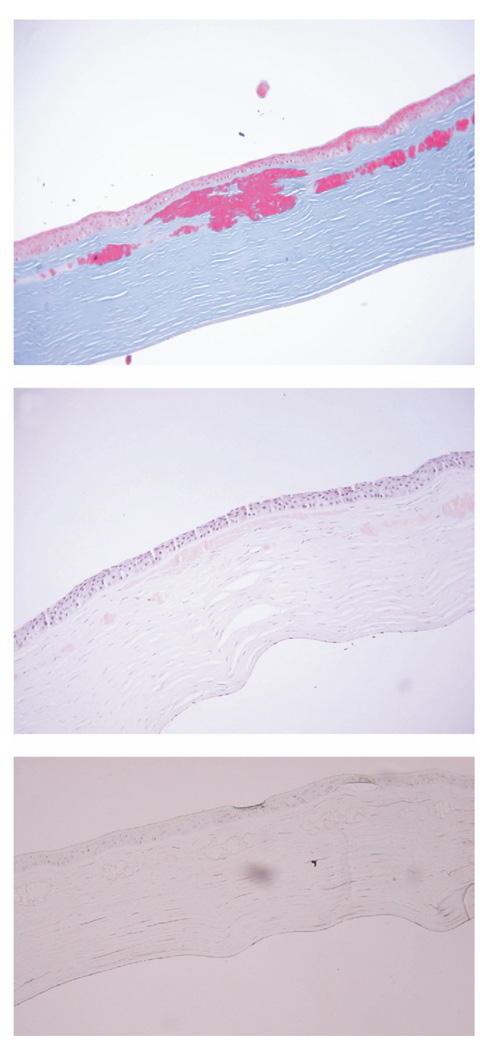
At one year post-PK, the patient was referred to the University of Texas Southwestern Medical Center at Dallas for evaluation. BCVA was 20/20 in the right eye, and 20/30 in the left eye with the patient complaining of significant glare. BAT performed in both eyes at high illumination levels yielded a BCVA of 20/20 in the right eye and 20/400 in the left. Slit-lamp examination revealed a clear corneal graft in the right eye, and anterior stromal and interface granular deposits involving the visual axis in the left eye (Figure 1). Confocal microscopy performed on the left eye showed the deposits to be solely localized anterior to and along the flap interface. The patient subsequently had PK performed in the left eye by her initial surgeon one month later. The recipient corneal button was sent to the University of Texas Southwestern Medical Center at Dallas for histopathological evaluation. Hematoxilin and eosin (H&E) staining, Periodic AcidSchiff (PAS), Congo red, thioflavin T, and the Masson trichrome staining were performed. On H&E and PAS staining, the corneal deposits were found just beneath the Bowman layer and extended down to and along the flap interface, where they were mainly concentrated (Figure 2, Top). No deposits were seen posterior to the flap interface, and none took up PAS stain (Figure 2, Bottom). But they stained deeply red with the Masson trichrome (Figure 3, Top), and weakly, but positively with Congo red, but failed to show apple-green birefringence on cross-polarized light, after Congo red staining (Figure 3, Middle). No fluorescence was exhibited on thioflavin T staining (Figure 3, Bottom).
FIGURE 1.
Avellino corneal dystrophy worsening after laser in situ keratomileusis (LASIK). (Left) Slit-lamp photography of the cornea of the left eye showing extensive granular deposits. (Right) The deposits are scattered in the anterior stroma, but are mainly concentrated in the LASIK flap interface.
FIGURE 2.
Avellino corneal dystrophy worsening after LASIK: histopathologic findings. (Top) Histologic section of the corneal recipient tissue (stain, hematoxylin and eosin; original magnification ×200). The deposits are seen in the sub-Bowman layer, anterior stroma, and the LASIK interface. The size of the deposits tends to increase as they approach the interface. (Bottom) Periodic Acid-Schiff section, with the deposits failing to stain (original magnification ×200).
FIGURE 3.
