Abstract
N-methyl-D-aspartate glutamate receptors (NMDAR) are a key route for Ca2+ influx into neurons important to both activity-dependent synaptic plasticity and, when uncontrolled, triggering events that cause neuronal degeneration and death. Among regulatory binding sites on the NMDAR complex is a glycine binding site, distinct from the glutamate binding site, which must be co-activated for NMDAR channel opening. We developed a novel glycine site partial agonist, GLYX-13, which is both nootropic and neuroprotective in vivo. Here, we assessed the effects of GLYX-13 on long-term synaptic plasticity and NMDAR transmission at Schaffer collateral-CA1 synapses in hippocampal slices in vitro. GLYX-13 simultaneously enhanced the magnitude of long-term potentiation (LTP) of synaptic transmission, while reducing long-term depression (LTD). GLYX-13 reduced NMDA receptor-mediated synaptic currents in CA1 pyramidal neurons evoked by low-frequency Schaffer collateral stimulation, but enhanced NMDAR currents during high-frequency bursts of activity, and these actions were occluded by a saturating concentration of the glycine site agonist D-serine. Direct two-photon imaging of Schaffer collateral burst-evoked increases in [Ca2+] in individual dendritic spines revealed that GLYX-13 selectively enhanced burst-induced NMDAR-dependent spine Ca2+ influx. Examining the rate of MK-801 block of synaptic versus extrasynaptic NMDAR-gated channels revealed that GLYX-13 selectively enhanced activation of burst-driven extrasynaptic NMDARs, with an action that was blocked by the NR2B-selective NMDAR antagonist ifenprodil. Our data suggest that GLYX-13 may have unique therapeutic potential as a learning and memory enhancer because of its ability to simultaneously enhance LTP and suppress LTD.
Keywords: glutamate receptor, learning, memory, N-methyl-D-aspartate receptor, partial agonist, glycine, synaptic plasticity
1. Introduction
N-methyl-D-aspartate receptors (NMDAR) have well established roles in synaptic plasticity, causing the induction of some forms of both LTP and LTD of synaptic strength (Collingridge et al., 1983; Dudek and Bear, 1992; Huang et al., 2005; Stanton et al., 2003, Stanton et al.2005). This receptor-ionophore complex is functionally involved in regulating glutamate-induced excitotoxic neuronal cell death, attenuating neuropathic pain in rat models measuring mechanical allodynia (Dickenson and Aydar, 1991; Laird et al., 1996), promoting the acquisition and extinction of conditioned fear (Miserendino et al., 1990; Falls et al., 1992), maintaining the epileptic state after kindling (Yeh et al., 1989), reducing the negative symptoms of schizophrenics (Heresco-Levy et al., 2002; Evins et al., 2002), and reducing morphine-induced tolerance (Quartaroli et al., 2001).
Although the stoichiometry of native NMDARs is still uncertain, recombinant NMDARs appear to consist of at least one NR1 and one NR2 subunit, and most evidence now suggests that NMDARs assemble as tetramers containing 2 NR1 and 2 NR2 subunits (whenever the NR3 subunit is not expressed; see Prybylowski and Wenthold, 2004; Paoletti and Neyton, 2007). There are multiple forms of both NR2 (A-D) and NR3 (A and B) subunits, and at least 8 splice variants of NR1. Moreover, NMDAR expression is developmentally regulated and differentially distributed in different brain regions. NMDARs are part of a family of ionotropic glutamate receptors that include kainate and (AMPA) receptors. Kainate and AMPA receptors are each ligand-gated ion channels that, upon activation, gate the flux of K+ and Na+ ions (though some AMPA receptor subunits confer significant Ca2+ permeability; Hollmann et al., 1991). In contrast, the NMDA receptor-ion channel complex opens a channel with significant Ca2+ permeability.
The NMDA receptor-ionophore complex is unique among glutamate receptors in requiring binding of both glutamate and glycine for activation. While glutamate binding during synaptic transmission causes channel opening, tonic binding of endogenous agonists of the glycine site is also necessary for glutamate-driven opening to occur (Forsythe et al., 1988; Kleckner and Dingledine, 1988). Moreover, there are various modulatory sites on the complex that can modulate function, including a Mg2+ site located within the channel that causes the voltage-dependence of NMDARs, and proton sensor, polyamine and Zn2+ sites found on various subunits (Mayer et al., 1992).
While there are many glutamate receptors found in the central nervous system, the strychnine-insensitive glycine site is unique to the NMDA receptor. This, together with the number of clinically relevant functions that NMDA receptors play, have made the glycine site an important target for drug development (see Wood, 2005 for review). For example, the glycine site partial agonist, D-cycloserine (DCS), has been shown to have cognitive-enhancing properties in vivo (Hood et al., 1989; Monaghan et al., 1988; Flood et al.,1992; Schuster and Schmidt, 1992; Thompson et al., 1992) as has the glycine prodrug, milacemide (Handelmann et al., 1989; Quartermain et al., 1991; Schwartz et al.,1991, Schwartz et al 1992; Finkelstein et al., 1994). Both of these compounds, however, appear to result in desensitization with chronic administration (Herting, 1991; Quartermainet al., 1994). Recently, Tuominen et al. (2005) found that glycine and D-serine, but not the partial agonist DCS, are effective at reducing some of the negative symptoms of schizophrenia when used to augment antipsychotic therapeutics.
Glyxins are a new family of glycine-site-specific, N-methyl-D-aspartate receptor modulators. They were generated from an amino acid sequence obtained from a hypervariable region of the light chain of a monoclonal antibody (MAb) with NMDAR-modulating properties (Stanton et al., 1987; Haring et al., 1991). One of the Glyxins, GLYX-13, is an amidated tetrapeptide, threonine-proline-proline-threonine, that readily crosses the blood-brain barrier. Pharmacological studies using rat hippocampal membrane preparations and monitoring NMDAR channel activation with the radiolabeled open channel blocker MK-801 suggest that GLYX-13 acts as a partial agonist at the glycine site of the NMDAR. Electrophysiological studies using xenopus oocyte preparations expressing murine NMDARs further support this observation (Moskal et al., 2005). GLYX-13 has also been found to enhance learning when injected into rats subjected to a hippocampus-dependent trace eyeblink paradigm (Moskal et al., 2005).
To characterize the actions of GLYX-13 on NMDAR modulation of long-term synaptic plasticity, we report here on studies measuring the effects of GLYX-13 on LTP and LTD of synaptic transmission at Schaffer collateral-CA1 synapses in hippocampal slices in vitro.
2. Methods
All experiments were conducted under an approved protocol from the Animal Care and Use Committee of New York Medical College, in compliance with National Institutes of Health guidelines.
2.1. Hippocampal slice preparation
Sprague-Dawley rats (12–18 days old; Taconic Farms) were deeply anesthetized with diethyl ether and decapitated. The brain was removed rapidly, submerged in ice-cold artificial cerebrospinal fluid (ACSF, 2–4°C), which contained (in mM): 124 NaCl, 4 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose; at pH 7.4, gassed continuously with 95% O2/5% CO2. The brain was hemisected, the frontal lobes cut off, and individual hemispheres glued using cyanoacrylate adhesive onto a stage immersed in ice-cold ACSF gassed continuously with 95% O2/5% CO2 during slicing. 300 µm thick coronal slices were cut using a Vibratome (DSK DTK-1000), and transferred to an interface holding chamber for incubation at room temperature for a minimum of one hr before commencing recording.
2.2. Extracellular recordings
Slices were transferred to an interface recording chamber and continuously perfused at 3 ml/min with oxygenated ACSF at 32 ± 0.5°C. Low resistance recording electrodes were made from thin-walled borosilicate glass (1–2 MΩ after filling with ACSF) and inserted into the apical dendritic region of the Schaffer collateral termination field in stratum radiatum of the CA1 region to record field excitatory postsynaptic potentials (fEPSPs). A bipolar stainless steel stimulating electrode (FHC Co.) was placed on Schaffer collateral-commissural fibers in CA3 stratum radiatum, and constant current stimulus intensity adjusted to evoke approximately half-maximal fEPSPs once each 30 sec (50–100 pA; 100 µs duration). fEPSP slope was measured by linear interpolation from 20–80% of maximum negative deflection, and slopes confirmed to be stable to within ± 10% for at least 10 min before commencing an experiment. Signals were recorded using a Multiclamp 700B amplifier and digitized with a Digidata 1322 (Axon Instruments, Foster City, CA). Data were analyzed using pClamp software (version 9, Axon Instruments) on an IBM-compatible personal computer.
2.3. Intracellular recordings
Patch pipettes were pulled from borosilicate glass (1B150F-4, World Precision Instruments) using a flaming/brown micropipette puller (P-97, Sutter Instruments). The composition of the patch pipette solution for NMDA current recordings was (in mM): 135 mM CsMeSO3 , 8 mM NaCl, 10 HEPES,2 Mg-ATP, 0.3 Na-GTP, 0.5 EGTA, 1 QX-314. The patch pipette solution pH was adjusted to 7.25 with CsOH, and had an osmolarity of 280 ± 10 mOsm. When filled with this solution, patch pipettes had tip resistances of 5–6 MΩ.
Whole cell patch clamp recordings were obtained from CA1 pyramidal neurons in slices in a fully submerged recording chamber at room temperature, and 95% O2/5% CO2 was passed over the perfusate to ensure an oxygenated local environment. The submerged recording chamber was mounted on a Zeiss Axioskop 2 FS upright microscope equipped with infrared differential interference contrast (DIC) optics. Pyramidal neurons of the hippocampal CA1 region were visualized with a 63x water immersion lens, and patched in the voltage-clamp configuration. Excitatory postsynaptic currents (EPSCs) were recorded using a MultiClamp 700B (Axon Instruments, Foster City, CA), with the low-pass filter setting at 1–3 kHz, series resistance was compensated in the voltage-clamp mode, and patched cells whose series resistance changed by more than 10% were rejected. Data were acquired with a 16-bit D/A interface (Digidata 1322A, Axon Instruments) stored on a PC-compatible computer and analyzed using PCLAMP software (v9, Axon Instruments).
