Abstract
FKBP52 is a tetratricopeptide repeat (TPR) protein that associates with steroid receptors in complexes containing heat shock protein (Hsp90). To investigate the role of FKBP52 in steroid-regulated physiology we generated FKBP52-deficient mice. FKBP52 (−/−) females are sterile due to a complete failure of implantation, a process that requires estrogen (ER) and progesterone receptor (PR). Since the uterus expresses two forms of PR, PR-A and PR-B, we investigated all three receptors as potential targets of FKBP52 action. FKBP52 (−/−) uteri showed a normal growth response to estradiol, and unaltered expression of genes controlled by ER and PR-B. In contrast, FKBP52 (−/−) uteri were unable to express two PR-A regulated genes, nor undergo decidualization in response to progesterone, suggesting that FKBP52 specifically regulates PR-A at this organ. Analysis of uterine PR heterocomplexes showed preferential association of FKBP52 with PR-A compared to PR-B. Loss of FKBP52 did not disrupt the PR-A/Hsp90 interaction, nor impair uterine PR-A hormone-binding function, demonstrating the essential role of FKBP52 in PR-A action to be down-stream of the hormone-binding event. Transcription studies in +/+ and −/− mouse embryonic fibroblast cells showed a near-complete loss of PR-A activity at MMTV and synthetic PRE promoters, although partial reductions of ER and PR-B were also observed. Partial disruptions of ovulation and mammary development were also found in FKBP52 (−/−) females. Taken as a whole, our results show FKBP52 to be an essential regulator of PR-A action in the uterus, while being a non-essential but contributory regulator of steroid receptors in the mammary and ovary. These data may now provide the basis for selective targeting of steroid-regulated physiology through TPR proteins.
Keywords: steroid receptor, progesterone, estrogen, fertility, FKBP52, tetratricopeptide repeat proteins
INTRODUCTION
Steroid receptors are hormone-activated transcription factors (1) controlling tissue-specific gene expression and a wide range of therapeutically important physiologies. Because of these properties, steroid receptors have been extensively studied at the level of gene expression (2–4). Yet other modes of steroid receptor regulation must exist, with heretofore-unknown impacts on physiology, as most members of this family occur in large heterogeneous complexes when not bound with hormone. Hormone-free complexes of glucocorticoid (GR), progesterone (PR), androgen (AR), and estrogen (ER) receptors all contain the heat shock protein Hsp90 and co-chaperone p23, as well as various tetratricopeptide repeat (TPR) proteins (5). To date, at least four TPR proteins have been found to associate with one steroid receptor type or another: FK506-binding protein 52 (FKBP52), FKBP51, cyclophilin 40 (Cyp40) and protein phosphatase 5 (PP5). Because the Hsp90 dimer of receptor complexes generates only one TPR-acceptor site (6, 7), at least four distinct heterocomplexes based on TPR protein content are possible for each steroid receptor. Although such heterogeneity implies differential function, almost nothing is known of how TPR proteins influence target cell responses to steroids. Indeed, a major unresolved question is whether all four TPRs contribute to the functioning of steroid receptors as a class, or whether individual TPRs preferentially regulate one receptor over another.
FKBP52 was the first TPR protein to be found with steroid receptors (8, 9) and the first to be cloned (10). Its structure contains a C-terminal site for peptidyl prolyl cis/trans isomerase (PPlase) activity, as well as three centrally located TPR domains - highly degenerate 34 amino acid sequences that mediate protein-protein interactions (11)- that are the site of interaction with Hsp90 (12–14). FKBP51 and Cyp40 share both of these domains with FKBP52, while PP5 shares only the TPR domain. FKBP52, FKBP51 and Cyp40 are also recognized as immunophilins, based on their ability to bind the immunosuppressive drugs FK506 and cyclosporine A (15–19). PP5 is not known to have this function.
Although interaction of FKBP52 with PR and AR complexes in vitro is long known (9, 20), most functional studies of FKBP52 action have been for GR and point to a role for FKBP52 in both hormone-binding function and subcellular trafficking of this receptor. Transfection of FKBP52 into S. cerevisiae (21) or over-expression in mammalian cells (22) caused potentiation of GR-mediated reporter gene expression that correlated with an increase in GR hormone-binding function. In the yeast system, a constitutively active GR mutant was not potentiated by FKBP52, suggesting that the effect is pre-transcriptional. In most cells, the majority of FKBP52 is nuclear, but that fraction of FKBP52 found in the cytoplasm localizes to microtubules and is found to co-purify with dynein (23, 24). The dynein/FKBP52 interaction occurs at the PPlase domain, suggesting that FKBP52 serves as an adaptor between dynein and the GR/Hsp90 complex that binds at the TPR-domain (7, 25). Indeed, a recent study by the Rein group showed that the PPlase domain of FKBP52 is essential for both dynein interaction and hormone-induced translocation of the GR (26). Further evidence for a role in translocation has been provided by a switching mechanism in which hormone causes displacement of FKBP51 by FKBP52 in GR complexes, leading to co-recruitment of dynein and movement of the newly-formed GR/Hsp90/FKBP52 complex to the nucleus of intact cells (27). Thus, it is now likely that differential incorporation of TPR proteins into steroid receptor complexes may form the basis for selective control of hormone binding, subcellular trafficking and, perhaps, other functions of steroid receptors.
To establish the relevance of these in vitro observations to physiological actions of steroid receptors, we generated FKBP52-deficient (−/−) mice. Surprisingly, no overt defects of GR-controlled physiology have been seen in these animals. Instead, the principal defect appears to be reduced fertility in males and sterility in females. Male infertility results from hypospadias due to compromised activity on the part of AR during development – a result that is highly similar to a recent report from the Smith laboratory who have independently established FKBP52 (−/−) mice (28). In this work, we analyze the underlying causes of sterility in FKBP52 (−/−) females, and show that it principally arises from a failure of implantation at the uterus due to compromised activity by the progesterone receptor A isoform (PR-A).
RESULTS
Targeted Disruption of FKBP52 Leads to Female Sterility
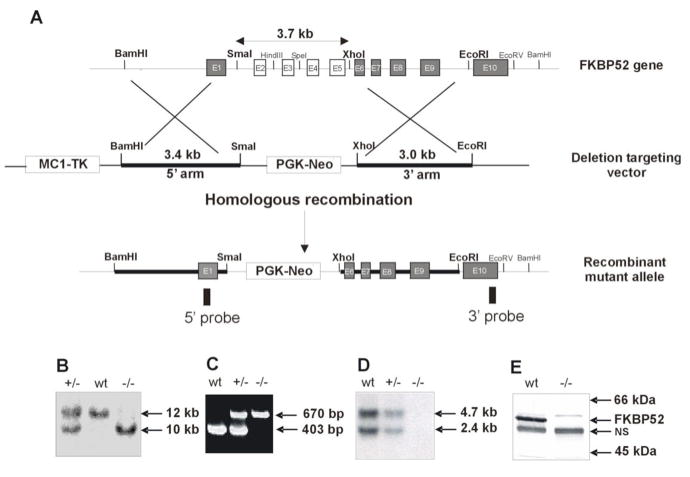
We generated FKBP52-deficient mice through targeted disruption of the FKBP52 gene at exons 2 through 5 (Fig. 1 A). Genomic Southern blot and PCR analyses were used to genotype targeted ES cells and mutant mice (Figs. 1B–C). Northern blot and Western blot analyses confirmed that FKBP52 expression was absent in FKBP52-deficient mice (Figs. 1D–E). Heterozygous (+/−) mice were viable and fertile and were inter-crossed to obtain homozygous (−/−) mutants. Male and female homozygous (−/−) mutants were equally presented in the litters and developed to adulthood.
Fig. 1.
Generation of FKBP52-deficient Mice. A, Genomic structure of the mouse FKBP52 gene, targeting vector, and FKBP52 mutant allele. B, Southern blot analysis of FKBP52 genotypes. C, PCR analysis of FKBP52 genotypes. D, Northern blot and E, Western blot analyses confirm the FKBP52 mutant allele to be null. Nonspecific band (NS) serves as a loading control.