Avellino corneal dystrophy worsening after LASIK: special histopathologic staining. (Top) Histologic section of the central corneal recipient tissue, staining deep red with the Masson trichrome stain (original magnification ×200). (Middle) The section stains weakly with Congo red (original magnification ×200). (Bottom) Staining with thioflavin T. No fluorescence is exhibited under the fluorescent microscope (original magnification ×200).
DISCUSSION
Recurrence of ACD as well as granular and lattice dystrophies has been documented after treatment by PTK and PRK.11,12 Worsening ACD has also been reported after LASIK.13–19 The deposits were described clinically and histologically to be located in the LASIK flap interface and the stroma anterior to it. Three reports have so far described histopathologic findings in eyes with ACD after LASIK. One of those reports, published by Aldave and associates, describes the findings of the right eye corneal graft of our patient.16 The findings consisted of eosinophilic materials staining positive with the Masson trichrome stain with no uptake with Congo red stain. Most of the deposits were described to be located in the LASIK interface, the stroma of the flap, and the anterior portion of the stromal bed. In the second report, by Lee and associates, the deposits were mainly seen in the interface, and they stained heavily with the Masson trichrome and with Congo red, interpreted by the authors as showing both granular and amyloid nature, respectively. However, electron microscopy performed by the same authors showed only granular features, not amyloid.14 The third report, by Chiu and associates, describes a similar clinical presentation with confirmatory deoxyribonucleic acids (DNA) analysis and histopathology showing positive staining with the Masson trichrome and negative Congo red staining.18
The histopathologic findings of our patient confirm as well as reconcile the histopathologic features of the previous reports and extend their findings farther. The deposits were not solely confined to the interface, but were present just underneath the Bowman layer and all along the underlying stroma anterior to the interface. However, deposits were heavily scattered throughout the interface, centrally, with no involvement of the stromal bed. The deposits stained weakly with Congo red, but did not exhibit birefringence with cross-polarized light, nor did they reveal fluorescence after thioflavin T staining. The absence of apple-green birefringence and lack of fluorescence on thioflavin T indicate that the beta-pleated sheet configuration, responsible for the birefringence, had not yet formed, and that the myriad of individual filaments are still in disarray, and therefore nonbirefringent.21–23 These findings are consistent with the report of Lee and associates, in which the deposits stained strongly with Congo red, but did not show amyloid features of parallel packing of fibrils on electron microscopy.14 Hence, collectively, these findings indicate that the deposits laid could harbor pre-amyloid features and could ultimately develop a similar amyloid picture as late stage ACD.
The mechanism for the worsening of ACD after LASIK remains elusive, much as the corneal dystrophies. After LASIK, the epithelial basement membrane and the Bowman layer remain intact, and hence there is minimal increase in TGF-beta and other inflammatory products, as opposed to PRK or PTK, where TGF-beta increases in the first few months postoperatively in the ablated area, and becomes undetectable only after six months.24–26 Hence, whether the keratoepithelin source is the epithelium or the keratocytes, the stimulation of the mutated keratoepithelin protein in ACD corneas after LASIK seems to be independent of TGF-beta. One prevailing theory for keratoepithelin-derived corneal dystrophy is that the mutated keratoepithelin protein, secreted predominantly by the epithelium, diffuses posteriorly through the Bowman membrane and aggregates with itself and possibly other molecules to form the final deposits characteristic of the corresponding disorder.2,9,10,27 An indirect evidence of the epithelial nature of keratoepithelin and its diffusion ability is the slow progression from anterior to posterior stroma of the lattice, granular, and Avellino corneal dystrophies, as well as the initial confinement of the recurrence to the