2.4. Calcium Imaging
A customized two-photon laser-scanning Olympus BX61WI microscope with a 60x/1.1 nA objective was used to detect Ca2+ signals. A Mai/Tai laser (Solid-State Laser, Mountain View, CA) tuned to 820 nm was used for excitation, and image acquisition was controlled by Olympus Fluoview FV300 software (Olympus America, Melville, NY). In the transfluorescence pathway, a 565 nm dichroic mirror was used to separate green and red fluorescence, passed through HQ525/50 and HQ605/50 emission filters, respectively, to eliminate transmitted or reflected excitation light (Chroma Technology, Rockingham, VT), and detected simultaneously by two photomultiplier tubes. Neurons were loaded with dyes through the patch pipette for 20 min before commencing image acquisition. Alexa Fluor-594 was used to outline neuronal dendritic structure, and Calcium Green to detect [Ca2+] changes. To measure [Ca2+] dynamics, fluorescence was collected by scanning at 4–5 Hz in a surface-scanning mode (XYT), or 1 kHz in XT mode, and averaged from specified structures to obtain F(t). Baseline fluorescence (F0) was the average of four images during control, ΔF/F was calculated as (ΔF/F) (t) = (F(t) − F0)/F0., and images were stored on a digitized data recorder (VR-10A/B, Introtech, New York, NY). No EGTA was added to the internal solution for [Ca2+] imaging.
2.5. Chemicals
All external and patch pipette solutions were made with deionized distilled water (resistance > 18 MΩ cm−2; Milli-Q system). The chemicals for making extra- and intracellular solutions were purchased from Sigma (St Louis, MO) and Fluka (New York, NY). Neurotransmitter receptor antagonists were purchased from Tocris (Ellisville, MO). Alexa Fluor-594 and Calcium Green were purchased from Molecular Probes (Eugene, OR).
2.6. Data Analysis
Electrophysiological data were analyzed initially with Clampfit (v9) (Axon Instruments, CA), and [Ca2+] image data evaluated using Fluoview FV300 (Olympus America, Melville, NY). Analyzed data were further processed and presented with Origin 6.1 (Microcal Software, Northampton, MA) and CorelDraw 10.0 (Corel, Ottawa, Ontario, Canada) programs. Statistical analysis were performed with SPSS (v11). Statistical data are presented as mean ± SEM if not indicated otherwise.
Results
3.1. GLYX-13 simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses
GLYX-13, a tetrapeptide (TPPT-amide) that enhances glutamate-mediated NMDA channel opening, also facilitates learning in rats of a hippocampus-dependent trace eyeblink conditioning paradigm (Moskal et al. 2005). Since LTP and LTD are thought to play important roles in learning and memory (Zhuo and Hawkins 1995; Riedel and Reymann 1996;Akhondzadeh 1999), we tested whether GLYX-13 affects the induction of LTP and LTD at Schaffer collateral-CA1 synapses. The occurrence of LTP and LTD at CA1 Schaffer collateral synapses was investigated in slices superfused with ACSF containing 10 µM bicuculline, and a cut was made between the hippocampal CA1 and CA3 regions to prevent propagation of seizure activity. LTP of fEPSPs was elicited by high frequency stimulation (HFS; three 100 Hz trains of 500 ms duration each, separated by 60 s intervals).
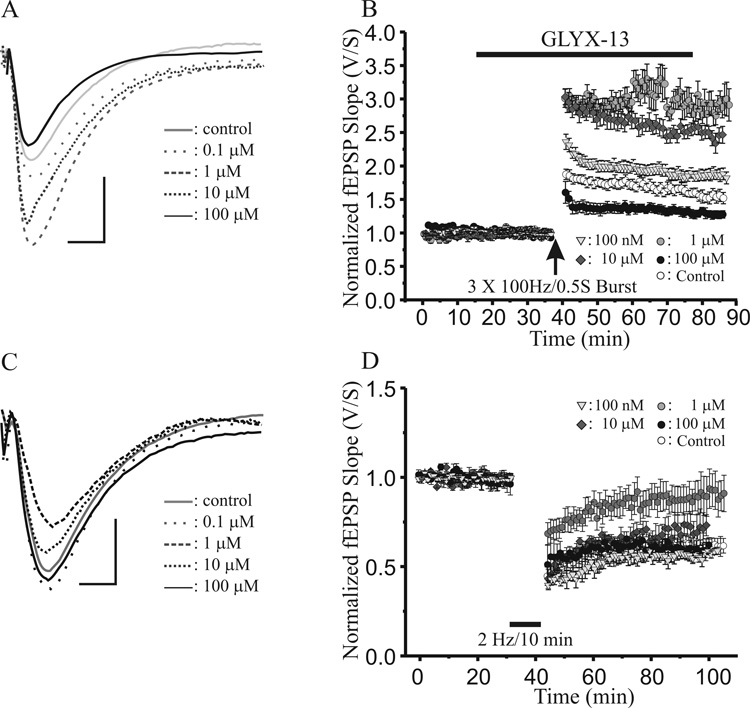
Figure 1A and B illustrate the dose-response relationship for the effects of GLYX-13 on the magnitude of LTP induced by HFS at Schaffer collateral-CA1 synapses. Compared to pre-tetanus baseline slopes, mean fEPSP slope increased to 190 ± 13% (n=15) 35 min after HFS in control, untreated slices (Fig 1B, open circles). Bath application of 100 nM (Fig 1B, grey triangles), 1 µM (Fig 1B, grey circles) or 10µM (Fig 1B, grey diamonds) GLYX-13 during HFS significantly enhanced the magnitude of LTP, with 100 nM GLYX-13 producing a 195 ± 9% increase in fEPSP slope (n=8; P<0.05, Student’s t-test compared to control LTP), 1µM GLYX-13 further enhancing LTP to 275 ± 24% of pre-tetanus baseline (n=8; P<0.05, Student’s t-test compared to control LTP), and 10 µM GLYX-13 resulting in 252 ± 11% LTP (n=6; P<0.05, Student’s t-test compared to control LTP). In contrast, increasing the concentration of GLYX-13 to 100µM converted the effect of GLYX-13 on LTP to a significant reduction in magnitude to 126 ± 7% of pre-tetanus baselines (Fig 1B, filled circles, n=8; P<0.05, Student’s t-test compared to control LTP).
Fig. 1.
0.1–10 µM GLYX-13 enhances the magnitude of long-term potentiation (LTP), while 1–10 µM GLYX-13 reduces long-term depression (LTD) of synaptic transmission at Schaffer collateral-CA1 synapses. A: Representative excitatory postsynaptic potentials (EPSP) after induction of control LTP (solid grey), compared to LTP in GLYX-13 at the indicated concentrations. (Calibration bar for A and C 0.5 mV/10 msec) B: Time course of experiments comparing LTP induced by a high frequency stimulus train (3×100Hz/500ms; arrow) at Schaffer collateral-CA1 synapses in control, untreated slices (open circles; n=15), compared to slices pretreated with 100 nM (triangle; n=8), 1 µM (grey circles; n=8), 10 µM (diamonds; n=6), or 100 µM (filled circles; n=8) GLYX-13 (solid bar). (Each point mean ± SEM of normalized field e.p.s.p. slope of n slices.) C: Representative excitatory postsynaptic potentials (EPSP) after induction of control LTP (solid grey), compared to LTP in GLYX-13 at the indicated concentrations. D: Time course of LTD induced by a low frequency stimulus train (2Hz/10min; solid bar) at Schaffer collateral-CA1 synapses in slices pre-treated with 100 nM (triangles; n=8), 1 µM (grey circles; n=7), 10µM (diamonds; n=6), or 100 µM (filled circles; n=6) GLYX-13 (solid bar), compared to control, untreated slices (open circles; n=16). (Each point mean ± SEM of normalized field e.p.s.p. slope of n slices.)
We next investigated the effects of the same concentrations of GLYX-13 on LTD induced by low frequency stimulation (LFS; 2 Hz/10 min). LFS (Fig 1D, solid bar) elicited LTD of Schaffer collateral synaptic transmission in field CA1, with mean fEPSP slope reduced to 58 ± 16% of pre-LFS baseline in control slices 30 min post-LFS (n=16; open circles). Perfusing slices with 100 nM GLYX-13 for 30 min prior to applying LFS (Fig 1D, grey triangles) did not significantly alter the magnitude of LTD compared to untreated control slices (n=8; P>0.20,Student’s t-test compared to LTD in control slices). An order of magnitude higher concentration of 1µM GLYX-13 markedly reduced the magnitude of LTD when perfused during LFS (Fig 1D, grey circles, n=8; mean fEPSP slope = 91.7 ± 6.1%, P<0.05, Student’s t-test compared to LTD in control slices). Further increasing the concentration of GLYX-13 to 10µM or 100µM no longer either significantly reduced or enhanced Schaffer collateral-CA1 LTD (Fig 1D). Thus, at low concentrations, GLYX-13 simultaneously acts as a promoter of the induction of LTP and a suppressor of LTD, while at high concentrations it becomes an suppressor of LTP and loses its modulation of LTD.