By breeding to (+/+) males, FKBP52-deficient females were found to be sterile, while fertility of (+/−) females was unimpaired (Table 1). To determine the cause of infertility, a series of morphological and histological analyses were performed on (−/−) ovaries and uteri. There were no apparent developmental abnormalities in these reproductive organs (data not shown), suggesting that FKBP52 is not required for their normal development. Mutant females had normal estrus cycle and could be plugged by wild-type male mice.
TABLE 1.
| Genotype | Number of Matings | Litter Size (Mean, SD) | Number Fertile | |
|---|---|---|---|---|
| Male | Female | |||
| +/+ | +/+ | 5 | 7.0, +/−1.2 | 5 |
| +/+ | +/− | 5 | 6.8, +/−1.8 | 5 |
| +/+ | −/− | 20 | 0 | 0 |
Failure of Progesterone-mediated Implantation in FKBP52−/− Females
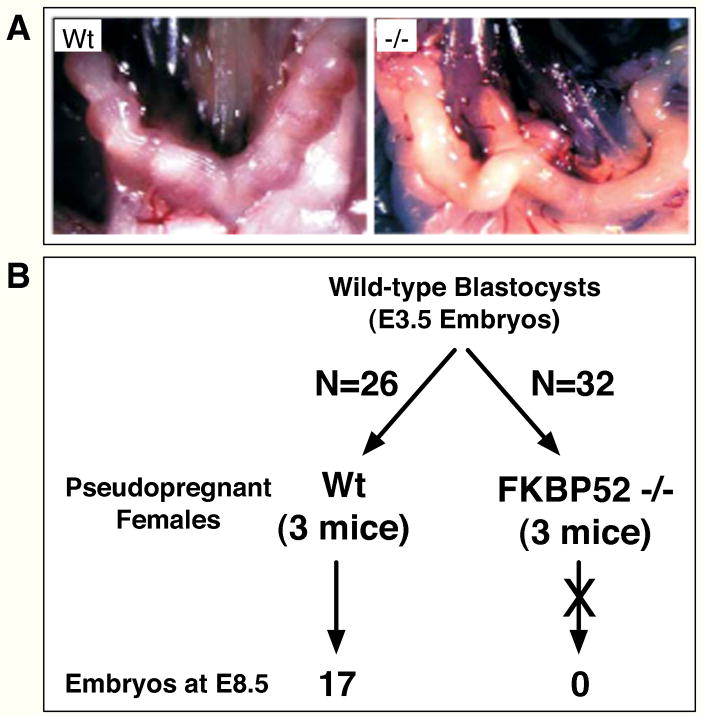
When FKBP52−/− females were mated with wild-type males, no implanted embryos could be found at day E6.5 of development (Fig. 2A). However, FKBP52−/− females or super-ovulated (−/−) females mated to wild-type males did result in the presence of 4-cell stage embryos in the oviducts (data not shown), implicating a failure of zygote implantation as the primary reason for sterility. To test this, we performed uterus-transfer experiments in which (+/+) blastocysts were implanted into uteri of pseudo-pregnant FKBP52 (−/−) and (+/+) females (Fig. 2B). Seventeen embryos (E8.5) out of 26 transferred blastocysts were recovered from (+/+) females, but not a single embryo was found (normal or abnormal) out of 32 blastocysts transferred to the (−/−) females, a strong indication that uteri of FKBP52-deficient females are not able to support implantation. These observations are consistent with recent work from Dey’s group describing an independent FKBP52 KO strain (29).
Fig. 2.
Failure of Implantation in the FKBP52-deficient Female. A, Morphological analysis of uteri from wild-type (Wt) and FKBP52-deficient (−/−) females mated to wild-type males show a complete lack of developing embryos in (−/−) females (E6.5). B, Uterus transfer analysis. Wild-type blastocysts (E3.5) were transferred to wild-type (N=3) and FKBP52-deficient (N=3) pseudo-pregnant females. At E8.5, 17 embryos were recovered from wild-type uteri out of 26 transferred blastocysts (65.4%). In contrast, 0 embryos were recovered from (−/−) uteri out of 32 blastocysts transferred (0%).
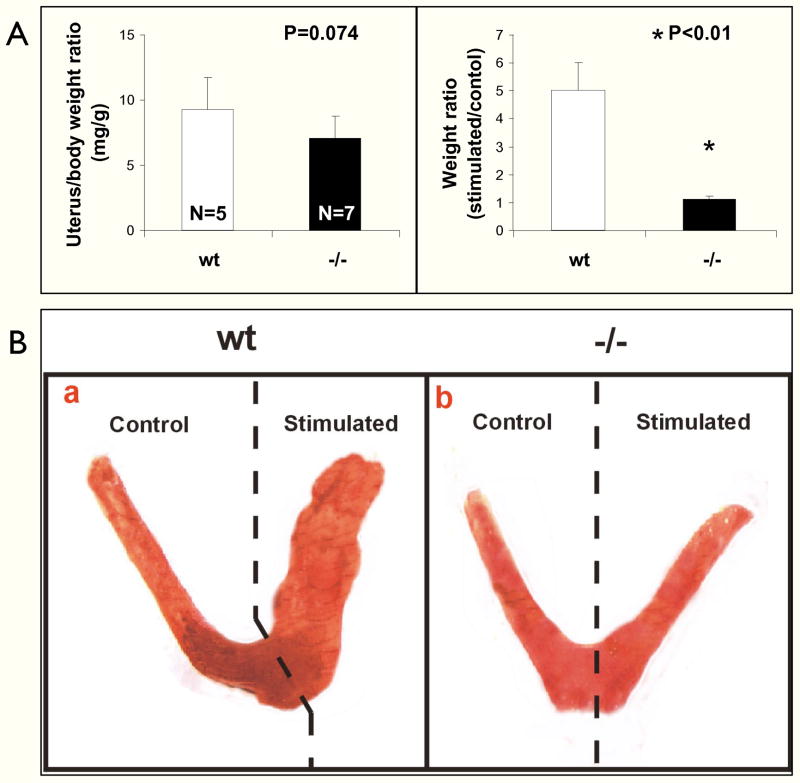
Based on these results, we reasoned that uterine insensitivity to progesterone, estrogen or both may be the reason for implantation failure. To assess the response to estrogens, ovariectomized FKBP52 (−/−) and (+/+) females were challenged with 17β-estradiol for 5 days. Although there was a slight trend of decreased uterine weight gain in response to estrogen (Fig. 3A), the difference between FKBP52 (−/−) and (+/+) females did not reach statistical significance, suggesting that the uterine response to estrogen in FKBP52-deficient females is largely normal. To assess the response to progesterone, decidualization experiments were performed on uteri from ovariectomized females. In contrast to the estrogen response, the results show a dramatic failure of decidualization by the (−/−) females in response to progesterone and implantation “trauma” (Figs. 3B and C), suggesting a strong inhibition of progesterone responsiveness in FKBP52 (−/−) females.
Fig. 3.
The FKBP52-deficient uterus responds normally to estrogen but fails to undergo decidualization in response to progesterone. A, Comparison of uterus weight following estrogen treatment in ovariectomized FKBP52-deficient and wild-type females. B, Decidualization response. FKBP52-deficient (N=10) and wild-type (N=10) females were ovariectomized and treated with sequential doses of 17β-estradiol and progesterone, as described in Methods. Implantation was mimicked by pricking of one uterine horn with a needle (stimulated). C, Quantitation of results from B.