epithelium after keratoplasty or PTK.28 Additional credits to this theory are provided by ultra-structural studies.27 In ACD, the granular lesions appear earliest and more superficially, whereas the lattice lesions usually develop at a later stage and are typically found more posteriorly in the cornea. Interestingly, in the case of lattice corneal dystrophy, keratoepithelin forms amyloid early in the course of the disease and more anteriorly in the stroma. The introduction of antibodies specific for the N terminal and C terminal portions of the keratoepithelin protein (KE-15 and KE-2, respectively) has elucidated this phenomenon and contributed significantly to our understanding of the different keratoepithelin-derived corneal dystrophies and the various clinical and histological situations engendered.2,10,29 It has been shown that keratoepithelins that bind both KE-15 and KE-2 tend to stain with the Masson trichrome and develop into granular deposits, while keratoepithelins exclusively binding to KE-2 are the ones which develop into amyloid deposits.9
Proteolysis in the epithelial area is believed to act on the N-terminal of keratoepithelins, leading to configurations that don’t bind to KE-15 and hence are prone to aggregate to form beta-pleated sheets.2,10,22,30 In a patient with classic lattice corneal dystrophy, frequent epithelial erosions occur with subsequent proteolysis, promoting the synthesis of both mutant and wild type keratoepithelin, leading to keratoepithelin that binds only to KE-2, and hence is receptive to aggregate and form amyloid.2 The quick surge in synthesis and proteolysis of keratoepithelin favors the molecules, which are now found in high concentration next to the epithelial entourage, to aggregate and form amyloid products early in life and in the anterior stroma. In ACD, the epithelial proteolysis is slow and mainly dependent on aging, as the erosions are infrequent and hence the amyloid deposits appear later in life than the granular ones, and are more posterior.2,9 After LASIK, however, the epithelium reactively secretes products like collagen III and undergoes a hyperplastic and hypertrophic response as documented by confocal microscopy.31–33 This epithelial reactivity could possibly result in an increase in keratoepithelin synthesis and proteolysis, leading to a relatively rapid development of granular and potentially pre-amyloid deposits. The clinical and histological findings in our case and the ones reported so far lend some credit to this theory. In addition, the total absence of the deposits posterior to the LASIK interface and the presence of the deposits in increasing number and size from the Bowman layer to the interface suggest a possible epithelial origin for the keratoepithelin. The LASIK interface being a large loose potential space, the aggregated keratoepithelin would tend to diffuse alongside of it, polymerizing even further to produce large deposits.
In summary, the pathophysiology of the worsening of Avellino corneal dystrophy after LASIK is likely to be similar to the original disease, but with an enhanced pace, possibly attributable to epithelial reactivity to keratocyte injury, and independent of TGF-beta. The staining with Congo red in our report and in a previously published one, in the absence of birefringence, or ultra-structural evidence of parallel packing of fibrils, suggests the production of amyloid-forming keratoepithelin configurations. The latter should be binding with KE-2, but not with KE-15. Immunohistochemical studies evaluating the binding of those two antibodies to the corneal deposits in ACD eyes after LASIK would shed more light on this intriguing phenomenon.
Acknowledgments
This study was supported in part by national eye institute (NEI) grants EY10738 (Dr Cavanagh) and EY016664, Bethesda, Maryland, The Pearle Vision Foundation, Dallas, Texas, and an unrestricted grant from Research to Prevent Blindness, Inc, New York, New York. The authors indicate no financial conflict of interest. Involved in study design (S.T.A., M.D.P., R.N.H., H.D.C., S.L.F.); material source (S.T.A., M.D.P., R.N.H., H.D.C., S.L.F.); data interpretation (S.T.A., R.N.H., J.P.M., H.D.C.); and preparation and review of the manuscript (S.T.A., H.D.C., J.P.M., H.D.C.). The study had Institutional Review Board approval and conformed to the Declaration of Helsinki and HIPAA regulation.