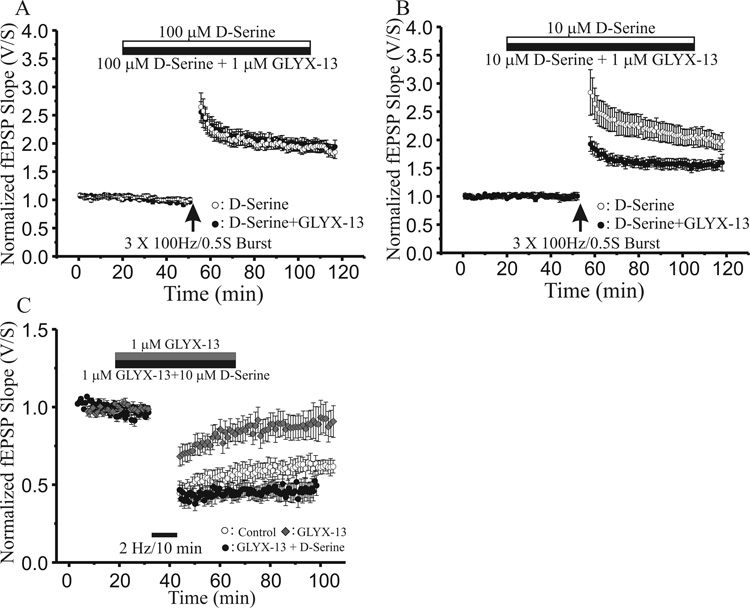
Since the enhancement of [3H]MK-801 binding to hippocampal membranes by GLYX-13 is competitively blocked by the glycine site antagonist 7-chlorokynurenic acid (Moskal et al.2001), we tested whether the effect of GLYX-13 on LTD could also be prevented by maximally activating NMDA glycine binding sites with the potent glycine site agonist D-serine (10 µM; Johnson and Ascher 1987; Kemp and Leeson 1993; Matsui et al., 1995). As shown in Figure 2A, adding 100 µM D-serine along with 1 µM GLYX-13 (filled circles; n=7) completely occluded the enhancement of LTP produced by GLYX-13 alone (open circles; n=7). Combining a ten-fold lower concentration of D-serine (10 µM) with 1 µM GLYX-13 (Fig 2B, filled circles; n=8) produced a partial occlusion of LTP compared to D-serine alone (Fig 2B, open circles; n=8), consistent with GLYX-13 being a partial agonist which can compete with and antagonize the actions of the full agonist D-serine. In contrast, the same 10 µM concentration of D-serine did fully occlude the reduction of LTD produced by 1 µM GLYX-13 (Fig 2C, filled circles; n=7). Taken together, these results indicate that both the enhancement of LTP and suppression of LTD produced by GLYX-13 are due to binding at the NMDA receptor glycine regulatory site, though the different affinities of D-serine in competing away these effects also suggests that the binding sites responsible for the two actions are different.
Fig. 2.
The NMDAR glycine site full agonist D-serine occludes the effects of GLYX-13 on both LTP and LTD. A: Time course of experiments comparing LTP induced by a high frequency stimulus train (3×100Hz/500ms; arrow) at Schaffer collateral-CA1 synapses in slices in presence of 100 µM D-serine alone (open circles; n=7), versus slices where 100 µM D-serine was coapplied with 1 µM GLYX-13 (filled circles; n=7). (Each point mean ± SEM of normalized field e.p.s.p. slope of n slices.) B: Time course of experiments comparing LTP induced by a high frequency stimulus train (3×100Hz/500ms; arrow) at Schaffer collateral-CA1 synapses in slices in presence of 10 µM D-serine alone (open circles; n=8), versus slices where 10 µM D-serine was co-applied with 1 µM GLYX-13 (filled circles; n=8). C: Time course of experiments comparing LTD induced by a low frequency stimulus train (2Hz/10min; solid bar) at Schaffer collateral-CA1 synapses in control slices (open circles; n=6), compared to slices bathed in 1 µM GLYX-13 alone (diamonds; n=7) or 1 µM GLYX-13 plus 10 µM D-serine (filled circles; n=7).
3.2. GLYX-13 exhibits concentration-dependent bidirectional modulation of NMDA currents in hippocampal CA1 pyramidal neurons
Activation of NMDA receptors is necessary for the induction of some forms of LTP and LTD (Kullmann, Erdemli et al. 1996; Stanton 1996; Kullmann, Asztely et al. 2000). Since GLYX-13 is a glycine site partial agonist, and has opposing effects on LTP and LTD, we hypothesized that this might be explained by opposing actions on the NMDA receptor-gated currents produced by the different stimulus patterns that elicit LTD versus LTP. To test this hypothesis, we obtained whole cell recordings from CA1 pyramidal neurons voltage clamped at −60mV, in slices perfused with ACSF containing 0 mM [Mg2+] and 3 mM [Ca2+], plus 10 µM bicuculline and 20 µM CNQX to pharmacologically isolate NMDAR-dependent EPSCs. EPSCs were elicited by stimulating Schaffer collateral fibers with single electrical pulses (80 µs duration) once every 30 s. NMDAR EPSCs were characterized by long rise and decay times, and were fully blocked by bath application of the NMDAR-specific antagonist D–2-amino-5-phosphonopentanoic acid (D-AP5; 50µM). Peak NMDA current ranged from 55.2 ± 16.3 to 106.8 ± 27.2 pA, with a mean rise time (10–90%) from 12.9 ± 1.2 ms to 27.5 ± 1.8 ms (n=15).
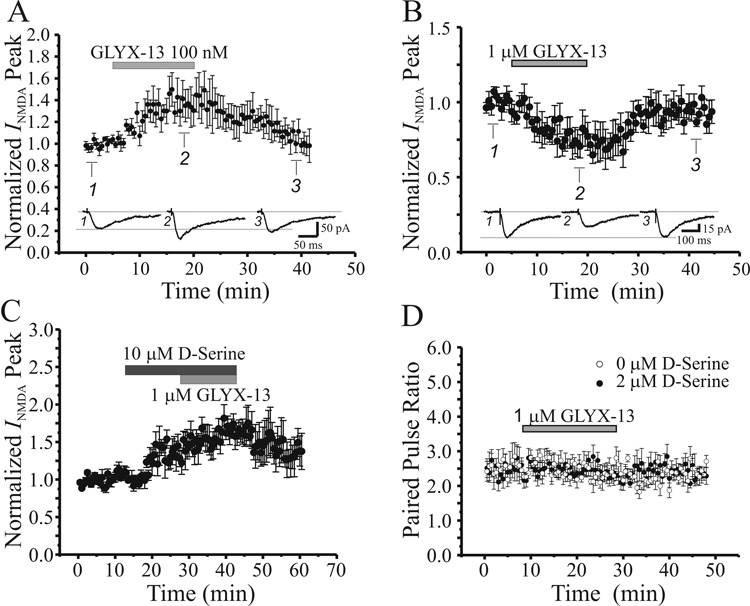
After isolating stable baseline NMDAR EPSCs, we tested the effect of GLYX-13 on these currents. As illustrated in Figure 3A, perfusing slices with 100 nM GLYX-13 for 15 min markedly and reversibly enhanced peak NMDAR currents, on average by 43% from 55.4 ± 4.8 to 79.4 ± 5.7 pA (n=8, P<0.05, paired t-test). In contrast, a 10-fold higher concentration of GLYX-13 (1 µM) for 15 min reduced peak NMDAR currents, on average by 28% from 45.3 ± 5.2 to 32.3 ± 5.2 pA (Fig 3B, n=8, P<0.05, paired t-test), an effect that also reversed upon washout of peptide. Prolonged exposure to 1 µM GLYX-13 for 40 min did not further decrease NMDAR currents, and 50 µM D-AP5 totally blocked these reduced NMDAR currents, confirming their origin (n=2, data not shown).
Fig. 3.
A low concentration of GLYX-13 enhances, and a 10-fold higher concentration reduces, postsynaptic NMDA receptor-mediated Schaffer collateral-evoked excitatory postsynaptic currents (EPSCs) by binding a D-serine sensitive site, without altering presynaptic transmission. A: Time course of the effect of GLYX-13 (100 nM; grey bar) on the NMDA component of Schaffer collateral-evoked EPSCs in CA1 pyramidal neurons (n=8). Each point is the mean ± SEM of EPSC peak amplitude of n cells. Insets: Signal-averaged sample EPSCs recorded at the times indicated. B: Time course of the effect of GLYX-13 (1 µM; grey bar) on the NMDA component of Schaffer collateral-evoked EPSCs in CA1 pyramidal neurons (n=8). Each point is the mean ± SEM of EPSC peak amplitude of n cells. Insets: Signal-averaged sample EPSCs recorded at the times indicated. C: Time course of the effect of GLYX-13 (1 µM; light grey bar) in the presence of D-serine (10 µM; dark grey bar) on the NMDA component of Schaffer collateral-evoked EPSCs in CA1 pyramidal neurons (n=8). Each point is the mean ± SEM of EPSC peak amplitude of n cells. D: Time course of the lack of effect of GLYX-13 (1 µM; grey bar) on paired-pulse ratio in normal ACSF (open circles; n=8) and in the presence of D-serine (2 µM; filled circles; n=7). Each point is the mean ± SEM of EPSC peak amplitude of n cells.
To test directly whether the inhibition of NMDAR EPSCs by GLYX-13 was likely to be due to binding to the glycine site on the NMDAR, we determined whether saturation of this site with the full glycine site agonist D-serine occludes this effect. As illustrated in figure 3C, application of 10 µM D-Serine, which itself enhanced NMDAR EPSCs by fully stimulating the glycine site, also occluded the GLYX-13 mediated inhibition, consistent with the peptide acting s a competitive antagonist at this glycine modulatory site.
Paired-pulse facilitation (PPF) is a phenomenon that is believed to occur in the presynaptic terminal, and alterations in PPF are one widely used method of testing whether alterations of synaptic transmission are presynaptic in locus (Zucker 1989; Neher 1998). We recorded PPF ratio of second to first EPSC amplitudes (100 ms interstimulus interval) from 8 pyramidal cells before and during bath application of GLYX-13 (1 µM). As illustrated in figure 3D, PPFs were quite stable and not altered over the period of bath application of GLYX-13, consistent with modulation of NMDAR EPSCs being a direct postsynaptic action on NMDARs.