Partial Disruption of Ovulation and Mammary Gland Alveologenesis in FKBP52 (−/−) Females
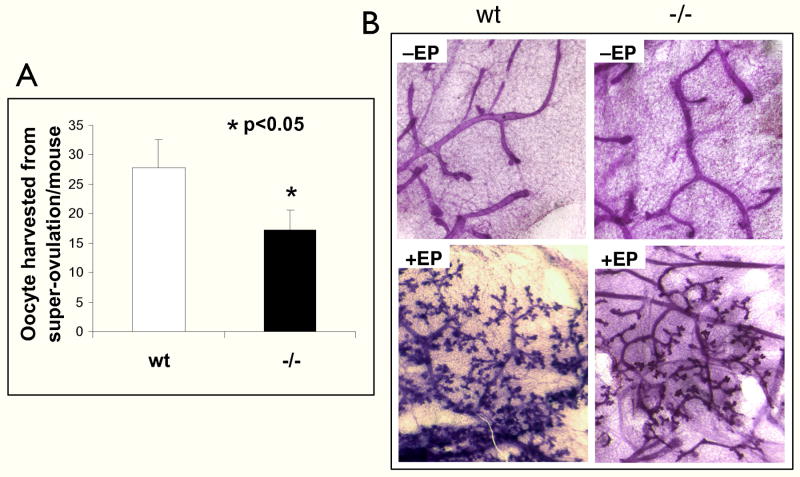
The prior results suggested that loss of FKBP52 in females primarily affected progesterone receptor activity, at least in the uterus. To test the involvement of FKBP52 in other PR-regulated tissues, we assessed responses to progesterone in the ovary and mammary. Super-ovulation experiments were performed to measure ovulation competency by treating virgin (3–4 week old) females with gonadotropins, followed by flushing of oocytes from the oviduct (Fig. 4A). Significantly fewer oocytes from PMSG/HCG-treated FKBP52 (−/−) females were obtained compared to age-matched litter-mate (+/+) females (17 vs 27 oocytes per female, respectively), suggesting that reduced ovulation is a contributory factor in the sterility phenotype of (−/−) females. Because this same test has been used in PRKO mice to establish the essential role of PR in normal ovulation (30), we can conclude that PR activity in the ovary is partially dependent on FKBP52. Mammary gland ductal development in the FKBP52 (−/−) animals was measured in response to administration of 17β-estradiol and progesterone for 14 days following ovariectomy. There was a moderate reduction in mammary gland tertiary sidebranching and alveologenesis in FKBP52-deficient females compared to wild-type controls (Fig. 4B). Like ovarian function, PRKO females have been used to show an essential role for PR in pregnancy-induced tertiary sidebranching (mimicked by E & P administration) (31). Thus, for both ovulation and mammary gland differentiation, it appears that FKBP52 contributes but is not essential to steroidal activity at these organs.
Fig. 4.
FKBP52 deficiency has moderate effects on ovarian and mammary glands functions. A, Partially reduced ovulation in FKBP52 (−/−) females. Young (3–4 weeks of age) FKBP52-deficient (n=8) and wild-type (n=7) females were subjected to a super-ovulation protocol, as described in Methods. B, Mammary gland tertiary ductal side-branching and alveologenesis is partially reduced in FKBP52 (−/−) females. Thoracic whole mounts of mammary glands from untreated (−EP) or 17β-estradiol and progesterone (+EP) treated wild-type (N=6) and FKBP52-deficient (N=10) mice.
Progesterone Failure in the Uterus of FKBP52 −/− Females is Selective for the PR-A Isoform
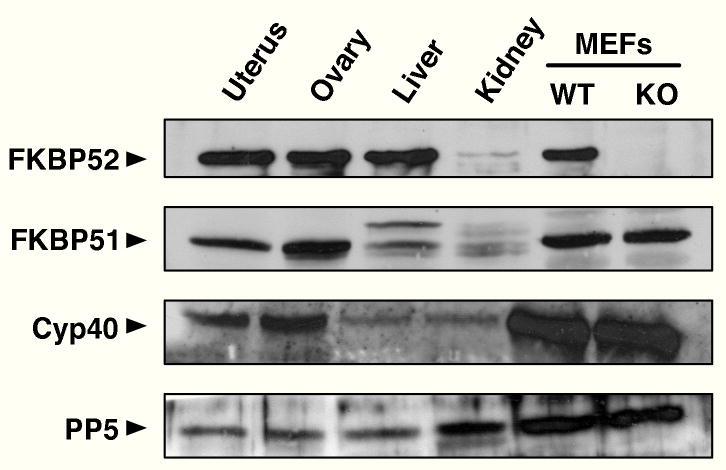
Progesterone receptors exist as two isoforms, PR-A and PR-B, with each isoform playing a critical but distinct role in uterine, ovarian and mammary gland function (32). The work of the Conneely laboratory has shown PR-B to be the essential isoform mediating response to progesterone in the mammary (33), while PR-A is essential to ovarian and uterine functions (34). Based on our results, it therefore appears that the FKBP52-deficient female more closely resembles the PR-A-deficient (PRAKO) rather than the PR-B-deficient (PRBKO) female, suggesting that FKBP52 may preferentially regulate the PR-A isoform in vivo. An alternative explanation, however, is that activities of both PR isoforms are controlled by the tissue expression profile of FKBP52. In other words, PR activity in the FKBP52-deficient female should inversely correlate with the expression levels of FKBP52 in WT animals. We have measured FKBP52 protein expression in various tissues (Fig. 5). (FKBP52 expression in the mammary is missing from this analysis due to the difficulty of selectively excising this tissue). About equal levels of FKBP52 were found in the uterus and ovary of WT females. Thus, expression levels of FKBP52 do not explain the tissue-specific differences seen in PR activities in the FKBP52 (−/−) females.
Fig. 5.
Western Blot Profile of TPR Proteins in Mouse Tissues and Cells. Expression levels of FKBP52, FKBP51, Cyp40 and PP5 were measured in lysates from uterus, ovary, liver and kidney of wild-type mice, as well as wild-type (WT) and FKBP52-deficient (KO) mouse embryonic fibroblast (MEF) cells. Analysis was performed by Western-blotting using lysates of equal protein content.
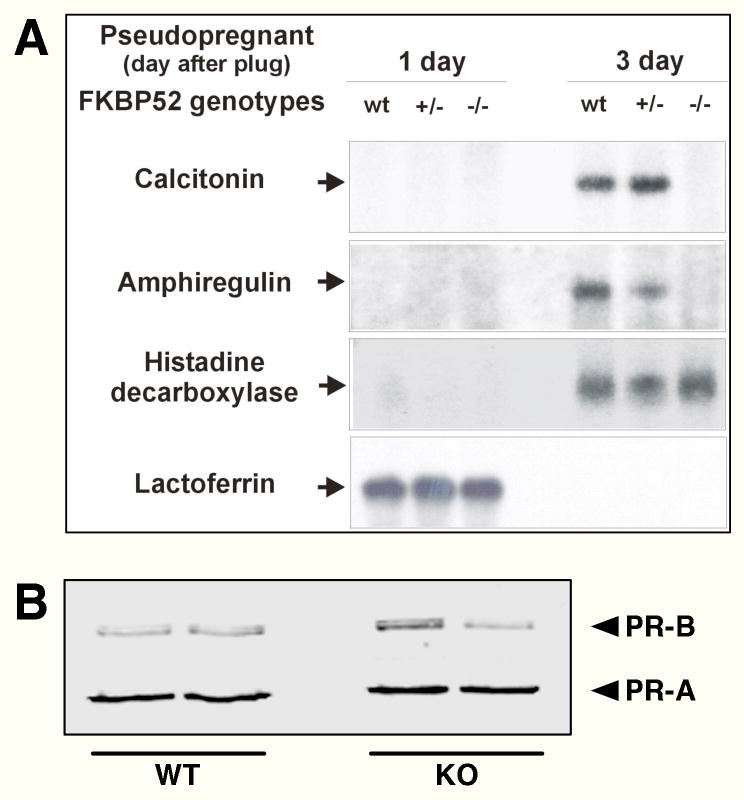
The above results suggest that FKBP52 control of PR action may be both receptor- and tissue-specific. To directly test the receptor-specificity of FKBP52 in the uterus, we asked whether ablation of FKBP52 only blocked PR-A signaling. This was done by measuring expression of uterine genes controlled by PR-A, PR-B and ER (Fig. 6). Calcitonin (Ct) and amphiregulin (Ar) are two PR-A targets that are up-regulated in uterus at implantation (3rd day post-fertilization after plug) (34–36). Histidine decarboxylase (Hdc) is PR-B target that is also up-regulated in uterus at implantation (34, 37). Lactoferrin (Lf) is a known target of estrogen receptor and is expressed in uterus at the pre-implantation stage and quickly down-regulated during implantation (38). As shown in Fig. 6A, the expression patterns of Lf and Hdc were normal in FKBP52-deficient uterine tissues. Only the Ct and Ar genes were affected by FKBP52 deficiency, showing no detectable expression levels in FKBP52-deficient uteri at the implantation stage. Lack of Ct and Ar expression was not due to reduced expression of PR-A, as normal levels of both PR-A and PR-B were detected in the uterus of (−/−) females (Fig. 6B). Thus, FKBP52 appears to be essential for PR-A activity at the uterus, but not for ER, nor its closely related isoform PR-B.