REFERENCES
- 1.Folberg R, Alfonso E, Croxatto JO, et al. Clinically atypical granular corneal dystrophy with pathologic features of lattice-like amyloid deposits. A study of these families. Ophthalmology. 1988;95:46–51. doi: 10.1016/s0161-6420(88)33226-4. [DOI] [PubMed] [Google Scholar]
- 2.Konishi M, Yamada M, Nakamura Y, Mashima Y. Immuno-histology of kerato-epithelin in corneal stromal dystrophies associated with R124 mutations of the BIGH3 gene. Curr Eye Res. 2000;21:891–896. doi: 10.1076/ceyr.21.5.891.5536. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui N, Afshari NA. The changing face of the genetics of corneal dystrophies. Curr Opin Ophthalmol. 2002;13:199–203. doi: 10.1097/00055735-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Holland EJ, Daya SM, Stone EM, et al. Avellino corneal dystrophy. Clinical manifestations and natural history. Ophthalmology. 1992;99:1564–1568. doi: 10.1016/s0161-6420(92)31766-x. [DOI] [PubMed] [Google Scholar]
- 5.Afshari NA, Mullally JE, Afshari MA, et al. Survey of patients with granular, lattice, avellino, and Reis-Bucklers corneal dystrophies for mutations in the BIGH3 and Gelsolin genes. Arch Ophthalmol. 2001;119:16–22. [PubMed] [Google Scholar]
- 6.Dolmetsch AM, Stockl FA, Folberg R, et al. Combined granular-lattice corneal dystrophy (Avellino) in a patient with no known Italian ancestry. Can J Ophthalmol. 1996;31:29–31. [PubMed] [Google Scholar]
- 7.El-Ashry MF, Abd El-Aziz MM, Larkin DF, et al. A clinical, histopathological, and genetic study of Avellino corneal dystrophy in British families. Br J Ophthalmol. 2003;87:839–842. doi: 10.1136/bjo.87.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto S, Okada M, Tsujikawa M, et al. The spectrum of beta ig-h3 gene mutations in Japanese patients with corneal dystrophy. Cornea. 2000;19:S21–S23. doi: 10.1097/00003226-200005001-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bron AJ. Genetics of the corneal dystrophies: what we have learned in the past twenty-five years. Cornea. 2000;19:699–711. doi: 10.1097/00003226-200009000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Korvatska E, Munier FL, Chaubert P, et al. On the role of kerato-epithelin in the pathogenesis of 5q31-linked corneal dystrophies. Invest Ophthalmol Vis Sci. 1999;40:2213–2219. [PubMed] [Google Scholar]
- 11.Dogru M, Katakami C, Nishida T, Yamanaka A. Alteration of the ocular surface with recurrence of granular/avellino corneal dystrophy after phototherapeutic keratectomy: report of five cases and literature review. Ophthalmology. 2001;108:810–817. doi: 10.1016/s0161-6420(00)00657-6. [DOI] [PubMed] [Google Scholar]
- 12.Dinh R, Rapuano CJ, Cohen EJ, Laibson PR. Recurrence of corneal dystrophy after excimer laser phototherapeutic keratectomy. Ophthalmology. 1999;106:1490–1497. doi: 10.1016/S0161-6420(99)90441-4. [DOI] [PubMed] [Google Scholar]
- 13.Jun RM, Tchah H, Kim TI, Stulting RD, et al. Avellino corneal dystrophy after LASIK. Ophthalmology. 2004;111:463–468. doi: 10.1016/j.ophtha.2003.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Lee WB, Himmel KS, Hamilton SM, et al. Excimer laser exacerbation of Avellino corneal dystrophy. J Cataract Refract Surg. 2007;33:133–138. doi: 10.1016/j.jcrs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Roh MI, Grossniklaus HE, Chung SH, et al. Avellino corneal dystrophy exacerbated after LASIK: scanning electron microscopic findings. Cornea. 2006;25:306–311. doi: 10.1097/01.ico.0000183536.07275.9a. [DOI] [PubMed] [Google Scholar]
- 16.Aldave AJ, Sonmez B, Forstot SL, et al. A clinical and histopathologic examination of accelerated TGFBIp deposition after LASIK in combined granular-lattice corneal dystrophy. Am J Ophthalmol. 2007;143:416–419. doi: 10.1016/j.ajo.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 17.Banning CS, Kim WC, Randleman JB, et al. Exacerbation of Avellino corneal dystrophy after LASIK in North America. Cornea. 2006;25:482–484. doi: 10.1097/01.ico.0000195949.93695.37. [DOI] [PubMed] [Google Scholar]