3.3. GLYX-13 exhibits frequency-dependent modulation of NMDAR currents and Ca2+ influx
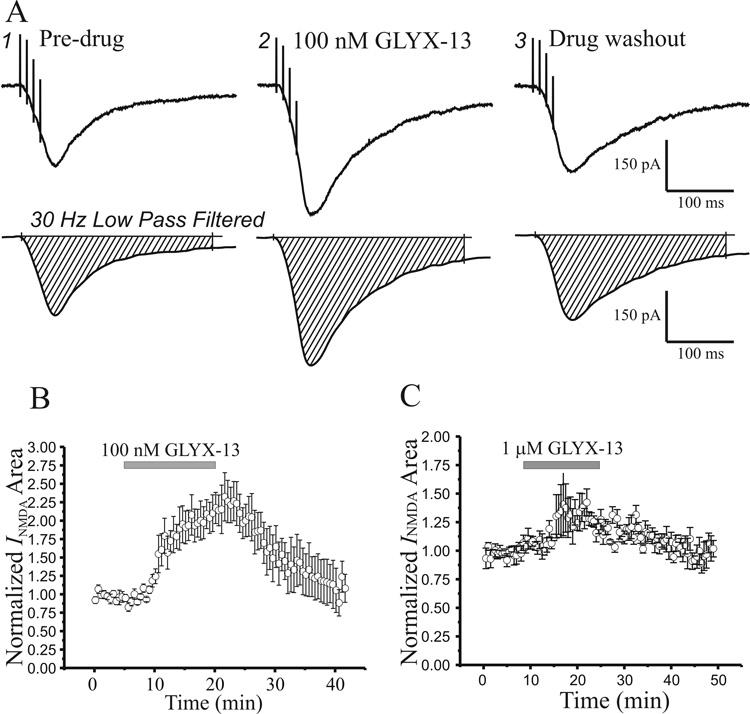
Many studies have found that the direction and magnitude of activity-dependent alterations in synaptic strength depends on both stimulus frequency and train duration. Since GLYX-13 exhibits the ability to simultaneously suppress the induction of LTD by low frequency stimulation, and enhance that of LTP by high frequency trains, we tested the effects of GLYX-13 on NMDAR-gated currents evoked by brief, high frequency bursts of stimuli (4 pulses/100 Hz, Fig 4A, top row). Because of the slow dynamics of NMDAR currents, we evaluated the change of NMDAR current area after 30 Hz low-pass filtering of the data to remove the much faster stimulus artifacts (Fig 4A, bottom row). In contrast to GLYX-13’s bidirectional effects on single-shock evoked NMDAR EPSCs, both 100 nM (Fig 4B) and 1 µM (Fig 4C) GLYX-13 increased burst-activated NMDAR current by 119 ± 21% (n=7) and 32.7 ± 8.3% (n=6), respectively, compared to pre-GLYX areas. Normalized NMDAR current areas were significantly enhanced by both concentrations of GLYX-13 (Fig 4B & C, n=6, P<0.05, ANOVA for Repeated Measures with Bonferroni correction for multiple comparisons).
Fig. 4.
GLYX-13 produces an enhancement of NMDA receptor-mediated summed EPSCs evoked by high-frequency burst stimulation. A: Sample EPSCs (top row) before, during application and 30 min after washout of GLYX-13 (100 nM) evoked by a burst of 4 Schaffer collateral stimuli given at a frequency of 100 Hz, and low-pass (30Hz) filtered EPSCs to eliminate stimulus artifacts for measurement (bottom row) of total NMDA current from CA1 pyramidal neurons in slices bathed in CNQX (20 µM) plus bicuculline (10 µM) in Mg2+-free ACSF. B: Time course of the effect of GLYX-13 (100 nM; grey bar) on total burst-induced NMDA EPSC area in normal ACSF (n=8). Each point is the mean ± SEM of EPSC peak amplitude normalized to starting amplitude in n cells. C: Time course of the effect of GLYX-13 (1 µM; grey bar; n=8) on burst-induced NMDA EPSC area in normal ACSF. Each point is the mean ± SEM of EPSC peak amplitude normalized to starting amplitude in n cells.
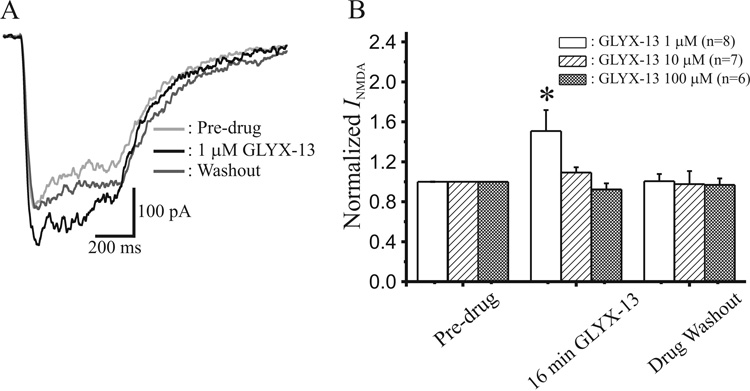
Examination of the effect of GLYX-13 on pharmacologically-isolated NMDAR currents induced by the same high-frequency Schaffer collateral stimulus train that we used to elicit LTP (100 Hz/500 msec) is shown in Fig 5. Similar to our findings with shorter high-frequency bursts, 1 µM GLYX-13 produced a significant 51 ± 21% (n=8) increase in NMDAR current, but 10 and 100µM concentrations did not significant alter evoked NMDAR currents (Fig 5B), confirming the low-dose selective enhancement of burst-induced NMDAR conductance by GLYX-13.
Fig. 5.
GLYX-13 also enhances NMDA receptor-gated conductances during longer high-frequency stimulus bursts of the type that elicits LTP. A: Sample low-pass (30Hz) filtered NMDAR-mediated EPSCs evoked by 100Hz/0.5sec bursts of Schaffer collateral stimulation in CA1 pyramidal neurons before (Ctl), during 16 min application of GLYX-13 (1 µM) and 30 min after washout. B: Mean ± SEM normalized current area of NMDAR EPSCs evoked by 50Hz/0.5sec Schaffer collateral stimulus trains before (Pre-drug), after 16 min application of GLYX-13 (16 min GLYX-13), and 30 min after drug washout, where 1 µM GLYX-13 significantly enhanced NMDAR EPSCs evoked by prolonged high-frequency trains of stimuli (*, P<0.05, paired t-test, n=# slices).
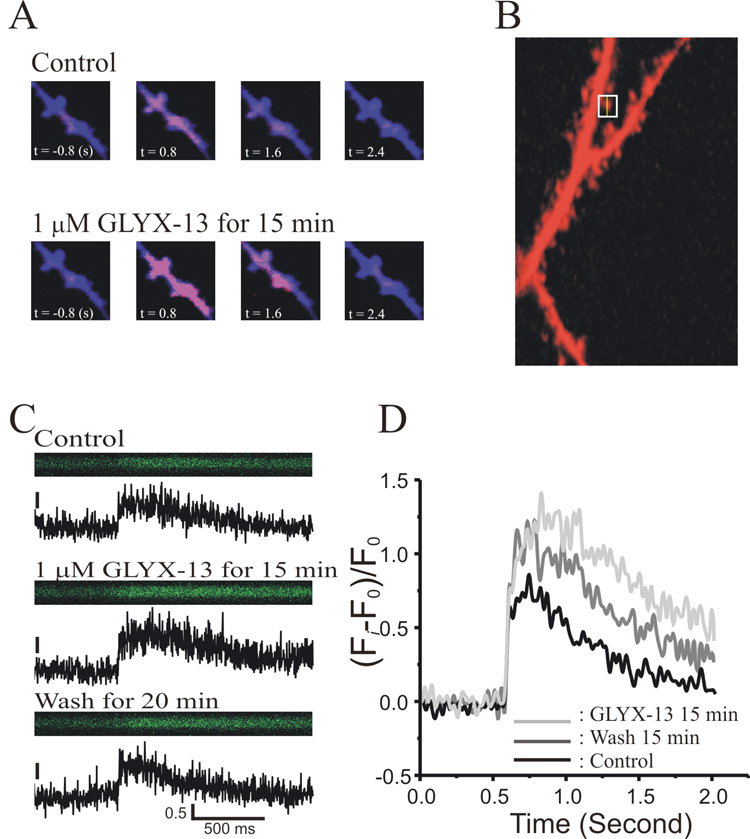
Since NMDAR-gated increases in postsynaptic dendritic spine [Ca2+] are necessary for the induction of both LTD and LTP (Lynch et al., 1983; Mulkey and Malenka, 1992; Neveu and Zucker, 1996), we used two-photon microscopy and the fluorescent indicator Calcium Green to directly image NMDAR-mediated [Ca2+] increases in local dendrites and spines evoked by each burst of 4 pulses given at a frequency of 100 Hz, applied once every 30 s. As illustrated in figure 6A, a 4-pulse burst induced NMDAR-mediated transient [Ca2+] increases lasting a few seconds. These transient [Ca2+] increases measured in dendritic spines (Fig 6B, line scan) were enhanced by bath application of 1 µM GLYX-13 (Fig 6C,D). Mean integral of these [Ca2+] signals (averages of 5–7 scans in each condition) were: pre-drug baseline = 0.65 ± 0.06, after 15–20 min bath application of GLYX-13 = 1.18 ± 0.05; and after 20 min drug-free wash = 0.88 ± 0.08, indicating that GLYX-13 significantly and reversibly potentiated NMDAR-mediated spine [Ca2+] increases induced by high frequency bursts of synaptic activation (n=9, F(2,16)=30.4, P<0.0001, ANOVA for Repeated Measures).
Fig. 6.
GLYX-13 enhances burst-induced NMDA receptor-dependent Ca2+ influx into individual dendritic spines of CA1 pyramidal neurons. A: Sample two-photon image of the increase in [Ca2+] in dendritic spines of a typical CA1 pyramidal neuron, measured with the indicator Calcium Green, in normal ACSF (top row) versus in GLYX-13 (1 µM; bottom row). B: Sample two-photon image of a CA1 pyramidal neuron dendrite using Alexafluor 594. The cross-section scanned in panel c is indicated in the box. C: Two-photon line scans of [Ca2+] in the single dendritic spine shown in b, in normal ACSF (Control), after 15 min in GLYX-13 (1 µM GLYX-13 for 15’), and 20 min after drug washout (Wash for 20’). D: Signal-averages of 5–7 line scans from a single dendritic spine of a CA1 pyramidal neuron in normal ACSF (Control), after 15 min in GLYX-13 (1 µM; GLYX-13 15’), and after 15 min washout (Wash for 15’).