Fig. 6.
Selective Abrogation of PR-A Regulated Genes in Uteri of FKBP52-deficient Females. A, Northern blot analysis of PR-A, PR-B and ER regulated gene expression at pre-implantation (1 day) and post-implantation (3 day) stages in pseudopregnant wild-type and FKBP52-deficient females. Expression of two PR-A regulated genes (calcitonin and amphiregulin) was observed at implantation stage in (+/+) and (+/−) controls, but was absent in FKBP52-deficient uteri. Expression of genes controlled by PR-B (histidine decarboxylase) and ER (lactoferrin) were unaffected in (−/−) uteri. B, Loss of PR-A regulated gene expression in (−/−) uteri is not due to loss of receptor. Western-blot analysis of PR-A and PR-B from wild-type and FKBP52-deficient (KO) uterine lysates. Results from two independent samples are shown.
Preferential Association of FKBP52 with Uterine PR-A Compared to PR-B and No Effect of FKBP52 Loss on PR Hormone-binding Function
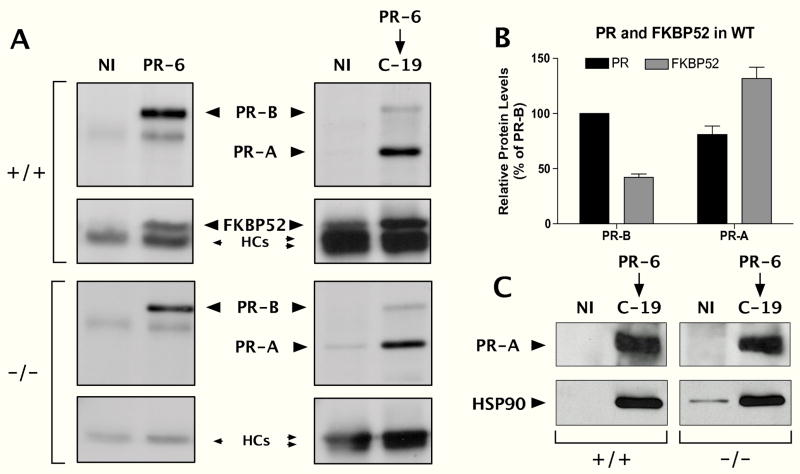
As a further test of the specificity model, we reasoned that selective regulation of PR-A over PR-B by FKBP52 would have to mean preferential association of FKBP52 with PR-A. To test this, we performed sequential co-immunoprecipitation of PR-B followed by PR-A complexes from wild-type and FKBP52-deficient uterine lysates (Fig. 7A). The first immunoprecipitation utilized an antibody specific to PR-B (PR-6), followed by a second immunoprecipitation of the PR-B-cleared uterine lysates with antibody reactive to PR-A/B (C-19). Samples were resolved on a single SDS-PAGE gel, transferred to Immobilon membrane and the membrane was cut at the 66kDa marker. Detection of the PRs (top half of membrane) was done by blotting with the C-19 antibody, making it possible to quantify relative yields of each PR isoform. Detection of FKBP52 (bottom half) was done with the HSP56 antibody. After blotting, the membrane halves were reassembled and exposed to film. This procedure resulted in a selective and approximately equal purification of each PR isoform from both the wild-type and FKBP52-deficient uterine lysates (Fig. 7A & B). Interestingly, the amount of receptor-associated FKBP52 in the wild-type lysates was greater for PR-A than for PR-B. Thus, a preferential interaction of FKBP52 with PR-A appears to occur, as predicted by the Receptor Specificity Model. Although both PR isoforms interacted with Hsp90 in wild-type uterine lysates (data not shown), we tested whether loss of FKBP52 leads to disruption of the PR-A/Hsp90 interaction. This was done by blotting for PR-A-associated Hsp90 in the wild-type and FKBP52-deficient uterine lysates (Fig. 7C). Results showed levels of Hsp90 to be unchanged.
Fig. 7.
Preferential Association of FKBP52 with Uterine PR-A. A, Analysis of FKBP52 content in PR-A and PR-B complexes of the uterus. Uterine lysates from FKBP52 (+/+) and (−/−) females were sequentially immunoadsorbed with antibody against PR-B (PR-6) and antibody against PR-A/B (C-19), followed by Western-blotting with C-19 to detect PR isoforms and HSP56 antibody to detect receptor-bound FKBP52. Nl, non-immune antibody. HC, immunoglobulin heavy chains. Panels for (+/+) and (−/−) co-IPs each represent equal exposures derived from a single autoradiograms. Note that HC bands in the PR-6/C-19 lanes are stronger because counter antibody used is anti-rabbit (to detect C-19), while PR-6 antibody is a mouse monoclonal. B, Quantitation of PR isoforms and receptor-bound FKBP52 from results of Panel A. Densitometric values were normalized as percent of PR-B and represent the mean (+/− SEM) of two experiments. C, Loss of FKBP52 does not alter the PR-A/Hsp90 interaction. PR-A complexes from (+/+) and (−/−) uterine lysates were immunoadsorbed as in Panel A, followed by Western-blotting for PR-A and Hsp90.
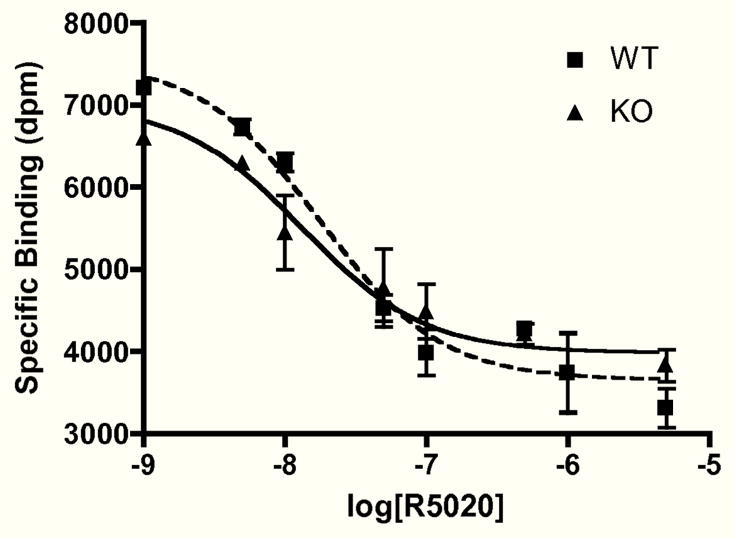
Because of the link between FKBP52 and ability of GR to bind hormone (21, 22), we measured PR hormone-binding function in whole and PR-B-cleared uterine lysates. There were no significant differences between wild-type and knock-out animals with respect to binding sites, dissociation constants, or EC50 values using both [3H]R5020 or [3H]progesterone (see Fig. 8 for a representative result). Thus, the uterine forms of PR-B and PR-A appear to have normal hormone-binding function in the absence of FKBP52.
Fig. 8.