- 18.Chiu EK, Lin AY, Folberg R, Saidel M. Avellino dystrophy in a patient after laser-assisted in situ keratomileusis surgery manifesting as granular dystrophy. Arch Ophthalmol. 2007;125:703–705. doi: 10.1001/archopht.125.5.703. [DOI] [PubMed] [Google Scholar]
- 19.Wan XH, Lee HC, Stulting RD, Kim T, et al. Exacerbation of Avellino corneal dystrophy after laser in situ keratomileusis. Cornea. 2002;21:223–226. doi: 10.1097/00003226-200203000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Streeten BW, Qi Y, Klintworth GK, et al. Immunolocalization of beta ig-h3 protein in 5q31-linked corneal dystrophies and normal corneas. Arch Ophthalmol. 1999;117:67–75. doi: 10.1001/archopht.117.1.67. [DOI] [PubMed] [Google Scholar]
- 21.Yanoff M, Fine BS. Ocular Pathology. St Louis, Missouri: Mosby; 2002. p. 279. [Google Scholar]
- 22.Sipe JD. Amyloidosis. Annu Rev Biochem. 1992;61:947–975. doi: 10.1146/annurev.bi.61.070192.004503. [DOI] [PubMed] [Google Scholar]
- 23.Mahalingam M, Palko M, Steinberg-Benjes L, Goldberg LJ. Amyloidosis of the auricular concha: an uncommon variant of localized cutaneous amyloidosis. Am J Dermatopathol. 2002;24:447–448. doi: 10.1097/00000372-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Lee JB, Choe CM, Kim HS, et al. Comparison of TGF-beta1 in tears following laser subepithelial keratomileusis and photorefractive keratectomy. J Refract Surg. 2002;18:130–134. doi: 10.3928/1081-597X-20020301-05. [DOI] [PubMed] [Google Scholar]
- 25.Kaji Y, Soya K, Amano S, et al. Relation between corneal haze and transforming growth factor-beta1 after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:1840–1846. doi: 10.1016/s0886-3350(01)01141-5. [DOI] [PubMed] [Google Scholar]
- 26.Long Q, Chu R, Zhou X, et al. Correlation between TGF-beta1 in tears and corneal haze following LASEK and epi-LASIK. J Refract Surg. 2006;22:708–712. doi: 10.3928/1081-597X-20060901-13. [DOI] [PubMed] [Google Scholar]
- 27.Akhtar S, Meek KM, Ridgway AE, et al. Deposits and proteoglycan changes in primary and recurrent granular dystrophy of the cornea. Arch Ophthalmol. 1999;117:310–321. doi: 10.1001/archopht.117.3.310. [DOI] [PubMed] [Google Scholar]
- 28.Lyons CJ, McCartney AC, Kirkness CM, et al. Granular corneal dystrophy. Visual results and pattern recurrence after lamellar or penetrating keratoplasty. Ophthalmology. 1994;101:1812–1817. doi: 10.1016/s0161-6420(94)31096-7. [DOI] [PubMed] [Google Scholar]
- 29.Stix B, Leber M, Bingemer P, et al. Hereditary lattice corneal dystrophy is associated with corneal amyloid deposits enclosing C-terminal fragments of keratoepithelin. Invest Ophthalmol Vis Sci. 2005;46:1133–1139. doi: 10.1167/iovs.04-1319. [DOI] [PubMed] [Google Scholar]
- 30.Takacs L, Boross P, Toszser J, et al. Transforming growth factor-beta induced protein, BetaIG-H3, is present in degraded form and altered localization in lattice corneal dystrophy type I. Exp Eye Res. 1998;66:739–745. doi: 10.1006/exer.1998.0471. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K. Interaction between injured corneal epithelial cells and stromal cells. Cornea. 2003;22:S35–S47. doi: 10.1097/00003226-200310001-00006. [DOI] [PubMed] [Google Scholar]
- 32.Patel SV, Erie JC, McLaren JW, Bourne WM. Confocal microscopy changes in epithelial and stromal thickness up to 7 years after LASIK and photorefractive keratectomy for myopia. J Refract Surg. 2007;23:385–392. doi: 10.3928/1081-597X-20070401-11. [DOI] [PubMed] [Google Scholar]
- 33.Dawson DG, Edelhauser HF, Grossniklaus HE. Long-term histopathologic findings in human corneal wounds after refractive surgical procedures. Am J Ophthalmol. 2005;139:168–178. doi: 10.1016/j.ajo.2004.08.078. [DOI] [PubMed] [Google Scholar]