3.4. Effects of GLYX-13 on NMDAR currents are due to actions at a D-serine sensitive binding site
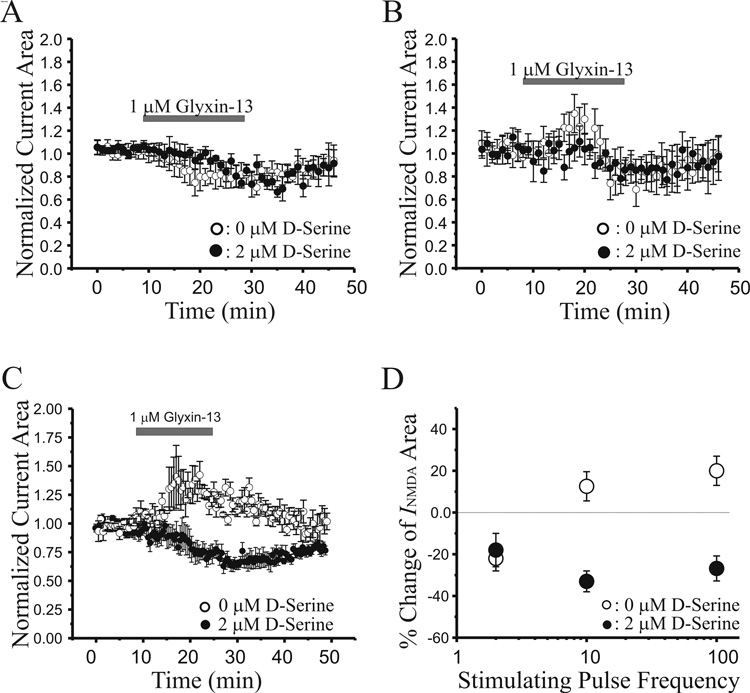
To test the hypothesis that GLYX-13 regulates NMDAR-mediated currents by acting on the glycine co-agonist site, we recorded NMDAR currents evoked by four pulses at different frequencies, while perfusing slices with 1 µM GLYX-13 in ACSF containing either 0 or 2 µM D-serine. GLYX-13 significantly reduced NMDAR currents from 0.98 ± 0.03 to 0.78 ± 0.06 (Fig 7A, n=6, P<0.05, paired t-test) in response to the first pulse of four applied at 2 Hz, the frequency we used to induce LTD. Although the decrease in NMDAR currents produced by 1 µM GLYX-13 was delayed in the presence of 2 µM D-serine, NMDAR current area was eventually lowered to the same level as in its absence (Fig 7A, 0.82 ± 0.08 vs 0.78 ± 0.06, P>0.1, paired t-test). As shown in figure 7B, GLYX-13 had biphasic effects on NMDAR currents evoked by the last stimulus of the 2 Hz burst, where current area initially increased from 1.02 ± 0.02 to 1.32 ± 0.19 (n=8, P<0.05, paired t-test), and later was reduced to 0.78 ± 0.08 (P<0.05, paired t-test). In contrast, NMDAR currents were monophasically reduced by 1 µM GLYX-13 in the presence of 2 µM D-serine (0.81 ± 0.06, n=9, P<0.05, paired t-test).
Fig. 7.
GLYX-13 selectively enhances NMDA receptor-gated conductances during later pulses in a short high-frequency stimulus burst. A: Time course of the effect of GLYX-13 (1 µM; grey bar) on the first pulse of a 4 stimulus, 2 Hz train, in normal ACSF (open circles; n=8) and in presence of D-serine (2 µM; filled circles; n=8). B: Time course of the effect of GLYX-13 (1 µM; grey bar) on the fourth pulse of a 4 stimulus, 2 Hz train, in normal ACSF (open circles; n=8) and in presence of D-serine (2 µM; filled circles; n=9). C: Time course of the effect of GLYX-13 (1 µM; grey bar) on the NMDA EPSC area evoked by a 4 stimulus, 100 Hz burst, in normal ACSF (open circles; n=8) and in presence of D-serine (2 µM; filled circles; n=8). D: Mean ± SEM percent change in the NMDA EPSC elicited by GLYX-13 (1 µM) as a function of Schaffer collateral stimulus frequency, in normal ACSF (open circles; n=8) and in D-serine (2 µM; filled circles; n=8).
The effect of GLYX-13 on NMDAR currents evoked by high frequency burst stimulation was quite different from its effect on low frequency responses. GLYX-13 (1 µM) applied in the absence of D-serine produced a marked increase in the NMDAR current area evoked by bursts of 4 stimuli applied at a frequency of 100 Hz (Fig 7C, 34 ± 8% increase, n=7, P<0.05, paired t-test). Co-application of GLYX-13 plus 2 µM D-serine completely occluded the potentiation of NMDAR currents by GLYX-13, converting it to a reduction similar in amplitude to that observed with low frequency synaptic activation (Fig 7C, 26 ± 5%, n=9, P<0.05, paired t-test). This effect of D-serine is consistent with the conclusion that the partial agonist GLYX-13 exhibits antagonist properties when competing with a saturating concentration of the full agonist, D-serine.
Figure 7D summarizes the frequency-dependent actions of GLYX-13, where low frequency evoked NMDAR-dependent currents were significantly reduced in amplitude, while those elicited by high frequency activation were enhanced. A significant interaction between D-Serine concentration and stimulus frequency was observed with Univariate ANOVA (F(2,31)=9.4, P<0.001), indicating that modulation of NMDAR-mediated currents by GLYX-13 depended on both synaptic stimulus frequency and extracellular D-Serine concentration.
3.5. Extrasynaptic NR2B-containing NMDA receptors are the likely targets of GLYX-13 responsible for potentiation of NMDAR currents
Glutamate released by low-frequency synaptic activation normally activates a subset of NMDARs termed “synaptic”, while higher-frequency activity is necessary to produce sufficient spillover of glutamate to activate an additional pool of receptors termed “extrasynaptic” (Asztely et al., 1997; Harris and Pettit, 2007). Previous studies suggest that the extrasynaptic pool of NMDARs may selectively include receptors containing NR2B subunits (Tovar and Westbrook, 1999; Brickley et al., 2003, but see also Thomas et al., 2006). Since GLYX-13 produces opposing actions on NMDAR-mediated currents, depending upon frequency of activation, we hypothesized that GLYX-13 suppression of low-frequency NMDAR current might be due to an action on synaptic NMDARs that lack NR2B, while its potentiation of high-frequency evoked NMDAR current might result from action on extrasynaptic NMDARs containing NR2B subunits.
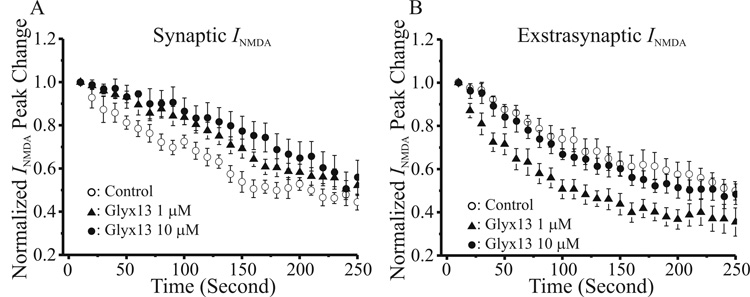
To test this hypothesis, we utilized the activity-dependent open-channel NMDAR blocker MK-801 to measure the effects of GLYX-13 on rates of onset of stimulus-evoked blockade of synaptic versus extrasynaptic NMDAR-gated channels. Figure 8A illustrates the rate of onset of MK-801 blockade of pharmacologically-isolated NMDAR-mediated EPSCs in CA1 pyramidal neurons evoked by single Schaffer collateral stimuli applied once each 10 sec. Both 1 µM and 10 µM GLYX-13, bath applied along with MK-801 prior to the start of stimulation, significantly slowed the rate of onset of blockade, consistent with a reduction in channel openings of synaptic NMDARs. Once the MK-801 blockade of single-shock evoked EPSCs had plateaued, indicating that all synaptic NMDARs activated by single stimuli are blocked, remaining extrasynaptic NMDARs were activated by applying 4 pulse/100 Hz bursts of Schaffer collateral stimulation once each 10 sec. In contrast to synaptic NMDARs, the rate of blockade by MK-801 of extrasynaptic EPSCs evoked by stimulus bursts was significantly enhanced by 1 µM GLYX-13, as shown in Fig 8B. These data suggest that extrasynaptic NMDARs may be the target of GLYX-13 that underlie enhancement of burst-evoked NMDAR-mediated current, and enhancement of LTP, while synaptic NMDARs are the target underlying the reduction of LTD.
Fig. 8.
GLYX-13 reduces openings of synaptic NMDA receptors, while increasing openings of extrasynaptic NMDA receptors. A: Time course of the blockade of NMDA receptor-dependent single shock-evoked synaptic EPSCs by the open channel blocker MK-801 (4 µM) in control slices (open circles), versus slices in the presence of 1 µM (filled triangles) or 10 µM (filled circles) GLYX-13. Each point is the mean ± SEM of EPSC peak amplitude normalized to starting amplitude in n cells. B: Time course of the blockade of burst-evoked (4 pulses/100Hz) extrasynaptic NMDA receptor-dependent EPSCs by MK-801 (4 µM), elicited once synaptic NMDA channel block had plateaued, in control slices (open circles), versus slices in the presence of 1 µM (filled triangles) or 10 µM (filled circles) GLYX-13. Each point is the mean ± SEM of EPSC peak amplitude normalized to starting amplitude in n cells.