No Alteration of PR-A Hormone-binding Function in FKBP52-deficient Uteri. Uterine lysates from wild-type (WT) and FKBP52-deficient (KO) females were depleted of PR-B by immunoadsorption with PR-6 antibody. Following depletion, a competition hormone-binding assay was performed using 20 nM [3H]R5020 and increasing amounts of unlabeled R5020. Values for specific hormone binding were analyzed by non-linear regression based on one-site competition. EC50 values were not statistically different between WT and KO samples. Analyses of PR-A and PR-B (whole uterine lysates) by saturation hormone binding using [3H]R5020 or [3H]progesterone also showed no differences between genotypes (data not shown).
Analysis of PR and ER Transcriptional Activities in (+/+) and (−/−) Mouse Embryonic Fibroblasts
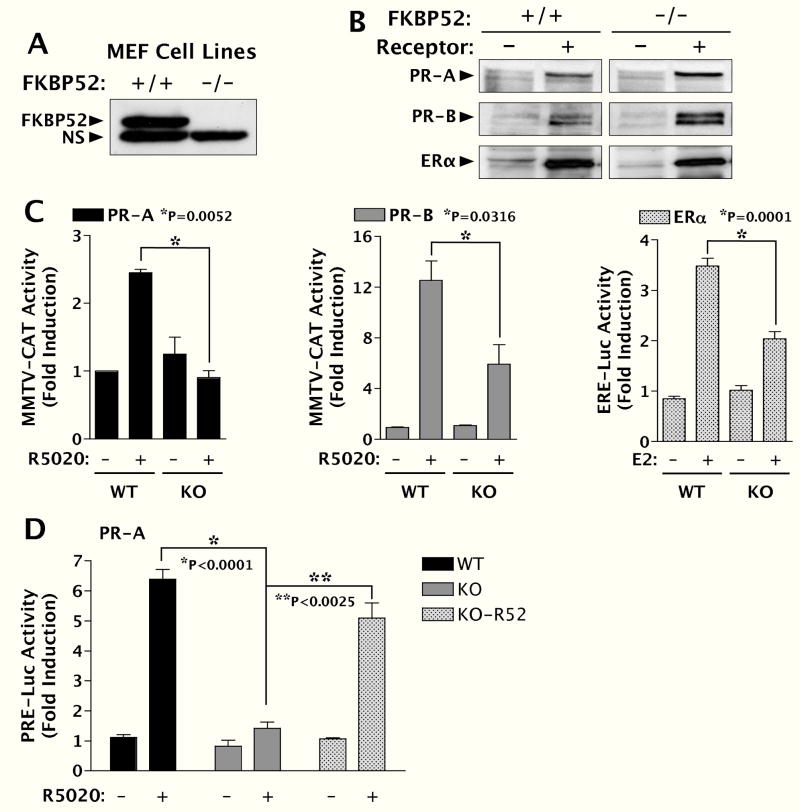
Since loss of FKBP52 had no affect on the hormone-binding function of PR-A, the major role of FKBP52 must be downstream of this function. To better measure the effect of FKBP52 on PR-A transcriptional activity, we established mouse embryonic fibroblast (MEF) cell lines from wild-type and FKBP52-deficient embryos (Fig. 9A). The MEF cells were transfected with expression constructs for mouse PR-A, mouse PR-B and human ERα (Fig. 9B) along with MMTV-CAT, ERE-Luc and PRE-Luc reporter genes. Results in the WT MEF cells show that PR-A activity was weaker at the MMTV promoter than PR-B (Fig. 9C), as previously demonstrated by the Hager laboratory (39). Even so, it can be seen that PR-A activity at MMTV was completely lost in the (−/−) MEF cells. Interestingly, PR-B activity was also affected in the (−/−) MEF cells but not to the same degree, with approximately 40% activity remaining. A similar result was found for ERα, for which approximately 50% activity remained at an ERE-Luc reporter in the (−/−) MEF cells (Fig. 9C). We speculate that partial abrogation of PR-B and ERα, as seen in the MEF cells, may form the basis for partial losses of ovary and mammary gland function seen in vivo. Because PR-A activity at the MMTV promoter was weak, we retested PR-A activity in the MEF cells using a PRE-Luc construct (Fig. 9D). This construct yielded much better activity for PR-A in the WT cells and once again there was a dramatic loss of activity in the KO cells. To eliminate any potential non-specific effect of the KO MEF cells on PR-A activity, we re-expressed FKBP52 in the KO cells and the results show a near-complete rescue of PR-A activity in these cells (Fig. 9D). Taken as a whole, the MEF transcriptional studies suggest that FKBP52 exerts relative, rather than absolute, effects on the activities by the PR isoforms and ER. As elaborated under Discussion, we propose that these results are consistent with the range of reproductive phenotypes seen in the (−/−) females.
Fig. 9.
Analysis of PR-A, PR-B and ERα transcription enhancement activities in FKBP52-deficient mouse embryonic fibroblast (MEF) cells. A, Western blot demonstration of presence (+/+) and absence (−/−) of FKBP52 in MEF cell lines. NS, non-specific. B, MEF cells transfected with mPR-A, mPR-B and ERα expression vectors were assayed for receptor expression by Western-blotting. C, Transcriptional enhancement activities in MEF cells of mPR-A and mPR-B at a MMTV-CAT reporter and ERα at an ERE-luciferase reporter. Values represent the means +/− SEM of four (PR-A and PR-B) or six (ERα) independent transfections. D, Activity of PR-A at a PRE-Luc reporter in WT and KO MEF cells and KO MEF cells re-expressing FKBP52 (KO-R52). Values represent the means +/− SEM of four independent transfections.
DISCUSSION
In recent years, the complexity of steroid receptor heterocomplexes based on TPR protein content has become apparent. This heterogeneity has contributed to two major competing hypotheses. Either all receptors are regulated (perhaps in distinct ways) by all four TPR proteins (FKBP52, FKBP51, Cyp40 and PP5), or some receptors are preferentially regulated by one TPR protein but not another. In this work, we provide evidence for the latter hypothesis through use of mice with targeted ablation of FKBP52. We show that FKBP52 plays an essential but receptor-specific role in female reproductive physiology. In particular, FKBP52 was found to selectively regulate the activity of PR-A in the uterus, while having a lesser role in the activity of PR-B or ERα in this tissue. At both ovary and mammary, loss of FKBP52 had a moderate effect on ovulation rates and ductal side-branching, respectively, suggesting that FKPB52 plays a contributory but not essential role in steroidal responses at these tissues.
We believe that key aspects of our data allow us to make the above conclusion. First, absence of FKBP52 leads to female sterility due to a selective failure of implantation (Fig. 2). Implantation failure was not due to a lack of estrogen stimulation (Fig. 3A). Rather, it resulted from an inability of the uterus to mount a decidualization response to progesterone (Fig. 3B), – a process known to be regulated by PR-A (34). Evidence for selective control of PR-A over PR-B by FKBP52 was provided by the observation that only PR-A regulated genes were affected in the uterus (Fig. 6) and that uterine PR-A receptors showed a preferential ability to interact with FKBP52 (Fig. 7). Lastly, studies in MEF cells showed a near-complete loss of PR-A transcriptional activity at heterologous reporters, while activities of PR-B and ERα were partially affected (Fig. 9). On this last point, one could legitimately wonder why partial loss of PR-B and, especially, ERα transcriptional activity should have no apparent affect on physiology in the uterus. One possibility is that half-transcriptional activity can be phenotypically neutral in the same way as half-gene dosage in heterozygotes is often neutral. For example, our (+/−) females show half dosage for FKBP52 RNA (Fig. 1D) and protein (data not shown), yet demonstrate no alteration in fertility (Table 1). On the other hand, partial loss of PR-B or ERα transcriptional activity may actually provide the basis for the partial responses seen in the mammary and ovary of the FKBP52-deficient female (Fig. 4). To reconcile these possibilities, transcriptional studies must move away from the generic MEF cells and reporter genes to WT and KO cell lines derived from the affected tissues (uterus, ovary, mammary) and the measurement of key endogenous genes. In this way, the actual extent to which FKBP52 controls PR-A, PR-B and ERα activities in each tissue can be determined.
With the seminal work of Lydon et al (30), in which genetic ablation of PR (A and B isoforms) was achieved to yield PRKO mice, the role of PR in reproductive physiology has become increasingly apparent. This work showed PR to be essential to reproductive responses at the uterus, ovary and mammary, as well as sexual behavior. More recent work by Conneely and coworkers has shown PR-A and PR-B to play distinct roles in these responses. For example, PRAKO mice were sterile, primarily due to a complete lack of implantation and a marked reduction in ovulation (34). PRAKO females, however, were normal with respect to hormone-induced ductal side-branching and lobuloalveologenesis at the mammary. In contrast, PRBKO females were fertile but showed greatly diminished mammary gland development in response to progesterone (33). Based on these reports, it appears that our FKBP52 (−/−) females show a strong resemblance to PRAKO animals. Although the FKBP52 (−/−) females have a moderately reduced mammary response to progesterone (Fig. 4B), they do show a complete failure of implantation (Fig. 2) and have uteri which are unable to mount a decidual response to hormone (Fig. 3), as also reported for PRAKO (34). In the PRAKO female ovulation was strongly reduced (but not eliminated), while the FKBP52 (−/−) animals showed a moderate but significant reduction for this parameter. On this point, our results differ in degree, but not, we would argue, with respect to overall physiology. Further evidence for this conclusion can be found in the PR- and ER-regulated genes of the uterus. Calcitonin and amphiregulin are two genes identified by Conneely as being regulated by PR-A (34). Expression of both of these genes was abrogated in FKBP52 (−/−) uteri on day three of pseudopregnancy (Fig. 6A). Conversely, genes controlled by PR-B (histidine decarboxylase) and ERα (lactoferrin) were unaffected (Fig. 6A). Interestingly, our data also support the claim of Conneely that progesterone-mediated repression of ER during the early stages of implantation is mediated by PR-B (34), as loss of lactoferrin expression at day three of pseudopregnancy was observed in the FKBP52 (−/−) uteri. Thus, uterine PR-B in the FKBP52 (−/−) females must be active with respect to this function.
Although our results are consistent with those of Conneely, a variety of reports have proposed PR-A to be the principal isoform responsible for inhibition of ER and other steroid receptors. The McDonnell group, in particular, has shown that ligand-activated PR-A can inhibit the transcriptional activities of ERα, PR-B, GR, AR and MR (40–42), while PR-B has no such activity. Interestingly, this inhibition is promoted by both agonist and antagonist and does not require a functional DNA-binding domain, suggesting that PR-A mediated repression acts by a distinct pathway from that which controls its own transactivation function. Why our results and those of Conneely should differ from the McDonnell laboratory is not clear. One possible explanation is that the inhibitory activity of PR-A is not universal, but tends to occur in cell lines (e.g., mammary-derived MCF cell lines) where PR-A transcriptional activity is not active (40). Conneely has clearly shown that PR-A has potent and selective transcriptional activity in uterine epithelial cells (34). Thus, in these cells the repressive activity may fall to PR-B. An alternative explanation in our system is that loss of FKBP52 only affects the transcriptional function of PR-A, leaving it free to repress ERα via its transrepressive function. We propose that proper resolution of these issues will require a thorough examination of the transactivation and transrepressive functions of each PR isoform in uterine epithelial cell lines derived from FKBP52 (+/+) and (−/−) females.
Our results have uncovered two interesting but unresolved issues concerning the role of FKBP52 in control of steroid receptor action in vivo. First, recent studies suggest that FKBP52 has a positive effect on the ability of glucocorticoid receptor to bind hormone (21, 22). In contrast, the closely-related TPR FKBP51 has the opposite effect of depressing GR hormone-binding ability and its subsequent transactivation function (26, 43, 44). Thus, we were surprised to find that loss of FKBP52 did not affect the ability of PR-A or PR-B to bind hormone (Fig. 8). A simple explanation may be that control of hormone-binding function by FKBP52 applies to some receptors (GR) but not others (PR). Alternatively, this effect may be tissue or cell-type dependent. In support of this, we (data not published) and others (28) have found that AR hormone-binding function is also not affected by FKBP52 loss. Yet, it is also true that the studies showing potentiation of GR hormone-binding function have relied on over-expression of FKBP52. Thus, if FKBP51, but not FKBP52, determines the basal level of this function, then down-regulation or knockout conditions for FKBP52 may have little or no affect. The second issue concerns our observation that FKBP52 loss seems to have a major effect in the uterus but only a moderate effect in the ovary. Since Conneely has shown PR-A to be essential to ovulation rates, our results suggest that FKBP52 plays a less essential role in PR-A activity in this organ. If so, then it is possible that another TPR may be the preferred co-chaperone of PR-A in the ovary. Our Western-blot analysis (Fig. 5) shows that all four TPRs (FKBP52, FKBP51, Cyp40 and PP5) are expressed in the ovary, and that two of these (FKBP51 and Cyp40) exist at higher levels in the ovary compared to the uterus. Thus, it will be interesting to see if genetic ablation of another TPR leads to a more dramatic phenotype at this organ. Indeed, a broader conclusion we can draw from our study is that FKBP52 is not the primary TPR regulating ERα or PR-B in vivo, suggesting that similar studies on the other TPRs may lead to alterations in ERα or PR-B controlled physiology.
Recently, Tranguch et al (29) made mice deficient in FKBP52 and showed that this protein plays an essential role in PR-mediated uterine receptivity to implantation, but they were not able to discriminate between PR-A and PR-B actions. We believe that our work provides an important advancement by showing that FKBP52 is essential to female reproductive physiology by primarily controlling the actions of PR-A in the uterus. Abrogation of PR-A activity at this organ through FKBP52 loss leads to a complete failure of implantation, while leaving other PR-regulated tissues only moderately affected. Thus, FKBP52 is now a potential target for development of non-steroidal birth-control drugs that avoid a myriad of estrogen- and progestin-based side effects. Of broader significance is the fact that other TPR proteins (e.g. FKBP51, Cyp40 and PP5) are known to act as steroid receptor co-chaperones. Because of the receptor- and tissue-selectively uncovered for FKBP52, it is now likely that each TPR will have a distinct profile of receptor- and tissue-specific actions, providing many new opportunities for selective intervention in steroid-based disease and physiology.
MATERIALS AND METHODS
Targeted deletion and generation of FKBP52-deficient mice
A FKBP52 genomic clone was isolated from a mouse 129SvEv genomic BAC library (RPCI-22 129 mouse library from BAC/PAC Resources, Children’s Hospital Oakland). The mouse FKBP52 gene contains ten exons (Figure 1A). Linearized targeting vector (25 ng) was electroporated into ES cells (CCE916 ES cell line), clones were selected in G418 and gancyclovir, DNA from the clones was analyzed by Southern blot, and targeted ES cell lines FK52-A22 and FK52-E14 were expanded and injected into blastocysts. Male chimeras were bred to C57BL/6J or 129SvEv females to generate F1 offspring. Mutant mice generated from both targeted ES cell lines had identical phenotype. Genotypes were determined by either Southern blot or PCR analyses. Primer sequences for FKBP52 genotyping were as follows: neo1-TGCCGCGCTGTTCTCCTCT, neo2-AAGCGGCCATTTTCCACCAT, fkbp52-forward-CTAAGCCATGGGGTGGAGATGTG, fkbp52-reverse-AAAGCTCTTCAGCCGCACTTCATAC. We also used PCR analysis of the sry gene to determine gender of the embryos. Primer sequences for sry genotyping are: forward primer GACTAGACATGTCTTAACATCTGTCC, reverse primer CCTATTGCATGGACTGCAG CTTATG. All animal experiments were carried out using a protocol approved by the Indiana University School of Medicine Institutional Animal Care and Research Advisory Committee.