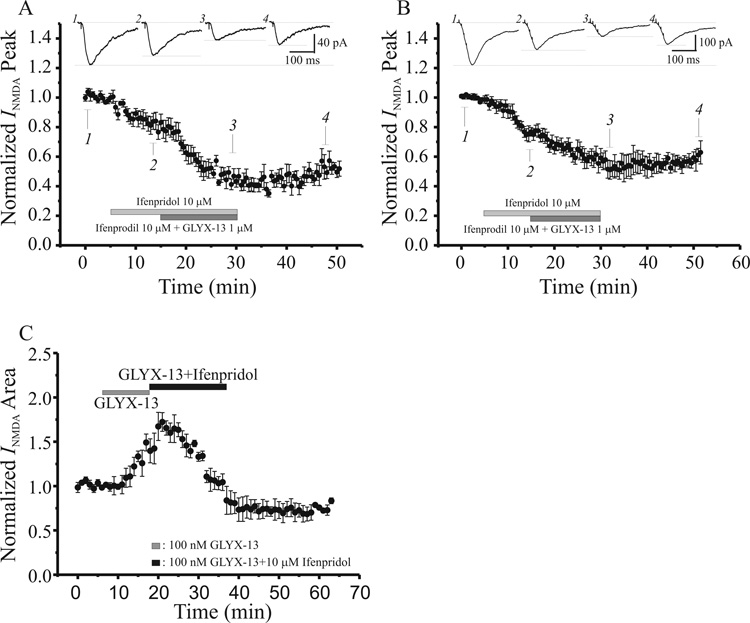
To test the hypothesis that it is NMDARs containing NR2B subunits that might be the targets of GLYX-13 specifically responsible for enhancing burst-induced Ca2+ influx and LTP, we utilized the NR2B-selective NMDAR antagonist ifenprodil. Figure 9 illustrates the effects of GLYX-13 (1 µM) applied in the presence of ifenprodil (10 µM). The reduction in single-shock evoked NMDAR EPSCs produced by GLYX-13 alone at this concentration (see Fig 3B) was not affected by co-application of ifenprodil (Fig 9A), suggesting that this reduction is mediated by actions on NMDARs that lack NR2B subunits. In contrast, the enhancement of burst-evoked NMDAR EPSCs produced by 100nM-1µM GLYX-13 (see Fig 4B and C), was converted by ifenprodil into a reduction in NMDAR currents (Fig 9B and C), supporting the hypothesis that GLYX-13 produces enhancement of NMDAR-mediated conductance by a selective action on the glycine site of extrasynaptically enriched NMDARs that contain NR2B subunits.
Fig. 9.
GLYX-13 enhances NMDA receptor-gated extrasynaptic conductances by acting on NR2B-containing NMDA receptors, while its suppression of synaptic NMDA currents is NR2Bindependent. A: Time course of the effect of GLYX-13 (1 µM; dark bar) on NMDA receptor-dependent single shock-evoked EPSCs in the presence of the NR2B-selective NMDA receptor antagonist ifenprodil (10 µM; light bar). The suppression of single-shock evoked NMDA currents by GLYX-13 is not affected by blockade of NR2B-containing NMDA receptors. (Insets are single shock-evoked NMDAR EPSCs at the indicated times) B: Time course of the effect of GLYX-13 (1 µM; dark bar) on NMDA receptor-dependent burst-evoked EPSCs in the presence of the NR2B-selective NMDA receptor antagonist ifenprodil (10 µM; light bar). The enhancement of burst-evoked NMDA EPSCs normal elicited by GLYX-13 is converted to a depression by blockade of NR2B-containiong NMDA receptors. (Insets are burst-evoked NMDAR EPSCs at the indicated times)
3.6. D-cycloserine has different effects on LTP and LTD than GLYX-13
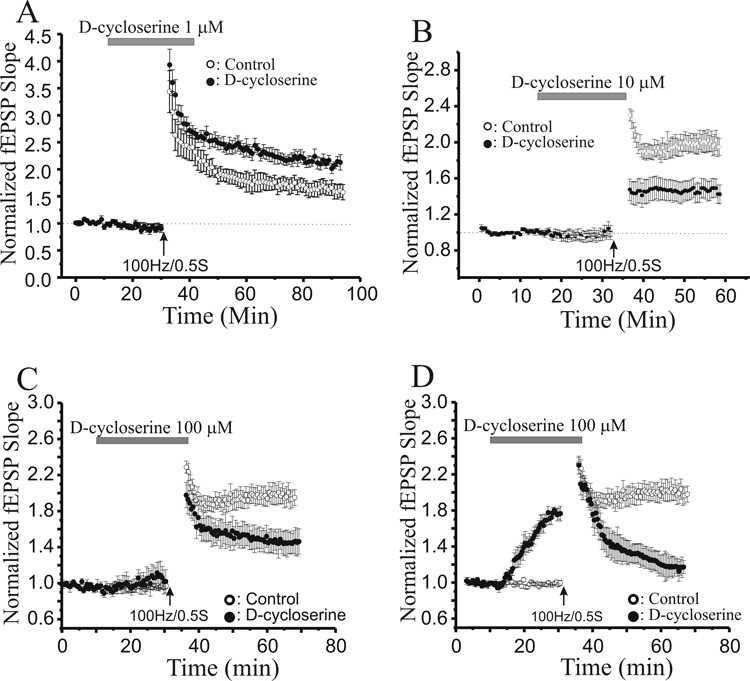
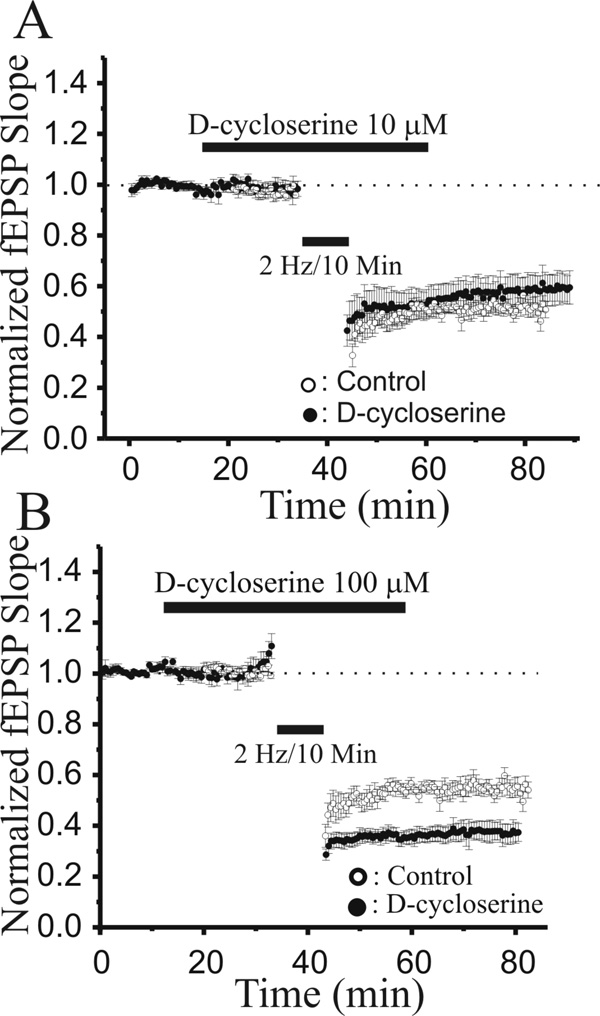
We compared the actions of GLYX-13 to those of another glycine site partial agonist, D-cycloserine (DCS). Bath application of a low concentration of DCS (1 µM) to hippocampal slices approximately doubled the amplitude of LTP induced by a sub-maximal tetanic Schaffer collateral stimulation (3×100Hz/500ms; Fig 10A, filled circles, n=10; P<0.05, Student’s t-test compared to control LTP). In contrast, a ten-fold higher concentration of DCS (10 µM) decreased the amplitude of LTP (Fig 10B, filled circles, n=8; P<0.05, Student’s t-test compared to control LTP), but did not significantly alter the magnitude of LTD induced by a low-frequency stimulus train (2 Hz/10 min; Fig 11A, filled circles, n=10). The behavior of LTP in slices treated with a 100 µM concentration of DCS fell into two groups. Half of slices exhibited no significant change in synaptic strength produced by 100 µM DCS alone, but when tetanic stimulation was applied, they showed a marked reduction in the amplitude of LTP (Fig 10C, filled circles, n=4). The other half of slices showed a marked increase in Schaffer collateral-evoked fEPSP amplitudes elicited by 100 µM DCS alone. In these slices, the same tetanic stimulation now elicited a frank depotentiation of synaptic strength to pre-LTP and pre-DCS amplitudes (Fig 10D, filled circles, n=5). This 100 µM concentration of DCS also produced a marked enhancement of the magnitude of LTD elicited by LFS of Schaffer collateral axons (Fig 11B, filled circles, n=8).
Fig. 10.
A low concentration of D-cycloserine (DCS, 1µM) enhances the magnitude of LTP, while a ten and hundred-fold higher concentrations of reduce LTP. A: Time course of the effect of 1 µM DCS on LTP induced by high frequency Schaffer collateral stimulus trains (3×100Hz/200ms; arrow) in slices pre-treated with DCS (1 µM; grey bar; filled circles; n=10), compared to control, untreated slices (open circles; n=8). B: Time course of the effect of 10 µM DCS on LTP induced by high frequency Schaffer collateral stimulus trains (3×100Hz/500ms; arrow) in slices pre-treated with DCS (10 µM; grey bar; filled circles; n=9), compared to control, untreated slices (open circles; n=8). C: Time course of the effect of 100 µM DCS on LTP induced by high frequency Schaffer collateral stimulus trains (3×100Hz/500ms; arrow) in slices where pre-treatment with DCS (100 µM; grey bar; filled circles; n=4) produced no effect alone, compared to control, untreated slices (open circles; n=8). D: Time course of the effect of 100 µM DCS on LTP induced by high frequency Schaffer collateral stimulus trains (3×100Hz/500ms; arrow) in slices where pre-treatment with DCS (100 µM; grey bar; filled circles; n=5) produced marked potentiation on its own, compared to control, untreated slices (open circles; n=8). (Each point is the mean ± SEM of normalized field e.p.s.p. slope of n slices.)
Fig. 11.
10 µM DCS did not alter the magnitude of LTD, while 100µM DCS enhanced LTD. A: Time course of the effect of 100 µM DCS on LTD induced by a low frequency stimulus train (2Hz/10min; solid bar) at Schaffer collateral-CA1 synapses in slices pre-treated with DCS (10 µM; grey bar; filled circles; n=10), compared to control, untreated slices (open circles; n=7). B: Time course of the effect of 100 µM DCS on LTD induced by a low frequency stimulus train (2Hz/10min; solid bar) at Schaffer collateral-CA1 synapses in slices pre-treated with DCS (100 µM; filled circles; n=8), compared to control, untreated slices (open circles; n=7). (Each point is the mean ± SEM of normalized field e.p.s.p. slope of n slices.)
4. Discussion
Previous studies suggest that the NMDAR glycine binding site could be an effective therapeutic target for nootropic agents, since it is an obligatory co-agonist site unique to NMDARs. D-cycloserine (DCS) is a glycine-site partial agonist which has been found to rescue NMDAR transmission in aged rats (Billard and Rouaud, 2007), facilitate learning acquisition in young adult rats (Thompson et al., 1992; Thompson and Disterhoft, 1997;), and enhance the induction of both LTP and LTD of synaptic transmission in the hippocampus (Rouaud and Billard, 2003; Billard and Rouaud, 2007). However, these observations have not consistently translated into positive cognitive outcomes in clinical trials (Laake and Oeksengaard, 2002; Duncan et al., 2007; but see Schwartz et al., 1996;Tsai et al., 1999).
Our previous studies have demonstrated that GLYX-13, like the antibody from which it was derived (Moskal et al., 2001), interacts with the glycine site on the NMDA receptor. GLYX-13 increases binding of MK-801 to rat brain homogenates, enhances NMDA receptor-mediated conductance (but to a lesser extent than glycine) in an oocyte expression system, and this action of GLYX-13 on NMDAR conductance is occluded by glycine and blocked by the glycine site antagonist 7-chlorokynurenic acid, all indicative of a partial agonist action on the glycine binding site (Moskal et al., 2005).
In the current study, GLYX-13 simultaneously enhanced the magnitude of LTP of Schaffer collateral-CA1 synaptic transmission, while reducing LTD. This is consistent with our observations that GLYX-13 reduced NMDAR-mediated synaptic currents in CA1 pyramidal neurons evoked by low-frequency Schaffer collateral stimulation in a frequency range that induced LTD, but enhanced NMDAR currents during high-frequency bursts of activity in the frequency range that elicited LTP. Direct two-photon imaging of Schaffer collateral burst-evoked increases in [Ca2+] in individual dendritic spines confirmed directly that GLYX-13 enhanced burst-induced NMDAR-dependent spine Ca2+ influx. The fact that GLYX-13 differentially affects NMDAR-gated conductance elicited by low and high frequency stimulation suggests there is activity-dependent release of endogenous glycine site ligands that compete with GLYX-13. Our previous study (Haring et al., 1991) with the antibody B6B21, from which GLYX-13 was derived, also demonstrated partial agonist activity at the glycine site, leading us to suggest then that the NMDA receptor glycine site is not saturated in vivo. Indeed, Nilsson et al. (1997) showed that systemic administration of glycine or D-serine can reduce MK-801 induced hyperactivity, consistent with the idea that further glycine-site activation of NMDARs is possible.
The pattern of actions of GLYX-13 was that low and intermediate concentrations (100 nM-10 µM) selectively enhanced LTP and, at 1 µM, suppressed LTD, while a higher concentration (100 µM) antagonized LTP and no longer altered LTD. In significant contrast, DCS at a low concentration (1 µM) enhanced the magnitude of LTP without affecting LTD, while higher DCS concentrations (10–100 µM) produced marked impairments in LTP, and 100 µM DCS did so while enhancing LTD. These patterns of action of DCS and GLYX-13 are quite different, though both compounds behave pharmacologically like partial agonists acting at the NMDAR glycine binding site. These differences may be only partially explained by postulating that GLYX-13 has higher agonist potency than DCS. Hrabetova et al. (2000) found that compounds that selectively block activation of NMDARs containing NR2A/B subunits show substantial selectivity for blocking LTP over LTD. Our data suggest that GLYX-13 has greater selectivity for NR2B subunit-containing NMDARs than DCS, and that this could explain its selective enhancement of LTP. Because DCS and GLYX-13 are structurally dissimilar, upon binding to the glycine site each may induce different conformational states of NMDARs (Erreger et al., 2005; Ianobe et al., 2005), resulting in different effects on NMDAR function. Physiological regulation of both LTP and LTD via the glycine site is likely to be important to learning and memory, and our data suggest unexplored therapeutic potential for new partial agonists at the glycine site to modulate cognitive function, promote learning and memory formation.
A growing number of studies suggest that both LTP and LTD of synaptic strength may play important roles in learning and long-term memory storage (Diamond et al., 2005; Kemp and Manahan-Vaughan, 2007). The basic concept of the Hebbian synapse posited that the selective strengthening of particular synaptic connections would enhance the likelihood of them being reactivated when a similar sensory input was supplied, leading to recall and re-experiencing of the stored pattern (Hebb, 1947). In this context, LTD of synaptic strength could serve to both balance overall synaptic strengths and to maximize the differences between strengthened patterns and background synaptic efficacy (Stanton and Sejnowski, 1989; Stanton, 1996). Thus, there is reason to hypothesize that simultaneous enhancement of the induction of LTP and suppression of LTD could be a potent nootropic mechanism, provided the overall stability of excitable neural networks is not compromised. A compound such as GLYX-13, that acts at a co-agonist binding site to modulate actions of glutamate and possesses non-linear effects on synaptic plasticity, could be an ideal nootropic agent, especially considering the recent evidence that the function of this binding site and NMDAR-dependent synaptic plasticity may be compromised during aging (Billard and Rouaud, 2007).
While the present study addresses only the effects of GLYX-13 on Schaffer collateral-CA1 synapses in the hippocampus, there are larger implications for the functional roles of both hippocampal and neocortical long-term plasticity. LTP and LTD in the hippocampal formation are believed to play important roles in promoting the acquisition and consolidation of long-term declarative, and particularly episodic, memories (Viskontas et al., 2000; Manns et al., 2003; Eichenbaum and Fortin, 2005). GLYX-13 could act on this structure to improve its function in memory storage. At the same time, it is also thought that long-lasting changes in synaptic strength in distributed cortical networks represent actual memories (Rioult-Pedotti et al., 2000;Martin and Morris, 2002), and GLYX-13 could simultaneously enhance the strength of memories by combining actions on hippocampal gating with direct effects on synapses actually storing the information.
In addition to the glycine site-dependent modulation by GLYX-13 of synaptic plasticity we outline here, we have previously shown that GLYX-13 has neuroprotective properties in vivo against stroke-induced delayed death of hippocampal CA1 pyramidal cells. In a carotid artery occlusion model in gerbils, intracerebroventricular administration of GLYX-13 1 hr prior to onset of stroke markedly reduced the magnitude of delayed death of CA1 pyramidal neurons 24 and 48 hr post-ischemia (Potter et al., 2002). It is fascinating to observe that an NMDAR modulator that promotes the induction of LTP can also be neuroprotective. This may not be a surprising property for a partial agonist which may enhance physiological NMDA receptor-mediated Ca2+ influx, but shift to acting as an antagonist under ischemic conditions, where release of both endogenous glutamate and glycine site agonists is certain to be much higher. However, studies by Liu et al. (2007) suggest that subunit identity may also be a relevant factor, since they found that activation of NMDA receptors containing NR2B subunits can promote excitotoxic neuronal damage, while those containing NR2A subunits promoted neuroprotection. Taken together, the properties of GLYX-13 suggest there is substantial therapeutic potential for compounds that can selectively modulate NMDA receptors containing particular subunits, and that partial agonists that selectively interact with glycine sites on NR2B subunits may simultaneously promote synaptic plasticity and confer neuronal resistance to excitoxicity.