Morphological and histological analyses
We examined the morphology and histology of multiple tissues and organs from female FKBP52-deficient mice and age-matched, litter-mate controls. Isolated tissue samples were fixed in 10% neutral-buffered formalin, paraffin embedded, sectioned (5 μm), and stained with haematoxylin and eosin.
Assessment of hormonal response
To assess the ovulatory function in FKBP52-deficient females, virgin females (3–4 weeks of age) were super-ovulated by administration of 5 IU of pregnant mare serum gonadotropin (PMSG) (Diosynth, Chicago, IL) and followed by 5 IU of human chorionic gonadotropin (hCG) (Pregnyl, Organon, Inc., West Orange, NJ) after 48 h to induce ovulation. Oocytes were harvested from oviducts 24 h following hCG administration.
To determine estrogen response in mice, wild-type and FKBP52 (−/−) females (8 week old) were subjected to an estrogen regimen 10 days after ovariectomy. Briefly, mice were primed for five days (days 11, 12, 13, 14 and 15) with subcutaneous injections of 100 ng 17β-estradiol in 0.1 ml sesame oil. Six hours after the fifth injection, uteri were dissected and weighed.
The decidualization response was performed as previously described (45). In brief, ovariectomized wild-type and FKBP52-deficient females (8 weeks of age) were administered in three continuous days with subcutaneous injections of 100 ng of 17β-estradiol (Sigma, St. Louis, MO) (dissolved in sesame oil), followed by two days without hormone treatment before another three-day course of hormone administration with progesterone (1 μg/day) and 17β-estradiol (6.7 ng/day). Six h after the third hormone injection, one horn of the uterus was traumatically stimulated by insertion of a burred needle into the uterus horn just proximal to the cervix and longitudinally scratching the entire length of the uterine horn. Another side of horn was not stimulated. Daily injections of progesterone were administered until 6 days post-stimulation. The uteri were isolated and the matched horns were cut to equal lengths and weighed.
Northern blot analysis of uterine genes
Total RNA was extracted from kidney and uteri with Trizol RNA kit (Invitrogen Corporation, Cartsbad, CA). Total RNA (20 μg) was denatured, separated by formaldehyde – agarose gel electrophoresis, transferred to nitrocellulose membrane, and cross-linked by UV irradiation (Spectrolinker, Spectronics Corp., Westerbury, NY ). cDNA fragments for probe (mouse FKBP52, Ct, Ar, Hdc, and Lf) were initially generated via RT-PCR and confirmed by sequence analysis. Standard hybridization and washing procedures were used throughout.
immunoprecipitation of PR-A and PR-B Uterine Heterocomplexes
Several uteri (4) from females of each genotype were pooled prior to tissue homogenization in 50 mM phosphate buffer, 20 mM sodium molybdate, 10% glycerol, pH 7.4 with protease inhibitor cocktail (Sigma, P2714) using a tissue disruptor (Tissue Tearor, Biospec Products, Inc). Lysates were centrifuged at 100,000 × g for 60 min and used fresh, without freezing or storage. The +/+ and −/− lysates were first immunoadsorbed to protein-A Sepharose in the complete absence of antibody in order to clear the lysates of FKBP52 that non-specifically bound the resin. The cleared lysates were then immunoadsorbed to protein-A Sepharose with PR-6 antibody specific to PR-B (Affinity Bioreagents, MA1-411) and corresponding non-immune IgG, as control. The same lysates (supernatants) were then immunoadsorbed a third time with appropriate non-immune IgG control or C-19 antibody recognizing both PR-A and PR-B (Santa Cruz, SC-538) in order to purify the remaining PR-A isoform. Samples were resolved on a single SDS-PAGE gel and Western-blotted using C-19 antibody to detect PR-A and PR-B, HSP56 antibody (Affinity Bioreagents, PA3-020) against FKBP52 or F-8 antibody (Santa Cruz, SC-13119) against Hsp90. It should be noted that use of C-19 antibody for the blotting step made it possible to measure relative yields of PR-A and PR-B, even though two distinct antibodies were used for the purification step. Western blotting was achieved in denaturing SDS gels (3), using a 7–14% acrylamide gradient in order to achieve maximal separation between FKBP52 and antibody heavy chains. Transfer of the samples to Immobilon-P® membranes (Millipore Corp.) and quantitative immuno-blotting were performed as previously described (4, 5), using appropriate 125I-conjugated counter antibodies, followed by autoradiography and densitometric scanning. Images provided in Fig. 7 were derived from a single autoradiogram film each for WT and KO co-IPs, ensuring equal exposure time for measurement PRA, PRB and FKBP52 yields.
PR hormone binding assay
Lysates from wild-type and FKBP52-deficient uteri were prepared as described above. The lysates were used for the binding assay without freezing. Both saturation and competition assays were performed using [3H]P4 or [3H]R5020. In each case, binding was allowed to occur for 20 h on ice. Nonspecific binding was determined in the presence of excess unlabeled hormone. Protein-bound radioactivity was isolated using 1% dextran-coated charcoal in 10 mM HEPES buffer (pH 7.4). Specific binding was normalized for protein content. Results shown in Fig. 8 represent a one-site competition analysis (GraphPad Prism software) using [3H]R5020.
Reporter Gene Assays in Mouse Embryonic Fibroblast Cells
Mouse embryonic fibroblasts (MEF) were isolated from WT and FKBP52 KO E13.5 embryos. Embryonic cells were cultured in DMEM with 15% FBS until confluence. Fibroblasts were the only cells that attached and proliferated. To generate immortalized MEF cells, primary cells were transfected with a vector for SV40-large T antigen. Transformed cells maintain normal MEF morphology with high proliferative activity. Re-expression of FKBP52 in FKBP52-deficient MEF cells was achieved with a pRSV expression method (46) using human FKBP52 cDNA (kind gift of Theo Rein). MEF cells were transiently transfected with expression vectors for mouse PR-A and mouse PR-B (kind gift of Dean Edwards) and human ERα (kind gift of Brian Rowan). MMTV-CAT and PRE-luciferase reporter constructs were used to assay for both PR-A and PR-B activity, while an ERE-luciferase reporter was used for ER. CAT enzyme activity was measured by the method Nordeen et al (6) using [3H]acetyl-CoA as substrate, while luciferase activities were measured using a commercial kit (Promega). All values were normalized for transfection efficiency by co-transfection with a CMV-driven galactosidase reporter.
Acknowledgments
This study was supported in part by National Institute of Health grants DK73402 (W.S., E.S.), HL70259 (W.S.), DK70127 (E.S., W.S.), DK43867 (E.S.), and the Riley Children’s Foundation (W.S.). We thank Dean Edwards for the gift of mouse PR-A and PR-B cDNAs, Theo Rein for the gift of human FKBP52 cDNA and Brian Rowan for the gift of human ERα cDNA.