Acknowledgements
This work is dedicated to the memories of Lewis N. Stanton, Sr., Gary L. Stanton and John M. Sarvey, and was supported in part by NIH grants R01-NS44421 (P.K.S.) and R43-MH071932 (J.R.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhondzadeh S. Hippocampal synaptic plasticity and cognition. J. Clin. Pharm. Ther. 1999;24:241–248. doi: 10.1046/j.1365-2710.1999.00231.x. [DOI] [PubMed] [Google Scholar]
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- Billard JM, Rouaud E. Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by D-cycloserine. Eur. J. Neurosci. 2007;25:2260–2268. doi: 10.1111/j.1460-9568.2007.05488.x. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG. NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J. Neurosci. 2003;23:4958–4966. doi: 10.1523/JNEUROSCI.23-12-04958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J. Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Campbell AM, Woodson JC. Competitive interactions between endogenous LTD and LTP in the hippocampus underlie the storage of emotional memories and stress-induced amnesia. Hippocampus. 2005;15:1006–1025. doi: 10.1002/hipo.20107. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Aydar E. Antagonism at the glycine site on the NMDA receptor reduces spinal nociception in the rat. Neurosci. Lett. 1991;121:263–266. doi: 10.1016/0304-3940(91)90700-4. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl. Acad. Sci. (U.S.A.) 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan EJ, Szilagyi S, Schwartz MP, Bugarski-Kirola D, Kunzova A, Negi S, Stephanides M, Efferen TR, Angrist B, Peselow E, Corwin J, Gonzenbach S, Rotrosen JP. Effects of D-cycloserine on negative symptoms in schizophrenia. Schizoph. Res. 2004;71:239–248. doi: 10.1016/j.schres.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Fortin NJ. Bridging the gap between brain and and behavior: cognitive and neural mechanisms of episodic memory. J. Exp. Anal. Behav. 2005;84:619–629. doi: 10.1901/jeab.2005.80-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Geballe MT, Dravid SM, Snyder JP, Wyllie DJ, Traynelis SF. Mechanism of partial agonism at NMDA receptors for a conformationally restricted glutamate analog. J. Neurosci. 2005;25:7858–7866. doi: 10.1523/JNEUROSCI.1613-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins AE, Amico E, Posever TA, Toker R, Goff DC. D-cycloserine added to risperidone in patients with primary negative symptoms of schizophrenia. Schizophrenia Res. 2002;56:19–23. doi: 10.1016/s0920-9964(01)00220-1. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJD, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J. Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Westbrook GL, Mayer ML. Modulation of excigtatory synaptic transmission by glycine and zinc in cultures of mouse hippocampal neurons. J. Neurosci. 1988;8:3733–3741. doi: 10.1523/JNEUROSCI.08-10-03733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring R, Stanton PK, Scheidler MA, Moskal JR. Glycine-like modulation of Nmethyl-D-asparate receptors by a monoclonal antibody that enhances long-term potentiation. J. Neurochem. 1991;57:323–332. doi: 10.1111/j.1471-4159.1991.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Recruiting extrasynaptic NMDA receptors augments synaptic signaling. J. Neurophysiol. 2007 Dec 5; doi: 10.1152/jn.01169.2007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. Wiley Press: NY; 1949. [Google Scholar]
- Heresco-Levy U, Ermilov M, Shimoni J, Shapira B, Silip G, Javitt DC. Placebo-controlled trail of D-cycloserine added to conventional neuroleptics, olanzapine, or risperidone in schizophrenia. Amer. J. Psych. 2002;159:480–482. doi: 10.1176/appi.ajp.159.3.480. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hrabetova S, Serrano P, Blace N, Tse HW, Skifter DA, Jane DE, Monaghan DT, Sacktor TC. Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J. Neurosci. 2000;20:RC81. doi: 10.1523/JNEUROSCI.20-12-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Zakharenko SS, Schoch S, Kaeser PS, Janz R, Sudhof TC, Siegelbaum SA, Kandel ER. Genetic evidence for a protein kinase-A presynaptic component in NMDA-receptor-dependent forms of long-term synaptic potentiation. Proc. Natl. Acad. Sci. (U.S.A.) 1992;102:9365–9370. doi: 10.1073/pnas.0503777102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianobe A, Furukawa H, Gouaux E. Mechanism of partial agonist action at the NR1 subunit of NMDA receptors. Neuron. 2005;47:71–84. doi: 10.1016/j.neuron.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature (London) 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kemp JA, Leeson PD. The glycine site of the NMDA receptor--five years on. Trends Pharmacol. Sci. 1993;14:20–25. doi: 10.1016/0165-6147(93)90108-v. [DOI] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine for activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F, et al. The role of mammalian ionotropic receptors in synaptic plasticity: LTP, LTD and epilepsy. Cell Mol. Life Sci. 2000;57:1551–1561. doi: 10.1007/PL00000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Erdemli G, et al. LTP of AMPA and NMDA receptor-mediated signals: evidence for presynaptic expression and extrasynaptic glutamate spill-over. Neuron. 1996;17:461–474. doi: 10.1016/s0896-6273(00)80178-6. [DOI] [PubMed] [Google Scholar]
- Laake K, Oeksengaard AR. D-cycloserine for Alzheimer's disease. Cochrane Database Syst. Rev. 2002;2 doi: 10.1002/14651858.CD003153. CD003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird JM, Mason GS, Webb J, Hill RG, Hargraves RJ. Effects of a partial agonist and a full antagonist acting at the glycine site of the NMDA receptor on inflammation-induced mechanical hyperalgesia in rats. Brit. J. Pharmacol. 1996;117:1487–1492. doi: 10.1111/j.1476-5381.1996.tb15311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic death both in vitro and in vivo. J. Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature (London) 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Squire LR. Semantic memory and the human hippocampus. Neuron. 2003;38:127–133. doi: 10.1016/s0896-6273(03)00146-6. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Morris RG. New life in an old idea: the synaptic plasticity and memory hypothesis revisited. Hippocampus. 2002;12:609–636. doi: 10.1002/hipo.10107. [DOI] [PubMed] [Google Scholar]
- Matsui T, Sekiguchi M, et al. Functional comparison of D-serine and glycine in rodents: the effect on cloned NMDA receptors and the extracellular concentration. J. Neurochem. 1995;65:454–458. doi: 10.1046/j.1471-4159.1995.65010454.x. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Benveniste M, Patneau DK, Vyklicky L., Jr Pharmacologic properties of NMDA receptors. Ann. Rev. NY Acad. Sci. 1992;648:194–204. doi: 10.1111/j.1749-6632.1992.tb24538.x. [DOI] [PubMed] [Google Scholar]
- Miserendino MJD, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature (London) 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Moskal JR, Kuo AG, Weiss C, Wood PL, Hanson AO, Kelso S, Harris RB, Disterhoft JF. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology. 2005;49:1077–1087. doi: 10.1016/j.neuropharm.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Moskal JR, Yamamoto H, et al. The use of antibody engineering to create novel drugs that target N-methyl-D-aspartate receptors. Curr. Drug Targets. 2001;2:331–345. doi: 10.2174/1389450013348399. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Neveu D, Zucker RS. Postsynaptic levels of [Ca2+]i needed to trigger LTD and LTP. Neuron. 1996;16:619–629. doi: 10.1016/s0896-6273(00)80081-1. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Carlsson A, Carlsson ML. Glycine and D-serine decrease MK-801-induced hyperactivity in mice. J. Neural Trans. 1997;104:1195–1205. doi: 10.1007/BF01294720. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr. Opin. Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Priestley T, Kemp JA. Agonist response kinetics of N-methyl-D-aspartate receptors in neurons cultured from rat cerebral cortex and cerebellum: evidence for receptor heterogeneity. Mol. Pharmacol. 1993;44:1252–1257. [PubMed] [Google Scholar]
- Prybylowski K, Wenthold RJ. N-methyl-D-aspartate receptors: subunit assembly and trafficking to the synapse. J. Biol. Chem. 2004;279:9673–9676. doi: 10.1074/jbc.R300029200. [DOI] [PubMed] [Google Scholar]
- Pussinen R, Nieminen S, Koivisto E, Haapalinna A, Riekkinen P, Sr, Sirvio J. Enhancement of intermediate-term memory by an alpha-1 agonist or a partial agonist at the glycine site of the NMDA receptor. Neurobiol. Learn. Mem. 1997;67:69–74. doi: 10.1006/nlme.1996.3738. [DOI] [PubMed] [Google Scholar]
- Quartaroli M, Fasdelli N, Gettelini L, Maraia G, Corsi M. GV196771A, an NMDA receptor/glycine site antagonist, attenuates mechanical allodynia in neuropathic rats and reduces tolerance induced by morphine in mice. Eur. J. Pharmacol. 2001;430:219–227. doi: 10.1016/s0014-2999(01)01278-x. [DOI] [PubMed] [Google Scholar]
- Riedel G, Reymann KG. Metabotropic glutamate receptors in hippocampal long-term potentiation and learning and memory. Acta Physiol. Scand. 1996;157:1–19. doi: 10.1046/j.1365-201X.1996.484231000.x. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rouaud E, Billard JM. D-cycloserine facilitates synaptic plasticity but impairs glutamatergic neurotransmission in rat hippocampal slices. Br. J. Pharmacol. 2003;140:1051–1056. doi: 10.1038/sj.bjp.0705541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BL, Hashtroudi S, Herting RL, Schwartz P, Deutsch SI. D-cycloserine enhances implicit memory in Alzheimer patients. Neurology. 1996;46:420–424. doi: 10.1212/wnl.46.2.420. [DOI] [PubMed] [Google Scholar]
- Stanton PK. LTD, LTP, and the sliding threshold for long-term synaptic plasticity. Hippocampus. 1996;6:35–42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM, Moskal JR. Inhibition of the production and maintenance of long-term potentiation in rat hippocampal slices by a monoclonal antibody. Proc. Natl. Acad. Sci. (U.S.A.) 1987;84:1684–1688. doi: 10.1073/pnas.84.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sejnowski TJ. Associative long-term depression in the hippocampus induced by hebbian covariance. Nature (London) 1989;339:215–218. doi: 10.1038/339215a0. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Winterer J, Bailey CP, Kyrozis A, Raginov I, Laube G, Veh RW, Nguyen CQ, Müller W. Long-term depression of presynaptic release from the readily-releasable vesicle pool induced by NMDA receptor-dependent retrograde nitric oxide. J. Neurosci. 2003;23:5936–5944. doi: 10.1523/JNEUROSCI.23-13-05936.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Winterer J, Zhang XL, Müller W. Imaging LTP of presynaptic release of FM1–43 from the rapidly-recycling vesicle pool of Schafffer collateral-CA1 synapses in rat hippocampal slices. Eur. J. Neurosci. 2005;22:2451–2461. doi: 10.1111/j.1460-9568.2005.04437.x. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Moskal JR, Disterhoft JF. Hippocampus-dependent learning facilitated by a monoclonal antibody or D-cycloserine. Nature (London) 1992;359:638–641. doi: 10.1038/359638a0. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Disterhoft JF. Age- and dose-dependent facilitation of associative eyeblink conditioning by D-cycloserine in rabbits. Behav. Neurosci. 1997;111:1303–1312. doi: 10.1037//0735-7044.111.6.1303. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J. Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai GE, Falk WE, Gunther J, Coyle JT. Improved cognition in Alzheimer's disease with short-term D-cycloserine treatment. Am. J. Psychiatry. 1999;156:467–469. doi: 10.1176/ajp.156.3.467. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, McAndrews MP, Moscovitch M. Remote episodic memory deficits in patients with unilateral temporal lobe epilepsy and excisions. J. Neurosci. 2000;20:5853–5857. doi: 10.1523/JNEUROSCI.20-15-05853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh GC, Bonhaus DW, Nadler JV, McNamara JO. N-methyl-D-aspartate receptor plasticity in kindling: quantitative and qualitative alterations in the N-methyl-D-aspartate receptor-channel complex. Proc. Natl. Acad. Sci. (USA) 1989;86:8157–8160. doi: 10.1073/pnas.86.20.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Hawkins RD. Long-term depression: a learning-related type of synaptic plasticity in the mammalian central nervous system. Rev. Neurosci. 1995;6:259–277. doi: 10.1515/revneuro.1995.6.3.259. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Ann. Rev. Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]