References
- 1.Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin RJ, Kao HY, Ordentlich P, Evans RM. The transcriptional basis of steroid physiology. Cold Spring Harb Symp Quant Biol. 1998;63:577–85. doi: 10.1101/sqb.1998.63.577. [DOI] [PubMed] [Google Scholar]
- 3.Ing NH, O’Malley BW. The steroid hormone receptor superfamily: molecular mechanisms of action. In: Weintraub BD, editor. Molecular Endocrinology: Basic Concepts and Clinical Correlations. Raven Press, Ltd; New York: 1995. pp. 195–215. [Google Scholar]
- 4.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 6.Renoir JM, Mercier-Bodard C, Hoffmann K, Le BS, Ning YM, Sanchez ER, Handschumacher RE, Baulieu EE. Cyclosporin A potentiates the dexamethasone-induced mouse mammary tumor virus-chloramphenicol acetyltransferase activity in LMCAT cells: a possible role for different heat shock protein-binding immunophilins in glucocorticosteroid receptor-mediated gene expression. Proc Natl Acad Sci U S A. 1995;92:4977–4981. doi: 10.1073/pnas.92.11.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverstein AM, Galigniana MD, Kanelakis KG, Radanyi C, Renoir JM, Pratt WB. Different regions of the immunophilin FKBP52 determine its association with the glucocorticoid receptor, hsp90, and cytoplasmic dynein. J Biol Chem. 1999;274:36980–36986. doi: 10.1074/jbc.274.52.36980. [DOI] [PubMed] [Google Scholar]
- 8.Nakao K, Myers JE, Faber LE. Development of a monoclonal antibody to the rabbit 8.5S uterin progestin receptor. Can J Biochem Cell Biol. 1984;63:33–40. doi: 10.1139/o85-005. [DOI] [PubMed] [Google Scholar]
- 9.Tai PK, Maeda Y, Nakao K, Wakim NG, Duhring JL, Faber LE. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986;25:5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- 10.Lebeau MC, Massol N, Herrick J, Faber LE, Renoir JM, Radanyi C, Baulieu EE. P59, an hsp 90-binding protein. Cloning and sequencing of its cDNA and preparation of a peptide-directed polyclonal antibody. J Biol Chem. 1992;267:4281–4284. [PubMed] [Google Scholar]
- 11.Goebl M, Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991;16:173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- 12.Sikorski RS, Boguski MS, Goebl M, Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990;60:307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- 13.Ratajczak T, Carrello A, Mark PJ, Warner BJ, Simpson RJ, Moritz RL, House AK. The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59) J Biol Chem. 1993;268:13187–13192. [PubMed] [Google Scholar]
- 14.Radanyi C, Chambraud B, Baulieu EE. The ability of the immunophilin FKBP59-HBI to interact with the 90-kDa heat shock protein is encoded by its tetratricopeptide repeat domain. Proc Natl Acad Sci U S A. 1994;91:11197–11201. doi: 10.1073/pnas.91.23.11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callebaut I, Renoir JM, Lebeau MC, Massol N, Burny A, Baulieu EE, Mornon JP. An immunophilin that binds M(r) 90,000 heat shock protein: main structural features of a mammalian p59 protein. Proc Natl Acad Sci U S A. 1992;89:6270–6274. doi: 10.1073/pnas.89.14.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peattie DA, Harding MW, Fleming MA, DeCenzo MT, Lippke JA, Livingston DJ, Benasutti M. Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 1992;89:10974–10978. doi: 10.1073/pnas.89.22.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tai PK, Albers MW, Chang H, Faber LE, Schreiber SL. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992;256:1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- 18.Yem AW, Tomasselli AG, Heinrikson RL, Zurcher-Neely H, Ruff VA, Johnson RA, Deibel MR., Jr The Hsp56 component of steroid receptor complexes binds to immobilized FK506 and shows homology to FKBP-12 and FKBP-13. J Biol Chem. 1992;267:2868–2871. [PubMed] [Google Scholar]
- 19.Kieffer LJ, Seng TW, Li W, Osterman DG, Handschumacher RE, Bayney RM. Cyclophilin-40, a protein with homology to the P59 component of the steroid receptor complex. Cloning of the cDNA and further characterization. J Biol Chem. 1993;268:12303–12310. [PubMed] [Google Scholar]
- 20.Veldscholte J, Berrevoets CA, Zegers ND, van der Kwast TH, Grootegoed JA, Mulder E. Hormone-induced dissociation of the androgen receptor-heat-shock protein complex: use of a new monoclonal antibody to distinguish transformed from nontransformed receptors. Biochemistry. 1992;31:7422–7430. doi: 10.1021/bi00147a029. [DOI] [PubMed] [Google Scholar]
- 21.Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith DF. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003;22:1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies TH, Ning YM, Sanchez ER. Differential Control of Glucocorticoid Receptor Hormone-Binding Function by Tetratricopeptide Repeat (TPR) Proteins and the Immunosuppressive Ligand FK506. Biochemistry. 2005;44:2030–2038. doi: 10.1021/bi048503v. [DOI] [PubMed] [Google Scholar]
- 23.Czar MJ, Owens-Grillo JK, Yem AW, Leach KL, Deibel MR, Jr, Welsh MJ, Pratt WB. The hsp56 immunophilin component of untransformed steroid receptor complexes is localized both to microtubules in the cytoplasm and to the same nonrandom regions within the nucleus as the steroid receptor. Mol Endocrinol. 1994;8:1731–1741. doi: 10.1210/mend.8.12.7708060. [DOI] [PubMed] [Google Scholar]
- 24.Perrot-Applanat M, Cibert C, Geraud G, Renoir JM, Baulieu EE. The 59 kDa FK506-binding protein, a 90 kDa heat shock protein binding immunophilin (FKBP59-HBI), is associated with the nucleus, the cytoskeleton and mitotic apparatus. J Cell Sci. 1995;108:2037–2051. doi: 10.1242/jcs.108.5.2037. [DOI] [PubMed] [Google Scholar]
- 25.Galigniana MD, Harrell JM, Murphy PJ, Chinkers M, Radanyi C, Renoir JM, Zhang M, Pratt WB. Binding of hsp90-associated immunophilins to cytoplasmic dynein: direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain. Biochemistry. 2002;41:13602–13610. doi: 10.1021/bi020399z. [DOI] [PubMed] [Google Scholar]
- 26.Wochnik GM, Ruegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 27.Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- 28.Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19:1654–1666. doi: 10.1210/me.2005-0071. [DOI] [PubMed] [Google Scholar]
- 29.Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 2005;102:14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CAJ, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 31.Lydon JP, Ge G, Kittrell FS, Medina D, O’Malley BW. Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res. 1999;59:4276–4284. [PubMed] [Google Scholar]
- 32.Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128:139–146. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- 33.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 35.Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol. 1995;9:691–705. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- 36.Zhu LJ, Cullinan-Bove K, Polihronis M, Bagchi MK, Bagchi IC. Calcitonin is a progesterone-regulated marker that forecasts the receptive state of endometrium during implantation. Endocrinology. 1998;139:3923–3934. doi: 10.1210/endo.139.9.6178. [DOI] [PubMed] [Google Scholar]
- 37.Paria BC, Das N, Das SK, Zhao X, Dileepan KN, Dey SK. Histidine decarboxylase gene in the mouse uterus is regulated by progesterone and correlates with uterine differentiation for blastocyst implantation. Endocrinology. 1998;139:3958–3966. doi: 10.1210/endo.139.9.6173. [DOI] [PubMed] [Google Scholar]
- 38.McMaster MT, Teng CT, Dey SK, Andrews GK. Lactoferrin in the mouse uterus: analyses of the preimplantation period and regulation by ovarian steroids. Mol Endocrinol. 1992;6:101–111. doi: 10.1210/mend.6.1.1738363. [DOI] [PubMed] [Google Scholar]
- 39.Lim CS, Baumann CT, Htun H, Xian W, Irie M, Smith CL, Hager GL. Differential localization and activity of the A- and B-forms of the human progesterone receptor using green fluorescent protein chimeras. Mol Endocrinol. 1999;13:366–375. doi: 10.1210/mend.13.3.0247. [DOI] [PubMed] [Google Scholar]
- 40.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter- specific represser of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 41.Wen DX, Xu YF, Mais DE, Goldman ME, McDonnell DP. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol. 1994;14:8356–8364. doi: 10.1128/mcb.14.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20:3102–3115. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84:663–669. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- 44.Denny WB, Prapapanich V, Smith DF, Scammell JG. Structure-function analysis of squirrel monkey FKBP51, a potent inhibitor of glucocorticoid receptor activity. Endocrinology. 2005;146:3194–3201. doi: 10.1210/en.2005-0027. [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O’Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 46.Zhao W, Zhong L, Wu J, Chen L, Qing K, Weigel-Kelley KA, Larsen SH, Shou W, Warrington KH. Srivastava A role of cellular FKBP52 protein in intracellular trafficking of recombinant adeno-associated virus 2 vectors. Virology. 2006 doi: 10.1016/j.virol.2006.04.042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]